Abstract
Objectives: The inhaled general anesthetic isoflurane has been shown to induce caspase-3 activation in vitro and in vivo. The underlying mechanisms and functional consequences of this activity remain unclear. Isoflurane can induce caspase-3 activation by causing accumulation of reactive oxygen species (ROS), mitochondrial dysfunction, and reduction in adenosine triphosphate (ATP) levels. This study aimed to investigate the protective effect of hydrogen, a novel antioxidant, against isoflurane-induced caspase-3 activation and cognitive impairment. Methods: H4 human neuroglioma cells overexpressing human amyloid precursor protein were treated with saline or hydrogen-rich saline (HS, 300 μM), with or without 2% isoflurane, for 6 h or 3 h. Western blot analysis, fluorescence assays, and a mitochondrial swelling assay were used to evaluate caspase-3 activation, levels of ROS and ATP, and mitochondrial function. The effect of the interaction of isoflurane (1.4% for 2 h) and HS (5 mL/kg) on cognitive function in mice was also evaluated using a fear conditioning test. Results: We found that HS attenuated isoflurane-induced caspase-3 activation. Moreover, HS treatment mitigated isoflurane-induced ROS accumulation, opening of mitochondrial permeability transition pores, reduction in mitochondrial membrane potential, and reduction in cellular ATP levels. Finally, HS significantly alleviated isoflurane-induced cognitive impairment in mice. Conclusions: Our results suggest that HS attenuates isoflurane-induced caspase-3 activation and cognitive impairment via inhibition of isoflurane-induced oxidative stress, mitochondrial dysfunction, and reduction in ATP levels. These findings warrant further research into the underlying mechanisms of this activity, and indicate that HS has the potential to attenuate anesthesia neurotoxicity.
Keywords: Hydrogen, isoflurane, caspase-3, cognitive impairment
Introduction
Alzheimer’s disease (AD) is the most common form of dementia among older adults, accounting for about two-thirds of all cases [1]. In vitro, animal, and human studies have demonstrated that some anesthetics are associated with changes in AD-related pathology, biomarkers of neuronal injury, and neurocognitive symptoms [1-11]. Findings from large retrospective studies and a randomized trial have indicated that exposure to surgery-related anesthesia may contribute to the development of AD, dementia, and progression of mild cognitive impairment [12-14]. However, other studies have found no association of prior exposure to general anesthesia during surgery with incident AD [15,16]. Given the inconsistent evidence, well-designed and adequately powered clinical studies with longer follow-up periods are required to establish a clear causal association between surgery, anesthesia, and AD. Meanwhile, mechanistic studies performed in cells and animals are equally important to investigate the underlying mechanisms and possible targeted interventions for anesthesia toxicity.
The commonly used inhaled anesthetic isoflurane has been shown to induce caspase-3 activation, increased β-amyloid peptide (Aβ) aggregation, tau protein phosphorylation, and learning and memory impairment [8,11,17-23]. Xie et al. [19-21,24-26] have shown that isoflurane can increase levels of reactive oxygen species (ROS), induce mitochondrial dysfunction (e.g., opening of mitochondrial permeability transition pores [mPTPs] and reduction in mitochondrial membrane potential [MMP]), and decrease ATP levels, which then may cause caspase-3 activation and lead to impairment of learning and memory.
Hydrogen, a novel and effective antioxidant, could selectively scavenge the two most aggressive ROS, OH- and ONOO-; moreover, there is substantial evidence that hydrogen provides neuroprotection against oxidative-stress-induced damage in neurological diseases such as AD and Parkinson’s disease, and in transient and permanent cerebral ischemia and spinal cord injury [27-29].
Our study was thus designed to determine whether HS could attenuate isoflurane-induced caspase-3 activation through a ROS-, mitochondria-, and ATP-associated mechanism in cultured H4 human neuroglioma cells overexpressing human amyloid precursor protein (H4-APP cells), and whether HS could ameliorate the cognitive impairment seen in rodents after isoflurane exposure. Our primary hypothesis was that HS would attenuate isoflurane-induced caspase-3 activation. Our secondary hypothesis was that HS would attenuate isoflurane-induced ROS accumulation, mitochondrial dysfunction, and ATP reduction, and would mitigate isoflurane-induced cognitive impairment.
Here, we aim to explore targeted intervention for isoflurane neurotoxicity and associated cognitive decline, and to further demonstrate the underlying mechanisms and related consequences of isoflurane-induced caspase-3 activation, using both cell culture and mouse studies.
Methods
IRB approval
All animal experiments were approved by the Ethics Committee for Animal Use of Shanghai Tenth People’s Hospital, Tongji University School of Medicine.
Cell line
H4 human neuroglioma cells stably transfected to express full-length human amyloid precursor protein (H4-APP cells) [19] were used in mechanistic studies to determine whether HS could attenuate isoflurane-induced cellular neurotoxicity. Cells were cultured in high-glucose Dulbecco’s modified Eagle’s medium containing 9% heat-inactivated fetal calf serum, 100 U/mL penicillin, 100 μg/mL streptomycin, and 2 mM L-glutamine, and were supplemented with 220 μg/mL G418.
Production of hydrogen-rich water
HS was prepared as described previously [27]. Briefly, H2 was dissolved in 0.9% saline for 6 h under high pressure (0.4 MPa) to a supersaturated level using an HS-producing apparatus (Diving Medicine Department, Second Military Medical University, Shanghai, China). The prepared HS was stored at 4°C. To confirm the concentration of hydrogen in the saline, gas chromatography was performed using the method described by Ohsawa et al. [27]. HS was freshly prepared every week to ensure that a concentration of at least 0.6 mmol/L was maintained.
Treatment of cells
Isoflurane was delivered from an anesthesia machine to a sealed plastic box in a 37°C incubator containing six-well plates or 96-well plates; six-well plates were seeded with one million cells in 1.5 mL cell culture media per well, and 96-well plates were seeded with 50,000 cells in 200 μL cell culture media per well, as described previously [19]. A Datex infrared gas analyzer (Puritan-Bennett, Tewksbury, MA, USA) was used to continuously monitor the delivered concentrations of carbon dioxide, oxygen, and isoflurane. Cells were treated with 2% isoflurane plus 21% O2 and 5% CO2 for 6 h for caspase-3 activation studies and ROS measurement, and for 3 h for studies of mPTP opening, MMP, and ATP levels, as described by Xie et al. [30] and Zhang et al. [19]. Isoflurane treatment durations were chosen according to findings from previous studies, which revealed that treatment with 2% isoflurane for 6 h induced caspase-3 activation and ROS accumulation, and treatment with 2% isoflurane for 3 h induced mPTP opening, MMP reduction, and decrease in ATP levels [19,30]. Cells were treated with HS (300 μM) [31] 30 min before isoflurane treatment.
