Abstract
Ginsenoside Rb1 (GRb1) is a major component of ginseng, which has been shown to ameliorate hyperglycemia in rodents and in humans with undetermined mechanisms. Here, we analyzed the molecular mechanisms by which GRb1 reduces the insulin resistance in high-fat diet (HFD)-induced mouse model for type 2 diabetes (T2D). HFD was applied for 4 weeks to induce T2D in mice, after which GRb1 was administrated and the effects on the fasting blood glucose, glucose tolerance and insulin sensitivity were analyzed. We found that HFD increased fasting blood glucose, glucose tolerance and reduced insulin sensitivity, which were all ameliorated by GRb1. GRb1 seemed to reduce the levels of 11β-Hydroxysteroid dehydrogenase type I (11β-HSD1) in liver and adipose tissue, to exert its anti-diabetes effects. Overexpression of 11β-HSD1 completely abolished the effects of GRb1 on HFD-induced increases in fasting blood glucose and glucose tolerance, and decreases in insulin sensitivity. Together, our data suggest that GRb1 may increase insulin sensitivity through suppressing 11β-HSD1 in treatment of T2D.
Keywords: Ginsenoside Rb1 (GRb1), type 2 diabetes (T2D), 11β-Hydroxysteroid dehydrogenase type I (11β-HSD1), insulin resistance
Introduction
Failure to maintain a tightly regulated blood glucose level results in a metabolic disease, called diabetes [1]. Among all diabetes cases, the majority is type 2 diabetes (T2D), in which the insulin loses its potent effects in regulating blood glucose, mostly by impaired insulin production and secretion and induction of insulin resistance in peripheral tissue [2-4]. The prevalence of T2D has risen enormously over the last decades and the final solution is still unavailable despite great advances that have been made in the past.
Glucocorticoid, as an antagonist for insulin, regulates multiple metabolic processes including central obesity, insulin resistance and glucose intolerance. 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) catalyses conversion of inactive cortisone to active cortisol in adipose tissue to enhance local effects of glucocorticoid and thus to trigger glucocorticoid-related obesity and T2D [5-8]. Previous studies have shown that 11β-HSD-1 activity is significantly increased in adipose tissue of obese animals and obese humans [9-12]. Mice that overexpress 11β-HSD1 showed increases in local glucocorticoid levels, and features of the obesity-associated metabolic disorders, e.g. dyslipidemia, insulin resistance, and glucose intolerance [13]. On the other hand, 11β-HSD1-knockout mice produced reduced glucocorticoid in adipose tissues and exhibited enhanced insulin sensitivity. Thus, 11β-HSD1 levels are associated with obesity, glucose intolerance and insulin resistance.
Ginseng is widely used herb in many medical approaches and has been used in treating T2D [14]. Ginseng has been shown to ameliorate hyperglycemia in rodents [15-18] and in humans [19,20]. Ginsenoside Rb1 (GRb1) is a major component of ginseng, has been found to have therapeutic effects in treating obese and diabetes [21-23]. However, the molecular mechanisms underlying the effects of Ginseng and GRb1 in such occasions are unknown. Here, we analyzed the molecular mechanisms by which GRb1 reduces the insulin resistance in high-fat diet (HFD)-induced mouse model for type 2 diabetes (T2D).
Materials and methods
Mouse treatment
All animal experiments were performed according to the Institutional guidance for Care and Use of Laboratory Animals, and the experimental protocols were approved by the Ethics Committee for Experimental Research from the First Hospital affiliated to Jinzhou Medical University. Female C57BL/C mice of 12 weeks of age were purchased from the National Resource Center of Model Mice (Nanjing, China). Mice were housed in Pathogen-free environment. The animals were randomly divided into two groups: the normal-diet group (ND) and the high-fat diet (HFD) group. After 4 weeks of ND or HFD, the mice of HFD group were i.p. administrated with 10 mg/kg GRb1 (Weikeqi Bioscience, China) every other day for 1 week. The control mice received saline of same frequency and same volume. AAV injection was through tail vein and the dose is 108 viral particles in 100 µl.
Generation of AAVs
AAV-CMV-11β-HSD1-2A-GFP (simplified as AAV-11β-HSD1) and AAV-GFP were prepared as has been previously described [24]. Briefly, a pAAV-CMV-GFP plasmid (Clontech, Mountain View, CA, USA), a packaging plasmid carrying the serotype 8 rep and cap genes and a helper plasmid carrying the adenovirus helper functions (Applied Viromics, LLC. Fremont, CA, USA) were co-transfected the HEK293 cells for generating AAVs, using Lipofectamine 2000 reagent (Invitrogen). The virus purification was done with CsCl density centrifugation and titration was determined by a quantitative densitometric dot-blot assay.
