Abstract
HoxB9, as a HOX family member, is known to play important roles in embryonic development. Recent studies have shown that HoxB9 is involved in cancer progression. However, little is known about the role of HoxB9 and the underlying mechanisms that suppress oral squamous cell carcinoma (OSCC) progression. In the present study, we used immunohistochemical staining to demonstrate that HoxB9 is over-expressed in OSCC cells and found that high levels of HoxB9 were significantly associated with shorter overall survival in patients with OSCC. Functional studies revealed that knocking down HoxB9 in OSCC cells using RNA interference decreased the migration and invasion of OSCC cells in vitro. Our mechanistic studies suggested that HoxB9 could stimulate the migration and invasion of OSCC cells by targeting EMT via the TGF-β1/Smad2/Slug signaling pathway. Collectively, these findings suggest the vital roles of HoxB9 in OSCC progression through its effects in promoting EMT.
Keywords: HoxB9, OSCC, EMT
Introduction
The identification of key molecular alterations in cancer has resulted in major advances in diagnosis and targeted therapies with validated biomarkers, heralding the advent of personalized medicine. However, oral squamous cell carcinoma (OSCC) lags behind in this regard because no consistent oncogenic drivers have been identified, and cetuximab is currently the only approved targeted therapeutic [1]. OSCC is the 6th most common cancer worldwide with an incidence exceeding 450,000 cases annually. Furthermore, survival rates have not significantly improved for several decades [2]. Thus, finding novel targets for therapeutic intervention as well as new biomarkers in OSCC is necessary and urgent.
HoxB9 is a member of the homeobox-containing (HOX) transcriptional factor family that includes 39 genes in humans and is classified into four different clusters: HOX A, B, C and D, all of which play important roles in embryonic development, especially in the patterning of the anterior-posterior axis [3,4]. In addition to their critical roles in development, increasing evidence has demonstrated that HOX family genes are associated with cancer progression [5,6]. HoxB9 was shown to induce angiogenesis, invasion and lung metastasis in breast cancer [7], was identified as an important prognostic factor for ovarian cancer [8] and pancreatic ductal adenocarcinoma [9], as well as for lung cancer patients [10]. These results strongly suggest that HoxB9 is involved in cancer progression and metastasis. Epithelial-mesenchymal transition (EMT) comprises a set of rapid changes in the cellular phenotype in which epithelial cells experience a molecular switch from a polarized, epithelial phenotype to a highly motile, non-polarized mesenchymal phenotype [11]. EMT is frequently observed at the invasive front of advanced tumors and is significantly correlated with metastasis in tumor progression [12,13]; however, whether HoxB9 could influence EMT in OSCC remains unclear.
In this study, we over-expressed HoxB9 in OSCC cells and found that high levels of HoxB9 are significantly associated with shorter overall survival in patients with OSCC. In addition, we discovered that downregulation of HoxB9 could significantly reduce cell migration in OSCC cells. These results could be attributed to the functions of HoxB9 in regulating epithelial-mesenchymal transition (EMT) process via the TGF-β1/Smad2/Slug signaling pathway.
Materials and methods
Ethics and patient tumor sections
Acquisition of tumor samples from oral squamous cell carcinoma patients who were enrolled in the current study was approved by the Ethics Committee of Second Xiangya Hospital of Central South University, Changsha, China. The procedures for handling human materials were in accordance with the ethical standards of the 1975 Declaration of Helsinki, which was revised in 1983. Primary OSCC tissue specimens (n = 50) were obtained from patients who underwent surgery at the Department of Oral and Maxillofacial Surgery of Second Xiangya Hospital. Adjacent matched normal mucosa samples (n = 50) were obtained at least 3 cm from the tumor tissues in the same group of patients and were used as controls. These patients underwent surgery between July 2010 and April 2011, and the follow-up period used for survival analyses ended in May 2016. No patients involved in this investigation received chemotherapy prior to surgery. The clinicopathological characteristics of the patients are summarized in Table 1.
Table 1.
Statistical analyses of factors associated with survival in OSCC patients withthe multivariate Cox proportional hazards model
| Overall survival | |||
|---|---|---|---|
|
| |||
| Variables | RR | 95% CI | P |
| HoxB9 expression | |||
| Positive VS negative | 2.472 | 1.367-4.469 | 0.003 |
| Gender | |||
| Male VS female | 1.017 | 0.512-2.020 | 0.438 |
| Age | |||
| < 60 VS ≥ 60 | 1.008 | 0.983-1.033 | 0.941 |
| Smoking | |||
| Yes VS no | 0.790 | 0.378-1.650 | 0.296 |
| Drinking | |||
| Yes VS no | 0.960 | 0.422-1.962 | 0.990 |
| Tumor stage | |||
| 3-4 VS 1-2 | 1.005 | 0.525-1.791 | 0.641 |
| Lymph node metastasis | |||
| + VS - | 1.676 | 1.230-2.282 | 0.001 |
| Clinical stage | |||
| 3-4 VS 1-2 | 1.433 | 1.034-1.986 | 0.037 |
| Histological type | |||
| Poor VS well-moderate | 1.312 | 0.986-1.744 | 0.062 |
CI, confidence interval.
Immunohistochemistry
All tumors used for this investigation were reviewed by at least two pathologists to confirm the diagnosis. After the sections were deparaffinized in xylene, they were rehydrated using an alcohol gradient. Endogenous peroxidase was quenched with 3% hydrogen peroxide in methanol at room temperature (25°C). The sections were placed in a 95°C solution of 0.01 M sodium citrate buffer (pH 6.0) for antigen retrieval. The primary antibody used for detecting HoxB9 was a rabbit anti-HoxB9 polyclonal antibody (Epitomics, Burlingame, CA, USA), which was applied overnight at 4°C at a 1:50 dilution. A PV9000 two-step method was used to develop the primary antibody with a polyclonal horseradish peroxidase (HRP)-conjugated antimouse/rabbit immunoglobulin (Ig)G (Zhongshan Jinqiao, Jiaxing, China). Detection was accomplished with a Dako Envision System (Dako, Glostrup, Denmark).
