Abstract
The aim of this study was to investigate the protective effects and underlying anti-oxidative molecular mechanism of lipoxin A4 (LA4) in rats with ischemia/reperfusion (I/R)-injured skeletal muscle. A rat model of I/R-injured skeletal muscle was obtained by subjecting rats to a 3-h ligation of the right femoral artery followed by 3 h of reperfusion. Treatment with LA4 significantly ameliorated histological damage scores in I/R-injured skeletal muscle. LA4 treatment resulted in remarkable decreases in the wet weight/dry weight ratio (W/D ratio), inflammatory response, oxidative stress, and cell apoptosis. In addition, treatment with LA4 was accompanied by a prominently enhanced nuclear accumulation of nuclear factor erythroid 2-related factor 2 (Nrf2) and expression of heme oxygenase 1 (HO-1) in the I/R-injured skeletal muscle. However, these protective effects were reversed by zinc protoporphyrin-IX (ZnPP), a specific HO-1 inhibitor. Our study shows that LA4 may have the potential as a therapeutic agent for I/R-injured muscle tissue via activation of the Nrf2/HO-1 signaling pathway.
Keywords: Ischemia-reperfusion injury, skeletal muscle, lipoxin A4, oxidative stress, apoptosis, inflammatory response, Nrf2/HO-1 signaling pathway
Introduction
Ischemia-reperfusion (I/R)-induced skeletal muscle injury is inevitable in peripheral vascular injury and reconstruction surgeries. It is also common in crush syndrome, free myocutaneous flap transfer, and acute osteofascial compartment syndrome, which could deteriorate limb function [1-3]. As a result, skeletal muscle releases metabolic byproducts and proinflammatory cytokines that may cause damage to remote, non-ischemic organs [4]. Skeletal muscle ischemia induces energy depletion and accumulation of metabolic products [5]. The injury can be worsened during the reperfusion period due to the generation of reactive oxygen species (ROS), proinflammatory cytokines, and cellular infiltration, all of which lead to additional tissue damage [1]. Although skeletal muscle is not as sensitive to warm ischemia as many other organs, prolonged ischemia and subsequent reperfusion injury in severe cases may result in loss of contractility, disability, and even limb amputation. Several agents, such as dexmedetomidine, curcumin, and hydrogen-rich saline, are effective in alleviating I/R-injured skeletal muscle [6-8].
Lipoxin A4 (LA4) is a bioactive product of arachidonic acid with anti-inflammatory properties that exerts inhibitory effects on neutrophilic infiltration and ROS generation [9,10]. Recent studies have shown that LA4 can protect several organs from I/R injury [11,12]. Nuclear factor erythroid 2-related factor 2 (Nrf2), a key transcriptional factor, regulates a wide array of genes for antioxidant and detoxification enzymes in response to oxidative and xenobiotic stress. Screening many Nrf2-antioxidant response element (ARE) activators for use in the treatment of I/R injury has shown that activation of Nrf2 signaling pathway plays a role in the protection of I/R injury in many organs [13]. In addition, LA4 enhances Nrf2 nuclear transcription and expression of heme oxygenase-1 (HO-1) in cultured cortical astrocytes exposed to oxidative stress injury [14]. However, the protective effects of LA4 on I/R-injured skeletal muscle have not been elucidated. Therefore, the aim of this study was to elucidate whether LA4 could exert novel protective effects on hind limb I/R injury. Because oxidative stress, inflammation, and apoptosis are implicated in I/R-injured skeletal muscle, the production of oxidative stress-related indicators, proinflammatory cytokines, and apoptosis-related proteins were examined. Potential mechanisms by which LA4 could exert a protective effect, like regulation of Nrf2 translocation and related pathways, were evaluated.
Materials and methods
Animals
Adult male Sprague-Dawley rats weighing 250-300 g were purchased from Sippr-bk Experimental Animal Center (Shanghai, China) and kept in an animal room under standard conditions of temperature (25°C), humidity (55-60%), and light-dark cycle (12 h/12 h). Rats were housed four per cage and had free access to food and water. The protocols used here were approved by the Animal Care and Use Committee of Fudan University (Shanghai, China) and are in accordance with United States National Institutes of Health guidelines.
Animal grouping
Rats were randomly assigned to the following six different groups according to previous protocols [15]: (1) sham; (2) I/R; (3) I/R + 0.1 μg/kg LA4; (4) I/R + 1 μg/kg LA4; (5) I/R + 10 μg/kg LA4; and (6) I/R + 10 μg/kg LA4 + zinc protoporphyrin IX (ZnPP). Rats in groups 1 and 2 were treated with the LA4 vehicle. LA4 (Cayman, Ann Arbor, MI, USA) was injected into the caudal vein 1 h before reperfusion. One hour before the onset of ischemia, ZnPP (Sigma-Aldrich, St. Louis, MO, USA) was injected intraperitoneally (1.5 mg/kg). Rats in groups 2 and 5 received ZnPP vehicle (saline) as a control.
Femoral artery I/R model
Rats were anesthetized with chloral hydrate (300 mg/kg, i.p.). The right groin was shaved, and all the subsequent surgical procedures were performed under sterilized conditions. A transverse groin incision was made, and the right groin vessels were exposed. The femoral artery was isolated and occluded with an atraumatic microvascular clamp. A rubber band was applied to the right greater trochanter after limb exsanguination to ensure interruption of the entire hind limb blood supply. After 3 h under these ischemic conditions, the clamp and rubber band were removed and blood reflow was allowed in the distal limb for an additional 3 h before sampling. A heat lamp was used to maintain each rat’s body temperature at 37 ± 0.5°C during the experiment.