Physiological assessments
Fasting blood glucose levels were measured using an Accu-Chek glucose meter (Roche, Indianapolis, IN, USA). For intraperitoneal glucose tolerance test (IPGTT), mice were fasted for 16 hours and injected with glucose (2 g/kg, i.p.). Blood glucose levels were measured at 15, 30, 60 and 120 minutes after injection. For insulin tolerance test, mice were fasted for 16 hours and injected with insulin (0.5 unit/kg, i.p.). Blood glucose levels were measured at 30, 60, 90 and 120 minutes after injection. For analysis of the rate of glycogen synthesis, rate of glycogen synthesis, 100 mg liver tissue was rinsed with cold phosphate-buffered saline (PBS) and solubilized by incubating with 1 mol/l KOH (0.5 ml) at 80°C for 30 min. After centrifugation, the supernatant was transferred to a new tube, 95% ethanol (550 µl) was added and the pellet was washed with ice-cold 95% ethanol and re-suspended with 300 µl ddH2O. Part of the solution (150 µl) was transferred in duplicate to scintillation vials, and 14C-radioactivity was counted. The activity per tissue mass was then normalized to the integral of the plasma specific activity of the 2-[14C] DG over the time of exposure, to yield a rate of incorporation into glycogen. For glucose up-take analysis, 100 mg skeletal mu-scle was added into 250 µl of 1N NaOH and incubated at 80°C for 30 min to digest the tissues. The solutions were neutralized with 250 µl of 1N HCl, and then centrifuged at 14,000 rpm for 15 min. An aliquot of the supernatant was precipitated by HClO4 and quantified by liquid scintillation counting to determine total tissue values (dpm) for the sum of 2-[14C] DG and 2-[14C] deoxyglucose phosphate (2-[14C] DGP). Another aliquot was deproteinized with 0.3N zinc sulfate (ZnSO4) and 0.3N barium hydroxide [Ba(OH)2] to precipitate 2-[14C] DG6P and quantify 2-[14C] DG in the supernatant. The value for the 2-[14C] DG in the supernatant (dpm) was subtracted from the total tissue 2-[14C] DG and 2-[14C] DGP (dpm) to calculate the glucose uptake rate as indicated by the skeletal muscle 2-[14C] DGP accumulation.
Immunohistochemistry
Mouse tissue were dissected out and fixed with 4% paraformaldehyde (Sigma-Aldrich) for 6 hours, and then cyro-protected in 30% sucrose for 24 hours. H&E staining was performed.
Western blot
Western blot was performed as previous described [24]. Primary antibodies for Western Blot are anti-11β-HSD1 and anti-β-actin (Cell Signaling, San Jose, CA, USA). Secondary antibody is HRP-conjugated anti-rabbit (Jackson ImmunoResearch Labs, West Grove, PA, USA). Images shown in the figure were representative from 5 repeats.
Flow cytometry
F4/80-based cell analysis was performed by flow cytometry, using dissociated adipose tissue cells that were labeled with PEcy7-conjugated anti-F4/80 antibodies (Becton-Dickinson Biosciences, San Jose, CA, USA). Flow cytometry was performed using a FACSAria (Becton-Dickinson Bioscien-ces) flow cytometer. Negative controls were applied to remove background noise and to confirm positive cells. Data were analyzed and quantified using Flowjo software (Flowjo LLC, Ashland, OR, USA).
Statistics
All values are depicted as mean ± standard deviation and are considered significant if P<0.05. All data were statistically analysed using one-way ANOVA with a Bonferroni correction, followed by Fisher’s Exact Test to compare 2 groups.
Results
Schematic of the experiments
Mice of 12 weeks of age were randomly divided into two groups: the normal-diet group (ND) and the highfat diet (HFD) group. After 4 weeks of ND or HFD, the mice of HFD group were administrated with GRb1 or control saline of same frequency and same volume, for one week before analysis (Figure 1).
Figure 1.
Schematic of the experiments. Mice of 12 weeks of age were randomly divided into two groups: the normal-diet group (ND) and the high-fat diet (HFD) group. After 4 weeks of ND or HFD, the mice of HFD group were administrated with GRb1 or control saline of same frequency and same volume, for one week before analysis.