Cell lysis and protein quantification
Harvested cell pellets were detergent-extracted on ice using an immunoprecipitation buffer (10 mM Tris-HCl pH 7.4, 150 mM NaCl, 2 mM EDTA, 0.5% Nonidet P-40) plus protease inhibitors (1 μg/mL aprotinin, 1 μg/mL leupeptin, 1 μg/mL pepstatin A) as described previously [19]. Lysates were collected, centrifuged at 13,000 rpm for 15 minutes, and quantified for total protein content using a bicinchoninic acid (BCA) protein assay kit (Pierce, Rockford, IL, USA).
Western blot analyses
Extracts from harvested cells were subjected to Western blot analyses as described previously [19]. Specifically, a caspase-3 antibody (1:1000 dilution; Cell Signaling Technology, Danvers, MA, USA) was used to recognize full-length caspase-3 (35-40 kDa) and caspase-3 fragments (17-20 kDa) resulting from cleavage at aspartate position 175. An anti-β-actin antibody (1:10,000, Sigma, St. Louis, MO, USA) was used to detect β-actin (42 kDa). Each lane in the Western blot represented an independent experiment, and results were averaged from six independent experiments. Signal intensity was analyzed using NIH Image software (ImageJ Version 1.49, U. S. National Institutes of Health, Bethesda, MD, USA). Western blots were quantified in 2 steps. First, we used β-actin levels to normalize protein levels (e.g., determining the ratio of the amount of caspase-3 fragment to that of β-actin) and to control for loading differences. Second, we presented protein levels in treated cells as a percentage of those in the control group, with the control group values set to 100%.
ROS measurement
An OxiSelect Intracellular ROS Assay Kit and an OxiSelect In Vitro ROS/RNS Assay Kit (Cell Biolabs, San Diego, CA, USA) were used to measure cellular ROS levels as described previously [19]. Briefly, cultured H4-APP cells were placed in a clear 96-well cell culture plate overnight in the incubator. We then added 2’,7’-dichlorofluorescein-diacetate (DCFH-DA) solution to the cells for 30 min. The DCFH-DA-loaded H4-APP cells were subsequently exposed to 2% isoflurane for 6 h. These treated cells were first lysed by adding 100 μL of cell lysis buffer, then mixed thoroughly and incubated for 5 min at room temperature. The lysate mixtures (150 μL each) were transferred to individual wells of a 96-well plate for fluorescence measurement. Finally, fluorescence was read with a fluorometric plate reader (Molecular Devices, Sunnyvale, CA, USA) at 480/530 nm.
Preparation of mitochondria
Mitochondria were isolated from H4-APP cells by standard differential centrifugation and resuspended in isolation buffer (0.2 M sucrose, 10 mM Tris-MOPS pH 7.4, 0.1 mM EGTA-Tris) and stored on ice. Mitochondria were used immediately after preparation. Mitochondria were isolated using a mitochondrial fractionation kit (Active Motif, Carlsbad, CA, USA). Mitochondrial protein content was quantified using a BCA protein assay kit (Pierce).
Mitochondrial swelling
The mitochondrial swelling assay was performed according to a previously published method [32]. Mitochondria (0.5 mg/mL) were incubated in experimental buffer (125 mM KCl, 10 mM Tris-MOPS pH 7.4, 1 mM Pi, 5 mM glutamate, 2.5 mM malate, 10 μM EGTA-Tris pH 7.4) Mitochondrial swelling caused by influx of solutes across the inner membrane was measured by recording the decrease in absorbance at 540 nm on a Beckman DU 640 spectrophotometer (Beckman, Brea, CA, USA).
Determination of MMP
MMP was quantified by tetraethylbenzimidazolylcarbocyanine iodide (JC-1) fluorescence ratio detection (Biotium, Hayword, CA) as described previously [11]. Specifically, H4-APP cells were seeded at a density of 50,000 per well in 96-well plates and placed in the incubator overnight. Cells were washed twice with 100 μL Dulbecco’s phosphate-buffered saline before isoflurane treatment. After treatment, cells were incubated with JC-1 reagents at 37°C for 15 min, then washed twice with Hank’s balanced salt solution (HBSS). Finally, fluorescence was read with a fluorometric plate reader (Fluoroskan Ascent FL, Thermo Fisher Scientific, Waltham, MA, USA) for red and green fluorescence (excitation 590 nm, emission 610 nm; and excitation 490 nm, emission 520 nm, respectively). MMP was calculated as the ratio of red fluorescence to green fluorescence. We also measured MMP using tetramethylrhodamine ethyl ester and perchlorate (TMRE; Sigma, St. Louis, MO, USA). TMRE is a cationic dye that is rapidly and reversibly accumulated by healthy mitochondria; thus, a decrease in levels of TMRE staining indicates reduction in MMP. TMRE studies were performed as described by Zhang et al. [19]. Briefly, after treatment, cells were stained with 100 nM TMRE for 30 min at 37°C. Cells were washed twice with HBSS, then analyzed under a fluorescence microscope (40× objective).
ATP measurement
We used the ATP Determination Kit (Invitrogen, Waltham, MA, USA) to detect ATP levels, as described previously [19,20]. Briefly, H4-APP cells were placed in six-well plates overnight in the incubator. Cells were then exposed to isoflurane treatment for 3 h. After treatment, the amount of fluorescence was measured and levels of ATP in the experimental samples were calculated from a standard curve constructed using samples containing known amounts of ATP.
Mice
Wild-type C57BL/6J mice (8 months old, The Jackson Laboratory, Bar Harbor, ME, USA) were randomly assigned to the anesthesia group or control group, then were further divided into the HS treatment group or control group. Mice were housed in a controlled environment (20-22°C; 12 h light/dark on a reversed light cycle) for 1 week prior to the studies. All animal experiments were carried out in accordance with the guidelines set forth by the National Institutes of Health, and all efforts were made to minimize the number of animals utilized in the studies. Power analyses used to establish experimental group sizes are described in the Statistical Analyses section.