At the end of the reperfusion period, the right anterior tibial and gastrocnemius muscles were harvested and washed with cold phosphate-buffered saline (PBS, 0.01 M, pH 7.4). The tibialis anterior muscles (n = 6 for each group) were used for analysis of tissue edema. Part of the medial heads of six gastrocnemius muscles was stored at -80°C for Western blot analysis. The other part of the medial heads of the six gastrocnemius muscles was homogenized using cold Tris-HCl buffer (pH 7.4). The homogenate was centrifuged at 3000 g for 20 min, and then the supernatant was collected. A bicinchoninic acid (BCA) protein assay reagent kit (Beyotime Biotechnology, Shanghai, China) was used to determine total protein concentrations. The lateral heads of the gastrocnemius muscle samples were fixed in 4% paraformaldehyde (n = 6 for each group) for hematoxylin and eosin (HE) staining and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining (Roche Life Sciences, Indianapolis, IN, USA).
Wet weight/dry weight ratio (W/D ratio) of muscular tissue
The tibialis anterior muscle samples were weighed (wet weight) immediately after being harvested from the right hind limb using an electronic balance (scale interval: 0.1 mg; R200D, Sartorius, Germany). The muscles were dehydrated at 60°C for 72 h and then reweighed (dry weight). Tissue edema was characterized by a W/D ratio as follows: W/D ratio = (wet weight/dry weight) × 100%.
Histological evaluation
Paramaldehyde-fixed muscle samples were processed after being embedded in paraffin, sectioned into 2-μm slices, and stained with HE. Sections were photographed from five random fields under 400 × magnification. Blinded observers scored morphological impairment according to previously published methods based on muscle fiber disorganization and degeneration and inflammatory cell infiltration [8].
TUNEL staining
Paraffin-embedded muscle samples were cut into 2-μm thick sections. The sections were stained according to TUNEL methods using an In Situ Cell Death Detection Kit according to the manufacturer’s protocols. Sections were observed under a light microscope (DWLB2; Leica, Hamburg, Germany). The average number of TUNEL-positive cells was determined from five randomized fields per section at 400 × magnitude by an observer who was blinded to the grouping. The results are expressed as a percentage of TUNEL-positive cells to total cells.
Neutrophilic infiltration
Neutrophilic infiltration was assessed by determining the activity of myeloperoxidase (MPO) using an immunofluorescent staining technique and detecting the H2O2-dependent oxidation of 3,3’-dimethoxybenzidine (MPO Detection Kit; Nanjing Jiancheng Biotechnology Institute, Nanjing, China). The MPO activity of the supernatant is expressed as units per gram protein. The absorbance was measured by spectrometry (Tacan M200, Salzburg, Austria) at 460 nm. Paraffin-embedded samples were cut into 2-μm thick sections. After antigen retrieval, sections were first incubated with 3% H2O2 for 10 min to block endogenous catalase and then with calf serum to block non-specific binding. Next, sections were incubated with anti-MPO antibody (1:100 diluted, rabbit polyclonal, Beyotime Biotechnology) at 4°C overnight. Alex flora 594 conjugated anti-rabbit secondary antibody (1:100) was used to mark the primary antibody, and 4’,6-diamidino-2-phenylindole (DAPI) was used to mark the nucleus. Sections were photographed using a fluorescence microscope (DWLB2, Hamburg, Leica) at 200 × magnitude.
Measurement of lipid peroxidation and antioxidase activities
Malondialdehyde (MDA) levels were used as an indicator of lipid peroxidation. MDA concentrations were measured using a commercially available kit (Nanjing Jiancheng Biotechnology Institute). The absorbance was measured by spectrometry (Tacan M200) at 532 nm. The concentration of MDA was expressed as nanomole per gram protein. The activities of total superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px) in the muscle homogenate were measured using kits (Nanjing Jiancheng Biotechnology Institute) according to the manufacturer’s instructions. SOD, CAT, and GSH-Px activities were expressed as unit per milligram protein.
Inflammatory cytokines
The levels of tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6) in the supernatants of the muscular tissue were measured by ELISA kits (ExCell Biology, Shanghai, China) according to the manufacturer’s instructions. The levels of cytokines were expressed as pg/ml protein.
Western blotting
Total proteins were extracted from the gastrocnemius muscle using a radio-immunoprecipitation assay (RIPA) lysate (Beyotime Biotechnology). Nuclear extracts were prepared using a nuclear and cytoplasmic protein extraction kit (Beyotime Biotechnology) for detection of nuclear expression of Nrf2. The concentrations of protein in samples were determined using a BCA protein assay kit. Equal amounts of protein samples were fractioned per line, electrophoresed in a 10% SDS-PAGE gel, and transferred to polyvinylidene difluoride membranes. The membranes were blocked with 5% fat-free milk. Membranes were blotted with primary antibodies for Nrf2 (1:500, goat polyclonal; Santa Cruz Biotechnology, Santa Cruz, CA, USA), HO-1 (1:500, rabbit polyclonal; Santa Cruz Biotechnology), Bcl-2 (1:500, rabbit polyclonal; Boster, Wuhan, China), Bax (1:500, rabbit polyclonal; Boster), β-actin (1:500, rabbit polyclonal; Guge Biological Technology Co., Wuhan, China), and histone H3 (1:500. rabbit polyclonal; Guge Biological Technology Co., Wuhan, China). Next, the membranes were washed with 1 × tris-buffered saline containing 0.01% (v/v) Tween-20 (TBS-T). The membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies (1:10000; Guge Biological Technology Co.). An enhanced chemiluminscence detection system was used to visualize the bands on the membranes. Band density was quantified using Image J software. The Nrf2 expression levels were normalized to histone H3, whereas the HO-1, Bcl-2, and Bax levels were expressed as a ratio of β-actin.
Statistical analysis
Statistical analyses were performed using SPSS 17.0 software (SPSS Inc., Chicago, IL, USA). Data were expressed as the mean ± standard error of the mean (SEM). The means of the different groups were compared by one-way analysis of variance (ANOVA) followed by Student-Newman-Keuls tests (for normally distributed data) or Kruskal-Wallis tests followed by Dunnett’s T3 tests (for data that were not normally distributed). A probability of P < 0.05 was considered statistically significant.
