Abstract
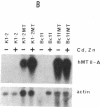
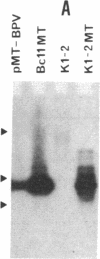
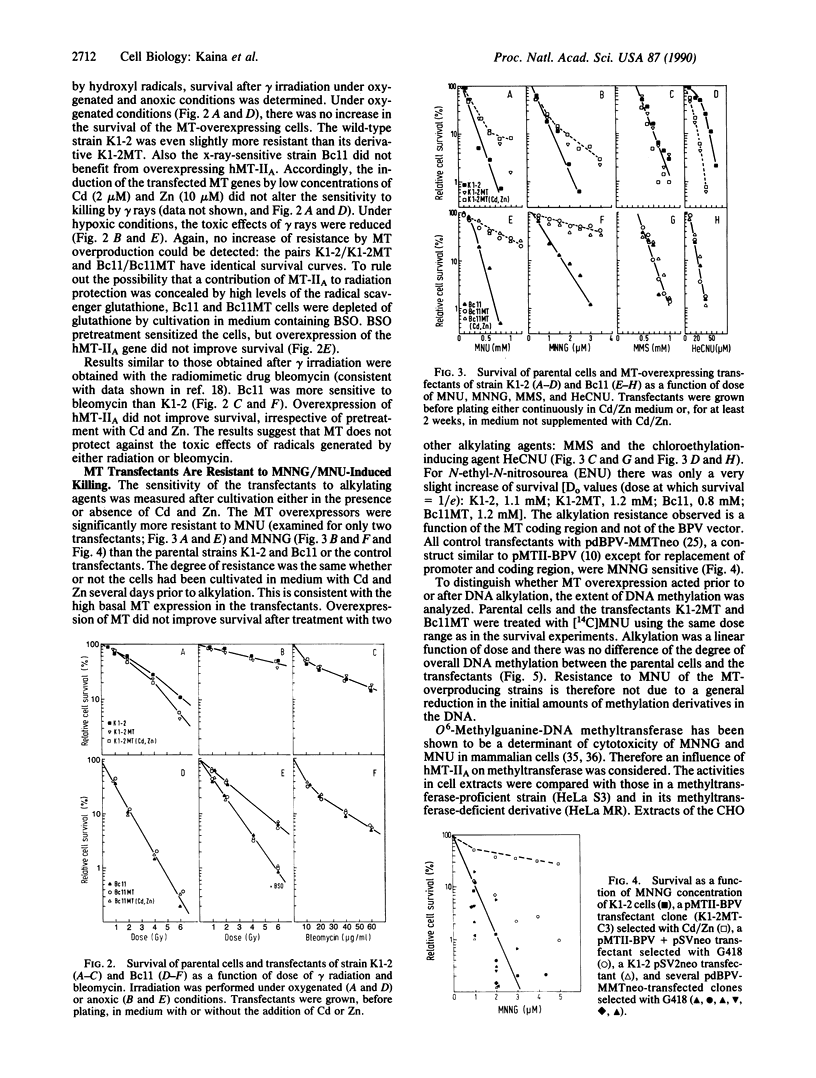
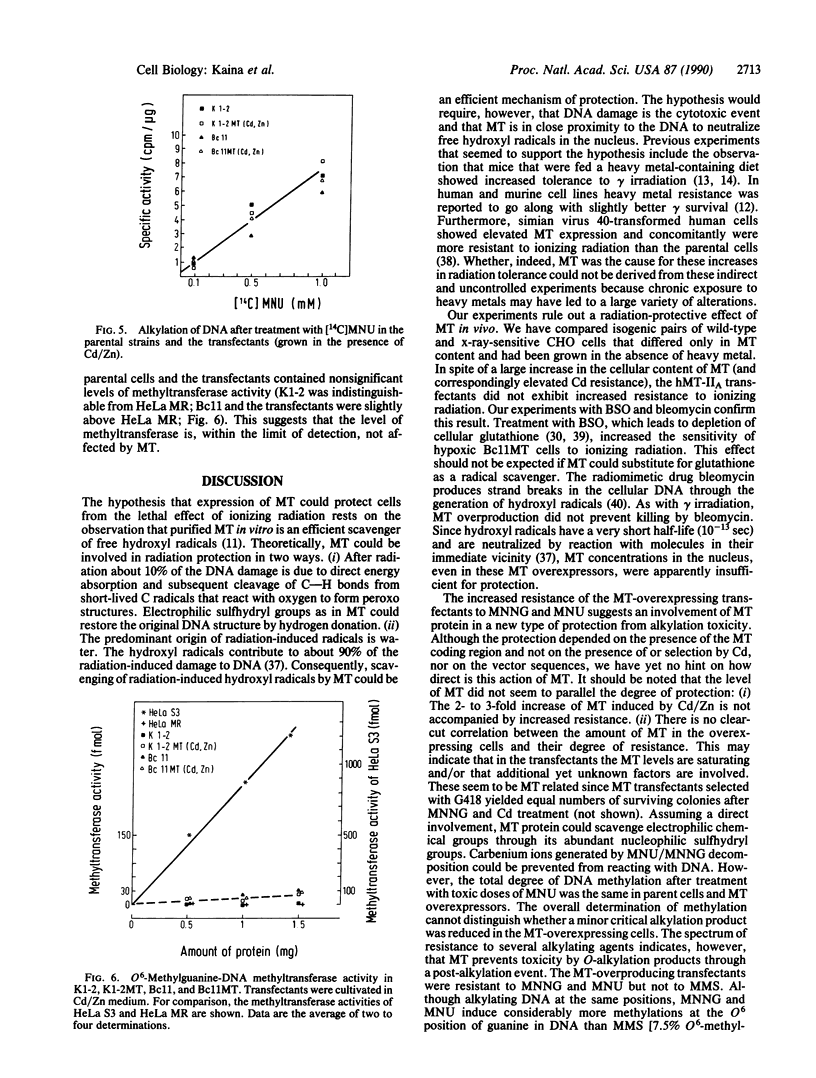
Experiments were designed to detect survival advantages that cells gain by overexpressing metallothionein (MT). Chinese hamster ovary K1-2 cells and an x-ray-sensitive derivative were transfected with a bovine papillomavirus (BPV)-linked construct carrying the human metallothionein IIA (hMT-IIA) gene. Transfectants survived 40-fold higher levels of cadmium chloride, harbored at least 30 copies of hMT-IIA, and contained 25- to 166-fold more MT than the parent cells. Even under conditions of reduced glutathione synthesis, the transfectants were not more resistant to the lethal effects of ionizing radiation and bleomycin than the parent cells. Thus free radicals generated by these agents cannot be scavenged efficiently by MT in vivo. The hMT-IIA transfectants, however, but not control transfectants harboring a BPV-MT promoter-neo construct, tolerated significantly higher doses of the alkylating agents N-methyl-N-nitrosourea and N-methyl-N'-nitro-N-nitrosoguanidine. Resistance and MT overexpression occurred irrespective of selection and cultivation in cadmium and zinc. There was no increase in resistance to methyl methanesulfonate and N-hydroxyethyl-N-chloroethylnitrosourea. MT did not affect the degree of overall DNA methylation after N-methyl-N-nitrosourea treatment nor the level of O6-methylguanine-DNA methyltransferase. The results suggest that MT participates as a cofactor or regulatory element in repair or tolerance of toxic alkylation lesions.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. A., Murphy M. P., Howell S. B. Metallothionein-mediated cisplatin resistance in human ovarian carcinoma cells. Cancer Chemother Pharmacol. 1987;19(2):149–154. doi: 10.1007/BF00254568. [DOI] [PubMed] [Google Scholar]
- Angel P., Pöting A., Mallick U., Rahmsdorf H. J., Schorpp M., Herrlich P. Induction of metallothionein and other mRNA species by carcinogens and tumor promoters in primary human skin fibroblasts. Mol Cell Biol. 1986 May;6(5):1760–1766. doi: 10.1128/mcb.6.5.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakka A., Endresen L., Johnsen A. B., Edminson P. D., Rugstad H. E. Resistance against cis-dichlorodiammineplatinum in cultured cells with a high content of metallothionein. Toxicol Appl Pharmacol. 1981 Nov;61(2):215–226. doi: 10.1016/0041-008x(81)90411-7. [DOI] [PubMed] [Google Scholar]
- Bakka A., Johnsen A. S., Endresen L., Rugstad H. E. Radioresistance in cells with high content of metallothionein. Experientia. 1982 Mar 15;38(3):381–383. doi: 10.1007/BF01949406. [DOI] [PubMed] [Google Scholar]