GRb1 attenuates HFD-induced increases in fasting blood glucose, glucose tolerance and HFD-induced reduction in insulin sensitivity
We found that HFD increased fasting blood glucose (Figure 2A), glucose tolerance (Figure 2B) and reduced insulin sensitivity (Figure 2C), which were all ameliorated by GRb1 (Figure 2A-C). Thus, GRb1 attenuates HFD-induced increases in fasting blood glucose, glucose tolerance and HFD-induced reduction in insulin sensitivity.
Figure 2.
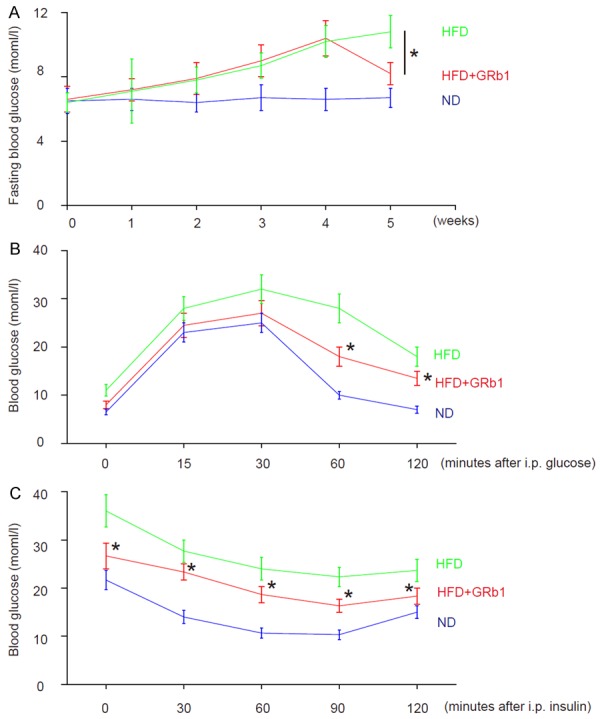
GRb1 attenuates HFD-induced increases in fasting blood glucose, glucose tolerance and HFD-induced reduction in insulin sensitivity. A. Fasting blood glucose. B. Glucose tolerance test. C. Insulin tolerance test. *P<0.05. N=10.
GRb1 reduces glucose production and increases glucose uptake
We found that GRb1 did not alter either food intake (Figure 3A) or body weight (Figure 3B), but significantly reduced glucose production in liver (Figure 3C), and significantly increased glucose uptake in skeletal muscle (Figure 3D). These data suggest that GRb1 may enhance insulin sensitivity in HFD-treated mice.
Figure 3.
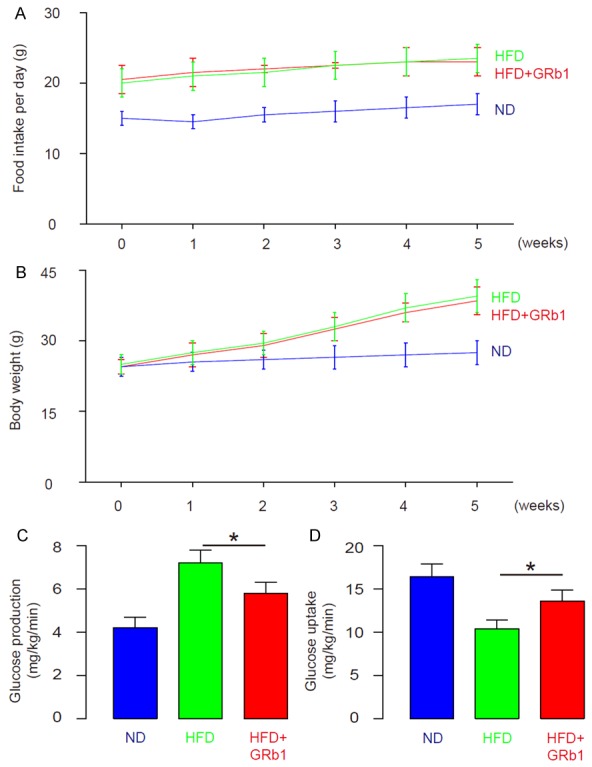
GRb1 reduces glucose production and increases glucose uptake. A. Food intake. B. Body weight. C. Glucose production in liver. D. Glucose uptake in skeletal muscle. *P<0.05. N=10.