Results
LA4 mitigates tissue edema in rats with I/R injury
The skeletal muscle W/D ratio in the I/R injury group was significantly increased (P < 0.01) compared with that in the control (sham) group (Figure 1). Treatments of I/R-injured rats with LA4 resulted in dose-dependent changes in tissue edema (Figure 1). The W/D ratio in the I/R-injury animals treated with the lowest dose (0.1 μg/kg) of LA4 did not differ significantly (P > 0.05) compared with that of animals in the untreated I/R-injury group. The W/D ratios of animals in the I/R injury groups treated with higher doses of LA4 (1 and 10 μg/kg) were significantly decreased (P < 0.01) compared with that of animals in the untreated I/R-injury group.
Figure 1.
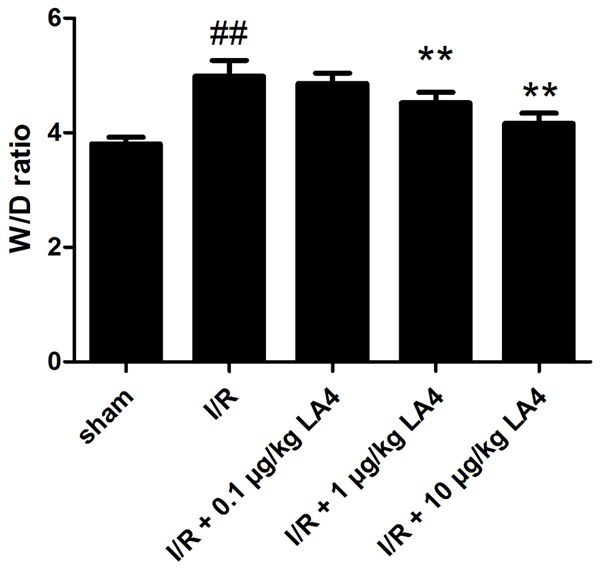
Effects of administration of LA4 on tissue edema in skeletal muscle with I/R injury. The data are presented as mean ± SD. ##P < 0.01 compared with that of sham group; **P < 0.01 compared with that of I/R group.
LA4 decreases histological damage scores of muscle tissue in rats with I/R injury
Muscular tissue injury was assessed based on the morphological changes detected with HE staining. Muscular fiber injury, sarcoplasm dissolution, neutrophil infiltration, and erythrocyte diapedesis were observed in the muscular tissue of the animals in the I/R group (Figure 2B), but not in that of the sham group (Figure 2A). Consequently, histological damage scores increased significantly in the I/R group versus the sham group (Figure 2F, P < 0.01). Treatment of I/R rats with LA4 significantly reduced muscle fiber injury in a dose-dependent manner, with less sarcoplasm dissolution and neutrophil infiltration (Figure 2C-E). As a result, LA4 decreased histological damage scores in the muscular tissue following I/R injury in a dose-dependent manner (Figure 2F).
Figure 2.
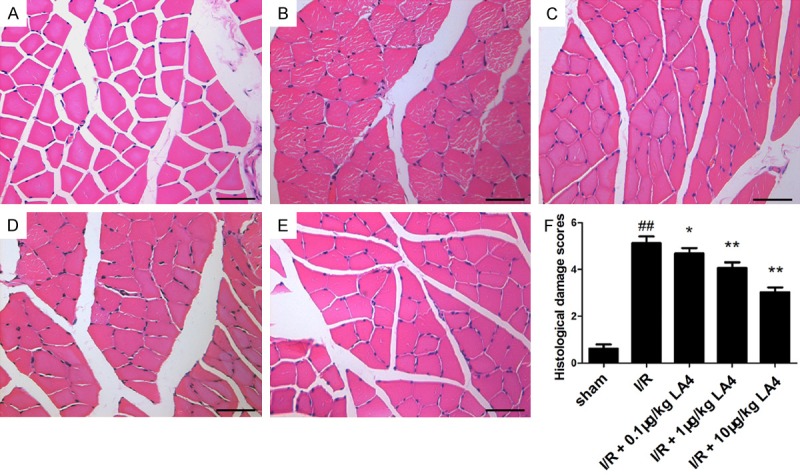
Representative photomicrographs of skeletal muscle tissue following HE staining (scale bar = 50 μm). A. Sham group. B. I/R group. C. I/R + 0.1 μg/kg LA4 group. D. I/R + 1 μg/kg LA4 group. E. I/R + 10 μg/kg LA4 group. F. Quantification of histological damage scores in different groups. ##P < 0.01 compared with that of sham group; *P < 0.05 and **P < 0.01 compared with that of I/R group.
LA4 suppresses skeletal muscle apoptosis in rats with I/R injury
Cell apoptosis of the skeletal muscle was assessed by TUNEL staining. The sham group showed few apoptosis positive cells (Figure 3A). The percentage of TUNEL-positive cells increased significantly in the I/R group versus the sham group (Figure 3F, P < 0.01). Treatment with LA4 dose-dependently decreased the percentage of TUNEL-positive cells. Furthermore, consistent with these TUNEL staining results, LA4 dose-dependently downregulated the expression of Bax and upregulated the expression of Bcl-2 (Figure 3G-I). Treatment with higher doses of LA4 (1 and 10 μg/kg), but not the lower dose (0.1 μg/kg), resulted in significant anti-apoptosis effects.
Figure 3.
