Abstract
Salivary adenoid cystic carcinoma (SACC) is a relatively uncommon epithelial-like malignancy that can occur in the head and neck region. Despite its slow growth, this aggressive salivary gland tumor frequently recurs and metastasizes to distant organs since lacking effective chemotherapy treatment. MicroRNAs are key regulators in tumor metastasis and progression, but their roles during SACC progression have not been illustrated. In current study, we demonstrate that miR-125a-5p is down-regulated in SACC and closely related to the metastasis and progression in human SACC specimens. In vitro, miR-125a-5p mimic can suppress SACC cell migration and invasion; while blocking miR-125a-5p can relieve the inhibition effect. By using dual-luciferase assay, we confirmed that miR-125a-5p directly targeted to p38 and tissue samples of patients indicated the negative correlation between miR-125a-5p and p38; clinical analysis also showed that low level expression of miR-125a-5p is closely associated with poor prognosis of SACC. Furthermore, down-regulation of miR-125a-5p triggered downstream p38/JNK/ERK activation. Taken together, our results indicate that down-regulation of miR-125a-5p promotes SACC progression through p38 signal pathway and miR-125a-5p can be a potential therapeutic target of SACC.
Keywords: miR-125a-5p, SACC, p38, metastasis, progression
Introduction
Adenoid cystic carcinoma (ACC) of the salivary gland is a rare malignancy, which accounts for 10% of salivary malignancies and only 1% of all head and neck tumors [1,2]. SACCs are characterized by their persistent slow but progressive clinical course, high rate of recurrence, and distant organs metastasis [3,4]. Despite the combination of complete surgical resection and adjuvant radiotherapy, the overall survival has not been improved and the majority of patients succumb to metastatic disease within a decade [5-7]. To date, numerous studies have identified factors which might be related to the prognosis and outcome of SACC, but little is known about the underlying molecular mechanisms which control SACC metastasis [8,9]. It is pivotal to reveal the process of SACC metastasis and identify the molecular factors that contribute to this process.
MicroRNAs (miRNAs) are a class of small, noncoding RNAs which have been identified as important regulators during many physiological and pathological processes, containing development, differentiation, proliferation and tumorigenesis [10,11]. As post-transcriptional gene regulators, miRNAs can repress messenger RNAs (mRNAs) gene translation via binding to the 3’-untranslated regions (3’-UTR) of target genes [12]. Many miRNAs have been characterized as oncogenes or tumor suppressor and are abnormally expressed in various cancers models, including SACC. The dysfunction of miRNA has closely correlated with tumor development and progression [13]. MiR-125a-5p has been previously reported as a key modulator during cell differentiation, and functions as tumor suppressor in multiple cancer types via targeting MMP11 and VEGF in hepatocellular carcinoma [14], HDAC4 in breast cancer [15], TAZ in glioblastoma [16], and ERBB2 in gastric cancer [17]. It is believed that the low expression of miR-125a-5p was associated with tumor metastasis in many tumors [18,19].
P38/MAPK signaling pathway has been regarded to regulate various physiological processes, including cell proliferation, differentiation and apoptosis [20-22]. Recently, increasing evidences have implicated that p38 signaling was activated during the tumorigenesis of many human malignancies progression [23,24]. Although the aberrant expression of p38 has been reported in several malignant tumors, the correlation between miR-125a-5p, p38 expression profiles and SACC progression has not been revealed yet. Therefore, the underlying molecular mechanism and the roles of miR-125a-5p, p38 in SACC should be elucidated.
In current study, we provided the evidences that low expression of miR-125a-5p promotes SACC cells migration and invasion in vitro. Furthermore, we identified that p38 was the transcript target of miR-125a-5p. We also demonstrated that the down-regulation of miR-125a-5p was closely associated with metastasis and poor prognosis in SACC patients.
Materials and methods
Cell lines and cell culture
The human SACC cell lines SACC-83 and SACC-LM were used in our study. SACC-83 and SACC-LM were purchased from Peking University (Beijing, China) and SACC-LM is a highly metastatic cell derived from lung metastasis of SACC-83 xenograft [25,26]. All cells were maintained in RPMI-1640 medium (Gibco, Rockville, MD) supplemented with 10% FBS (Invitrogen, Carlsbad, CA) at 37°C with 5% CO2.
MiRNA microarray analysis
MiRNA microarray analysis were performed in SACC-83, SACC-LM and SACC-LMTGF-β cells as described previously [27]. A heat map demonstrating the expression levels of total miRNAs, which are differentially expressed in SACC-83 VS SACC-LM VS SACC-LMTGF-β, was created using DMVS 2.0 software (Chipscreen Biosciences, Shenzhen, China).
RNA extraction, reverse transcription, and semi-quantitative and quantitative PCR (qPCR)
Total RNA was extracted from tissues or cells using the Trizol reagent (Invitrogen, Carlsbad, California, USA), and the reverse transcription was performed using the Prime Script™ RT reagent kit according to the manufacturer’s instructions (Takara Biotechnology, China). RT-PCR products were analyzed via 2.0% agarose gel electrophoresis and stained with ethidium bromide for visualization using ultraviolet light. Quantitative real-time PCR was performed using the miScript SYBR Green PCR Kit (Qiagen, China) by LightCycler 480 (Roche, Basel, Switzerland) in triplicate in three independent experiments and the relative expression of miR-125a-5p was normalized to U6. QPCR primers are from the Qiagen miScript Primer Assay as following: hsa-miR-222-3p (MS00007609), hsa-miR-3607-3p (MS00022953), hsa-miR-221-3p (MS00003857), hsa-miR-125a-5p (MS00003423), hsa-miR-148b-3p (MS00031458), hsa-miR-126-3p (MS00003430), hsa-miR-625-3p (MS00032046), hsa-miR-1275 (MS00037786), hsa-miR-589-5p (MS00010255), hsa-miR-210-3p (MS00003801), hsa-miR-145-3p (MS00008708) and hsa-U6 small nuclear RNA (MS00033740) respectively.
Patients and specimens
In this study, a total of 106 patients with SACC were recruited. Paired, adjacent non-neoplastic tissue (ANT) samples of salivary glands were obtained from 20 of these patients. None of the patients received any preoperative chemotherapy or radiotherapy prior to surgery and all patients were histopathologically and clinically diagnosed at the Department of Oral and Maxillofacial Surgery, Sun Yat-Sen Hospital, Sun Yat-Sen University between 2003 and 2012 with giving informed consent. Tumors were classified according to the histologic classification of salivary gland tumors by WHO and the clinicopathological features of the patients are summarized in Table 1. All of the SACC samples were from parotid gland tissues and the adjacent non-neoplastic parotid gland tissues were used as control which were separated distant from tumor area at least 2 cm. The histological diagnosis of all samples were performed and verified by two independent pathologists. This study was approved by the Institutional Ethics Committee of the Sun Yat-Sen Hospital, Sun Yat-Sen University, China.