Cell lines, antibodies and reagent
The human OSCC cell lines Cal27 and SCC-25 were purchased from the Shanghai Cell Bank of the Chinese Academy of Sciences and were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS) (Gibco) and incubated at 37°C in a humidified atmosphere containing 5% CO2. When the cells reached 80-90% confluency, they were passed by dissociation with 0.25% trypsin-EDTA solution (Gibco) for 1-2 min. Chemical reagents for the experiments were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise specified. Two different HoxB9 siRNA sequences were purchased from Sigma-Aldrich. Primary antibody against human HoxB9 was purchased from Epitomics (Burlingame, CA, USA). E-cadherin, N-cadherin, vimentin, Snail, α-catenin and Slug were purchased from Cell Signaling Technology (Danvers, MA).
HoxB9 siRNA transfection
To further analyze the role of HoxB9 in OSCC malignancy, Cal27 and SCC-25 cells were transfected with HoxB9 siRNA using Lipofectamine2000 (Invitrogen, Carlsbad, CA). The HoxB9 siRNA sequence used is 5’-CCATTTCTGGGACGCTTAGCA-3’, and the non-targeted control sequence is 5’-CTGAGCGTGGCTACTCCTTC-3’. After a 48-h transfection, the cells were harvested for Western blot analysis.
Wound healing assay
Cells were seeded in six-well plates to 100% confluency. After serum starving for 10 h, a wound was induced by scratching the cell cultures with a 5 μl pipette tip. Following three rinses with PBS to remove the detached cells, the adherent cells were cultured in medium without serum. Images of four random fields of each well were captured immediately and again after 3 h and 6 h using a microscope (Nikon Corporation, Tokyo, Japan) at ×10 magnification. The width of the wound at these specific locations was visualized on each plate to quantify the rate of cell migration.
Transwell invasion assays
Transwells (6.5 mm) with polycarbonate membrane inserts with an 8-μm pore (Corning, Albany, NY) were embedded with 120 μg of Matrigel (BD Biosciences, San Jose, CA, USA) and 100 μg of gelatin (Sigma-Aldrich, St Louis, MO, USA) in DMEM. Either Cal27 or SCC-25 cells (1×105 per well) in serum-free medium were added into the Matrigel-embedded inserts (the top chambers), and the inserts were placed into chambers containing 10% FBS media. After incubation for 36 h at 37°C, the remaining cells in the upper chamber were carefully removed with a cotton swab, and the cells that had invaded through the Matrigel were stained with hematoxylin, photographed and quantified.
Cell immunofluorescence
Cal27 or SCC-25 cells were seeded onto coverslips at a density of 105/mL and cultured in a 6-well plate for 24 h with the indicated treatment. After treatment, the cells were washed twice with PBS and fixed with 4% paraformaldehyde for 30 minutes. Then, cells were permeabilized with 0.2% Triton X-100 in PBS for 15 minutes and blocked with non-immune goat serum for 60 minutes at room temperature. Then, the cells were incubated at 4°C overnight with corresponding primary antibody at dilutions recommended by the manufacturers. After a PBS washout, PerCP-Cy5.5-conjugated secondary antibody (1:200, Jackson Immuno-Research, USA) was used to detect the proteins, and DAPI was used for counterstaining nuclei. The coverslips were mounted on microscope slides with anti-fade mounting media (Molecular Probes, Carlsbad, CA, USA) and photographed on a fluorescence microscope (Leica).
Western blot analysis
Cal27 and SCC-25 cell lines were transfected with HoxB9 siRNA (100 nM, Sigma-Aldrich) using HiPerFect transfection reagent (Qiagen, Germantown, MD). Then, the cells were lysed, and after the protein concentration was measured by using the bicinchoninic acid (BCA) method, total protein was separated on a 12% gel by using SEMS polyacrylamide gel electrophoresis and transferred onto polyvinyldinefluoride membranes (Millipore, Billerica, MA). The membranes were blocked with 5% nonfat dry milk in Tris-buffered saline with 0.05% Tween 20 (TBST) for 1 h at room temperature and then incubated overnight at 4°C with specialized antibodies. Afterwards, the membranes were washed three times, incubated with secondary antibody for 1 h at room temperature and visualized with enhanced chemiluminescence.
Statistical analysis
HoxB9 expression differences among the various subgroups were determined using the Kruskal-Wallis rank sum test. Patient survival was calculated using Kaplan-Meier analysis, and comparisons were made using the log-rank test. Univariate and multivariate Cox analyses were used to examine the significance of other factors related to survival. Student’s t-test was used for paired studies. The data were analyzed and visualized using GraphPad Prism 5.0. *, **, and *** indicated P < 0.05, P < 0.01 and P < 0.001, respectively.