Mouse anesthesia
Mice (ten per group) were randomized by weight and sex into either anesthesia groups that received 1.4% isoflurane plus 100% oxygen for 2 h, or control groups that received 100% oxygen for 2 h, at identical flow rates and in identical anesthetizing chambers (20×20×7 cm). The induction flow rate was 2 L/min for the first 3 min (for induction), then 0.2 L/min afterward (for maintenance). Isoflurane and oxygen concentrations were measured continuously using a gas analyzer (Ohmeda, GE Healthcare, Tewksbury, MA, USA). The temperature of the anesthetizing chamber was controlled using a DC Temperature Control System (FHC, Bowdoinham, ME, USA), a feedback-based system for monitoring and controlling temperature, to maintain the rectal temperature of the mice at 37±0.5°C. For the interaction studies, HS (5 mL/kg) or saline was administered intraperitoneally to the mice 30 min before isoflurane anesthesia. The concentration of HS was chosen according to previous studies [33,34].
Fear conditioning test
Fear conditioning is a very sensitive and non-effort-dependent test of learning and memory [35]. The fear conditioning test (FCT; Stoelting Co., Wood Dale, IL, USA) was performed exactly as described in previous studies [19,36]. In short, the pairing phase of the FCT was performed 2 h after isoflurane anesthesia. Each mouse was first allowed to explore the chamber for a 180-sec habituation period. A 2-Hz pulsating tone (80 dB, 3600 Hz) persisting for 60 sec was then presented. A mild foot shock (0.8 mA for 0.5 sec) was administered during the last 2 sec of the tone presentation. The first context test was performed 30 min after pairing, and the first tone test was performed 90 min after pairing. During both the context test and the tone test, each mouse was allowed to stay in the chamber for a total of 390 sec. No tone was played during the context test; however, during the tone test, the 2-Hz pulsating tone was played during the second 180 sec, with no foot shock. In both the context test and the tone test, learning and memory function was assessed by measuring the amount of time during which the mouse demonstrated freezing behavior, which was defined as “absence of movement except for respiration” [35] during the test period (the second 180 sec). The second and third context and tone tests were performed at 48 h and 7 days after anesthesia, respectively. Freezing behavior was analyzed using an Any-Maze video tracking system (Stoelting Co., Wood Dale, IL, USA) with the following settings: freezing on threshold, 10 sec; freezing off threshold, 20 sec; minimum freezing duration, one sec.
Statistical analyses
Data were expressed as mean ± standard deviation (SD). To determine sample sizes, a power calculation was performed using information collected from a preliminary study that was conducted under the same conditions. The preliminary data indicated that, assuming a two-sided Student’s t test, sample sizes of six and 10 for the in vitro and vivo studies, respectively, would lead to 90% power and 95% significance. Two-way analysis of variance (ANOVA) was used to assess the interaction of HS with isoflurane, and to test the hypothesis that HS would mitigate the effects of isoflurane on caspase-3 activation, ROS, mPTP opening, MMP, ATP levels, and freezing time. Post-hoc analyses were conducted if the main effects were found to be statistically significant. The cutoff P value was Bonferroni-adjusted to correct for subset analysis, e.g., comparing the level of isoflurane-mediated caspase-3 activation between HS and saline treatments. The nature of the hypothesis testing was two-tailed. P values less than 0.05 were considered statistically significant. SAS software, version 9.1 (Cary, NC, USA) and GraphPad Prism software, version 5 (La Jolla, CA, USA) were used to analyze the data.
Results
HS attenuates isoflurane-induced caspase-3 activation
Caspase-3 immunoblotting (Figure 1A) showed that treatment with 2% isoflurane plus saline for 6 h (lanes 3) increased the intensity of the caspase-3 fragment band compared with the control condition plus saline (lanes 1). Treatment with the control condition plus HS for 6 h (lanes 2) did not significantly alter the level of caspase-3 fragment compared with the control condition plus saline (lanes 1; quantitative data not shown). However, treatment with isoflurane plus 300 μM HS (lanes 4) led to a smaller increase in the caspase-3 fragment band compared with isoflurane plus saline (lanes 3). There were no significant differences in the levels of full-length caspase-3 or β-actin among the above treatments (data not shown). Quantification of the Western blots based on the ratio of caspase-3 fragments to full-length caspase-3 (Figure 1B) showed that treatment with 2% isoflurane plus saline for 6 h induced more caspase-3 activation compared with the control condition plus saline. Two-way ANOVA revealed a significant interaction between the effects of group (control condition vs. isoflurane) and treatment (saline vs. HS) on caspase-3 activation (F=5.649, P=0.0085). A post-hoc Bonferroni test showed that treatment with 2% isoflurane plus saline for 6 hours induced more caspase-3 activation compared with the control condition plus saline (P=0.0303), and treatment with isoflurane plus HS led to less caspase-3 activation compared with isoflurane plus saline (P=0.028).
Figure 1.
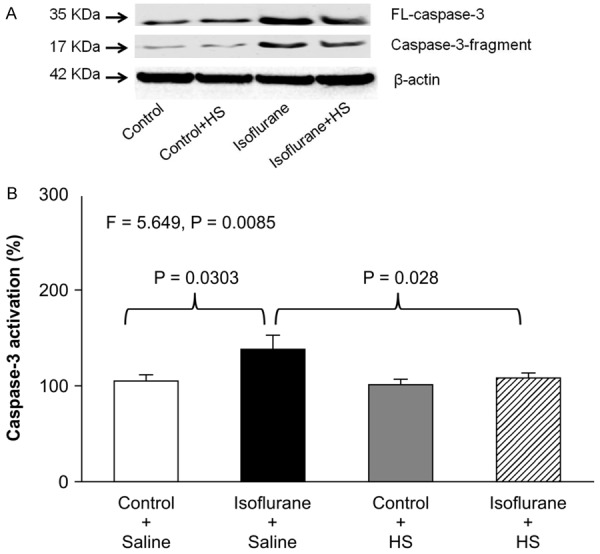
HS attenuates isoflurane-induced caspase-3 activation in H4-APP cells. A: Treatment with 2% isoflurane plus saline for 6 h (lanes 3) induces caspase-3 activation compared with the control condition plus saline (lanes 1) in H4-APP cells. Treatment with the control condition plus HS (lanes 2) does not induce caspase-3 activation compared with the control condition plus saline (lanes 1). Treatment with 2% isoflurane plus 300 μM HS for 6 h (lanes 4) induces less caspase-3 activation compared with treatment with 2% isoflurane plus saline (lanes 3). There is no substantial difference in the levels of full-length caspase-3 and β-actin among the above treatments. B: Quantification of the Western blots shows that treatment with 2% isoflurane plus saline for 6 h (black bar), but not the control condition plus 300 μM HS (gray bar), induces caspase-3 activation compared with the control condition plus saline (white bar) in H4-APP cells. Treatment with 300 μM HS (striped bar) attenuates the isoflurane-induced caspase-3 activation. N=6 in each group. FL: full length, APP: amyloid precursor protein, HS: hydrogen-rich saline.
