Abstract
Objective: This study aimed to explore the role of the transforming growth factor-β/mitogen activated protein kinase (TGF-β/MAPK) signaling pathway in the effects of bone marrow mesenchymal stem cells (BMSCs) on urinary control and interstitial cystitis in a rat model of urinary bladder transplantation. Methods: A urinary bladder transplantation model was established using Sprague-Dawley rats. Rats were assigned to normal (blank control), negative control (phosphate-buffered saline injection), BMSCs (BMSC injection), sp600125 (MAPK inhibitor injection), or protamine sulfate (protamine sulfate injection) groups. Immunohistochemistry, urodynamic testing, hematoxylin-eosin staining, Western blotting, enzyme-linked immunosorbent assay, and MTT assay were used to assess BMSC growth, the kinetics of bladder urinary excretion, pathological changes in bladder tissue, bladder tissue ultrastructure, the expression of TGF-β/MAPK signaling pathway-related proteins, levels of inflammatory cytokines, and the effects of antiproliferative factor on cell proliferation. Results: Compared with normal, negative control, BMSCs, and sp600125 groups, rats in the PS group exhibited decreased discharge volume, maximal micturition volume, contraction interval, and bladder capacity but increased residual urine volume, bladder pressure, bladder peak pressure, expression of TGF-β/MAPK signaling pathway-related proteins, levels of inflammatory cytokines, and growth inhibition rate. Levels of inflammatory cytokines and the growth inhibition rate were positively correlated with the expression of TGF-β/MAPK signaling pathway-related proteins. Conclusions: Our findings demonstrate that the TGF-β/MAPK signaling pathway mediates the beneficial effects of BMSCs on urinary control and interstitial cystitis.
Keywords: TGF-β/MAPK signaling pathway, bone marrow mesenchymal stem cells, interstitial cystitis, urinary control, bladder transplantation
Introduction
Interstitial cystitis (IC), also referred to as bladder pain syndrome or painful bladder syndrome, is a debilitating chronic inflammatory bladder disorder [1] that mainly affects women and is characterized by symptoms such as frequency, nocturia, urinary urgency, and pelvic and suprapubic pain [2]. IC symptoms are reportedly widespread among women in the United States, suggesting that IC may be underdiagnosed, and are associated with considerable disability [3]. One study reports that the overall prevalence of IC is 306/100,000 women, with the highest prevalence (464/100,000) among women aged 40-59 years old, and two-thirds of those with moderate to high risk for IC have poor quality of life [4]. IC patients typically experience pain, pressure, or discomfort caused by bladder filling and may present with urinary frequency and urgency, indicating poor urinary control [5]. Despite advances in our understanding of pelvic anatomy and the refinement of surgical techniques, loss of urinary control after radical prostatectomy remains a significant source of patient morbidity and diminishes quality of life [6], and effective treatments are currently lacking. However, bone marrow mesenchymal stem cells (BMSCs), the differentiation of which requires suitable stimuli such as transforming growth factor-β (TGF-β), can improve remodeling and integration with the host surface zone and thereby help restore urinary bladder after transplantation [7].
As a multifunctional cytokine that regulates embryogenesis and tissue homeostasis, TGF-β controls the growth and differentiation of a large variety of cells [8]. Members of the TGF-β family, including TGF-β1, 2, and 3 that are encoded by different genes, exhibit diverse tissue distribution patterns and perform different biological activities in the body [9]. Tyagi et al. report that TGF-β1 expression is elevated in tissue biopsies of IC patients and that TGF-β1 can be utilized for the diagnosis and treatment of IC [10]. TGF-β-related agents stimulate the mitogen activated protein kinase (MAPK) signal transduction pathway, which is highly conserved across eukaryotic cells [10]. The MAPK family of signal transduction proteins includes extracellular signal-regulated kinases (ERKs), c-Jun N-terminal kinases (JNKs), and p38, which can convert extracellular signals [11]. Previous studies show that activation of MAPK signaling promotes proliferation and inflammation-associated malignant transformation, and MAPKs are regarded as promising targets for treating diseases [12,13]. Importantly, the MAPK signaling pathway is sufficient to direct differentiation of mouse BMSCs into hepatocytes, which could be beneficial for clinical application [14]. Although activation of the MAPK/TGF-β signaling pathway is associated with hepatic fibrosis [15], few studies have described its relationship with urinary control or IC. Thus, the aim of our study was to investigate the role of the TGF-β/MAPK signaling pathway in the effect of BMSCs on urinary control and IC.