Table 1.
Correlation of miR-125a-5p and p38 expression in tissues with patients’ clinicopathological variables in 106 cases of SACC
| Clinical variables | miR-125a-5p expression | P38 expression | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| High (n=39) | Low (n=67) | P value | High (n=63) | Low (n=43) | P value | |
| Gender | 0.842 | 0.807 | ||||
| Male | 20 | 37 | 35 | 22 | ||
| Female | 19 | 30 | 28 | 21 | ||
| Age | 0.639 | 0.698 | ||||
| ≥50 | 23 | 35 | 33 | 25 | ||
| <50 | 16 | 32 | 30 | 18 | ||
| Tumor stage | 0.458 | 0.086 | ||||
| T1+T2 | 28 | 42 | 37 | 33 | ||
| T3+T4 | 11 | 25 | 26 | 10 | ||
| TNM stage | 0.173 | 0.112 | ||||
| I+II | 27 | 36 | 33 | 30 | ||
| III+IV | 12 | 31 | 30 | 13 | ||
| Lymph node metastasis | 0.039 | 0.02 | ||||
| N0 | 37 | 52 | 48 | 41 | ||
| N1 | 2 | 15 | 15 | 2 | ||
| Distant metastasis | 0.032 | 0.033 | ||||
| M0 | 26 | 31 | 28 | 29 | ||
| M1 | 13 | 36 | 35 | 14 | ||
| Survival status | 0.0066 | 0.0005 | ||||
| Survival | 26 | 25 | 21 | 30 | ||
| Death | 13 | 42 | 42 | 13 | ||
Transfection
MiR-125a-5p mimics and antisense oligonucleotides (ASOs) were purchased from GenePharma (Shanghai, China) and Transfection was performed in 6-well plates using Lipofectamine 3000 (Invitrogen) following the manufacturer’s instructions in SACC cells.
Boyden chamber assay
A total of 1×105 cells were plated into the upper chamber of a polycarbonate transwell filter chamber (Corning, New York, NY, USA) and incubated for 20 h. For invasion assay, the upper chamber was coated with Matrigel (R&D, Minneapolis, MN, USA). The non-invading cells were gently removed with a soft cotton swab, and the cells that had invaded to the bottom chamber were fixed, stained, photographed and counted.
Luciferase reporter assay
The miR-125a-5p response element (wild type or mutated) of the 3’-UTR of p38 was cloned into pMir-REPORT plasmid downstream of luciferase reporter gene. Luciferase activities were measured using a luciferase assay kit (Promega, Madison, WI, USA), and target effect was evaluated as relative luciferase activity of the reporter vector with target sequence over the one without target sequence.
Western blotting
Cells were lysed in sample buffer and equal amounts of proteins were resolved by 10% polyacrylamide SDS gels (SDS-PAGE), transferred on polyvinylidene fluoride (PVDF) membranes (Amersham Pharmacia Biotech), probed with antibodies as followed: anti-p38 (SantCruz, 1:1000), anti-phospho-p38, JNK, phospho-JNK, ERK, phospho-ERK, β-actin (CST, 1:1000), or GAPDH (1:3000, Proteintech, Chicago, IL, USA), and then with peroxidase-conjugated secondary antibody (1:3000, Proteintech). Then, the signals were visualized by enhanced chemiluminescence kit (GE, Fairfield, CT, USA) according to the manufacturer’s instructions.
In-situ hybridization
This assay was performed according to the manufacturer’s protocol (Exiqon, Vedbaek, Denmark). Briefly, after demasking, 40 nM of linearized, double DIG-labeled miRNA-specific LNA probes (Exiqon) in commercial hybridization solution (BioChain Institute, Inc.) was added. Probes were hybridized to the slides overnight at 58°C for use with miR-125a-5p probes. Digoxigenins were recognized by a specific anti-DIG antibody that was directly conjugated with alkaline phosphatase. Slides were subsequently washed, counterstained with Nuclear Fast Red (Vector Laboratories, Inc.) for 5 minutes, rinsed, air dried, and clarified in xylene.
Immunohistochemistry
For immunohistochemistry, the formalin-fixed, paraffin-embedded samples were dewaxed in xylene and rehydrated through graded alcohol baths. After immersed in citrate buffer (pH 6.0) for antigen retrieval and blocked with bovine serum albumen (BSA) Tris-HCI buffer, the slides were incubated with p38 antibody (SantCruz, 1:100) at 4°C overnight. After washing with PBS, the sections were incubated with the peroxidase-labeled secondary antibody (EnVision/HRP system; DAKO) for 30 min at room temperature Diaminobenzidine (Dako, Carpinteria, CA, USA) was used as a chromogen and the nuclei were counterstained with haematoxylin. The tissue sections were observed under a Zeiss AX10-Imager A1 microscope e (Carl Zeiss, Thornwood, NY) and all images were captured using AxioVision 4.7 microscopy software (Carl Zeiss, Thornwood, NY).
Statistical analysis
All statistical analyses were carried out using SPSS 19.0 statistical software (SPSS, Chicago, IL, USA). Each experiment was repeated three times, with all the data presented as the mean ± standard deviation. The Chi-square and Fisher’s exact tests were used to analyze the relationship between miR-125a-5p, p38 expression and clinicopathologic features. Comparisons were performed with student’s t-test and one-way ANOVA and correlation analysis was evaluated with Pearson correlation analysis. Survival curves were plotted by the Kaplan-Meier method and compared using the log-rank test. Survival data were evaluated using univariate and multivariate Cox regression analyses. P<0.05 was considered statistically significant in all cases.
Results
MiRNA differentially expressed among SACC cell lines
To evaluate the effect and function of miRNAs during SACC proliferation and development, we compared miRNA expression profiles in untreated SACC-83, SACC-LM, and SACC-LM cells treated with TGF-β (SACC-LMTGF-β) using miRNA microarrays. Of the examined miRNAs, expression levels of 12 miRNAs varied remarkably and consistently among the SACC-83, SACC-LM, and SACC-LMTGF-β cells (Figure 1A, 1B). To further verify the 12 miRNAs expression alternation, quantitative real time PCR was performed in SACC-83, SACC-LM and SACC-LMTGF-β cells and miR-125a-5p was observed to be down-regulated in SACC-LM and SACC-LMTGF-β cells, comparing with SACC-83 cells (Figure 1C).
Figure 1.