HS attenuates isoflurane-induced increases in ROS levels
Isoflurane has been shown to increase ROS levels in vitro and in vivo, which may lead to caspase-3 activation [11,26]. Therefore, we investigated whether treatment with 300 μM HS was able to mitigate isoflurane-induced ROS accumulation in H4-APP cells. Two-way ANOVA of results from the quantitative ROS assay (Figure 2) showed that there was a significant interaction between the effects of group (control condition vs. isoflurane) and treatment (saline vs. HS) on ROS levels (F=32.38, P<0.001). A post-hoc Bonferroni test showed that isoflurane significantly increased ROS levels compared with the control condition (P<0.001) and treatment with isoflurane plus HS led to lower ROS levels compared with isoflurane plus saline (P<0.001). Moreover, HS treatment decreased baseline ROS levels in the control condition. Taken together, these data suggest that HS could alleviate isoflurane-induced ROS accumulation.
Figure 2.
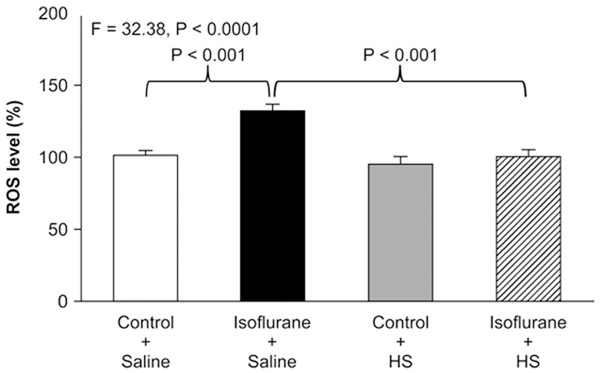
HS attenuates isoflurane-induced ROS accumulation in H4-APP cells. Fluorescence staining for ROS shows that treatment with 2% isoflurane plus saline for 6 h (black bar) increases ROS levels compared with the control condition plus saline (white bar). Treatment with isoflurane plus HS (striped bar) leads to lower ROS levels compared with treatment with isoflurane plus saline (black bar). N=6 in each group. ROS: reactive oxygen species, APP: amyloid precursor protein, HS: hydrogen-rich saline.
HS attenuates isoflurane-induced opening of mPTP
To assess mPTP opening, we measured mitochondrial swelling in response to Ca2+. Mitochondria from both isoflurane-treated and control-treated H4-APP cells showed swelling in response to Ca2+, with mitochondria from isoflurane-treated cells showing more swelling than those from control cells (Figure 3). Notably, mitochondria from cells treated with isoflurane plus HS were more resistant to Ca2+-induced swelling and permeability transition than were mitochondria from cells treated with isoflurane plus saline (Figure 3).
Figure 3.
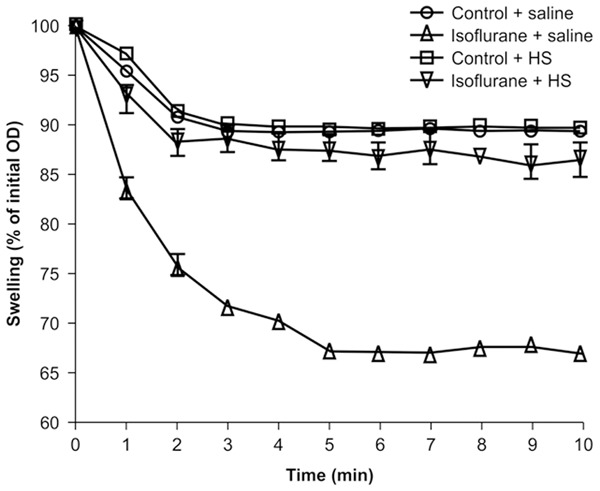
HS attenuates isoflurane-induced opening of mPTP. Data are shown as the relative percent of the initial OD at an absorbance of 540 nm. Mitochondria from H4-APP cells treated with both the isoflurane condition and the control condition show swelling in response to Ca2+, with mitochondria from isoflurane-treated cells showing greater swelling than those from control cells. Mitochondria from cells treated with isoflurane plus HS are more resistant to Ca2+-induced swelling and permeability transition than those from cells treated with isoflurane plus saline.
HS attenuates isoflurane-induced reduction in MMP
Cytochemistry staining with TMRE, an indicator of MMP (Figure 4A), showed that treatment with 2% isoflurane plus saline for 3 h (panel b) decreased MMP detected by confocal microscopy compared with the control condition (panel a) in H4-APP cells. Moreover, HS inhibited the isoflurane-induced reduction in MMP, as evidenced by greater TMRE staining in cells treated with isoflurane plus HS (panel d) compared with those treated with isoflurane plus saline (panel b). JC-1 fluorescence ratio detection (Figure 4B) showed that treatment with 2% isoflurane plus saline for 3 h decreased MMP compared with treatment with the control condition plus saline. Two-way ANOVA revealed a significant interaction between the effects of group (control condition vs. isoflurane) and treatment (saline vs. HS) on MMP (F=17.25, P<0.0001). A post-hoc Bonferroni test showed that treatment with isoflurane led to a smaller MMP compared with the control condition (P<0.0001), and treatment with isoflurane plus HS led to a higher MMP compared with treatment with isoflurane plus saline (P<0.01).
Figure 4.