Materials and methods
Ethics statement
All animal experimental designs were approved by the animal ethics committee of our hospital, and all studies strictly conformed to animal use and protection guidelines issued by the International Association for the Study of Pain [16].
Study subjects and the extraction and identification of BMSCs
A total of 60 (30 males and 30 females) 4-month-old clean healthy Sprague-Dawley (SD) rats (Shanghai SLAC Laboratory Animal Co., Ltd., Shanghai, China) between 200-250 g were included in the study. The rats were maintained on a normal circadian rhythm with free access to food and water in a quiet room with good ventilation at 21-23°C and humidity of 60 ± 5%. Two percent pentobarbital sodium (Wuhan Boster Biological Engineering Co., Ltd, Hubei, China) was used to anaesthetize the rats. The skin was then disinfected, and the tibias were removed under aseptic conditions and washed with phosphate-buffered saline (PBS). The epiphysis was cut off to expose the marrow cavity, and Dulbecco’s modified Eagle’s medium (GE Healthcare Life Sciences HyClone Laboratories, Logan, UT, USA) containing 10% fetal bovine serum (FBS, Gibco Company, Grand Island, NY, USA), 0.1 U/L penicillin, and 0.1 μg/L streptomycin (GE Healthcare Life Sciences HyClone Laboratories, Logan, UT, USA) was used to wash out the bone marrow. A single-cell suspension was made and centrifuged for 5 min at 1000 rpm. The cells were re-suspended, inoculated into the culture bottle, and cultured at 37°C. The culture medium was replaced after 48 h and again every 3 d. Cells in a good growth state were obtained after the third passage and digested with 0.25% trypsin (Gibco Company, Grand Island, NY, USA) and centrifuged for 5 min at 1000 rpm at 4°C. Cells were washed with PBS containing 1% bovine serum albumin (BSA) and counted. Monoclonal antibodies CD34, CD44, CD45, CD90, and CD105 (1:100, Abcam Inc., Cambridge, MA, USA) were added into the tube successively. The same type of negative control was established for each sample, and the tubes were incubated on ice for 45 min in the dark. Cells were washed three times to remove unconjugated antibody, and 500 μL PBS containing 1% BSA was added to re-suspend the cells, which were detected and analyzed by flow cytometry (Becton, Dickinson and Company, Franklin Lakes, NJ, USA). BMSCs (third passage) plus BrdU (final concentration: 10 μmol/L, Beijing Zhongsheng Rutai Biotechnology Co., Ltd., Beijing, China) were cultured for 48 h, and the concentration of BrdU-labeled BMSCs was adjusted to 2 × 105/mL.
Experimental groups
Rats were randomly assigned into five groups with 6 rats per group: normal (blank control), negative control (NC; bladder transplantation + PBS injection), BMSCs (bladder transplantation + BMSC injection), sp600125 (bladder transplantation + MAPK inhibitor injection), and protamine sulfate (PS; bladder transplantation + PS injection). The treatments for rats in each group are shown in Table 1. After rats were anesthetized with 2% pentobarbital sodium (Wuhan Boster Biological Engineering Co., Ltd, Hubei, China), the abdomen was disinfected, a midline incision was made, and the omentum majus was removed from the abdominal cavity. A full bladder was immediately transplanted into the omentum majus with distal reentry and stitched with a 8-0 Prolene suture (Huatuo Medicines Co., Ltd, Anhui, China). After fixation, the abdomen was closed with sutures, and rats were treated with 30,000 units of penicillin (Sigma-Aldrich Chemical Company, St. Louis, MO, USA) by intramuscular injection for preventing infection. One month after bladder transplantation, 3 mL/kg of 10% chloral hydrate was injected into the abdominal cavity to anaesthetize and disinfect the rats. For rats in the NC group, after lubrication with sterile paraffin oil, PE-50 tubing was introduced into the bladder through a catheter, and 0.5 mL PBS (30 mg/mL) was irrigated into the bladder and preserved for 30 min after draining out the bladder urine. For rats in the PS group, 0.5 mL PS was used to irrigate the bladder in the same manner. For rats in the sp600125 group, 300 mg/kg sp600125 solution was injected into the abdominal cavity 2 h before the irrigation of PS into the bladder. For rats in the BMSCs group, the lower abdomen was cut lengthwise with a 1-cm incision. BMSC cell sap (10 μL) was extracted using a micro-injector (Hamilton Company, Reno, NV, USA) and injected into the tunica muscularis vesicae urinariae. The incision was closed with sutures, and rats were intramuscularly injected with 30,000 units of penicillin (Sigma-Aldrich Chemical Company, St. Louis, MO, USA) for preventing infection. All rats were treated three times a week for 4 weeks, and carbon dioxide was used to sacrifice rats 48 h after the last treatment.