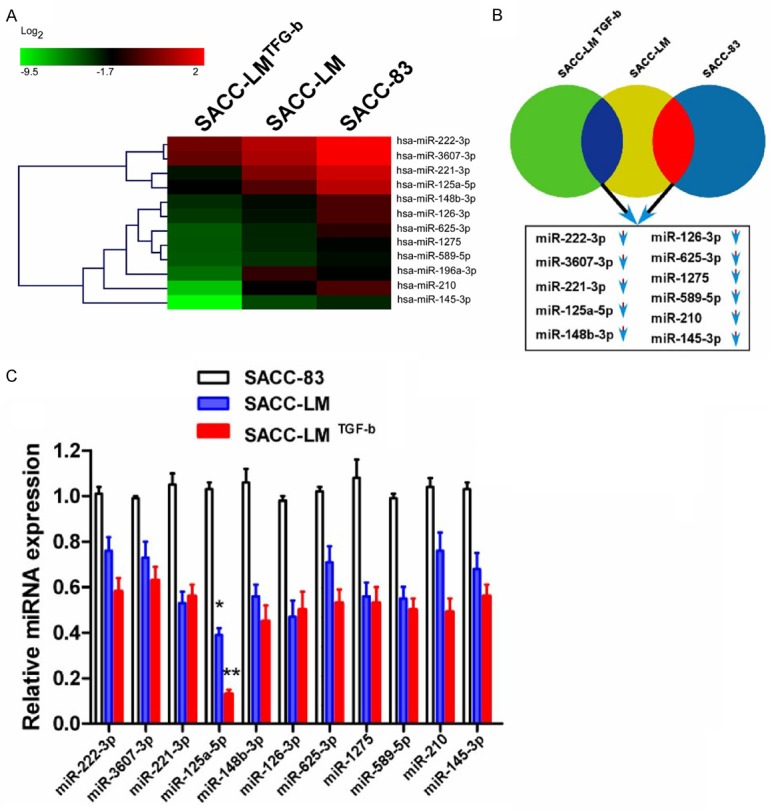
miRNA expression profiles in SACC cell lines. A. SACC cell lines (SACC-83 and SACC-LM) were treated for 48 h with or without TGF-β (10 ng ml-1). miRNA expression profiles were analyzed by microarray. Data are presented as the ratio of miRNA levels in treated versus untreated cells. B. Venn diagram showing down-regulated miRNAs in SACC-83 and SACC-LM cells treated with TGF-β compared to thosein untreated SACC-83 and SACC-LM cells. C. The expression of select miRNAs 48 h after TGF-β treatment was confirmed by qPCR. Three independent experiments were performed in triplicate. The relative expression of miR-125a-5p was normalized to U6 expression (*P<0.05; **P<0.01).
MiR-125a-5p was down-regulated in SACC tissues and associated with an aggressive phenotype
Down-regulation of miR-125a-5p is closely associated with progression in multiple human cancers. However, its expression status in SACC remains unknown. To investigate the miR-125a-5p expression, qPCR was performed using eight cases of paired primary SACC tissues and ANTs (Figure 2A). miR-125a-5p was down-regulated in SACC tissues. Comparative analysis of miR-125a-5p expression was also performed that also indicated that the expression of miR-125a-5p was inhibited in all eight human primary SACC tissues compared with their matched ANTs using qPCR (Figure 2B). Additionally, using qPCR, 20 cases of paired primary SACC samples and matched ANTs were analyzed. Most of these cases had much lower miR-125a-5p expression levels in the tumor tissues than in the ANTs (Figure 2C). Thus, miR-125a-5p was down-regulated in primary SACC tissues.
Figure 2.
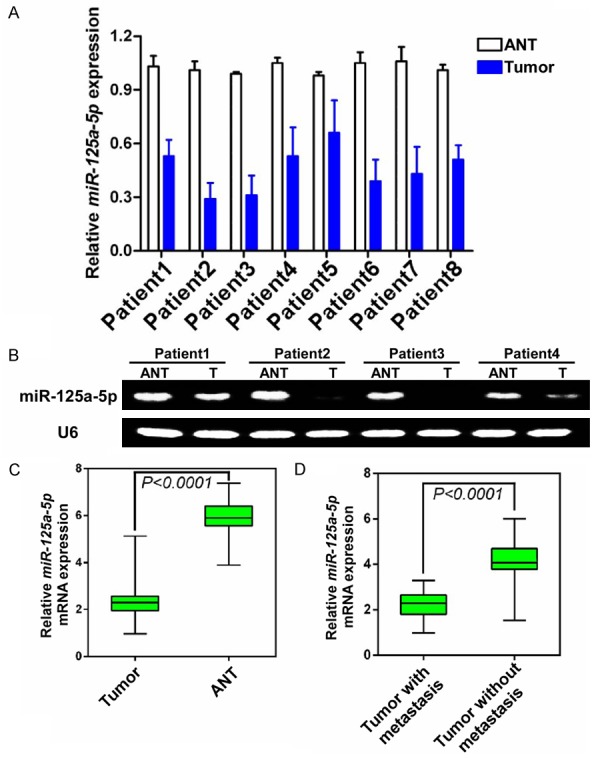
miR-125a-5p was down-regulated in primary SACC tissues and low miR-125a-5p expression was associated with an aggressive phenotype. (A) The expression ofmiR-125a-5p was lower in primary SACC tumors compared to the paired adjacent non-neoplastic tissues (ANT) from the same patient as measured by qPCR and Reverse transcription-PCR analysis (B). U6 was used as a loading control. (C) Expression levels of miR-125a-5p in 30 paired SACC tissues and ANTs. Differential expression is shown in a box plot. The mean level of miR-125a-5p expression in SACC tissues was significantly lower than in ANTs (P<0.0001). (D) Comparison of miR-125a-5p expression levels in SACC tissues with and without metastasis. The mean level of miR-125a-5p expression in SACC tissues with metastasis was significantly lower than in SACC tissues without distant metastasis (P<0.0001).
Since metastasis is the main cause of SACC-related mortality, the relationship between miR-125a-5p expression and SACC metastasis status was evaluated in 40 SACC tissues that were divided into groups characterized by tumors with or without metastasis. The expression level of miR-125-5p in SACCs with metastasis was significantly lower than in SACCs without metastasis, indicating that lower miR-125a-5p expression levels are positively associated with a metastatic phenotype (Figure 2D, P<0.0001).