- Beach L. R., Palmiter R. D. Amplification of the metallothionein-I gene in cadmium-resistant mouse cells. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2110–2114. doi: 10.1073/pnas.78.4.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan M. W., Chilton M. D. Multiple transcripts of T-DNA detected in nopaline crown gall tumors. J Mol Appl Genet. 1982;1(6):539–546. [PubMed] [Google Scholar]
- Brennand J., Margison G. P. Reduction of the toxicity and mutagenicity of alkylating agents in mammalian cells harboring the Escherichia coli alkyltransferase gene. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6292–6296. doi: 10.1073/pnas.83.17.6292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieter H. H., Müller L., Abel J., Summer K. H. Determination of Cd-thionein in biological materials: comparative standard recovery by five current methods using protein nitrogen for standard calibration. Toxicol Appl Pharmacol. 1986 Sep 30;85(3):380–388. doi: 10.1016/0041-008x(86)90345-5. [DOI] [PubMed] [Google Scholar]
- Erickson L. C., Laurent G., Sharkey N. A., Kohn K. W. DNA cross-linking and monoadduct repair in nitrosourea-treated human tumour cells. Nature. 1980 Dec 25;288(5792):727–729. doi: 10.1038/288727a0. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine). J Biol Chem. 1979 Aug 25;254(16):7558–7560. [PubMed] [Google Scholar]
- Guichard M., Lespinasse F., Malaise E. P. Influence of buthionine sulfoximine and misonidazole on glutathione level and radiosensitivity of human tumor xenografts. Radiat Res. 1986 Jan;105(1):115–125. [PubMed] [Google Scholar]
- Hager L. J., Palmiter R. D. Transcriptional regulation of mouse liver metallothionein-I gene by glucocorticoids. Nature. 1981 May 28;291(5813):340–342. doi: 10.1038/291340a0. [DOI] [PubMed] [Google Scholar]
- Hamer D. H. Metallothionein. Annu Rev Biochem. 1986;55:913–951. doi: 10.1146/annurev.bi.55.070186.004405. [DOI] [PubMed] [Google Scholar]
- Henner W. D., Grunberg S. M., Haseltine W. A. Sites and structure of gamma radiation-induced DNA strand breaks. J Biol Chem. 1982 Oct 10;257(19):11750–11754. [PubMed] [Google Scholar]
- Herrlich P., Angel P., Rahmsdorf H. J., Mallick U., Pöting A., Hieber L., Lücke-Huhle C., Schorpp M. The mammalian genetic stress response. Adv Enzyme Regul. 1986;25:485–504. doi: 10.1016/0065-2571(86)90030-0. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Ishizaki K., Tsujimura T., Yawata H., Fujio C., Nakabeppu Y., Sekiguchi M., Ikenaga M. Transfer of the E. coli O6-methylguanine methyltransferase gene into repair-deficient human cells and restoration of cellular resistance to N-methyl-N'-nitro-N-nitrosoguanidine. Mutat Res. 1986 Sep;166(2):135–141. doi: 10.1016/0167-8817(86)90011-8. [DOI] [PubMed] [Google Scholar]