GRb1 reduces visceral fat index in HFD-treated mice
We found that HFD significantly increased the visceral fat cell size (Figure 4A, 4B), and significantly increased the F4/80+ cells in the fat tissue (Figure 4C, 4D). However, GRb1 treatment significantly reduced the visceral fat cell size in HFD-treated mice (Figure 4A, 4B), and significantly decreased the F4/80+ cells in the fat tissue in HFD-treated mice (Figure 4C, 4D). Thus, GRb1 reduces visceral fat index in HFD-treated mice.
Figure 4.
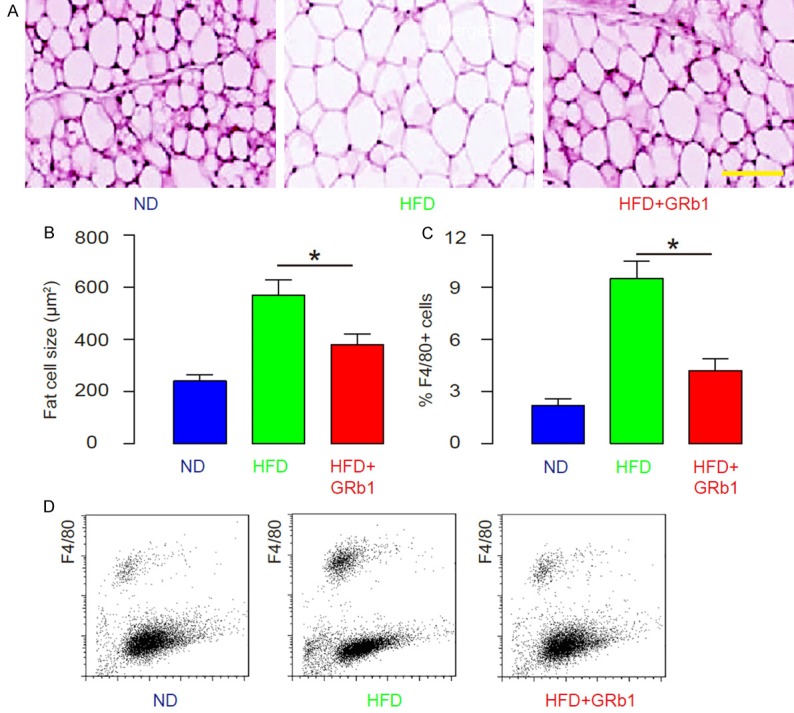
GRb1 reduces visceral fat index in HFD-treated mice. (A, B) The visceral fat cell size, by representative images (A), and by quantification (B). (C, D) The F4/80+ cells in the fat tissue analyzed by flow cytometry, shown by quantification (C), and by representative flow charts (D). *P<0.05. N=10. Scale bar is 50 µm.
GRb1 decreases 11β-HSD1 levels in HFD-treated mice
We found that 11β-HSD1 levels significantly increased in liver (Figure 5A) and in adipose tissue (Figure 5B) in HFD-treated mice. In order to examine the role of 11β-HSD1 in GRb1-treated HFD-mice, we generated AAV vectors that carry 11β-HSD1 and GFP, AAV-CMV-Pax4-2A-GFP (simplified as AAV-11β-HSD1). AAV-CMV-GFP (simplified as AAV-GFP) was used as a control (Figure 5C). The quality of AAV-11β-HSD1 was confirmed in Western blot (Figure 5D). Then, a single viral infusion through tail vein were given at the time of GRb1 (Figure 5E).
Figure 5.
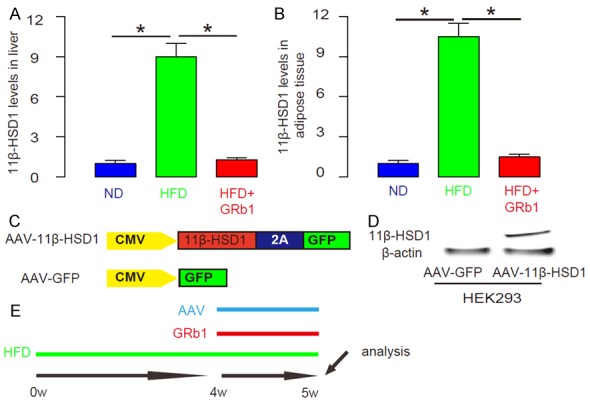
GRb1 decreases 11β-HSD1 levels in HFD-treated mice. (A, B) The Western blot for 11β-HSD1 levels in liver (A) and in adipose tissue (B) in HFD-treated mice. (C) AAV-CMV-Pax4-2A-GFP was generated to carry 11β-HSD1 and GFP, AAV-CMV-Pax4-2A-GFP (simplified as AAV-11β-HSD1). AAV-CMV-GFP was used as a control (simplified as AAV-GFP). (D) Western blot for AAV-11β-HSD1. (E) Schematic of the experiment. A single viral infusion through tail vein were given at the time of GRb1. *P<0.05. N=10.