Reduction of miR-125a-5p promotes SACC cells migration and invasion
As miR-125a-5p expression was negatively associated with metastasis in SACC patients, we further investigate the effect of miR-125a-5p on the migration and invasion of SACC cell. Transfection of SACC-83 cells with miR-125a-5p inhibitor but not the non-relevant lin4 inhibitor can significantly decrease miR-125a-5p expression (Figure 3A, P<0.01). Meanwhile, the downregulation of miR-125a-5p by inhibitor remarkably promotes the migration and invasion abilities in SACC-83 cells (Figure 3B, P<0.05). Additionally, transfection of the high lung metastatic cells with miR-125a-5p mimics can specifically increase miR-125a-5p expression levels in SACC-LM cell (Figure 3C, P<0.01). We next investigated whether ectopic expression of miR-125a-5p attenuated the invasiveness in SACC cells. Transfection of miR-125a-5p mimics remarkably inhibited the migration and invasion capabilities of SACC-LM cells (Figure 3D, P<0.01).
Figure 3.
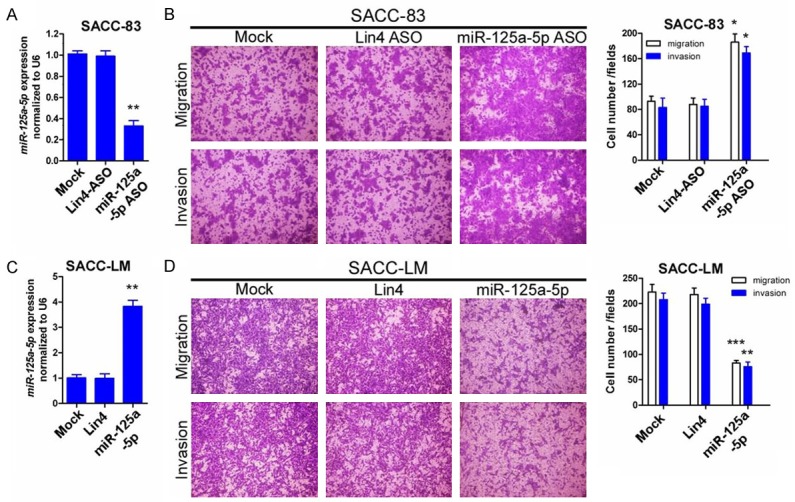
Altered expression of miR-125a-5p modifies SACC-cell migration and invasion in vitro. A. A miR-125a-5p antisense oligo (AGO) was used in SACC-83 cells to inhibitmiR-125a-5p expression. Inhibition of miR-125a-5p expression was measured by qPCR. B. Inhibition of miR-125a-5p expression in SACC-83 cells promoted migration and invasion. C. miR-125a-5p mimics were used in SACC-LM cells to overexpress miR-125a-5p. Overexpression was examined by qPCR. D. Overexpression of miR-125a-5p in SACC-LM cells attenuated migration and invasion. Representative images and quantification of the indicated invaded and migrated cells. Lin4 expression was used as an internal control (*P<0.05; **P<0.01; ***P<0.0001).
MiR-125a-5p inhibits the invasiveness of SACC cells by targeting p38
miRNAs can regulate gene expression by binding to the 3’-untranslated region (3’-UTR) of target genes through sequence homology. Using miRNA target prediction software (TargetScan), we found that miR-125a-5p can bind to the 3’-UTR of p38 (Figure 4A). To confirm whether p38 is a target of miR-125a-5p, luciferase reporter assays were performed to evaluate the relative luciferase activities in SACC cells transfected with a reporter plasmid carrying a miR-125a-5p target sequence (p38 3’-UTR). As we shown that transfection of miR-125a-5p mimics significantly reduced the luciferase activity both in SACC-LM and SACC-83 cells. However, when the target gene 3’-UTR sequences was mutated (p38 3’-UTR mut), transfection of miR-125a-5p mimics failed to trigger the relative luciferase activity, implying that miR-125a-5p can suppresses p38 expression by binding to its 3’-UTR in SACC cells (Figure 4B). To further verify the binding, the p38 expression in SACC-83 and SACC-LM were examined by WB in the presence of miR-125a-5p inhibitor and mimics, respectively. We found that miR-125a-5p inhibitor can upregulate p38 expression in SACC-83 cells, while miR-125a-5p mimics can inhibit the expression level of p38 in SACC-LM cells (Figure 4C and 4D).
Figure 4.
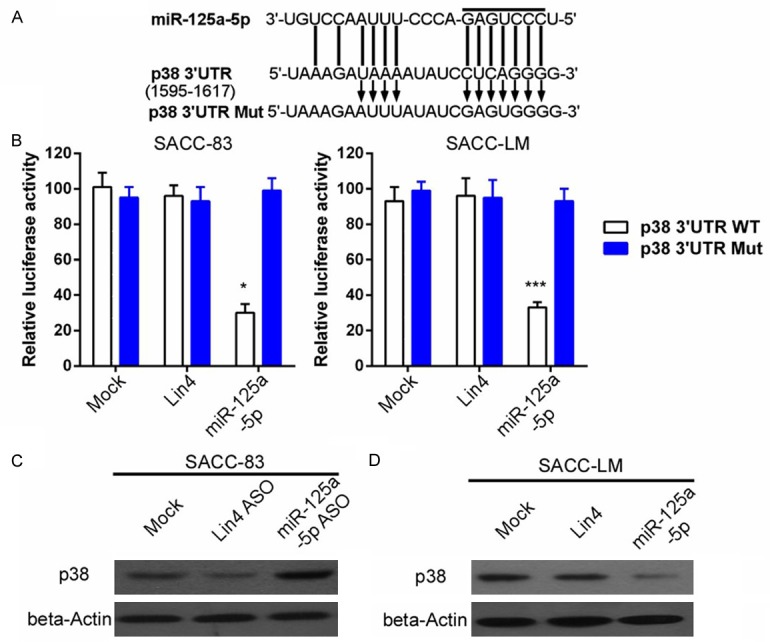
miR-125a-5p directly targets the 3’-UTR of p38. A. The predicted miR-125a-5p binding sites (seed sequence) of the wild-type and mutated p38 3’UTR. B. Luciferase reporter assays for SACC cells transfected with pRL-TK vectors carrying the p38-3’UTRor p38-Mut-3’UTR in the absence or presence of miR-125a-5p mimics (*P<0.05; ***P<0.001). C and D. SACC-LM and SACC-83 cells were transduced with miR-125a-5p mimics and miR-125a-5p antisense oligonucleotides (ASO), respectively. p38 protein expression was measured with beta-actin as the loading control.