Results
HoxB9 expression is upregulated in oral squamous cell carcinoma (OSCC)
We examined HoxB9 expression in 50 oral squamous cell carcinoma tissue samples using immunohistochemical staining and compared that to paired normal mucosa samples. The results indicated that HoxB9 immunoreactivity was fairly weak in normal mucosa, whereas OSCC samples showed strong protein expression in the tumor cells, especially in the nucleus (Figure 1A). Through quantification, we found that HoxB9 expression in epithelial dysplasia and OSCC is strongly positive compared to that of normal oral mucosa (P < 0.05 and P < 0.001, respectively. Figure 1B). We plotted the overall survival for HoxB9 using Kaplan-Meier curves. Based on the follow-up data of the 50 OSCC patients, we analyzed whether HoxB9 expression affected overall survival (OS). The Kaplan-Meier survival curves showed that patients with more HoxB9-positive expression had a significantly poorer 5-year OS (P < 0.01; Figure 1C). What’s more, the multivariate Cox regression analyses revealed that HoxB9 expression was an independent prognostic factor for poor OS (P = 0.003; Table 2). Interestingly, the relationship between HoxB9 expression and the clinicopathological characteristics was statistically analyzed and showed a direct association between HoxB9 expression and lymph node metastasis (P < 0.001; Table 2). To further explore whether HoxB9 is associated with OSCC progression, we compared HoxB9 expression to the different Grades, T categories and N categories of the HNSCC (Supplementary Figure 3A-C). Unfortunately, no differences were found among the different Grades or T categories. However, the difference between positive lymph node metastasis (N+) and negative lymph node metastasis (N-) reached statistical significance. Protein and mRNA expression levels of HoxB9 were examined in eight fresh tumor samples (four N+ and four N-) by Western blot and real-time PCR, respectively. Both HoxB9 protein levels and mRNA levels in N+ patients were generally much higher than those in N-patients (Figure 1D and 1E).
Figure 1.
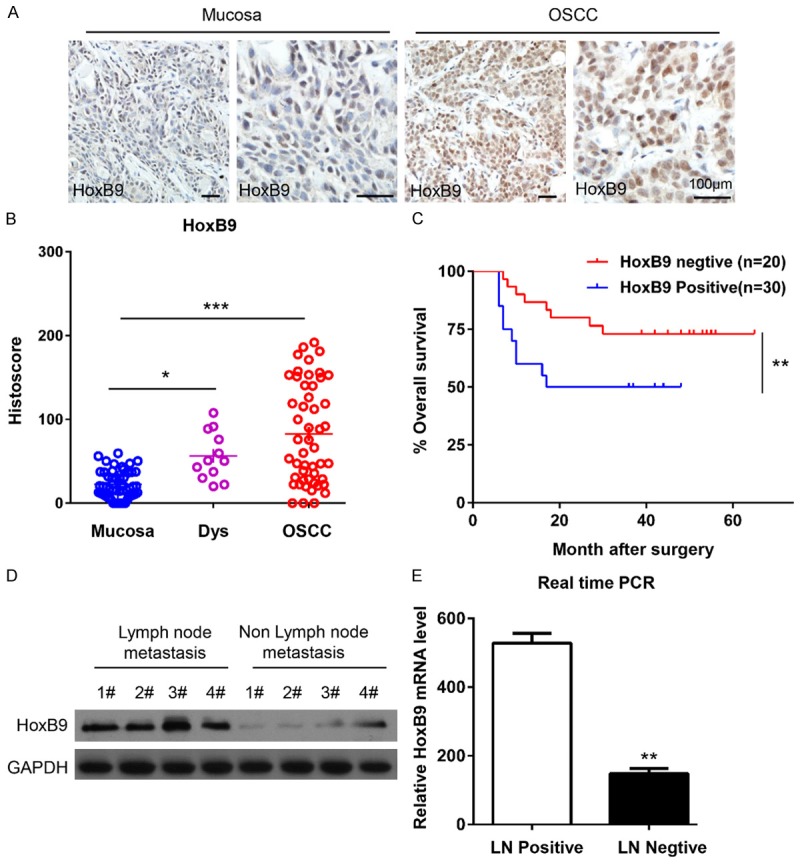
HoxB9 expression was up-regulated in oral squamous cell carcinoma (OSCC). A. Representative immunohistochemical staining of HoxB9 in human OSCC tissue (right panel) compared with normal mucosa (left panel; scale bars = 100 μm). B. Quantification of HoxB9 expression levels in human mucosa, dysplastic tissue and OSCC tissue (*P < 0.05; ***P < 0.001; one-way ANOVA with GraphPad Prism 5.0). C. Kaplan-Meier curve of overall survival of 50 patients with OSCC stratified by the expression level of HoxB9. The duration of survival was measured from the beginning of the treatment to the time of death or at the final follow-up (60 months). The cumulative survival for patients with HoxB9-positive OSCC was significantly lower than that for patients with HoxB9-negative OSCC (**P < 0.01; one-way ANOVA with GraphPad Prism 5.0). D. Western blot analysis of the protein expression of HoxB9 in patients with or without lymph node metastasis, GAPDH was used as a loading control. E. The relative mRNA levels of HoxB9 were detected by RT-PCR in patients with or without lymph node metastasis. The data are presented as the means ± SEM. One-way ANOVA with post-Dunnett analysis was performed using GraphPad Prism 5.0. **P < 0.01 versus the control group (n = 3).
Table 2.