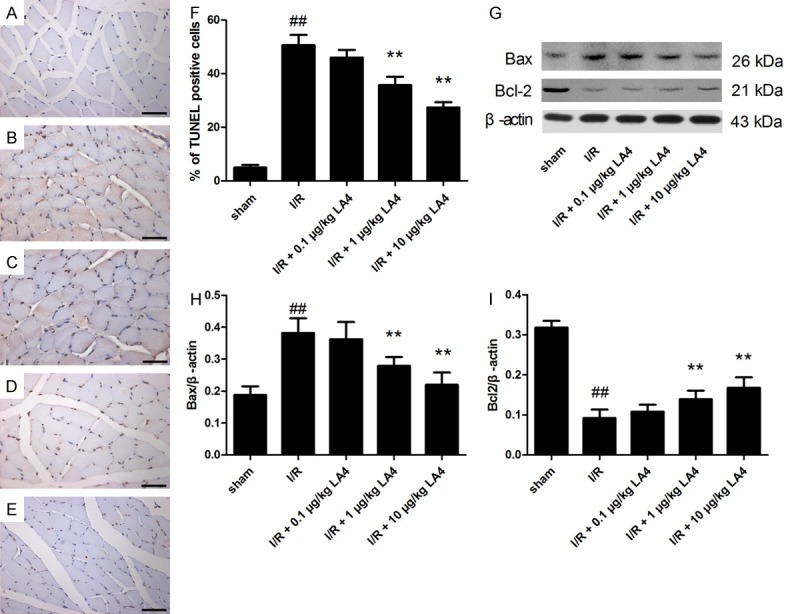
Protective effects of LA4 on muscle apoptosis in rats with I/R injury evaluated by TUNEL staining and Western blotting. (A) Sham group. (B) I/R group. (C) I/R + 0.1 μg/kg LA4 group. (D) I/R+ 1 μg/kg LA4 group. (E) I/R + 10 μg/kg LA4 group. (F) Quantification of apoptosis cells. (G) Blots of Bax and Bcl-2. (H, I) Semi-quantification of protein levels. Data are presented as mean ± SD. Scale bar = 50 μm (A-E). ##P < 0.01 compared with that of sham group; **P < 0.01 compared with that of I/R group.
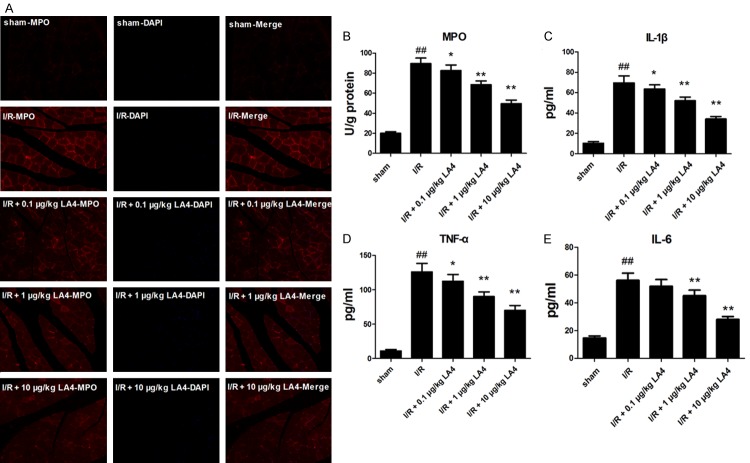
LA4 inhibits skeletal muscle neutrophilic infiltration and inflammatory response in rats with I/R injury
Neutrophilic infiltration was assessed by determining MPO activity using immunofluorescent staining against MPO. The activity of MPO in the I/R group significantly increased compared with that of sham group (Figure 4B, P < 0.01), whereas treatment with 0.1, 1, and 10 μg/kg LA4 markedly decreased or ameliorated the increase in the MPO activity compared with that of I/R group. According to double-immunofluorescent staining results, LA4 treatment significantly decreased the number of MPO positive cells (Figure 4A). Concurrently, levels of TNF-α, IL-1β, and IL-6 were significantly increased in the I/R group compared with the levels in the sham group (Figure 4C-E, P < 0.01). Like its effects on MPO activity, LA4 treatment reduced levels of inflammatory cytokines in a dose-dependent manner compared with those in the I/R group. In particular, LA4 (1 and 10 μg/kg) treatments significantly reduced the IL-6 levels.
Figure 4.
Effect of LA4 treatment on I/R-injured skeletal muscle inflammatory responses. A. Immunofluorescent staining of MPO and DAPI in skeletal muscle sections (magnitude 200 ×) showing LA4 treatment decreased the number of MPO-positive cells. B-E. Changes in MPO activity and concentrations of TNF-α, IL-1β, and IL-6 in skeletal muscle tissue showing increased MPO activity, levels of TNF-α, IL-1β, and IL-6 in the muscle tissue after I/R injury, and significant reduction in this response following treatment with LA4. ##P < 0.01 compared with sham group; *P < 0.05 and **P < 0.01 compared with the I/R group.
LA4 attenuates skeletal muscle oxidative stress in rats with I/R injury
Skeletal muscle oxidative stress status was assessed by MDA level and SOD, CAT, and GSH-Px activities. The MDA level increased and SOD, CAT, and GSH-Px activities decreased significantly in the I/R-injured skeletal muscle group compared with that of sham group (Figure 5, P < 0.01). Additionally, treatment with LA4 (1 or 10 μg/kg) significantly decreased the tissue MDA level and increased the tissue SOD, CAT, and GSH-Px activities compared with that of the I/R group (Figure 5).
Figure 5.
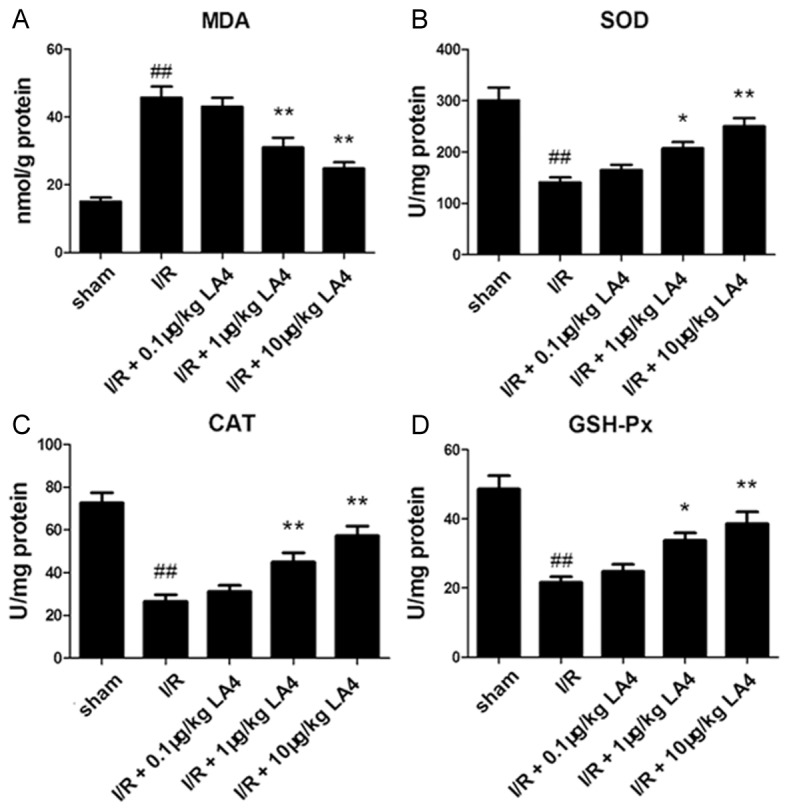
Changes in MDA, SOD, CAT, and GSH-Px in muscle tissue (A-D). The content of MDA increases in the muscular tissue after I/R injury, the activities of SOD, CAT, and GSH-Px decrease, and LA4 treatment reverses these injury-induced changes. ##P < 0.01 compared with the sham group; *P < 0.05 and **P < 0.01 compared with the I/R group.