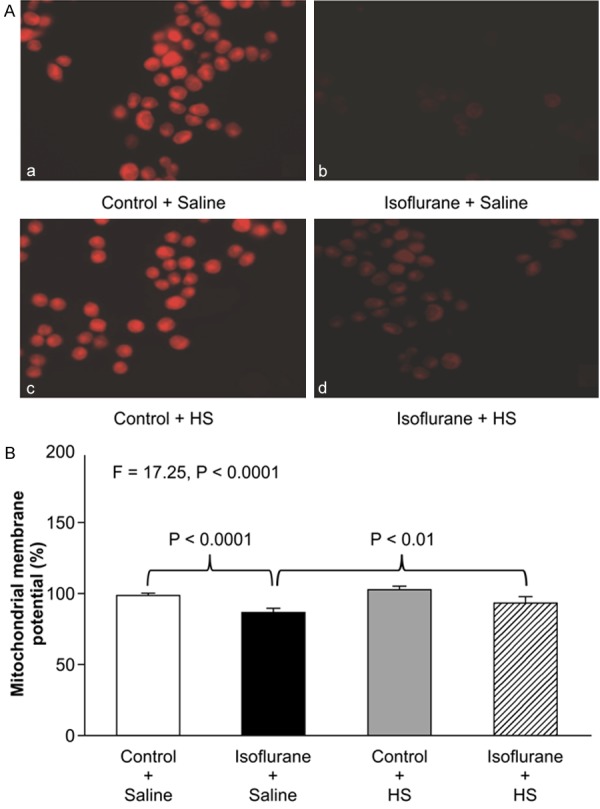
HS attenuates the isoflurane-induced reduction in mitochondrial membrane potential in H4-APP cells. A: Staining with tetramethylrhodamine ethyl ester and perchlorate, a MMP-dependent fluorescent indicator, shows that treatment with 2% isoflurane plus saline for 3 h (panel b) decreases MMP levels compared with the control condition plus saline (panel a). Treatment with 2% isoflurane plus 300 μM HS for 3 h (panel d) attenuates the isoflurane-induced MMP reduction (panel b). B: Tetraethylbenzimidazolylcarbocyanine iodide (JC-1) fluorescence analysis shows that treatment with 2% isoflurane plus saline for 3 h (black bar) decreases MMP compared with the control condition plus saline (white bar) in H4-APP cells. Treatment with 300 μM HS for 3 h (gray bar) does not significantly alter MMP compared with the control condition plus saline (white bar). Treatment with 2% isoflurane plus 300 μM HS for 3 h (striped bar) leads to a smaller reduction in MMP compared with treatment with 2% isoflurane plus saline (black bar). N=6 in each group. MMP: mitochondrial membrane potential, HS: hydrogen-rich saline.
HS attenuates isoflurane-induced reduction in ATP levels
Two-way ANOVA showed a significant interaction between the effect of group (control condition vs. isoflurane) and treatment (saline vs. HS) on ATP levels (F=7.475, P=0.0027; Figure 5). A post-hoc Bonferroni test showed that ATP levels were lower in H4-APP cells treated with isoflurane than in those treated with the control condition (P=0.0388), and ATP levels were higher in cells treated with isoflurane plus HS than in those treated with isoflurane plus saline (P=0.014). Taken together, these results suggest that HS alleviated isoflurane-indu-ced mitochondrial dysfunction, including both mPTP opening and reduction of MMP and ATP, in H4-APP cells.
Figure 5.
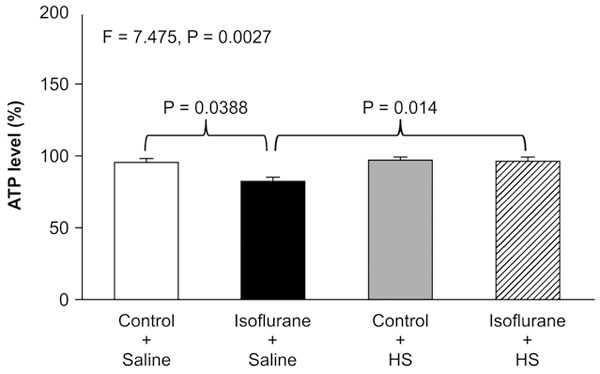
HS attenuates isoflurane-induced reduction in ATP levels in H4-APP cells. Treatment with 2% isoflurane plus saline for 3 h (black bar) decreases ATP levels compared with the control condition plus saline (white bar). Treatment with 300 μM HS (gray bar) does not significantly alter ATP levels compared with the control condition plus saline (white bar). Moreover, treatment with 2% isoflurane plus 300 μM HS for (striped bar) leads to a smaller reduction in ATP levels compared with treatment with 2% isoflurane plus saline (black bar). N=6 in each group. ATP: adenosine triphosphate, HS: hydrogen-rich saline.
HS ameliorates isoflurane-induced cognitive impairment
As the in vitro studies suggested that HS could alleviate isoflurane-induced cytotoxicity, we then assessed whether HS would ameliorate isoflurane-induced cognitive impairment in mice. Anesthesia with 1.4% isoflurane for 3 h induced cognitive deficit in mice, as evidenced by decreased freezing time in the FCT context test at 7 days (two-way ANOVA, F=17.66, P<0.0001; post-hoc Bonferroni test, P<0.0001; Figure 6A) and in the FCT tone test at 2 days (two-way ANOVA, F=7.893, P=0.0006; post-hoc Bonferroni test, P<0.0001; Figure 6B). A post-hoc Bonferroni test showed that freezing times were greater for mice treated with isoflurane plus HS than for those treated with isoflurane plus saline in the FCT context test at 7 days (P=0.015; Figure 6A) and in the FCT tone test at 2 days (P=0.004; Figure 6B). These findings suggest that HS has the ability to mitigate isoflurane-induced cognitive decline in mice.
Figure 6.
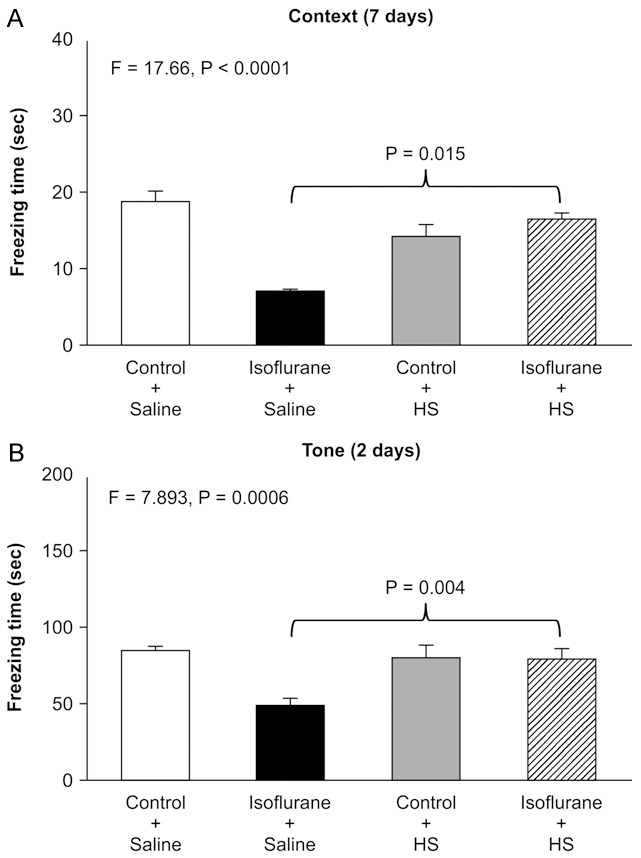
HS Ameliorates isoflurane-induced cognitive impairment. Anesthesia with 1.4% isoflurane for 2 h induces cognitive deficit in mice, as evidenced by decreased freezing time in the FCT context test at 7 days (two-way ANOVA, F=8.032, P=0.008; post-hoc Bonferroni test, P=0.034) and in the FCT tone test at 2 days (two-way ANOVA, F=10.050, P=0.003; post-hoc Bonferroni test, P= 0.041). A post-hoc Bonferroni test showed that freezing times were greater for mice treated with isoflurane plus HS than for those treated with isoflurane plus saline in the FCT context test at 7 days (P=0.015) and in the FCT tone test at 2 days (P=0.004).