Association between the patient’s clinicopathological characteristics and HoxB9 expression in 50 OSCC patients
| Clinicopathological features | No. | HOXB9 | Expression | P |
|---|---|---|---|---|
| Negative (%) | Positive (%) | |||
| Gender | 0.895 | |||
| Male | 37 | 15 | 22 | |
| Female | 13 | 5 | 8 | |
| Age, years | 0.904 | |||
| < 60 | 32 | 13 | 19 | |
| ≥ 60 | 18 | 7 | 11 | |
| Smoking | 0.405 | |||
| Yes | 31 | 11 | 20 | |
| No | 19 | 9 | 10 | |
| Drinking | 0.815 | |||
| Yes | 21 | 8 | 13 | |
| No | 29 | 12 | 17 | |
| Tumor stage | 0.239 | |||
| 3-4 | 20 | 6 | 14 | |
| 1-2 | 30 | 14 | 16 | |
| Clinical stage | 0.028 | |||
| 3-4 | 27 | 7 | 20 | |
| 1-2 | 23 | 13 | 10 | |
| Lymph node metastasis | 0.006 | |||
| + | 19 | 3 | 16 | |
| - | 31 | 17 | 14 | |
| Histological type | 0.523 | |||
| Poor | 4 | 1 | 3 | |
| Well-Moderate | 46 | 19 | 27 |
Knockdown of HoxB9 decreases migration and invasion of OSCC cell lines
To determine whether the activity of HoxB9 is involved in invasion and/or migration of OSCC, we first detected the HoxB9 expression in OSCC cell lines. As shown in Figure 2A, HoxB9 expression was up-regulated in OSCC cell lines (Cal27, SCC25, FaDu, SCC4, and SCC23) compared with that in normal keratinocytes (OKC). The Cal27 and SCC25 cell lines were selected because they had the highest content of HoxB9. siRNAs were designed to knock down HoxB9 (Figure 2B). We employed wound healing assays to examine the cytological effect of HoxB9 on the migratory ability of OSCC cells and transwell invasion assays to determine the effect of HoxB9 downregulation on malignant progression and metastasis. The results show that knockdown of HoxB9 notably decreased the cell motility of the Cal27 (Figure 2C) and SCC-25 (Supplementary Figure 1B) cell lines, and the number of migratory cells was quite different between the respective control groups and the HoxB9 siRNA-treated groups for both the Cal27 (Figure 2D) and SCC-25 (Supplementary Figure 1C) cell lines.
Figure 2.
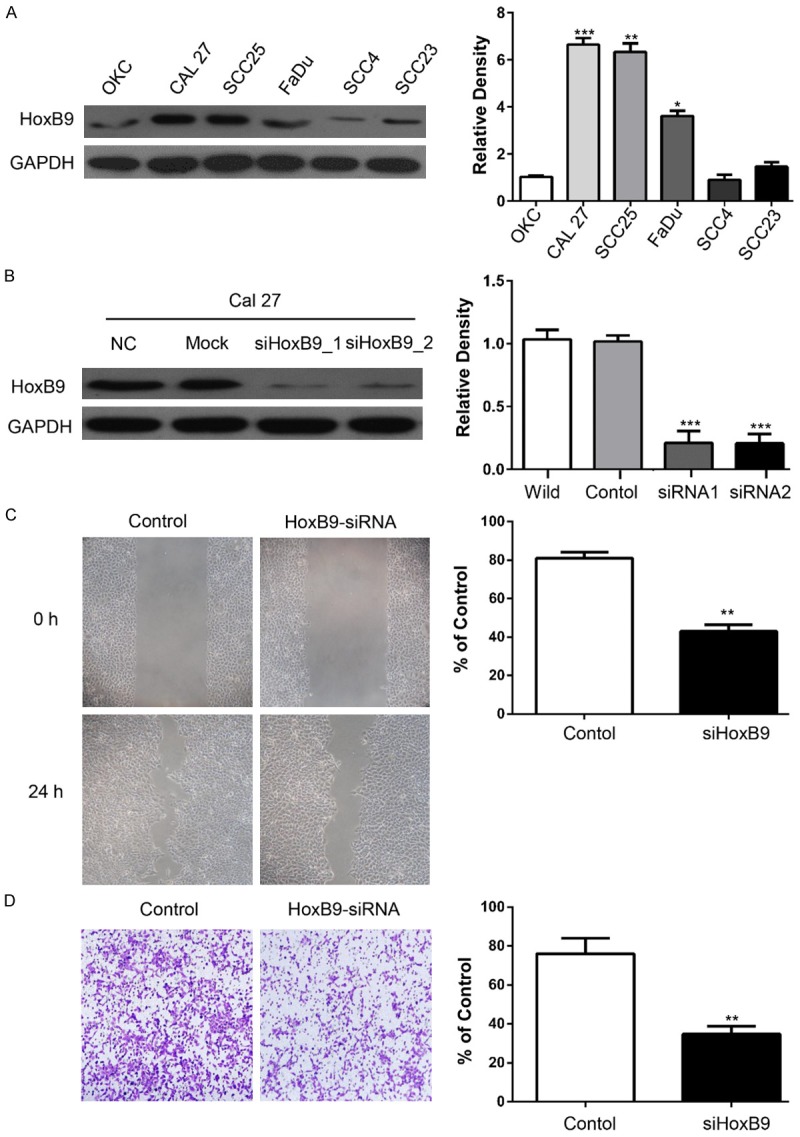
Knockdown of HoxB9 decreases the migration and invasion of OSCC cell lines. A. Western blot analysis was performed to assess the expression levels of HoxB9 in a normal keratinocyte cell line (OKC) and in OSCC cell lines. GAPDH served as a loading control, and the relative densities were calculated by using Image J. The data are presented as the mean of three independent experiments. **P < 0.01, ***P < 0.001. B. Knockdown of HoxB9 by two different siRNAs in the Cal27 cell line with GAPDH as the loading control. The relative densities were calculated by using Image J, and the data are presented as the mean of three independent experiments. ***P < 0.001. C. Wound healing assay showed that knockdown of HoxB9 suppressed the cell motility of the Cal27 cell line, and quantification of the wound closure shows that the difference is statistically significant (mean ± SD; **P < 0.01, Student’s t-test with GraphPad Prism 5.0); D. Transwell assay showed that the migration abilities of Cal27 cells were impaired after knocking down HoxB9 compared with those of the control group. Quantification of the cell numbers was performed by using the Image J “cell counter” module (mean ± SEM; **P < 0.01, Student’s t-test with GraphPadPrism 5.0 (n = 3).