Low miR-125a-5p expression is associated with metastasis and poor prognosis in SACC patients
To further investigate the clinicopathological and prognostic significance of miR-125a-5p expression in patients with SACC, the clinical significance of miR-125a-5p expression in patient prognosis and SACC metastasis were evaluated. In situ hybridization and immunohistochemical staining demonstrated that miR-125a-5p expression was lower and p38 expression was higher in primary SACCs with metastasis compared with those samples without metastasis (Figure 5A). Correlation analysis also showed the inverse correlation between miR-125a-5p and p38 expression in SACCs (Figure 5B, r=-0.8472, P<0.0001). Next, we analyzed the association between miR-125a-5p expression and the clinicopathologic status of SACC patients. As shown in Table 1, miR-125a-5p and p38 expression strongly correlated with lymph node metastasis (P=0.039, P=0.02), distant metastasis (P=0.032, P=0.033) and survival (P=0.0066, P=0.0005) in SACC patients, respectively. However, the analysis data indicated that miR-125a-5p and p38 expression was not correlated with age, gender, tumor size or TNM stage. Tumors with distant metastasis expressed low levels of miR-125a-5p. Conversely, p38 expression was positively correlated with distant metastasis in SACC patients.
Figure 5.
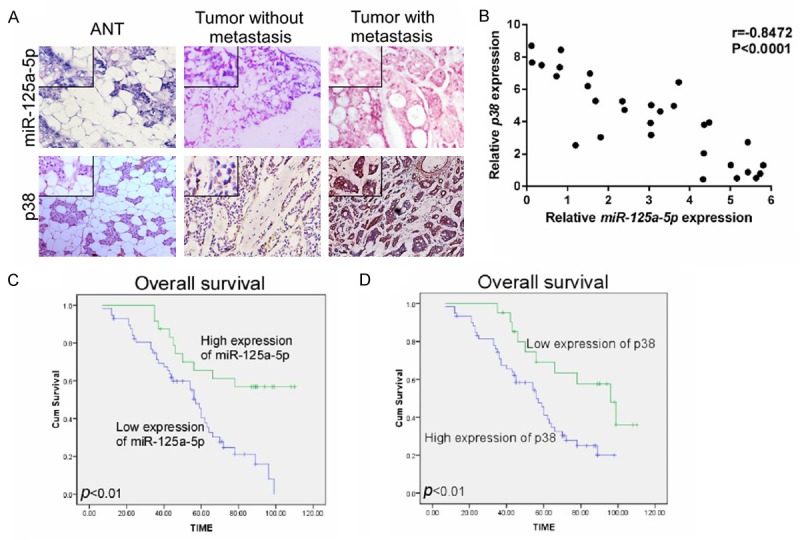
Expression levels of miR-125a-5p and p38 were negatively correlated in SACC tissues; low expression of miR-125a-5p and high expression of p38 were associated with poor prognosis. A. The expression of miR-125a-5p and p38 were determined in primary SACC tissue samplesby in situ hybridization and immunohistochemistry. Inserts show higher magnification;cation. B. The correlation between miR-125a-5p and p38 expression was analyzed indicating that miR-125a-5p expression was negatively related to p38 expression. C and D. Kaplan-Meier survival analysis of the association between miR-125a-5p and p38 expression and 10-year overall survival for SACC patients (log-rank test; P<0.01, P<0.01, respectively).
Furthermore, Kaplan-Meier analysis and the log-rank test were generated to evaluate the correlation between miR-125a-5p expression and survival of the SACC patients. As shown in Figure 5C, the length of overall survival time varied significantly different between patients with low and high miR-125a-5p expression (P<0.01), with the high miR-125a-5p expression group having a longer overall survival time, compared with those with low level expression of miR-125a-5p. In contrast, high p38 expression was associated with a high rate of poor survival in the SACC patients (Figure 5D). When multivariate Cox analyses was carried out to determine whether miR-125a-5p expression is an independent prognostic factor of SACC patient outcomes, as Table 2 shows, low level expression of miR-125a-5p was regarded as an independent prognostic factors for poor survival of SACC patients (P=0.003). Taken together, our data suggest that miR-125a-5p might be a potential biomarker for the prognosis of SACC patients.
Table 2.
Multivariate analysis of factors associated with lung metastasis survival of patients with SACC
| Clinical variables | HR | 95% CI | P value |
|---|---|---|---|
| Gender (male vs female) | 1.4 | 0.7-1.9 | 0.35 |
| Age (≥50 vs <50 ) | 0.9 | 0.6-1.5 | 0.21 |
| Tumor size (≥3 cm vs <3 cm) | 1.3 | 0.6-3.0 | 0.09 |
| TNM stage (III+V vs I+II) | 1.5 | 0.7-2.3 | 0.09 |
| Node metastasis (N1 vs N0) | 1.9 | 0.8-3.1 | 0.13 |
| Distant metastasis (M1 vs M0) | 0.8 | 0.6-1.7 | 0.13 |
| P38 expression (high vs low) | 1.6 | 0.9-2.8 | 0.08 |
| miR-125a-5p expression (high vs low) | 0.5 | 0.3-0.9 | 0.003 |
MiR-125a-5p regulates SACC progression through p38/JNK/ERK signal pathway
All the results above suggested that reduction of miR-125a-5p is closely associated with poor prognosis in patients of SACC; however, the underlying molecular mechanism which mediated this effect was little known and should be elucidated. It has been abundantly described that the phosphorylation of p38 can trigger downstream JNK-ERK pathway activation, which promotes multiple types of tumor development and progression. Therefore, the phosphorylation of p38/JNK/ERK was detected by WB using miR-125a-5p inhibitor in SACC-83 and the results shown in the Figure 6A, the expression level of phosphorylated p38/JNK/ERK were increased. In contrast, there is no change with the expression of total JNK and ERK. Meanwhile, when miR-125a-5p mimics were used, the activation of phosphorylated p38, JNK and ERK were inhibited in SACC-LM and with no change about total JNK/ERK expression (Figure 6B). Given the pivotal role and function of p38/JNK/ERK in tumor promotion of multiple types of cancer, we hypothesize that the p38/JNK/ERK signal pathway plays a strong role in SACC development and progression.
Figure 6.
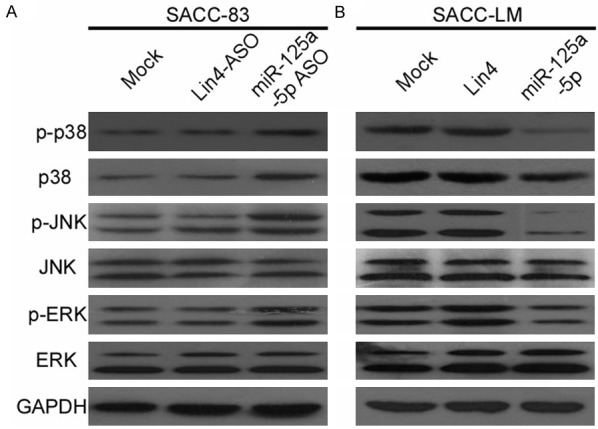
miR-125a-5p targets the p38/JNK/ERK pathway. A and B. Western blot analysis of phosphorylated p38, total p38, phosphorylated JNK, total JNK, phosphorylated ERK, and total ERK protein in SACC-LM and SACC-83 cells transduced with miR-125a-5p antisense oligonucleotides (ASO) and mimics. GAPDH was loaded as an internal control.