Discussion
In the current study, we evaluated the interaction between isoflurane and HS on caspase-3 activation, ROS accumulation, mitochondrial function, ATP levels, and cognitive change. We found that HS treatment ameliorated isoflurane-induced caspase-3 activation in H4-APP cells (Figure 1). These findings suggest that HS could mitigate the cytotoxicity of isoflurane, and could thus serve as a targeted intervention for anesthetic neurotoxicity.
Studies have convincingly demonstrated that hydrogen has cytoprotective effects in different cell types and disease models, including AD [37,38], ischemia-reperfusion injury [31], drug toxicity [39], and trauma [40]. Hong et al. [34] showed that hydrogen seemed to reduce neuronal apoptosis through regulation of the Bcl-2 family and caspase-3 in acute brain injury after subarachnoid hemorrhage. The neuronal protective effect of hydrogen against isoflurane observed in our study warrants future studies to assess HS as a potential therapeutic candidate in for treating and preventing anesthesia neurotoxicity.
ROS accumulation, mitochondrial dysfunction, and reduction in ATP levels have all been proposed as underlying mechanisms of isoflurane-induced caspase-3 activation [19,20,41]. Hydrogen, a small gas molecule, is capable of penetrating cell membranes and entering the cytosol, mitochondria, and nucleus to selectively scavenge the reactive oxygen species OH- and ONOO-, thereby preventing their interference in normal metabolism and signal transmission [27]. The application of saline containing a therapeutic dose of hydrogen (HS) is a useful method of delivering molecular hydrogen [33]. Here, we utilized HS to investigate the possible mechanisms of isoflurane-induced caspase-3 activation. We found that HS ameliorated isoflurane-induced effects including accumulation of ROS (Figure 2), opening of mPTP (Figure 3), reduction in MMP (Figure 4), and reduction in ATP levels (Figure 5) in H4-APP cells. This study provides evidence that HS inhibits caspase-3 activation by inhibiting oxidative stress and maintaining mitochondrial function. Moreover, these data suggest that ROS and mitochondrial dysfunction may be upstream mechanisms of isoflurane-induced caspase-3 activation.
Further, we demonstrated that HS attenuated isoflurane-induced cognitive decline in mice. These data suggest that HS could mitigate the neurobehavioral impairment induced by anesthesia. Previously, Li et al. [37] showed that HS improved memory function in a rat model of amyloid-β-induced AD. Taken together, these findings indicate that the benefits of HS may not be limited to a specific cognitive disease.
This study has several limitations. First, we did not assess dose-dependent effects of HS on caspase-3 activation and cognitive function. It is likely that different concentrations of HS may have different effects on isoflurane-induced caspase-3 activation, increases in ROS levels, mitochondrial dysfunction, and reductions in ATP levels. Runtuwene et al. [42] showed that high-content hydrogen water (0.8 mM H2 saturation) exhibited a stronger anti-oxidative effect than natural hydrogen water (NHW, 0.125 mM hydrogen), and increased the survival rate of cancer-bearing mice to a greater degree. Second, we did not measure caspase-3 activation in vivo. Wang et al. showed that HS reduced caspase-3 overexpression in spinal cord injury model rats [40]. Thus, future studies are required to determine whether isoflurane-induced caspase-3 activation is causally associated with neurodegeneration. Third, we did not determine whether HS could ameliorate isoflurane-induced cognitive decline as measured via other behavioral methods. Future studies should include other methods (e.g., the Morris water maze) to further assess whether treatment with HS can improve isoflurane-induced neurobehavioral deficits. Nevertheless, the aim of the present study was to establish a system and to generate a concept. In the future, we will use this established system to systematically investigate the interaction of HS (as well as other antioxidant agents; e.g., carbon monoxide and hydrogen sulfide) and isoflurane (as well as other anesthetics; e.g., sevoflurane) on neurotoxicity and neurobehavioral decline.
In conclusion, we found that HS attenuated isoflurane-induced cytotoxicity, including caspase-3 activation, ROS accumulation, mPTP opening, decreases in MMP, and reduction in ATP levels, in H4-APP cells. Moreover, HS alleviated isoflurane-induced cognitive impairment in mice. Pending further studies, these results suggest that HS might mitigate isoflurane-induced caspase-3 activation via ROS-, mitochondria-, and ATP-associated mechanisms, thus inhibiting the isoflurane-induced cognitive deficit. The findings of the current study may help guide the design of new therapies to address the underlying mechanisms of anesthetic neurotoxicity, and develop targeted interventions for anesthesia neurotoxicity.
Acknowledgements
Isoflurane was generously provided by Department of Anesthesiology, Shanghai Tenth People’s Hospital, Tongji University School of Medicine. We also appreciate the information that all authors of the publications included in this study provided. This work was supported by the Fundamental Research Funds for the Central Universities (1501219104) to Dan Chen, the Shanghai Health and Family Planning Committee (20154Y0011) to Cheng Li, the Xinchen Youth Anesthesiologists Foundation to Cheng Li, Natural Science Foundation of Shanghai (16ZR1426400) to Cheng Li and National Natural Science Foundation of China (81600921) to Cheng Li.
Disclosure of conflict of interest
None.