HoxB9 regulates the transition between epithelial and mesenchymal phenotypes in OSCC cells
To further confirm the relationship between HoxB9 and EMT progression, we employed siRNA to knock down HoxB9 and detect alterations of the EMT as indicated by detecting putative EMT markers in vitro via Western blotting. After knockdown of HoxB9, the expression levels of E-cadherin and α-catenin were up-regulated, while N-cadherin and vimentin were downregulated in each of the HoxB9-silenced groups compared with the negative control groups (Figure 3A and Supplementary Figure 2A). Furthermore, we detected the morphological expression of the cell lines by using immunofluorescence. Similar results were observed in SCC-25 cell lines: vimentin and N-cadherin were decreased while E-cadherin and α-catenin were increased after knockdown of HoxB9 (Figure 3B and Supplementary Figure 2B). These results suggested that HoxB9 may play a role in the EMT of OSCC.
Figure 3.
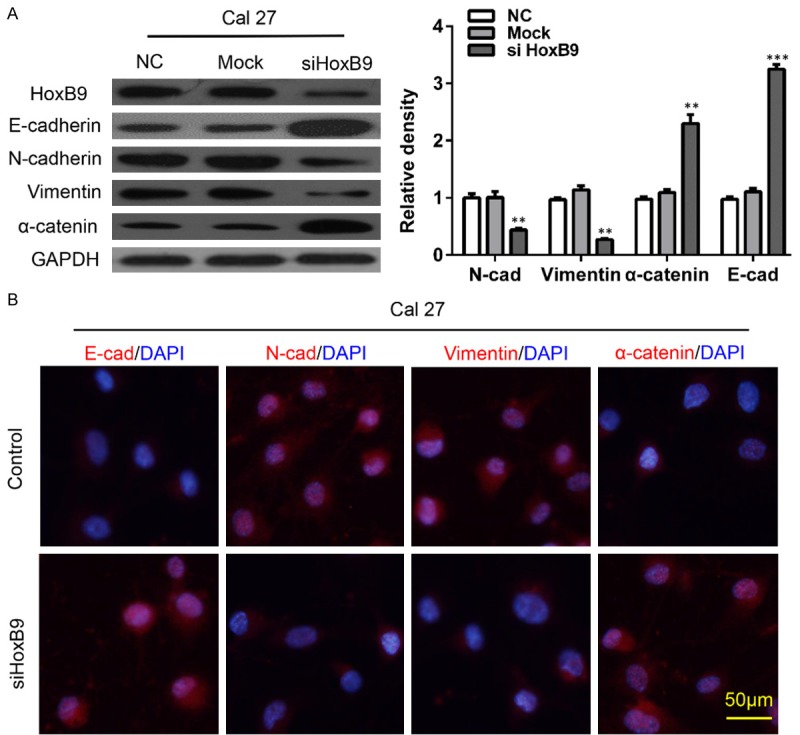
HoxB9 regulates the transition between epithelial and mesenchymal phenotypes in OSCC cells. A. Cal27 cells were treated with siRNA targeting HoxB9, and the E-cadherin, N-cadherin, vimentin and α-catenin levels were determined. GAPDH was used as an internal standard for protein loading. The values are presented as the means ± SEM. One-way ANOVA with post-Dunnett analysis was performed using GraphPad Prism 5.0. **P < 0.01 versus the control group (n = 3). B. Cal27 cells were treated with siRNA targeting HoxB9, and the representative immunofluorescence levels of E-cadherin, N-cadherin, vimentin and α-catenin were determined (Scale bars = 50 μm).
HoxB9s regulates the migration of OSCC cells by targeting EMT via the TGF-β1/Smad2/Slug signaling pathway
To further confirm the relationship between HoxB9 and EMT progression, we employed siRNA to knock down the HoxB9 and detect alterations of the EMT as indicated by detecting putative EMT markers in vitro via Western blotting. The members of multifunctional cytokine TGF-β1 superfamily exert important functions that control cell proliferation, apoptosis, differentiation, and aging. TGF-β1 is the most widely used inducer of EMT for in vitro studies and acts by inducing EMT via a Smad2-dependent pathway. Thus, we explored the possibility that TGF-β1 acts downstream of HoxB9 to control EMT in OSCC cells. Knockdown of HoxB9 in Cal27 cells significantly reduced TGF-β1 protein expression (Figure 4A), which was consistent with the results in SCC-25 cells (Figure 4B). To investigate the involvement of TGF-β1-dependent signaling in HoxB9-induced EMT, we examined the expression of phospho-Smad2. Consistent with the observed TGF-β1 activation, phospho-Smad2 expression was downregulated in Cal27 and SCC-25 cells with HoxB9 knockdown. Previous studies have shown that TGF-β1 promotes invasion by inducing EMT via induction of transcriptional repressors such as Slug and Snail [14,15]. Thus, we measured the expression of these transcriptional repressors in OSCC cells. As shown in Figure 4A, Hox9B knockdown significantly suppressed Slug and Snail expression levels. These results suggested that HoxB9 regulates the migration of OSCC cells by targeting EMT via the TGF-β1/Smad2/Slug signaling pathway.
Figure 4.