Discussion
SACC is a rare salivary gland malignant tumor that has a high rate of distant-organ metastasis and recurrence despite its slow, but persistent, growth [3,28]. Even when treated with effective therapeutic drugs, most patients still succumb to metastasis [29,30]. Therefore, to develop novel therapeutic strategies to improve patient survival, it is essential to determine the underlying mechanisms that regulate SACC development and progression.
miRNAs play pivotal roles in the invasion and metastasis of multiple malignances. However, expression profiles and the dysregulation of miRNAs in the development and progression of SACC have not been characterized. In the current study, differential miRNA expression profiles were compared among three SACC cell lines with metastatic potential. The down-regulation of miR-125a-5p was observed in a SACC cell line with high metastatic potential (SACC-LM). The decrease in expression was further verified by qPCR. miR-125a-5p is down-regulated in hepatocellular carcinoma tissues and cancer cell lines, and ectopic overexpression of miR-125a-5p can attenuate the proliferation and invasion of hepatocellular carcinoma cells by directly targeting MMP11 and VEGF. In gastric cancer cells, miR-125a-5p suppresses gastric cancer metastasis and progression by regulating the oncogene ERBB2 [17]. Furthermore, a combination of a miR-125a-5p inhibitor and trastuzumab can severely impair tumor proliferation. miR-125a-5p can also regulate breast cancer tumorigenesis by targeting HDAC4 [31]. In nasopharyngeal carcinoma, up-regulation of miR-125a-5p can improve tumor-cell sensitivity to and the effectiveness of gefitinib, both in vitro and in vivo [32].
Here, we observed that miR-125a-5p inhibited the migration and invasion of SACC cells in vitro. Additionally, the down-regulation of miR-125a-5p was involved in SACC metastasis. Therefore, miR-125a-5p may regulate the metastasis of SACC and function as a tumor suppressor in SACC by targeting p38, an oncogene identified in multiple tumor models [33-36]. Overexpression of p38 is strongly associated with tumor progression and poor prognosis in numerous tumors [22]. Furthermore, the integration and crosstalk between p38 MAPK families and JNK signals can affect tumor proliferation, differentiation, survival, and migration [37,38]. Deregulated p38/MAPK signaling in colon cancer cells is associated with metastasis to liver and lung [20,39]. Activation of p38 MAPK promotes metastasis in gastric adenocarcinoma and bladder cancer via up-regulation of MMP2 and MMP9 [40]. Inhibition of p38 MAPK signaling sensitizes cells to apoptosis by activating the JNK pathway in breast cancer and colon cancer cell lines [41]. The dysregulation of p38 MAPK also mediates osteosarcoma cell metastasis through epithelial mesenchymal transition (EMT) induced by TGF-β [23]. Tumor-cell dissemination and distant metastasis is the main cause of mortality in SACC patients [42]. Therefore, revealing the underlying mechanisms of dissemination and metastasis will greatly assist in developing more effective treatments for SACC patients.
miR-125a-5p was down-regulated in an aggressive phenotype of SACC cells, as determined by miRNA microarray analysis and confirmed by qPCR analysis. Furthermore, this down-regulation was consistent in multiple SACC cell lines and tissue samples. Using miRNA mimics and an inhibitor, we found that down-regulation of miR-125a-5p promotes SACC-cell migration and invasion, while up-regulation of miR-125a-5p attenuates tumor-cell motility. Furthermore, p38 was identified as the target of miR-125a-5p using microRNA target prediction software and was confirmed by dual-luciferase assay. This negative correlation was analyzed by fluorescence in situ hybridization (FISH) and immunohistochemistry. Clinical analysis also showed that low expression levels of miR-125a-5p were closely associated with poor prognosis of SACC patients. The activation of the p38/JNK/ERK signal pathway under the regulation of miR-125a-5p may be the cause.
In summary, our data showed that miR-125a-5p should be further studied as a novel metastasis-related prediction biomarker in SACC patients. Our findings provide a strong rationale for the potential use of miR-125a-5p as a therapeutic target and prognostic biomarker for SACC.
Acknowledgements
This work was supported by International Cooperation Project of Science and Technology Research Program of Guangdong Province (2010B050700005), Science and Technology Planning Project of Guangdong Province (2012B031800029) and Natural Science Foundation of Guangdong Province (2014A030313025).
Disclosure of conflict of interest
None.