Overexpression of 11β-HSD1 levels abolishes the effects of GRb1 on HFD-induced increases in fasting blood glucose, glucose tolerance and HFD-induced reduction in insulin sensitivity
We found that injection of AAV-11β-HSD1 completely abolished the effects of GRb1 on HFD-induced increases in fasting blood glucose (Figure 6A), glucose tolerance (Figure 6B) and HFD-induced decreases in insulin sensitivity (Figure 6C).
Figure 6.
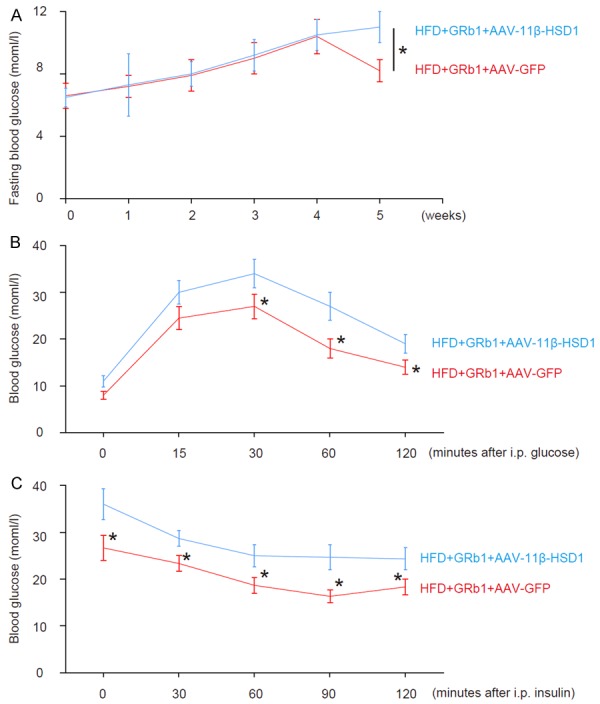
Overexpression of 11β-HSD1 levels abolishes the effects of GRb1 on HFD-induced increases in fasting blood glucose, glucose tolerance and HFD-induced reduction in insulin sensitivity. A. Fasting blood glucose. B. Glucose tolerance test. C. Insulin tolerance test. *P<0.05. N=10.
Overexpression of 11β-HSD1 levels abolishes the effects of GRb1 on glucose production, glucose uptake and visceral fat index
We found that injection of AAV-11β-HSD1 completely abolished the effects of GRb1 on HFD-induced increases in glucose production in liver (Figure 7A), on HFD-induced reduction in glucose uptake in skeletal muscle (Figure 7B), on visceral fat cell size (Figure 7C, 7D), and on F4/80+ cells in adipose tissue (Figure 7E, 7F). Together, our data suggest that GRb1 may increase insulin sensitivity through suppressing 11β-HSD1.
Figure 7.
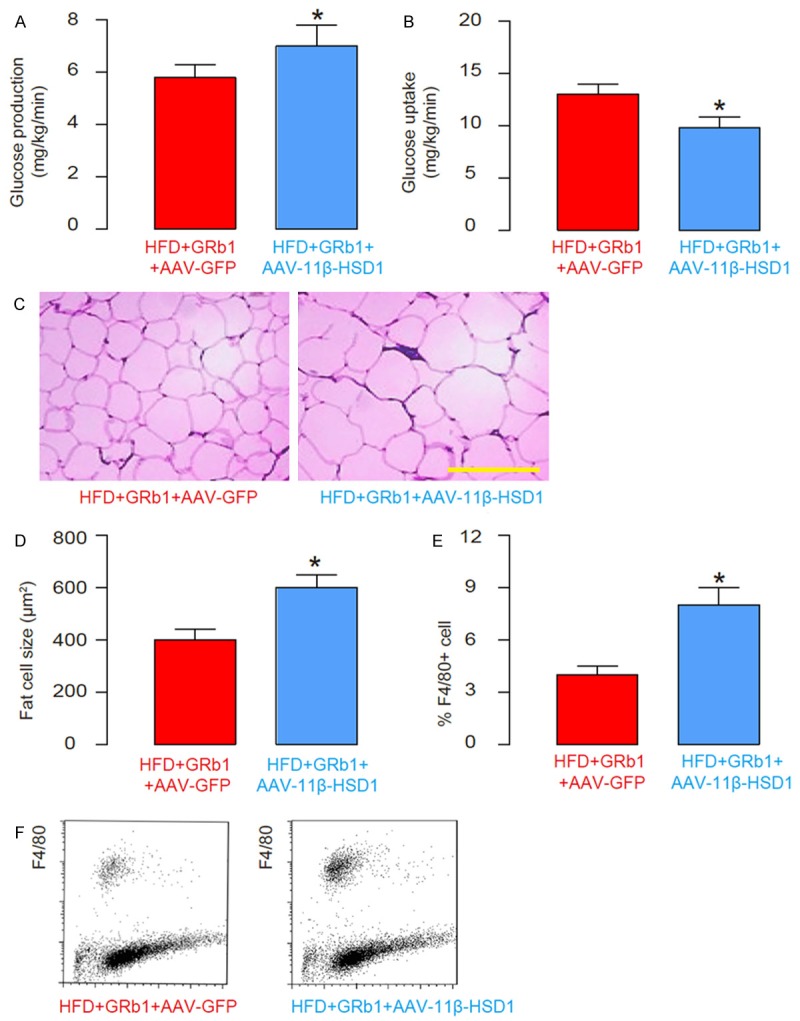
Overexpression of 11β-HSD1 levels abolishes the effects of GRb1 on glucose production, glucose uptake and visceral fat index. (A) Glucose production in liver. (B) Glucose uptake in skeletal muscle. (C, D) The visceral fat cell size, by representative images (C), and by quantification (D). (E, F) The F4/80+ cells in the fat tissue analyzed by flow cytometry, shown by quantification (E), and by representative flow charts (F). *P<0.05. N=10. Scale bar is 50 µm.
Discussion