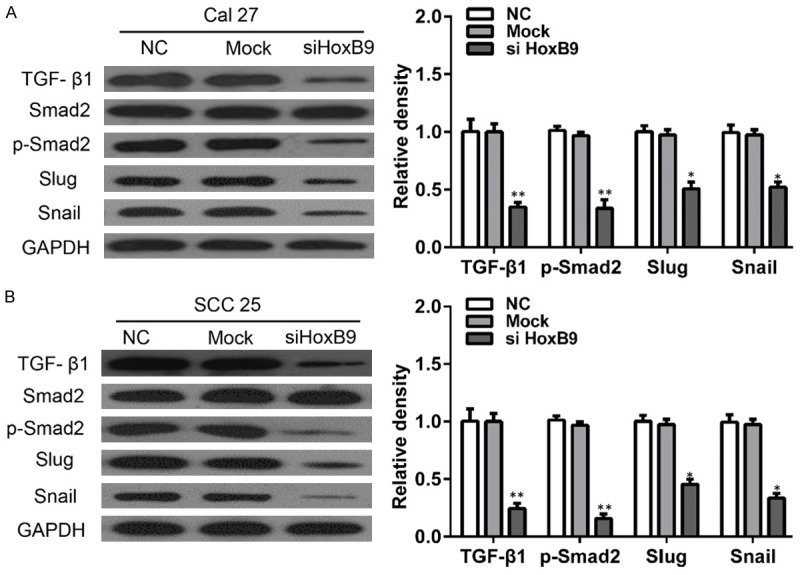
HoxB9 regulates TGF-β1 expression in OSCC cells. A. Cal27 cells were treated with siRNA targeting HoxB9, and the TGF-β1, Smad2, p-Smad2, Slug and Snail levels were determined. GAPDH was used as an internal standard for protein loading. B. SCC-25 cells were treated with siRNA targeting HoxB9, and the TGF-β1, Smad2, p-Smad2, Slug and Snail levels were determined. GAPDH was used as an internal standard for protein loading. The values are presented as the means ± SEM. One-way ANOVA with post-Dunnett analysis was performed using GraphPad Prism 5.0. *P < 0.05, **P < 0.01 versus the control group (n = 3).
Discussion
EMT is frequently observed at the invasive front of advanced tumors and is significantly correlated with metastasis in tumor progression [12,13]. What’s more, as a tumor-associated antigen, HoxB9 is also associated with cancer progression [16-18]. In this study, we used immunohistochemical staining to demonstrate that HoxB9 is over-expressed in OSCC and that high levels of HoxB9 are significantly associated with shorter overall survival in patients with OSCC. Furthermore, in vitro functional studies suggested that knockdown of HoxB9 in OSCC cells decreases cell migration and invasion. Additionally, HoxB9 may promote OSCC EMT by activating the TGF-β1/Smad2/Slug signaling pathway.
HoxB9 has been reported to be mediated via ERK5 signaling and BMI1 [19] and is an important prognostic factor for many cancers [9,20,21]. Silencing HoxB9 is associated with downregulation of CD56 and extrathyroidal extension of papillary thyroid carcinoma tumors [22] as well as the induction of tumor invasion and metastasis of breast and lung cancers [21]. Recently, a report showed that decreased expression of HoxB9 is related to poor overall survival in patients with gastric carcinoma, identifying an opposing role of HoxB9 in cancer [23], which suggested that HoxB9 may play diverse roles during cancer progression under various circumstances. In our study, we demonstrated that HoxB9 was over-expressed in OSCC and that high levels of HoxB9 were significantly associated with shorter overall survival in patients with OSCC.
The EMT is a key event for cancerous cells to acquire the capabilities of migration and invasion [24,25]. These processes are temporally and spatially regulated in a strict manner by the expression and activation of many signaling molecules [26-28]. Recent reports have suggested that Slug is the key molecule regulating EMT in cancer [29,30], and a number of studies reported that Slug-mediated epithelial-mesenchymal transition plays an important role in metastasis and apoptosis [31-33]. In addition, HoxB9 upregulation of cell-cell adhesion proteins including E-cadherin, Claudin-1, ZO-1 and Occludin has been identified in colon cancer [34]. Furthermore, HoxB9 was also found to induce EMT in breast cancer by activating the Wnt signaling pathway [7]. These results suggested that HoxB9 was associated with the EMT process. To better elucidate the invasive and metastatic mechanisms of HoxB9, the effect of HoxB9 knockdown on the EMT was investigated. The present study revealed that HoxB9 indeed plays a role in promoting EMT in OSCC, which may be related with the TGF-β1/Smad2/Slug signaling pathway.
Transforming growth factor-β1 (TGF-β1) is one of the critical growth factors that regulate tumor invasion and metastasis through the epithelial-mesenchymal transition (EMT) [35,36]. Dysregulation of TGF-β1 had been known to contribute to the progression of pancreatic cancer [37]; however, the molecular mechanism is not fully elucidated. In the present study, we observed that TGF-β1 levels were decreased after HoxB9 knockdown. To investigate the involvement of TGF-β1-dependent signaling in HoxB9-induced EMT, we examined the expression of phospho-Smad2, Consistent with the changes in TGF-β1 activation, phospho-Smad2 expression was downregulated in Cal27 and SCC-25 cells with HoxB9 knockdown.
Of note, HoxB9 expression levels were not correlated with the OSCC sequence in the TNM classifications. The reason could be the inadequate number of patients included in the cohort. Therefore, the relationship between HoxB9 expression levels and the progression of OSCC remains to be elucidated using a larger patient cohort in future studies.
In conclusion, this study confirmed that HoxB9 is over-expressed in OSCC and that high levels of HoxB9 are significantly associated with shorter overall survival in patients with OSCC. Knockdown of HoxB9 inhibits the EMT process via the TGF-β1/Smad2/Slug signaling pathway, which plays an important role in this process. This finding supports the possibility of HoxB9 and/or its associated molecules as targets for anti-metastatic therapy against OSCC.