Table 1.
Treatment regimens of rats in each group
| Group | Treatment regimen |
|---|---|
| Normal | Normal rats, no treatment |
| NC | Bladder transplantation, 0.5 mL PBS perfusion |
| PS | Bladder transplantation, 0.5 mL 30 mg/kg PS perfusion |
| BMSCs | Bladder transplantation, PS perfusion, intraperitoneal injection of BMSCs |
| sp600125 | Bladder transplantation, PS perfusion, intraperitoneal injection of 30 mg/kg sp600125 solution |
Note: NC, negative control; PBS, phosphate-buffered saline; PS, protamine sulfate; BMSCs, bone marrow mesenchymal stem cells.
Immunohistochemistry (IHC)
IHC was used to identify dye-positive cells in bladder tissue. Four weeks after cell transplantation, 10% chloral hydrate (3 mL/kg) was injected into the abdominal cavity to anaesthetize and disinfect the rats, and bladder tissue was removed. The wound was closed with sutures, and rats were returned to the animal colony after waking. Bladder tissue was fixed in 4% paraformaldehyde, dehydrated using graded ethanol, waxed and embedded, and cut into slices (5 μm). Slices were hydrated with conventional dewaxing, soaked in 3% H2O2 in the dark to remove endogenous catalase, and antigen was used for repair. Slices were incubated in 0.5% Triton after washing with PBS-Tween, incubated with 2 mol/L hydrochloric acid, and 1% BSA was added to remove heterogenetic antigen. Slices were incubated with BrdU antibody (mouse monoclonal antibody, 1:100, Abcam Inc., Cambridge, MA, USA) for 2 h at 37°C. Slices were then treated with kit 1 (Beijing Zhongshan Jinqiao Biotechnology Co., Ltd., Beijing, China) for 20 min at 37°C and kit 2 (Beijing Zhongshan Jinqiao Biotechnology Co., Ltd., Beijing, China) for 20 min. Diaminobenzidine was added for coloration, and the slices were lightly stained with hematoxylin for 30 s (Wuhan Boster Biological Engineering Co., Ltd, Hubei, China). An Olympus CX23 microscope was used to observe the growth of transplanted cells.
Urodynamic testing
Urodynamic testing was performed using a urine tester (Laborie, Mississauga, ON, Canada) 12 h after drinking when the bladder was empty. Rats were anesthetized via hypodermic injection with 2% pentobarbital sodium (Wuhan Boster Biological Engineering Co., Ltd, Hubei, China) before urine testing. Free uroflowmetry was first used to determine residual urine volume, followed by filling cystometry and pressure-flow experiments. Bladder perfusion was conducted using a 3M micro-perfusion pump that injected normal saline into the bladder a rate of 50 mL/min through an epidural catheter. The detection indexes included discharge volume (total amount of urination/micturition frequency), residual urine volume (amount of perfusion-total amount of urination), maximal micturition volume (longest filling time × perfusion rate), bladder pressure, bladder peak pressure, contraction interval, and bladder capacity.
Hematoxylin-eosin (HE) staining
HE staining was used to observe mucosal epithelial defects in bladder tissue and the infiltration condition of bladder interstitial inflammation. Tissue was fixed with 4% paraformaldehyde, dehydrated using graded ethanol, embedded in paraffin (Thermo Fisher Scientific Inc., Waltham, MA, USA), and cut into slices (5 μm). Slices underwent conventional dewaxing and were stained by HE (Wuhan Boster Biological Engineering Co., Ltd, Hubei, China) for 10 min. After washing in running water for 15 min, 1% alcohol solution was used for differentiation. Slices were washed, dehydrated using graded ethanol, and then hematoxylin was added to re-dye for 2 min. After dehydration, transparency, and sealing with resinene (Wuhan Boster Biological Engineering Co., Ltd, Hubei, China), an Olympus CX31 microscope was used to observe pathological changes in the tissue.