References
- 1.Ho AS, Kannan K, Roy DM, Morris LG, Ganly I, Katabi N, Ramaswami D, Walsh LA, Eng S, Huse JT, Zhang J, Dolgalev I, Huberman K, Heguy A, Viale A, Drobnjak M, Leversha MA, Rice CE, Singh B, Iyer NG, Leemans CR, Bloemena E, Ferris RL, Seethala RR, Gross BE, Liang Y, Sinha R, Peng L, Raphael BJ, Turcan S, Gong Y, Schultz N, Kim S, Chiosea S, Shah JP, Sander C, Lee W, Chan TA. The mutational landscape of adenoid cystic carcinoma. Nat Genet. 2013;45:791–798. doi: 10.1038/ng.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stephens PJ, Davies HR, Mitani Y, Van Loo P, Shlien A, Tarpey PS, Papaemmanuil E, Cheverton A, Bignell GR, Butler AP, Gamble J, Gamble S, Hardy C, Hinton J, Jia M, Jayakumar A, Jones D, Latimer C, McLaren S, McBride DJ, Menzies A, Mudie L, Maddison M, Raine K, Nik-Zainal S, O’Meara S, Teague JW, Varela I, Wedge DC, Whitmore I, Lippman SM, McDermott U, Stratton MR, Campbell PJ, El-Naggar AK, Futreal PA. Whole exome sequencing of adenoid cystic carcinoma. J Clin Invest. 2013;123:2965–2968. doi: 10.1172/JCI67201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li J, Perlaky L, Rao P, Weber RS, El-Naggar AK. Development and characterization of salivary adenoid cystic carcinoma cell line. Oral Oncol. 2014;50:991–999. doi: 10.1016/j.oraloncology.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frierson HF Jr, Moskaluk CA. Mutation signature of adenoid cystic carcinoma: evidence for transcriptional and epigenetic reprogramming. J Clin Invest. 2013;123:2783–2785. doi: 10.1172/JCI69070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drier Y, Cotton MJ, Williamson KE, Gillespie SM, Ryan RJ, Kluk MJ, Carey CD, Rodig SJ, Sholl LM, Afrogheh AH, Faquin WC, Queimado L, Qi J, Wick MJ, El-Naggar AK, Bradner JE, Moskaluk CA, Aster JC, Knoechel B, Bernstein BE. An oncogenic MYB feedback loop drives alternate cell fates in adenoid cystic carcinoma. Nat Genet. 2016;48:265–272. doi: 10.1038/ng.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu H, Du L, Wang R, Wei C, Liu B, Zhu L, Liu P, Liu Q, Li J, Lu SL, Xiao J. High frequency of loss of PTEN expression in human solid salivary adenoid cystic carcinoma and its implication for targeted therapy. Oncotarget. 2015;6:11477–11491. doi: 10.18632/oncotarget.3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang YL, Liu X, Gao SY, Feng H, Jiang YP, Wang SS, Yang J, Jiang J, Ma XR, Tang YJ, Chen Y, Liang XH. WIP1 stimulates migration and invasion of salivary adenoid cystic carcinoma by inducing MMP-9 and VEGF-C. Oncotarget. 2015;6:9031–9044. doi: 10.18632/oncotarget.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ivanov SV, Panaccione A, Brown B, Guo Y, Moskaluk CA, Wick MJ, Brown JL, Ivanova AV, Issaeva N, El-Naggar AK, Yarbrough WG. TrkC signaling is activated in adenoid cystic carcinoma and requires NT-3 to stimulate invasive behavior. Oncogene. 2013;32:3698–3710. doi: 10.1038/onc.2012.377. [DOI] [PubMed] [Google Scholar]
- 9.Jiang Y, Feng X, Zheng L, Li SL, Ge XY, Zhang JG. Thioredoxin 1 mediates TGF-beta-induced epithelial-mesenchymal transition in salivary adenoid cystic carcinoma. Oncotarget. 2015;6:25506–25519. doi: 10.18632/oncotarget.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Leva G, Garofalo M, Croce CM. MicroRNAs in cancer. Annu Rev Pathol. 2014;9:287–314. doi: 10.1146/annurev-pathol-012513-104715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin S, Gregory RI. MicroRNA biogenesis pathways in cancer. Nat Rev Cancer. 2015;15:321–333. doi: 10.1038/nrc3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ameres SL, Zamore PD. Diversifying microRNA sequence and function. Nat Rev Mol Cell Biol. 2013;14:475–488. doi: 10.1038/nrm3611. [DOI] [PubMed] [Google Scholar]
- 13.Pencheva N, Tavazoie SF. Control of metastatic progression by microRNA regulatory networks. Nat Cell Biol. 2013;15:546–554. doi: 10.1038/ncb2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bi Q, Tang S, Xia L, Du R, Fan R, Gao L, Jin J, Liang S, Chen Z, Xu G, Nie Y, Wu K, Liu J, Shi Y, Ding J, Fan D. Ectopic expression of MiR-125a inhibits the proliferation and metastasis of hepatocellular carcinoma by targeting MMP11 and VEGF. PLoS One. 2012;7:e40169. doi: 10.1371/journal.pone.0040169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsieh TH, Hsu CY, Tsai CF, Long CY, Chai CY, Hou MF, Lee JN, Wu DC, Wang SC, Tsai EM. miR-125a-5p is a prognostic biomarker that targets HDAC4 to suppress breast tumorigenesis. Oncotarget. 2015;6:494–509. doi: 10.18632/oncotarget.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuan J, Xiao G, Peng G, Liu D, Wang Z, Liao Y, Liu Q, Wu M, Yuan X. MiRNA-125a-5p inhibits glioblastoma cell proliferation and promotes cell differentiation by targeting TAZ. Biochem Biophys Res Commun. 2015;457:171–176. doi: 10.1016/j.bbrc.2014.12.078. [DOI] [PubMed] [Google Scholar]
- 17.Nishida N, Mimori K, Fabbri M, Yokobori T, Sudo T, Tanaka F, Shibata K, Ishii H, Doki Y, Mori M. MicroRNA-125a-5p is an independent prognostic factor in gastric cancer and inhibits the proliferation of human gastric cancer cells in combination with trastuzumab. Clin Cancer Res. 2011;17:2725–2733. doi: 10.1158/1078-0432.CCR-10-2132. [DOI] [PubMed] [Google Scholar]
- 18.Kim JK, Noh JH, Jung KH, Eun JW, Bae HJ, Kim MG, Chang YG, Shen Q, Park WS, Lee JY, Borlak J, Nam SW. Sirtuin7 oncogenic potential in human hepatocellular carcinoma and its regulation by the tumor suppressors MiR-125a-5p and MiR-125b. Hepatology. 2013;57:1055–1067. doi: 10.1002/hep.26101. [DOI] [PubMed] [Google Scholar]
- 19.Liu S, Ye D, Xu D, Liao Y, Zhang L, Liu L, Yu W, Wang Y, He Y, Hu J, Guo W, Wang T, Sun B, Song H, Yin H, Liu J, Wu Y, Zhu H, Zhou BP, Deng J, Zhang Z. Autocrine epiregulin activates EGFR pathway for lung metastasis via EMT in salivary adenoid cystic carcinoma. Oncotarget. 2016;7:25251–63. doi: 10.18632/oncotarget.7940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Urosevic J, Garcia-Albeniz X, Planet E, Real S, Cespedes MV, Guiu M, Fernandez E, Bellmunt A, Gawrzak S, Pavlovic M, Mangues R, Dolado I, Barriga FM, Nadal C, Kemeny N, Batlle E, Nebreda AR, Gomis RR. Colon cancer cells colonize the lung from established liver metastases through p38 MAPK signalling and PTHLH. Nat Cell Biol. 2014;16:685–694. doi: 10.1038/ncb2977. [DOI] [PubMed] [Google Scholar]