Acknowledgements
This work was supported by National Natural Science Foundation of China (grant no. 81500832).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Markovic A, Chung CH. Current role of EGF receptor monoclonal antibodies and tyrosine kinase inhibitors in the management of head and neck squamous cell carcinoma. Expert Rev Anticancer Ther. 2012;12:1149–1159. doi: 10.1586/era.12.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sacco AG, Cohen EE. Current treatment options for recurrent or metastatic head and neck squamous cell carcinoma. J. Clin. Oncol. 2015;33:3305–3313. doi: 10.1200/JCO.2015.62.0963. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Fernandez J. The genesis and evolution of homeobox gene clusters. Nat Rev Genet. 2005;6:881–892. doi: 10.1038/nrg1723. [DOI] [PubMed] [Google Scholar]
- 4.Abate-Shen C. Deregulated homeobox gene expression in cancer: cause or consequence? Nat Rev Cancer. 2002;2:777–785. doi: 10.1038/nrc907. [DOI] [PubMed] [Google Scholar]
- 5.Chang Q, Zhang L, He C, Zhang B, Zhang J, Liu B, Zeng N, Zhu Z. HOXB9 induction of mesenchymal-to-epithelial transition in gastric carcinoma is negatively regulated by its hexapeptide motif. Oncotarget. 2015;6:42838–42853. doi: 10.18632/oncotarget.5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu SY, Rupaimoole R, Shen F, Pradeep S, Pecot CV, Ivan C, Nagaraja AS, Gharpure KM, Pham E, Hatakeyama H, McGuire MH, Haemmerle M, Vidal-Anaya V, Olsen C, Rodriguez-Aguayo C, Filant J, Ehsanipour EA, Herbrich SM, Maiti SN, Huang L, Kim JH, Zhang X, Han HD, Armaiz-Pena GN, Seviour EG, Tucker S, Zhang M, Yang D, Cooper LJ, Ali-Fehmi R, Bar-Eli M, Lee JS, Ram PT, Baggerly KA, Lopez-Berestein G, Hung MC, Sood AK. A miR-192-EGR1-HOXB9 regulatory network controls the angiogenic switch in cancer. Nat Commun. 2016;7:11169. doi: 10.1038/ncomms11169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayashida T, Takahashi F, Chiba N, Brachtel E, Takahashi M, Godin-Heymann N, Gross KW, Vivanco M, Wijendran V, Shioda T, Sgroi D, Donahoe PK, Maheswaran S. HOXB9, a gene overexpressed in breast cancer, promotes tumorigenicity and lung metastasis. Proc Natl Acad Sci U S A. 2010;107:1100–1105. doi: 10.1073/pnas.0912710107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelly Z, Moller-Levet C, McGrath S, Butler-Manuel S, Kierzek AM, Pandha H, Morgan R, Michael A. The prognostic significance of specific HOX gene expression patterns in ovarian cancer. Int J Cancer. 2016;139:1608–17. doi: 10.1002/ijc.30204. [DOI] [PubMed] [Google Scholar]
- 9.Ma SR, Wang WM, Huang CF, Zhang WF, Sun ZJ. Anterior gradient protein 2 expression in high grade head and neck squamous cell carcinoma correlated with cancer stem cell and epithelial mesenchymal transition. Oncotarget. 2015;6:8807–8821. doi: 10.18632/oncotarget.3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darda L, Hakami F, Morgan R, Murdoch C, Lambert DW, Hunter KD. The role of HOXB9 and miR-196a in head and neck squamous cell carcinoma. PLoS One. 2015;10:e0122285. doi: 10.1371/journal.pone.0122285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 12.Ye LY, Chen W, Bai XL, Xu XY, Zhang Q, Xia XF, Sun X, Li GG, Hu QD, Fu QH, Liang TB. Hypoxia-Induced epithelial-to-mesenchymal transition in hepatocellular carcinoma induces an immunosuppressive tumor microenvironment to promote metastasis. Cancer Res. 2016;76:818–830. doi: 10.1158/0008-5472.CAN-15-0977. [DOI] [PubMed] [Google Scholar]
- 13.Brabletz T. To differentiate or not-routes towards metastasis. Nat Rev Cancer. 2012;12:425–436. doi: 10.1038/nrc3265. [DOI] [PubMed] [Google Scholar]
- 14.Wu ZQ, Li XY, Hu CY, Ford M, Kleer CG, Weiss SJ. Canonical Wnt signaling regulates Slug activity and links epithelial-mesenchymal transition with epigenetic breast cancer 1, Early Onset (BRCA1) repression. Proc Natl Acad Sci U S A. 2012;109:16654–16659. doi: 10.1073/pnas.1205822109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strippoli R, Loureiro J, Moreno V, Benedicto I, Perez Lozano ML, Barreiro O, Pellinen T, Minguet S, Foronda M, Osteso MT, Calvo E, Vazquez J, Lopez Cabrera M, del Pozo MA. Caveolin-1 deficiency induces a MEK-ERK1/2-Snail-1-dependent epithelial-mesenchymal transition and fibrosis during peritoneal dialysis. EMBO Mol Med. 2015;7:102–123. doi: 10.15252/emmm.201404127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhussupova A, Hayashida T, Takahashi M, Miyao K, Okazaki H, Jinno H, Kitagawa Y. An E2F1-HOXB9 transcriptional circuit is associated with breast cancer progression. PLoS One. 2014;9:e105285. doi: 10.1371/journal.pone.0105285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwon OS, Oh E, Park JR, Lee JS, Bae GY, Koo JH, Kim H, Choi YL, Choi YS, Kim J, Cha HJ. GalNAc-T14 promotes metastasis through Wnt dependent HOXB9 expression in lung adenocarcinoma. Oncotarget. 2015;6:41916–41928. doi: 10.18632/oncotarget.6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuan R, Wang K, Hu J, Yan C, Li M, Yu X, Liu X, Lei J, Guo W, Wu L, Hong K, Shao J. Ubiquitin-like protein FAT10 promotes the invasion and metastasis of hepatocellular carcinoma by modifying beta-catenin degradation. Cancer Res. 2014;74:5287–5300. doi: 10.1158/0008-5472.CAN-14-0284. [DOI] [PubMed] [Google Scholar]