Involvement of activation of the Nrf2/HO-1 signaling pathway in the protective effects of LA4 in rats with I/R-injured skeletal muscle
To further explore the mechanism of the protective effects of LA4, the Nrf2/HO-1 defense pathway was investigated. Western blot analysis indicated that Nrf2 nuclear translocation was increased by LA4 treatment in a dose-dependent manner in I/R-injured muscle (Figure 6A, 6B). In addition, quantification of Nrf2 and HO-1 levels showed that LA4 dose-dependently activated the Nrf2/HO-1 signaling pathway (Figure 6A, 6C). Administration of ZnPP 1 h before I/R injury, to block the Nrf2/HO-1 signaling pathway, resulted in partial elimination of the protective effects of LA4 treatment on characteristics of skeletal muscle I/R injury, including edema and histologic damage scores (Figure 6D-H).
Figure 6.
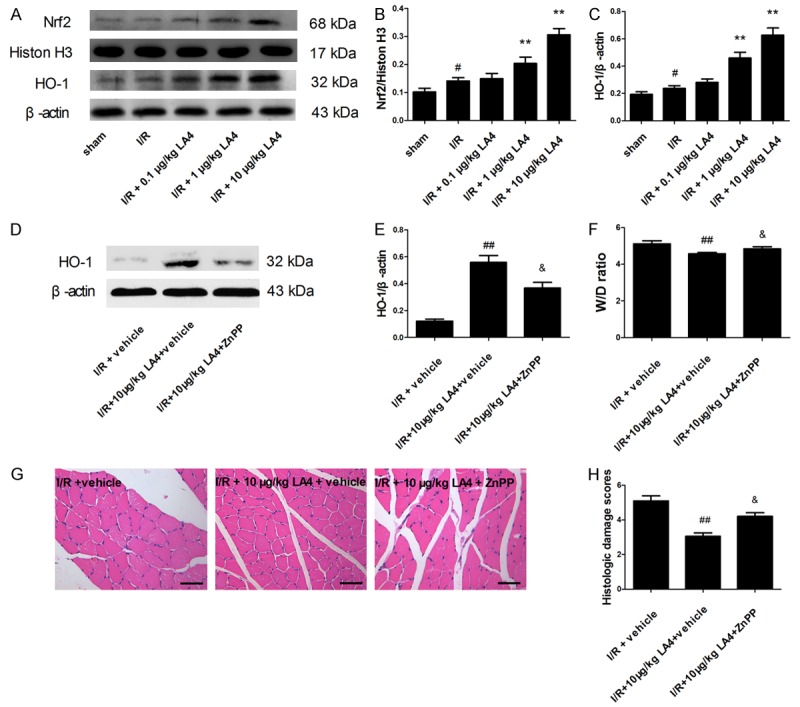
Involvement of activation of the Nrf2/HO-1 signaling pathway in the protective effects of LA4 in rats with skeletal muscle I/R injury. A. Protein expression of Nrf2 and HO-1. B, C. Quantification of Nrf2 and HO-1 levels showing LA4 dose dependently activated the Nrf2/HO-1 signaling pathway. D, E. ZnPP administered 1 h before ischemia and HO-1 protein expression determined by Western blotting. F. Skeletal muscle edema. G. Representative images of HE staining from I/R + vehicle group, I/R + 10 μg/kg LA4 + vehicle group, and I/R + 10 μg/kg LA4 + ZnPP group (scale bar = 50 μm). H. Histological damage scores. Results are expressed as mean ± SD. #P < 0.05 compared with sham group; **P < 0.01 compared with I/R group; ##P < 0.01 compared with I/R + vehicle group; &P < 0.05 compared with IR + 10 μg/kg LA4 + vehicle group.
Discussion
I/R injury of skeletal muscles is a common pathological process that is inevitably involved in peripheral vascular injury and reconstruction [1]. Ischemia/reperfusion (I/R) injury of skeletal muscles is a common pathological process in reconstructive surgeries. In this study, we showed that LA4 significantly reduces I/R-induced skeletal morphologic injury, tissue edema, oxidative stress injury, inflammatory response, and apoptosis. LA4 treatment of rats with I/R-injured skeletal muscle also resulted in prominently enhanced nuclear accumulation of Nrf2 and expression of HO-1. These results suggest that strategies to administer LA4 may represent a novel and efficacious treatment for I/R-injured muscle tissue.
An additional effect of the LA4 treatment in our study was enhanced Nrf2 and HO-1 expression, which impacts the process of inflammation. Inflammatory reactions triggered by skeletal muscle cell damage remove dead cells and matrix debris and are believed to be critical for infarct healing, vascularization, and skeletal muscle function restoration [16]. Puntel and co-workers [17] demonstrated that the activity of MPO could be used as an indicator of neutrophilic infiltration in skeletal muscle injury. Another factor in inflammatory reactions is infiltrated neutrophils that could release a mass of inflammatory cytokines, such as TNF-α, IL-1β, and IL-6 [18]. A previous study demonstrated that LA4 served as an endogenous anti-inflammatory mediator in several diseases [19]. In addition, inactivation of the phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT) signaling pathway in lipopolysaccharide (LPS)-stimulated lung injury and down regulation of nuclear factor κB (NF-κB) in LPS-stimulated macrophages are involved in the anti-inflammatory mechanisms of LA4 [20,21]. In this study, we also showed that LA4-treated I/R-injured rats exhibited lower levels of inflammatory cytokines and less neutrophilic infiltration in reperfused skeletal muscle than I/R-injured animals without LA4 treatment. These findings suggest that the anti-inflammatory properties of LA4 could be a previously unrevealed mechanism underlying LA4-mediated skeletal muscle protection.