References
- 1.Hussain M, Berger M, Eckenhoff RG, Seitz DP. General anesthetic and the risk of dementia in elderly patients: current insights. Clin Interv Aging. 2014;9:1619–1628. doi: 10.2147/CIA.S49680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bilotta F, Doronzio A, Stazi E, Titi L, Fodale V, Di Nino G, Rosa G. Postoperative cognitive dysfunction: toward the Alzheimer’s disease pathomechanism hypothesis. J Alzheimers Dis. 2010;22(Suppl 3):81–89. doi: 10.3233/JAD-2010-100825. [DOI] [PubMed] [Google Scholar]
- 3.Dong Y, Zhang G, Zhang B, Moir RD, Xia W, Marcantonio ER, Culley DJ, Crosby G, Tanzi RE, Xie Z. The common inhalational anesthetic sevoflurane induces apoptosis and increases beta-amyloid protein levels. Arch Neurol. 2009;66:620–631. doi: 10.1001/archneurol.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perucho J, Rubio I, Casarejos MJ, Gomez A, Rodriguez-Navarro JA, Solano RM, De Yebenes JG, Mena MA. Anesthesia with isoflurane increases amyloid pathology in mice models of Alzheimer’s disease. J Alzheimers Dis. 2010;19:1245–1257. doi: 10.3233/JAD-2010-1318. [DOI] [PubMed] [Google Scholar]
- 5.Tang JX, Baranov D, Hammond M, Shaw LM, Eckenhoff MF, Eckenhoff RG. Human Alzheimer and inflammation biomarkers after anesthesia and surgery. Anesthesiology. 2011;115:727–732. doi: 10.1097/ALN.0b013e31822e9306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whittington RA, Virag L, Gratuze M, Petry FR, Noel A, Poitras I, Truchetti G, Marcouiller F, Papon MA, El Khoury N, Wong K, Bretteville A, Morin F, Planel E. Dexmedetomidine increases tau phosphorylation under normothermic conditions in vivo and in vitro. Neurobiol Aging. 2015;36:2414–2428. doi: 10.1016/j.neurobiolaging.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C, Ho YS, Ng OT, Irwin MG, Chang RC, Wong GT. Dexmedetomidine directly increases tau phosphorylation. J Alzheimers Dis. 2015;44:839–850. doi: 10.3233/JAD-142238. [DOI] [PubMed] [Google Scholar]
- 8.Li C, Liu S, Xing Y, Tao F. The role of hippocampal tau protein phosphorylation in isoflurane-induced cognitive dysfunction in transgenic APP695 mice. Anesth Analg. 2014;119:413–419. doi: 10.1213/ANE.0000000000000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang H, Dong Y, Zhang J, Xu Z, Wang G, Swain CA, Zhang Y, Xie Z. Isoflurane induces endoplasmic reticulum stress and caspase activation through ryanodine receptors. Br J Anaesth. 2014;113:695–707. doi: 10.1093/bja/aeu053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ribeiro PO, Antunes LM, Nunes CS, Silva HB, Cunha RA, Tome AR. The effects of different concentrations of the alpha2-Adrenoceptor agonist medetomidine on basal excitatory synaptic transmission and synaptic plasticity in hippocampal slices of adult mice. Anesth Analg. 2015;120:1130–1137. doi: 10.1213/ANE.0000000000000636. [DOI] [PubMed] [Google Scholar]
- 11.Zhang B, Tian M, Zhen Y, Yue Y, Sherman J, Zheng H, Li S, Tanzi RE, Marcantonio ER, Xie Z. The effects of isoflurane and desflurane on cognitive function in humans. Anesth Analg. 2012;114:410–415. doi: 10.1213/ANE.0b013e31823b2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen CW, Lin CC, Chen KB, Kuo YC, Li CY, Chung CJ. Increased risk of dementia in people with previous exposure to general anesthesia: a nationwide population-based case-control study. Alzheimers Dement. 2014;10:196–204. doi: 10.1016/j.jalz.2013.05.1766. [DOI] [PubMed] [Google Scholar]
- 13.Chen PL, Yang CW, Tseng YK, Sun WZ, Wang JL, Wang SJ, Oyang YJ, Fuh JL. Risk of dementia after anaesthesia and surgery. Br J Psychiatry. 2014;204:188–193. doi: 10.1192/bjp.bp.112.119610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y, Pan N, Ma Y, Zhang S, Guo W, Li H, Zhou J, Liu G, Gao M. Inhaled sevoflurane may promote progression of amnestic mild cognitive impairment: a prospective, randomized parallel-group study. Am J Med Sci. 2013;345:355–360. doi: 10.1097/MAJ.0b013e31825a674d. [DOI] [PubMed] [Google Scholar]
- 15.Sprung J, Jankowski CJ, Roberts RO, Weingarten TN, Aguilar AL, Runkle KJ, Tucker AK, McLaren KC, Schroeder DR, Hanson AC, Knopman DS, Gurrieri C, Warner DO. Anesthesia and incident dementia: a population-based, nested, case-control study. Mayo Clin Proc. 2013;88:552–561. doi: 10.1016/j.mayocp.2013.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seitz DP, Shah PS, Herrmann N, Beyene J, Siddiqui N. Exposure to general anesthesia and risk of Alzheimer’s disease: a systematic review and meta-analysis. BMC Geriatr. 2011;11:83. doi: 10.1186/1471-2318-11-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie Z, Dong Y, Maeda U, Moir RD, Xia W, Culley DJ, Crosby G, Tanzi RE. The inhalation anesthetic isoflurane induces a vicious cycle of apoptosis and amyloid beta-protein accumulation. J Neurosci. 2007;27:1247–1254. doi: 10.1523/JNEUROSCI.5320-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie Z, Culley DJ, Dong Y, Zhang G, Zhang B, Moir RD, Frosch MP, Crosby G, Tanzi RE. The common inhalation anesthetic isoflurane induces caspase activation and increases amyloid beta-protein level in vivo. Ann Neurol. 2008;64:618–627. doi: 10.1002/ana.21548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Xu Z, Wang H, Dong Y, Shi HN, Culley DJ, Crosby G, Marcantonio ER, Tanzi RE, Xie Z. Anesthetics isoflurane and desflurane differently affect mitochondrial function, learning, and memory. Ann Neurol. 2012;71:687–698. doi: 10.1002/ana.23536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Dong Y, Wu X, Lu Y, Xu Z, Knapp A, Yue Y, Xu T, Xie Z. The mitochondrial pathway of anesthetic isoflurane-induced apoptosis. J Biol Chem. 2010;285:4025–4037. doi: 10.1074/jbc.M109.065664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miao HH, Zhen Y, Ding GN, Hong FX, Xie ZC, Tian M. Ginsenoside Rg1 attenuates isoflurane-induced caspase-3 activation via inhibiting mitochondrial dysfunction. Biomed Environ Sci. 2015;28:116–126. doi: 10.3967/bes2015.014. [DOI] [PubMed] [Google Scholar]