Some Chinese traditional medicine have been shown to have pronounced therapeutic effects on a number of different diseases. However, the mixture manner of these Chinese traditional medicine prevents a precise determination of the molecular mechanisms underlying their therapeutic effects. GRb1 is a purified and defined item from Ginseng with reported effects in protection and treatment of obesity, insulin resistance and T2D. Here, we showed that in a well-defined T2D model, HFD, application of GRb1 attenuated the increases in fasting blood glucose, glucose intolerance and insulin insensitivity. Since the alterations of glucose synthesis in liver, glucose update in muscle and visceral fat index by HFD were all attenuated by GRb1 without changes in either food intake or body weight, the insulin resistance induced by HFD appeared to be ameliorated.
Among all the factors that are associated with pathogenesis of obesity, insulin resistance and T2D, 11β-HSD1 was specifically detected as an altered one by HFD and by GRb1. 11β-HSD1 triggers the activation of glucocorticoid to antagonize the insulin effects. Previous studies have shown that 11β-HSD1 suppresses the insulin sensitivity by downregulating the expression of insulin-signaling pathway associated proteins, e.g. PI3K, Akt and GLUT4. Thus, the effects of GRb1 may affect insulin resistance status directly through modification of cortisol by 11β-HSD1. These hypothesis were confirmed in a loss-of-function experiment, in which forced expression of 11β-HSD1 diminished the effects of GRb1 in this model. The infection of the mouse tissue by AAV will turn on the transgene within 2 days [25-27], which is sufficient to examine the effects of the transgene.
In future, the direct regulation of 11β-HSD1 by GRb1 should be analyzed in vitro to fully understand the molecular signaling pathways that are involved. Here, we provide compelling evidence to demonstrate a role of GRb1 in treating obesity, insulin resistance and T2D. These results may be fundamental for generating GRb1-based therapy for T2D.
Acknowledgements
This work was supported by Liaoning Province Natural Science Fund Program (NO: 201602308).
Disclosure of conflict of interest
None.