It is now becoming clearer that I/R injury induces skeletal muscle cell apoptosis that may lead to muscle cell death and muscle contraction function loss. Skeletal muscle cell apoptosis begins at early stages of the reperfusion period [6]. Previous studies showed that LA4 might exert a biphasic effect on cell apoptosis. Wu and colleagues reported that high doses of LA4 might induce apoptosis in intestinal fibroblasts [22]. However, at low concentrations, LA4 protects the macrophage against I/R-induced apoptosis [23]. In accordance with these studies, our study showed that LA4 remarkably reduced the ratio of apoptosis muscle cells, increased the expression of Bcl-2, and down regulated the expression of Bax, suggesting a critical role of LA4 in regulating mitochondria-mediated apoptosis. These results indicate that an anti-apoptotic effect of LA4 may be involved in the successful treatment of I/R-induced limb injuries.
Skeletal muscle tissue damage, cell death, and apoptosis are associated with I/R injury in experimental rats [1,24,25]. Overproduction of ROS has been observed in I/R injured organs [26]. Excessive production of ROS could initiate lipid peroxidation, inactivate anti-oxidative stress-related proteins, and aggravate I/R injury [27,28]. MDA has been used as an indicator of oxidative stress during I/R injury in many organs, including kidneys and muscles [29,30]. Antioxidants such as SOD, CAT, and GSH-Px have been used as indicators of oxidative stress in skeletal muscle [31,32]. Our results suggest that LA4 could significantly ameliorate I/R injury-induced skeletal muscular oxidative stress by reducing MDA production and enhancing SOD, CAT, and GSH-Px activities. Similarly, the anti-oxidative properties of LA4 have been reported in testis I/R injury and many other oxidative stress-related diseases [33,34]. These results suggest that the anti-oxidative stress effect of LA4 may be protective in cases of limb I/R injury.
Under normal conditions, Nrf2 is bound to Keap-1 in the cytoplasm. In response to oxidative stress, Nrf2 has been reported to be released from Keap-1, to translocate to the nucleus, to bind to AREs, and subsequently to induce transcriptions of anti-oxidative genes [35,36]. We found that nuclear-translocated Nrf2, as well as its downstream target HO-1, increased under I/R injury conditions. LA4 further increased nuclear translocation of Nrf2 and expression of HO-1 in a dose-dependent manner in reperfused skeletal muscle tissue. In addition to anti-oxidative stress effects, enhanced expression of Nrf2 and HO-1 is also associated with cell anti-apoptosis and anti-inflammatory effects [37-39]. In our study, the co-administration of ZnPP (HO-1 inhibitor) and LA4 diminished the protective effects induced by LA4 under oxidative stress in limb I/R injury. Our results suggest that LA4 protected skeletal muscle against I/R injury in part through activation of the Nrf2/HO-1 defense pathway. Previously, Wu and co-workers [14] found that LA4 increases nuclear translocation of Nrf2 in vitro in cultured cortical astrocytes under oxidative stress. Also, Han and colleagues [11] found that LA4 activates the Nrf2 pathway during intestinal I/R injury in vivo. These in vivo and in vitro reports support our results, that the mechanism of action of LA4 in treating I/R-induced skeletal muscle injuries could be activation of the Nrf2 signaling pathway in different cell types.
Limb ischemia primarily occurs during a sudden accident, so pretreatment is not possible due to the unpredictable nature of vascular injury events. Hence, administration of LA4 at the reperfusion moment or post-reperfusion has the greatest potential for clinical application in cases of limb I/R injury. The administration of LA4 in our study was around the time of reperfusion initiation, so the time mode was clinically relevant. However, our study still has some limitations. First, the optimal time of administration of LA4 during limb I/R injury was not established. In a previous experiment conducted by Zhao and co-workers [40], post-injury treatment with LA4 exerted a better protective effect than pretreatment on ultrastructure and Na+-K+-ATPase in I/R-injured heart in rats. Future studies will focus on the optimal administration time of LA4 during skeletal muscle I/R injury. Moreover, our study only showed that activation of Nrf2/HO-1 signaling pathway was involved in the protective effects of LA4 on I/R-injured skeletal muscle. Future studies will focus on whether other signaling pathways are involved in the protective effects of LA4 in skeletal muscle I/R injury.
Conclusions
Our study demonstrates that LA4 significantly ameliorated histological damage scores in I/R-injured muscle tissue. LA4 treatment resulted in remarkably decreased wet weight/dry weight ratios, inflammatory responses, oxidative stress, and cell apoptosis accompanied by prominently enhanced nuclear accumulation of Nrf2 and expression of H0-1 in the I/R-injured muscle tissue. These protective effects were reversed by zinc protoporphyrin-IX (ZnPP), a specific HO-1 inhibitor. Our study also showed that the mechanism of action of LA4 may be activation of the Nrf2/HO-1 signaling pathway. LA4 has potential to be an important therapeutic agent for I/R-injured muscle tissue.
Acknowledgements
This work was supported by the “Dawn” Program of Shanghai Education Commission, China (Grant NO. 15SG34).
Disclosure of conflict of interest
None.
Authors’ contribution
Haiyang Zong designed and performed experiments, analysed data and wrote the manuscript. Xinghui Li designed and performed experiments on inflammatory cytokines measurement. Haodong Lin performed experiments on enzyme activity assay. Chunlin Hou, and Fenfen Ma proposed the idea and supervised the project.