- 21.Hiratsuka S, Duda DG, Huang Y, Goel S, Sugiyama T, Nagasawa T, Fukumura D, Jain RK. C-X-C receptor type 4 promotes metastasis by activating p38 mitogen-activated protein kinase in myeloid differentiation antigen (Gr-1)-positive cells. Proc Natl Acad Sci U S A. 2011;108:302–307. doi: 10.1073/pnas.1016917108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer. 2009;9:537–549. doi: 10.1038/nrc2694. [DOI] [PubMed] [Google Scholar]
- 23.Odagiri H, Kadomatsu T, Endo M, Masuda T, Morioka MS, Fukuhara S, Miyamoto T, Kobayashi E, Miyata K, Aoi J, Horiguchi H, Nishimura N, Terada K, Yakushiji T, Manabe I, Mochizuki N, Mizuta H, Oike Y. The secreted protein ANGPTL2 promotes metastasis of osteosarcoma cells through integrin alpha5beta1, p38 MAPK, and matrix metalloproteinases. Sci Signal. 2014;7:ra7. doi: 10.1126/scisignal.2004612. [DOI] [PubMed] [Google Scholar]
- 24.Yamashita M, Fatyol K, Jin C, Wang X, Liu Z, Zhang YE. TRAF6 mediates Smad-independent activation of JNK and p38 by TGF-beta. Mol Cell. 2008;31:918–924. doi: 10.1016/j.molcel.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guan X, Qiu W, He R. [The selection of highly lung metastatic salivary adenoid cystic carcinoma clone] . Zhonghua Kou Qiang Yi Xue Za Zhi. 1996;31:74–77. [PubMed] [Google Scholar]
- 26.Li SL. [Establishment of a human cancer cell line from adenoid cystic carcinoma of the minor salivary gland] . Zhonghua Kou Qiang Yi Xue Za Zhi. 1990;25:29–31. 62. [PubMed] [Google Scholar]
- 27.Sun L, Yao Y, Liu B, Lin Z, Lin L, Yang M, Zhang W, Chen W, Pan C, Liu Q, Song E, Li J. MiR-200b and miR-15b regulate chemotherapy-induced epithelial-mesenchymal transition in human tongue cancer cells by targeting BMI1. Oncogene. 2012;31:432–445. doi: 10.1038/onc.2011.263. [DOI] [PubMed] [Google Scholar]
- 28.Su BH, Qu J, Song M, Huang XY, Hu XM, Xie J, Zhao Y, Ding LC, She L, Chen J, Lin LS, Lin X, Zheng DL, Lu YG. NOTCH1 signaling contributes to cell growth, anti-apoptosis and metastasis in salivary adenoid cystic carcinoma. Oncotarget. 2014;5:6885–6895. doi: 10.18632/oncotarget.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brayer KJ, Frerich CA, Kang H, Ness SA. Recurrent fusions in MYB and MYBL1 define a common, transcription factor-driven oncogenic pathway in salivary gland adenoid cystic carcinoma. Cancer Discov. 2016;6:176–187. doi: 10.1158/2159-8290.CD-15-0859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen W, Cao G, Yuan X, Zhang X, Zhang Q, Zhu Y, Dong Z, Zhang S. Notch-1 knockdown suppresses proliferation, migration and metastasis of salivary adenoid cystic carcinoma cells. J Transl Med. 2015;13:167. doi: 10.1186/s12967-015-0520-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsieh TH, Hsu CY, Tsai CF, Long CY, Chai CY, Hou MF, Lee JN, Wu DC, Wang SC, Tsai EM. miR-125a-5p is a prognostic biomarker that targets HDAC4 to suppress breast tumorigenesis. Oncotarget. 2014;6:494–509. doi: 10.18632/oncotarget.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Y, Li Z, Wu L, Wang Z, Wang X, Yu Y, Zhao Q, Luo F. MiRNA-125a-5p: a regulator and predictor of gefitinib’s effect on nasopharyngeal carcinoma. Cancer Cell Int. 2014;14:24. doi: 10.1186/1475-2867-14-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kennedy NJ, Cellurale C, Davis RJ. A radical role for p38 MAPK in tumor initiation. Cancer Cell. 2007;11:101–103. doi: 10.1016/j.ccr.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 34.Han J, Sun P. The pathways to tumor suppression via route p38. Trends Biochem Sci. 2007;32:364–371. doi: 10.1016/j.tibs.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 35.Koul HK, Pal M, Koul S. Role of p38 MAP kinase signal transduction in solid tumors. Genes Cancer. 2013;4:342–359. doi: 10.1177/1947601913507951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.del Barco Barrantes I, Nebreda AR. Roles of p38 MAPKs in invasion and metastasis. Biochem Soc Trans. 2012;40:79–84. doi: 10.1042/BST20110676. [DOI] [PubMed] [Google Scholar]
- 37.Chesi G, Hegde RN, Iacobacci S, Concilli M, Parashuraman S, Festa BP, Polishchuk EV, Di Tullio G, Carissimo A, Montefusco S, Canetti D, Monti M, Amoresano A, Pucci P, van de Sluis B, Lutsenko S, Luini A, Polishchuk RS. Identification of p38 MAPK and JNK as new targets for correction of Wilson disease-causing ATP7B mutants. Hepatology. 2016;63:1842–59. doi: 10.1002/hep.28398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu X, Zhang CS, Lu C, Lin SC, Wu JW, Wang ZX. A conserved motif in JNK/p38-specific MAPK phosphatases as a determinant for JNK1 recognition and inactivation. Nat Commun. 2016;7:10879. doi: 10.1038/ncomms10879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ehyai S, Dionyssiou MG, Gordon JW, Williams D, Siu KW, McDermott JC. A p38 Mitogen-activated protein kinase-regulated myocyte enhancer factor 2-beta-catenin interaction enhances canonical wnt signaling. Mol Cell Biol. 2015;36:330–346. doi: 10.1128/MCB.00832-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kumar B, Koul S, Petersen J, Khandrika L, Hwa JS, Meacham RB, Wilson S, Koul HK. p38 mitogen-activated protein kinase-driven MAPKAPK2 regulates invasion of bladder cancer by modulation of MMP-2 and MMP-9 activity. Cancer Res. 2010;70:832–841. doi: 10.1158/0008-5472.CAN-09-2918. [DOI] [PubMed] [Google Scholar]
- 41.Rosenthal DT, Iyer H, Escudero S, Bao L, Wu Z, Ventura AC, Kleer CG, Arruda EM, Garikipati K, Merajver SD. p38gamma promotes breast cancer cell motility and metastasis through regulation of RhoC GTPase, cytoskeletal architecture, and a novel leading edge behavior. Cancer Res. 2011;71:6338–6349. doi: 10.1158/0008-5472.CAN-11-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laurie SA, Ho AL, Fury MG, Sherman E, Pfister DG. Systemic therapy in the management of metastatic or locally recurrent adenoid cystic carcinoma of the salivary glands: a systematic review. Lancet Oncol. 2011;12:815–824. doi: 10.1016/S1470-2045(10)70245-X. [DOI] [PubMed] [Google Scholar]