- Jeggo P. A., Kemp L. M., Holliday R. The application of the microbial "tooth-pick" technique to somatic cell genetics, and its use in the isolation of X-ray sensitive mutants of Chinese hamster ovary cells. Biochimie. 1982 Aug-Sep;64(8-9):713–715. doi: 10.1016/s0300-9084(82)80116-8. [DOI] [PubMed] [Google Scholar]
- Kaina B., Aurich O. Dependency of the yield of sister-chromatid exchanges induced by alkylating agents on fixation time. Possible involvement of secondary lesions in sister-chromatid exchange induction. Mutat Res. 1985 May;149(3):451–461. doi: 10.1016/0027-5107(85)90163-0. [DOI] [PubMed] [Google Scholar]
- Kaina B., Van Zeeland A. A., Backendorf C., Thielmann H. W., Van de Putte P. Transfer of human genes conferring resistance to methylating mutagens, but not to UV irradiation and cross-linking agents, into Chinese hamster ovary cells. Mol Cell Biol. 1987 May;7(5):2024–2030. doi: 10.1128/mcb.7.5.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M., Cathala G., Nguyen-Huu M. C. Expression and regulation of a human metallothionein gene carried on an autonomously replicating shuttle vector. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4040–4044. doi: 10.1073/pnas.80.13.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M., Haslinger A., Holtgreve H., Richards R. I., Krauter P., Westphal H. M., Beato M. Characterization of DNA sequences through which cadmium and glucocorticoid hormones induce human metallothionein-IIA gene. Nature. 1984 Apr 5;308(5959):513–519. doi: 10.1038/308513a0. [DOI] [PubMed] [Google Scholar]
- Karin M., Herschman H. R. Characterization of the metallothioneins induced in HeLa cells by dexamethasone and zinc. Eur J Biochem. 1980 Jun;107(2):395–401. doi: 10.1111/j.1432-1033.1980.tb06042.x. [DOI] [PubMed] [Google Scholar]
- Karin M., Imbra R. J., Heguy A., Wong G. Interleukin 1 regulates human metallothionein gene expression. Mol Cell Biol. 1985 Oct;5(10):2866–2869. doi: 10.1128/mcb.5.10.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M. Metallothioneins: proteins in search of function. Cell. 1985 May;41(1):9–10. doi: 10.1016/0092-8674(85)90051-0. [DOI] [PubMed] [Google Scholar]
- Karran P., Lindahl T., Griffin B. Adaptive response to alkylating agents involves alteration in situ of O6-methylguanine residues in DNA. Nature. 1979 Jul 5;280(5717):76–77. doi: 10.1038/280076a0. [DOI] [PubMed] [Google Scholar]
- Kataoka H., Hall J., Karran P. Complementation of sensitivity to alkylating agents in Escherichia coli and Chinese hamster ovary cells by expression of a cloned bacterial DNA repair gene. EMBO J. 1986 Dec 1;5(12):3195–3200. doi: 10.1002/j.1460-2075.1986.tb04629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley S. L., Basu A., Teicher B. A., Hacker M. P., Hamer D. H., Lazo J. S. Overexpression of metallothionein confers resistance to anticancer drugs. Science. 1988 Sep 30;241(4874):1813–1815. doi: 10.1126/science.3175622. [DOI] [PubMed] [Google Scholar]