References
- 1.Weir GC, Bonner-Weir S. Islets of langerhans: The puzzle of intraislet interactions and their relevance to diabetes. J Clin Invest. 1990;85:983–987. doi: 10.1172/JCI114574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matveyenko AV, Butler PC. Relationship between beta-cell mass and diabetes onset. Diabetes Obes Metab. 2008;10(Suppl 4):23–31. doi: 10.1111/j.1463-1326.2008.00939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiao X, Gittes GK. Concise review: New insights into the role of macrophages in beta-cell proliferation. Stem Cells Transl Med. 2015;4:655–658. doi: 10.5966/sctm.2014-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gunasekaran U, Gannon M. Type 2 diabetes and the aging pancreatic beta cell. Aging. 2011;3:565–575. doi: 10.18632/aging.100350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akiyama N, Akiyama Y, Kato H, Kuroda T, Ono T, Imagawa K, Asakura K, Shinosaki T, Murayama T, Hanasaki K. Pharmacological evaluation of adipose dysfunction via 11beta-hydroxysteroid dehydrogenase type 1 in the development of diabetes in diet-induced obese mice with cortisone pellet implantation. J Pharmacol Exp Ther. 2014;349:66–74. doi: 10.1124/jpet.113.210716. [DOI] [PubMed] [Google Scholar]
- 6.Goldberg FW, Dossetter AG, Scott JS, Robb GR, Boyd S, Groombridge SD, Kemmitt PD, Sjogren T, Gutierrez PM, deSchoolmeester J, Swales JG, Turnbull AV, Wild MJ. Optimization of brain penetrant 11beta-hydroxysteroid dehydrogenase type i inhibitors and in vivo testing in diet-induced obese mice. J Med Chem. 2014;57:970–986. doi: 10.1021/jm4016729. [DOI] [PubMed] [Google Scholar]
- 7.Park JS, Rhee SD, Jung WH, Kang NS, Kim HY, Kang SK, Ahn JH, Kim KY. Anti-diabetic and anti-adipogenic effects of a novel selective 11beta-hydroxysteroid dehydrogenase type 1 inhibitor in the diet-induced obese mice. Eur J Pharmacol. 2012;691:19–27. doi: 10.1016/j.ejphar.2012.06.024. [DOI] [PubMed] [Google Scholar]
- 8.Stimson RH, Andrew R, McAvoy NC, Tripathi D, Hayes PC, Walker BR. Increased whole-body and sustained liver cortisol regeneration by 11beta-hydroxysteroid dehydrogenase type 1 in obese men with type 2 diabetes provides a target for enzyme inhibition. Diabetes. 2011;60:720–725. doi: 10.2337/db10-0726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turban S, Liu X, Ramage L, Webster SP, Walker BR, Dunbar DR, Mullins JJ, Seckl JR, Morton NM. Optimal elevation of beta-cell 11beta-hydroxysteroid dehydrogenase type 1 is a compensatory mechanism that prevents high-fat diet-induced beta-cell failure. Diabetes. 2012;61:642–652. doi: 10.2337/db11-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gogebakan O, Andres J, Biedasek K, Mai K, Kuhnen P, Krude H, Isken F, Rudovich N, Osterhoff MA, Kintscher U, Nauck M, Pfeiffer AF, Spranger J. Glucose-dependent insulinotropic polypeptide reduces fat-specific expression and activity of 11beta-hydroxysteroid dehydrogenase type 1 and inhibits release of free fatty acids. Diabetes. 2012;61:292–300. doi: 10.2337/db10-0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Man TY, Michailidou Z, Gokcel A, Ramage L, Chapman KE, Kenyon CJ, Seckl JR, Morton NM. Dietary manipulation reveals an unexpected inverse relationship between fat mass and adipose 11beta-hydroxysteroid dehydrogenase type 1. Am J Physiol Endocrinol Metab. 2011;300:E1076–1084. doi: 10.1152/ajpendo.00531.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wamil M, Battle JH, Turban S, Kipari T, Seguret D, de Sousa Peixoto R, Nelson YB, Nowakowska D, Ferenbach D, Ramage L, Chapman KE, Hughes J, Dunbar DR, Seckl JR, Morton NM. Novel fat depot-specific mechanisms underlie resistance to visceral obesity and inflammation in 11 beta-hydroxysteroid dehydrogenase type 1-deficient mice. Diabetes. 2011;60:1158–1167. doi: 10.2337/db10-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masuzaki H, Paterson J, Shinyama H, Morton NM, Mullins JJ, Seckl JR, Flier JS. A transgenic model of visceral obesity and the metabolic syndrome. Science. 2001;294:2166–2170. doi: 10.1126/science.1066285. [DOI] [PubMed] [Google Scholar]