References
- 1.Wang WZ, Baynosa RC, Zamboni WA. Therapeutic interventions against reperfusion injury in skeletal muscle. J Surg Res. 2011;171:175–182. doi: 10.1016/j.jss.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 2.Via AG, Oliva F, Spoliti M, Maffulli N. Acute compartment syndrome. Muscles Ligaments Tendons J. 2015;5:18–22. [PMC free article] [PubMed] [Google Scholar]
- 3.Wang WZ, Baynosa RC, Zamboni WA. Update on ischemia-reperfusion injury for the plastic surgeon: 2011. Plast Reconstr Surg. 2011;128:685e–692e. doi: 10.1097/PRS.0b013e318230c57b. [DOI] [PubMed] [Google Scholar]
- 4.Blaisdell FW. The pathophysiology of skeletal muscle ischemia and the reperfusion syndrome: a review. Cardiovasc Surg. 2002;10:620–630. doi: 10.1016/s0967-2109(02)00070-4. [DOI] [PubMed] [Google Scholar]
- 5.Lindsay TF, Liauw S, Romaschin AD, Walker PM. The effect of ischemia reperfusion on adenine-nucleotide metabolism and xanthine-oxidase production in skeletal-muscle. J Vasc Surg. 1990;12:8–15. doi: 10.1067/mva.1990.19946. [DOI] [PubMed] [Google Scholar]
- 6.Huang TL, Wang WC, Tu C, Yang ZY, Bramwell D, Sun XJ. Hydrogen-rich saline attenuates ischemia-reperfusion injury in skeletal muscle. J Surg Res. 2015;194:471–480. doi: 10.1016/j.jss.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 7.Dong X, Xing QZ, Li Y, Han XC, Sun LX. Dexmedetomidine protects against ischemia-reperfusion injury in rat skeletal muscle. J Surg Res. 2014;186:240–245. doi: 10.1016/j.jss.2013.07.052. [DOI] [PubMed] [Google Scholar]
- 8.Avci G, Kadioglu H, Sehirli AO, Bozkurt S, Guclu O, Arslan E, Muratli SK. Curcumin protects against ischemia/reperfusion injury in rat skeletal muscle. J Surg Res. 2012;172:E39–E46. doi: 10.1016/j.jss.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 9.Serhan CN, Chiang N. Endogenous pro-resolving and anti-inflammatory lipid mediators: a new pharmacologic genus. Br J Pharmacol. 2008;153:S200–S215. doi: 10.1038/sj.bjp.0707489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nascimento-Silva V, Arruda MA, Barja-Fidalgo C, Fierro IM. Aspirin-triggered lipoxin A(4) blocks reactive oxygen species generation in endothelial cells: a novel antioxidative mechanism. Thromb Haemost. 2007;97:88–98. [PubMed] [Google Scholar]
- 11.Han X, Yao WF, Liu ZP, Li HB, Zhang ZJ, Hei ZQ, Xia ZY. Lipoxin A4 preconditioning attenuates intestinal ischemia reperfusion injury through Keap1/Nrf2 pathway in a lipoxin a4 receptor independent manner. Oxid Med Cell Longev. 2016;2016:9303606. doi: 10.1155/2016/9303606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao QF, Hu XT, Shao L, Wu GW, Du J, Xia J. Lipoxin A4 attenuates myocardial ischemia reperfusion injury via a mechanism related to downregulation of GRP-78 and caspase-12 in rats. Heart Vessels. 2014;29:667–678. doi: 10.1007/s00380-013-0418-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Espinosa-Diez C, Miguel V, Mennerich D, Kietzmann T, Sanchez-Perez P, Cadenas S, Lamas S. Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol. 2015;6:183–197. doi: 10.1016/j.redox.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu L, Li HH, Wu Q, Miao S, Liu ZJ, Wu P, Ye DY. Lipoxin A4 activates Nrf2 pathway and ameliorates cell damage in cultured cortical astrocytes exposed to oxygen-glucose deprivation/reperfusion insults. J Mol Neurosci. 2015;56:848–857. doi: 10.1007/s12031-015-0525-6. [DOI] [PubMed] [Google Scholar]
- 15.Yu DS, Li MW, Tian YQ, Liu J, Shang J. Luteolin inhibits ROS-activated MAPK pathway in myocardial ischemia/reperfusion injury. Life Sci. 2015;122:15–25. doi: 10.1016/j.lfs.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 16.Rigamonti E, Touvier T, Clementi E, Manfredi AA, Brunelli S, Rovere-Querini P. Requirement of inducible nitric oxide synthase for skeletal muscle regeneration after acute damage. J Immunol. 2013;190:1767–1777. doi: 10.4049/jimmunol.1202903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Puntel GO, Carvalho NR, Amaral GP, Lobato LD, Silveira SO, Daubermann MF, Barbosa NV, Rocha JB, Soares FA. Therapeutic cold: an effective kind to modulate the oxidative damage resulting of a skeletal muscle contusion. Free Radic Res. 2011;45:125–138. doi: 10.3109/10715762.2010.517252. [DOI] [PubMed] [Google Scholar]
- 18.Bonaventura A, Montecucco F, Dallegri F. Cellular recruitment in myocardial ischaemia/reperfusion injury. Eur J Clin Invest. 2016;46:590–601. doi: 10.1111/eci.12633. [DOI] [PubMed] [Google Scholar]
- 19.Canny GO, Lessey BA. The role of Lipoxin A4 in endometrial biology and endometriosis. Mucosal Immunol. 2013;6:439–450. doi: 10.1038/mi.2013.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang Y, Cheng Y, Lian QQ, Yang L, Qi W, Wu DR, Zheng X, Liu YJ, Li WJ, Jin SW, Smith FG. Contribution of CFTR to alveolar fluid clearance by lipoxin A4 via PI3K/Akt pathway in LPS-induced acute lung injury. Mediators Inflamm. 2013;2013:862628. doi: 10.1155/2013/862628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang YH, Wang HM, Cai ZY, Xu FY, Zhou XY. Lipoxin A4 Inhibits NF-κB activation and cell cycle progression in RAW264.7 Cells. Inflammation. 2014;37:1084–1090. doi: 10.1007/s10753-014-9832-2. [DOI] [PubMed] [Google Scholar]