- 19.Nagel S, Burek C, Venturini L, Scherr M, Quentmeier H, Meyer C, Rosenwald A, Drexler HG, MacLeod RA. Comprehensive analysis of homeobox genes in Hodgkin lymphoma cell lines identifies dysregulated expression of HOXB9 mediated via ERK5 signaling and BMI1. Blood. 2007;109:3015–3023. doi: 10.1182/blood-2006-08-044347. [DOI] [PubMed] [Google Scholar]
- 20.Calvo R, West J, Franklin W, Erickson P, Bemis L, Li E, Helfrich B, Bunn P, Roche J, Brambilla E, Rosell R, Gemmill RM, Drabkin HA. Altered HOX and WNT7A expression in human lung cancer. Proc Natl Acad Sci U S A. 2000;97:12776–12781. doi: 10.1073/pnas.97.23.12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seki H, Hayashida T, Jinno H, Hirose S, Sakata M, Takahashi M, Maheswaran S, Mukai M, Kitagawa Y. HOXB9 expression promoting tumor cell proliferation and angiogenesis is associated with clinical outcomes in breast cancer patients. Ann Surg Oncol. 2012;19:1831–1840. doi: 10.1245/s10434-012-2295-5. [DOI] [PubMed] [Google Scholar]
- 22.Kim JH, Kim YH, Han JH, Lee KB, Sheen SS, Lee J, Soh EY, Park TJ. Silencing of homeobox B9 is associated with down-regulation of CD56 and extrathyroidal extension of tumor in papillary thyroid carcinoma. Hum Pathol. 2012;43:1221–1228. doi: 10.1016/j.humpath.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 23.Sha S, Gu Y, Xu B, Hu H, Yang Y, Kong X, Wu K. Decreased expression of HOXB9 is related to poor overall survival in patients with gastric carcinoma. Dig Liver Dis. 2013;45:422–429. doi: 10.1016/j.dld.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer. 2013;13:97–110. doi: 10.1038/nrc3447. [DOI] [PubMed] [Google Scholar]
- 26.Kang FB, Wang L, Jia HC, Li D, Li HJ, Zhang YG, Sun DX. B7-H3 promotes aggression and invasion of hepatocellular carcinoma by targeting epithelial-to-mesenchymal transition via JAK2/STAT3/Slug signaling pathway. Cancer Cell Int. 2015;15:45. doi: 10.1186/s12935-015-0195-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yadav A, Kumar B, Datta J, Teknos TN, Kumar P. IL-6 promotes head and neck tumor metastasis by inducing epithelial-mesenchymal transition via the JAK-STAT3-SNAIL signaling pathway. Mol Cancer Res. 2011;9:1658–1667. doi: 10.1158/1541-7786.MCR-11-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tam WL, Weinberg RA. The epigenetics of epithelial-mesenchymal plasticity in cancer. Nat Med. 2013;19:1438–1449. doi: 10.1038/nm.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo W, Keckesova Z, Donaher JL, Shibue T, Tischler V, Reinhardt F, Itzkovitz S, Noske A, Zurrer-Hardi U, Bell G, Tam WL, Mani SA, van Oudenaarden A, Weinberg RA. Slug and Sox9 cooperatively determine the mammary stem cell state. Cell. 2012;148:1015–1028. doi: 10.1016/j.cell.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu B, Wei J, Hu Z, Shan C, Wang L, Zhang C, Yang X, Yang X, Lei D. Slug silencing inhibited perineural invasion through regulation of EMMPRIN expression in human salivary adenoid cystic carcinoma. Tumour Biol. 2016;37:2161–2169. doi: 10.1007/s13277-015-4043-5. [DOI] [PubMed] [Google Scholar]
- 31.Bu LL, Zhao ZL, Liu JF, Ma SR, Huang CF, Liu B, Zhang WF, Sun ZJ. STAT3 blockade enhances the efficacy of conventional chemotherapeutic agents by eradicating head neck stemloid cancer cell. Oncotarget. 2015;6:41944–41958. doi: 10.18632/oncotarget.5986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu JF, Mao L, Bu LL, Ma SR, Huang CF, Zhang WF, Sun ZJ. C4.4A as a biomarker of head and neck squamous cell carcinoma and correlated with epithelial mesenchymal transition. Am J Cancer Res. 2015;5:3505–3515. [PMC free article] [PubMed] [Google Scholar]
- 33.Fan TF, Bu LL, Wang WM, Ma SR, Liu JF, Deng WW, Mao L, Yu GT, Huang CF, Liu B, Zhang WF, Sun ZJ. Tumor growth suppression by inhibiting both autophagy and STAT3 signaling in HNSCC. Oncotarget. 2015;6:43581–43593. doi: 10.18632/oncotarget.6294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhan J, Niu M, Wang P, Zhu X, Li S, Song J, He H, Wang Y, Xue L, Fang W, Zhang H. Elevated HOXB9 expression promotes differentiation and predicts a favourable outcome in colon adenocarcinoma patients. Br J Cancer. 2014;111:883–893. doi: 10.1038/bjc.2014.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oft M, Heider KH, Beug H. TGFbeta signaling is necessary for carcinoma cell invasiveness and metastasis. Curr Biol. 1998;8:1243–1252. doi: 10.1016/s0960-9822(07)00533-7. [DOI] [PubMed] [Google Scholar]
- 36.Bhowmick NA, Ghiassi M, Bakin A, Aakre M, Lundquist CA, Engel ME, Arteaga CL, Moses HL. Transforming growth factor-beta1 mediates epithelial to mesenchymal transdifferentiation through a RhoA-dependent mechanism. Mol Biol Cell. 2001;12:27–36. doi: 10.1091/mbc.12.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ellermeier J, Wei J, Duewell P, Hoves S, Stieg MR, Adunka T, Noerenberg D, Anders HJ, Mayr D, Poeck H, Hartmann G, Endres S, Schnurr M. Therapeutic efficacy of bifunctional siRNA combining TGF-beta1 silencing with RIG-I activation in pancreatic cancer. Cancer Res. 2013;73:1709–1720. doi: 10.1158/0008-5472.CAN-11-3850. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