- 14.Franz MJ, Bantle JP, Beebe CA, Brunzell JD, Chiasson JL, Garg A, Holzmeister LA, Hoogwerf B, Mayer-Davis E, Mooradian AD, Purnell JQ, Wheeler M, American Diabetes A. Evidence-based nutrition principles and recommendations for the treatment and prevention of diabetes and related complications. Diabetes Care. 2003;26(Suppl 1):S51–61. doi: 10.2337/diacare.26.2007.s51. [DOI] [PubMed] [Google Scholar]
- 15.Kimura M, Waki I, Tanaka O, Nagai Y, Shibata S. Pharmacological sequential trials for the fractionation of components with hypoglycemic activity in alloxan diabetic mice from ginseng radix. J Pharmacobiodyn. 1981;4:402–409. doi: 10.1248/bpb1978.4.402. [DOI] [PubMed] [Google Scholar]
- 16.Kimura M, Waki I, Chujo T, Kikuchi T, Hiyama C, Yamazaki K, Tanaka O. Effects of hypoglycemic components in ginseng radix on blood insulin level in alloxan diabetic mice and on insulin release from perfused rat pancreas. J Pharmacobiodyn. 1981;4:410–417. doi: 10.1248/bpb1978.4.410. [DOI] [PubMed] [Google Scholar]
- 17.Attele AS, Zhou YP, Xie JT, Wu JA, Zhang L, Dey L, Pugh W, Rue PA, Polonsky KS, Yuan CS. Antidiabetic effects of panax ginseng berry extract and the identification of an effective component. Diabetes. 2002;51:1851–1858. doi: 10.2337/diabetes.51.6.1851. [DOI] [PubMed] [Google Scholar]
- 18.Yun SN, Moon SJ, Ko SK, Im BO, Chung SH. Wild ginseng prevents the onset of high-fat diet induced hyperglycemia and obesity in icr mice. Arch Pharm Res. 2004;27:790–796. doi: 10.1007/BF02980150. [DOI] [PubMed] [Google Scholar]
- 19.Sotaniemi EA, Haapakoski E, Rautio A. Ginseng therapy in non-insulin-dependent diabetic patients. Diabetes Care. 1995;18:1373–1375. doi: 10.2337/diacare.18.10.1373. [DOI] [PubMed] [Google Scholar]
- 20.Vuksan V, Sievenpiper JL, Wong J, Xu Z, Beljan-Zdravkovic U, Arnason JT, Assinewe V, Stavro MP, Jenkins AL, Leiter LA, Francis T. American ginseng (panax quinquefolius l. ) attenuates postprandial glycemia in a time-dependent but not dose-dependent manner in healthy individuals. Am J Clin Nutr. 2001;73:753–758. doi: 10.1093/ajcn/73.4.753. [DOI] [PubMed] [Google Scholar]
- 21.Yu X, Ye L, Zhang H, Zhao J, Wang G, Guo C, Shang W. Ginsenoside rb1 ameliorates liver fat accumulation by upregulating perilipin expression in adipose tissue of db/db obese mice. J Ginseng Res. 2015;39:199–205. doi: 10.1016/j.jgr.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Y, Yu Y, Szabo A, Han M, Huang XF. Central inflammation and leptin resistance are attenuated by ginsenoside rb1 treatment in obese mice fed a high-fat diet. PLoS One. 2014;9:e92618. doi: 10.1371/journal.pone.0092618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen L, Xiong Y, Wang DQ, Howles P, Basford JE, Wang J, Xiong YQ, Hui DY, Woods SC, Liu M. Ginsenoside rb1 reduces fatty liver by activating amp-activated protein kinase in obese rats. J Lipid Res. 2013;54:1430–1438. doi: 10.1194/jlr.M035907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen L, Zhang J, Zhang Z, Chu Y, Song B, Cai W. Pax4 expression does not transduce pancreatic alpha cells to beta cells. Cell Physiol Biochem. 2015;36:1735–1742. doi: 10.1159/000430146. [DOI] [PubMed] [Google Scholar]
- 25.Xiao X, Guo P, Prasadan K, Shiota C, Peirish L, Fischbach S, Song Z, Gaffar I, Wiersch J, El-Gohary Y, Husain SZ, Gittes GK. Pancreatic cell tracing, lineage tagging and targeted genetic manipulations in multiple cell types using pancreatic ductal infusion of adeno-associated viral vectors and/or cell-tagging dyes. Nat Protoc. 2014;9:2719–2724. doi: 10.1038/nprot.2014.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grieger JC, Choi VW, Samulski RJ. Production and characterization of adeno-associated viral vectors. Nat Protoc. 2006;1:1412–1428. doi: 10.1038/nprot.2006.207. [DOI] [PubMed] [Google Scholar]
- 27.Koerber JT, Maheshri N, Kaspar BK, Schaffer DV. Construction of diverse adeno-associated viral libraries for directed evolution of enhanced gene delivery vehicles. Nat Protoc. 2006;1:701–706. doi: 10.1038/nprot.2006.93. [DOI] [PubMed] [Google Scholar]