- 22.Wu SH, Lu C, Dong L, Zhou GP, He ZG, Chen ZQ. High dose of lipoxin A4 induces apoptosis in rat renal interstitial fibroblasts. Prostaglandins Leukot Essent Fatty Acids. 2005;73:127–137. doi: 10.1016/j.plefa.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Prieto P, Cuenca J, Traves PG, Fernandez-Velasco M, Martin-Sanz P, Bosca L. Lipoxin A4 impairment of apoptotic signaling in macrophages: implication of the PI3K/Akt and the ERK/Nrf-2 defense pathways. Cell Death Differ. 2010;17:1179–1188. doi: 10.1038/cdd.2009.220. [DOI] [PubMed] [Google Scholar]
- 24.Murphy AD, McCormack MC, Bichara DA, Nguyen JT, Randolph MA, Watkins MT, Lee RC, Austen WG. Poloxamer 188 protects against ischemia-reperfusion injury in a murine hind-limb model. Plast Reconstr Surg. 2010;125:1651–1660. doi: 10.1097/PRS.0b013e3181ccdbef. [DOI] [PubMed] [Google Scholar]
- 25.Primeau AJ, Adhihetty PJ, Hood DA. Apoptosis in heart and skeletal muscle. Can J Appl Physiol. 2002;27:349–395. doi: 10.1139/h02-020. [DOI] [PubMed] [Google Scholar]
- 26.Bagheri F, Khori V, Alizadeh AM, Khalighfard S, Khodayari S, Khodayari H. Reactive oxygen species-mediated cardiac-reperfusion injury: Mechanisms and therapies. Life Sci. 2016;165:43–55. doi: 10.1016/j.lfs.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 27.Tian XP, Yin YY, Li X. Effects and mechanisms of Acremoniumterricola milleretal mycelium on liver fibrosis induced by carbon tetrachloride in rats. Am J Chin Med. 2011;39:537–550. doi: 10.1142/S0192415X11009019. [DOI] [PubMed] [Google Scholar]
- 28.Lee JM, Li J, Johnson DA, Stein TD, Kraft AD, Calkins MJ, Jakel RJ, Johnson JA. Nrf2, a multi-organ protector? FASEB J. 2005;19:1061–1066. doi: 10.1096/fj.04-2591hyp. [DOI] [PubMed] [Google Scholar]
- 29.Liu DJ, Shang HP, Liu Y. Stanniocalcin-1 protects a mouse model from renal ischemia-reperfusion injury by affecting ROS-mediated multiple signaling pathways. Int J Mol Sci. 2016:17. doi: 10.3390/ijms17071051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takhtfooladi HA, Takhtfooladi MA, Karimi P, Asl HA, Mobarakeh Mobarakeh SZ. Influence of tramadol on ischemia-reperfusion injury of rats’ skeletal muscle. Int J Surg. 2014;12:963–968. doi: 10.1016/j.ijsu.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 31.Ekinci Akdemir FN, Gulcin I, Karagoz B, Soslu R. Quercetin protects rat skeletal muscle from ischemia reperfusion injury. J Enzyme Inhib Med Chem. 2016;31:162–166. doi: 10.1080/14756366.2016.1193735. [DOI] [PubMed] [Google Scholar]
- 32.Zhao ZH, Zheng XW, Fang F. Ganoderma lucidum polysaccharides supplementation attenuates exercise-induced oxidative stress in skeletal muscle of mice. Saudi J Biol Sci. 2014;21:119–123. doi: 10.1016/j.sjbs.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou XL, Yang QS, Ni SZ, Tu XP, Zhao Y, Xu B, Pan ZQ, Shen J. Protective effects of lipoxin A4 in testis injury following testicular torsion and detorsion in rats. Mediators Inflamm. 2014;2014:898056. doi: 10.1155/2014/898056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Han JQ, Liu CL, Wang ZY, Liu L, Cheng L, Fan YD. Anti-inflammatory properties of lipoxin A4 protect against diabetes mellitus complicated by focal cerebral ischemia/reperfusion injury. Neural Regen Res. 2016;11:636–640. doi: 10.4103/1673-5374.180750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aboonabi A, Singh I. Chemopreventive role of anthocyanins in atherosclerosis via activation of Nrf2-ARE as an indicator and modulator of redox. Biomed Pharmacother. 2015;72:30–36. doi: 10.1016/j.biopha.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 36.Chen B, Lu YR, Chen YN, Cheng JQ. The role of Nrf2 in oxidative stress-induced endothelial injuries. J Endocrinol. 2015;225:R83–R99. doi: 10.1530/JOE-14-0662. [DOI] [PubMed] [Google Scholar]
- 37.Li WG, Khor TO, Xu CJ, Shen GX, Jeong WS, Yu S, Kong AN. Activation of Nrf2-antioxidant signaling attenuates NF kappa B-inflammatory response and elicits apoptosis. Biochem Pharmacol. 2008;76:1485–1489. doi: 10.1016/j.bcp.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peng ZM, Geh E, Chen L, Meng QH, Fan YX, Sartor M, Shertzer HG, Liu ZG, Puga A, Xia Y. Inhibitor of kappaB kinase beta regulates redox homeostasis by controlling the constitutive levels of glutathione. Mol Pharmacol. 2010;77:784–792. doi: 10.1124/mol.109.061424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang YJ, Zhang LY, Wu JH, Di CX, Xia ZW. Heme oxygenase-1 exerts a protective role in ovalbumin-induced neutrophilic airway inflammation by inhibiting Th17 cell-mediated immune response. J Biol Chem. 2013;288:34612–34626. doi: 10.1074/jbc.M113.494369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao QF, Shao L, Hu XT, Wu GW, Du J, Xia J, Qiu HX. Lipoxin a4 preconditioning and postconditioning protect myocardial ischemia/reperfusion injury in rats. Mediators Inflamm. 2013;2013:231351. doi: 10.1155/2013/231351. [DOI] [PMC free article] [PubMed] [Google Scholar]