- Kraker A., Schmidt J., Krezoski S., Petering D. H. Binding of cis-dichlorodiammine platinum(II) to metallothionein in Ehrlich cells. Biochem Biophys Res Commun. 1985 Jul 31;130(2):786–792. doi: 10.1016/0006-291x(85)90485-1. [DOI] [PubMed] [Google Scholar]
- Law M. F., Byrne J. C., Howley P. M. A stable bovine papillomavirus hybrid plasmid that expresses a dominant selective trait. Mol Cell Biol. 1983 Nov;3(11):2110–2115. doi: 10.1128/mcb.3.11.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai S., Stein B., van den Berg S., Kaina B., Lücke-Huhle C., Ponta H., Rahmsdorf H. J., Kraemer M., Gebel S., Herrlich P. Mechanisms of the ultraviolet light response in mammalian cells. J Cell Sci. 1989 Dec;94(Pt 4):609–615. doi: 10.1242/jcs.94.4.609. [DOI] [PubMed] [Google Scholar]
- Matsubara J., Tajima Y., Karasawa M. Metallothionein induction as a potent means of radiation protection in mice. Radiat Res. 1987 Aug;111(2):267–275. [PubMed] [Google Scholar]
- Matsubara J., Tajima Y., Karasawa M. Promotion of radioresistance by metallothionein induction prior to irradiation. Environ Res. 1987 Jun;43(1):66–74. doi: 10.1016/s0013-9351(87)80058-0. [DOI] [PubMed] [Google Scholar]
- Myrnes B., Norstrand K., Giercksky K. E., Sjunneskog C., Krokan H. A simplified assay for O6-methylguanine-DNA methyltransferase activity and its application to human neoplastic and non-neoplastic tissues. Carcinogenesis. 1984 Aug;5(8):1061–1064. doi: 10.1093/carcin/5.8.1061. [DOI] [PubMed] [Google Scholar]
- Pegg A. E. Formation and metabolism of alkylated nucleosides: possible role in carcinogenesis by nitroso compounds and alkylating agents. Adv Cancer Res. 1977;25:195–269. doi: 10.1016/s0065-230x(08)60635-1. [DOI] [PubMed] [Google Scholar]
- Samson L., Derfler B., Waldstein E. A. Suppression of human DNA alkylation-repair defects by Escherichia coli DNA-repair genes. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5607–5610. doi: 10.1073/pnas.83.15.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Thornalley P. J., Vasák M. Possible role for metallothionein in protection against radiation-induced oxidative stress. Kinetics and mechanism of its reaction with superoxide and hydroxyl radicals. Biochim Biophys Acta. 1985 Jan 21;827(1):36–44. doi: 10.1016/0167-4838(85)90098-6. [DOI] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R., Urlaub G., Chasin L. DNA-mediated transfer of the adenine phosphoribosyltransferase locus into mammalian cells. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1373–1376. doi: 10.1073/pnas.76.3.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]