- 22.Gentry KR, Steele LM, Sedensky MM, Morgan PG. Early developmental exposure to volatile anesthetics causes behavioral defects in Caenorhabditis elegans. Anesth Analg. 2013;116:185–189. doi: 10.1213/ANE.0b013e31826d37c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng Y, Levy RJ. Subclinical carbon monoxide limits apoptosis in the developing brain after isoflurane exposure. Anesth Analg. 2014;118:1284–1292. doi: 10.1213/ANE.0000000000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y, Dong Y, Xu Z, Xie Z. Propofol and magnesium attenuate isoflurane-induced caspase-3 activation via inhibiting mitochondrial permeability transition pore. Med Gas Res. 2012;2:20. doi: 10.1186/2045-9912-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun Y, Zhang Y, Cheng B, Dong Y, Pan C, Li T, Xie Z. Glucose may attenuate isoflurane-induced caspase-3 activation in H4 human neuroglioma cells. Anesth Analg. 2014;119:1373–1380. doi: 10.1213/ANE.0000000000000383. [DOI] [PubMed] [Google Scholar]
- 26.Cheng B, Zhang Y, Wang A, Dong Y, Xie Z. Vitamin C Attenuates isoflurane-induced caspase-3 activation and cognitive impairment. Mol Neurobiol. 2015;52:1580–9. doi: 10.1007/s12035-014-8959-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K, Katsura K, Katayama Y, Asoh S, Ohta S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13:688–694. doi: 10.1038/nm1577. [DOI] [PubMed] [Google Scholar]
- 28.Hong Y, Chen S, Zhang JM. Hydrogen as a selective antioxidant: a review of clinical and experimental studies. J Int Med Res. 2010;38:1893–1903. doi: 10.1177/147323001003800602. [DOI] [PubMed] [Google Scholar]
- 29.Han L, Tian R, Yan H, Pei L, Hou Z, Hao S, Li YV, Tian Q, Liu B, Zhang Q. Hydrogen-rich water protects against ischemic brain injury in rats by regulating calcium buffering proteins. Brain Res. 2015;1615:129–138. doi: 10.1016/j.brainres.2015.04.038. [DOI] [PubMed] [Google Scholar]
- 30.Xie Z, Dong Y, Maeda U, Alfille P, Culley DJ, Crosby G, Tanzi RE. The common inhalation anesthetic isoflurane induces apoptosis and increases amyloid beta protein levels. Anesthesiology. 2006;104:988–994. doi: 10.1097/00000542-200605000-00015. [DOI] [PubMed] [Google Scholar]
- 31.Wei R, Zhang R, Xie Y, Shen L, Chen F. Hydrogen suppresses hypoxia/reoxygenation-induced cell death in hippocampal neurons through reducing oxidative stress. Cell Physiol Biochem. 2015;36:585–598. doi: 10.1159/000430122. [DOI] [PubMed] [Google Scholar]
- 32.Schinzel AC, Takeuchi O, Huang Z, Fisher JK, Zhou Z, Rubens J, Hetz C, Danial NN, Moskowitz MA, Korsmeyer SJ. Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc Natl Acad Sci U S A. 2005;102:12005–12010. doi: 10.1073/pnas.0505294102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo SX, Jin YY, Fang Q, You CG, Wang XG, Hu XL, Han CM. Beneficial effects of hydrogen-rich saline on early burn-wound progression in rats. PLoS One. 2015;10:e0124897. doi: 10.1371/journal.pone.0124897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hong Y, Shao A, Wang J, Chen S, Wu H, McBride DW, Wu Q, Sun X, Zhang J. Neuroprotective effect of hydrogen-rich saline against neurologic damage and apoptosis in early brain injury following subarachnoid hemorrhage: possible role of the Akt/GSK3beta signaling pathway. PLoS One. 2014;9:e96212. doi: 10.1371/journal.pone.0096212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- 36.Xu Z, Dong Y, Wang H, Culley DJ, Marcantonio ER, Crosby G, Tanzi RE, Zhang Y, Xie Z. Age-dependent postoperative cognitive impairment and Alzheimer-related neuropathology in mice. Sci Rep. 2014;4:3766. doi: 10.1038/srep03766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li J, Wang C, Zhang JH, Cai JM, Cao YP, Sun XJ. Hydrogen-rich saline improves memory function in a rat model of amyloid-beta-induced Alzheimer’s disease by reduction of oxidative stress. Brain Res. 2010;1328:152–161. doi: 10.1016/j.brainres.2010.02.046. [DOI] [PubMed] [Google Scholar]
- 38.Wang C, Li J, Liu Q, Yang R, Zhang JH, Cao YP, Sun XJ. Hydrogen-rich saline reduces oxidative stress and inflammation by inhibit of JNK and NF-kappaB activation in a rat model of amyloid-beta-induced Alzheimer’s disease. Neurosci Lett. 2011;491:127–132. doi: 10.1016/j.neulet.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 39.Nakashima-Kamimura N, Mori T, Ohsawa I, Asoh S, Ohta S. Molecular hydrogen alleviates nephrotoxicity induced by an anti-cancer drug cisplatin without compromising anti-tumor activity in mice. Cancer Chemother Pharmacol. 2009;64:753–761. doi: 10.1007/s00280-008-0924-2. [DOI] [PubMed] [Google Scholar]
- 40.Wang JL, Zhang QS, Zhu KD, Sun JF, Zhang ZP, Sun JW, Zhang KX. Hydrogen-rich saline injection into the subarachnoid cavity within 2 weeks promotes recovery after acute spinal cord injury. Neural Regen Res. 2015;10:958–964. doi: 10.4103/1673-5374.158361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang H, Xu Z, Wu A, Dong Y, Zhang Y, Yue Y, Xie Z. 2-deoxy-D-glucose enhances anesthetic effects in mice. Anesth Analg. 2015;120:312–319. doi: 10.1213/ANE.0000000000000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Runtuwene J, Amitani H, Amitani M, Asakawa A, Cheng KC, Inui A. Hydrogen-water enhances 5-fluorouracil-induced inhibition of colon cancer. Peer J. 2015;3:e859. doi: 10.7717/peerj.859. [DOI] [PMC free article] [PubMed] [Google Scholar]