Western blotting
After bladder tissue was prepared, total protein was extracted from cells using the Trizol method (Invitrogen Inc., Carlsbad, CA, USA), and a bicinchoninic acid kit (Beyotime Biotechnology Co., Shanghai, China) was used to determine protein concentration. Protein samples were boiled at 100°C for 10 min after the addition of sample buffer and then added to each well (30 μg). A total of 10% polyacrylamide gel (Wuhan Boster Biological Engineering Co., Ltd, Hubei, China) was added for electrophoresis, and the protein was separated in spacer gel at 60 V for 45 min and in separation gel at 120 V for 1 h. Samples were wet-transferred onto a polyvinylidene fluoride membrane at 250 mA for 2 h with constant current and incubated in 5% BSA for 1 h at room temperature. Samples were incubated with primary antibodies TGF-β (ab31013), Smad2/3 (ab207447), p-Smad2/3 (ab63399), connective tissue growth factor (CTGF; ab6992), JNK (ab208035), p-JNK (ab124956), c-jun (ab32137), p-c-jun (ab32385), ERK1/2 (ab17942), p-ERK1/2 (ab200807), p38 (ab31828), p-p38 (ab47363), or β-actin (ab8226) (all 1: 1000, Abcam Inc., Cambridge, MA, USA) overnight at 4°C. After washing with TRIS buffer solution-Tween (TBS-T) for 5 min three times, corresponding secondary antibodies were added for 1 h at room temperature. Samples were washed again with TBS-T for 5 min three times. Finally, Roche ECL chemiluminescence reagent was used for development, and Image J software was used for gray value analysis.
Enzyme-linked immunosorbent assay (ELISA)
An ELISA kit (eBioscience, Inc. San Diego, CA, USA) was placed at room temperature for 20 min to prepare the cleaning solution. After dissolving, 100 μL of standard solution was added to the reaction plate to create a standard curve. A total of 100 μL test sample was added to the reaction well and incubated for 90 min at 37°C. After washing, 100 μL biotin antibody working fluid was added and incubated for another 60 min at 37°C. Samples were washed again, treated with 100 μL working fluid with enzyme binding reagent in the dark and incubated for 30 min at 37°C. After washing the plate three times, 100 μL substrate was added and incubated in the dark for 15 min at 37°C, and stop solution was quickly added to terminate the reaction. Universal enzyme marker (Synergy 2, Omega Bio-tek Inc, Norcross, GA, USA) was used to measure optical density (OD) values at a wavelength of 450 nm within 3 min. Expression of inflammatory factors including interleukin (IL)-2, IL-4, IL-10, tumor necrosis factor-α (TNF-α), and interferon-γ (INF-γ) in tail vein serum were analyzed.
Methyl thiazolyl tetrazolium (MTT) assay
Human bladder epithelium cells were obtained from patients with ureteral calculus without history of bladder disease. Cells were cold-digested and cultured with rat urine, and the inhibitory effect of anti-proliferation factor (APF) on cell proliferation was analyzed using MTT assay. Cells were inoculated into 96-well plates (5 × 103/mL/well). After 24 h of culture, 20 μL MTT solution (5 mg/mL; Sigma-Aldrich Chemical Company, St. Louis, MO, USA) was added to each well and incubated for 4 h at 37°C. The culture medium was aspirated, and cells were treated with 150 μL dimethyl sulfoxide (Sigma-Aldrich Chemical Company, St. Louis, MO, USA). Four duplicated wells were set up for each group, and electrophoresis was used to measure OD values at a wavelength of 490 nm. Cell growth inhibition rate (%) was calculated as (1-mean OD valuetreatment group/mean OD valuecontrol group) × 100%.
Statistical analysis
Statistical analysis was conducted using SPSS version 21.0 (SPSS Inc., Chicago, IL, USA). Data are reported as mean ± standard error of the mean. Pairwise comparisons were performed using t tests, and multiple group comparisons were performed using one-way analysis of variance (ANOVA). Frequency data were analyzed using Chi-square tests and presented as count (percentage). Pearson correlation coefficients were calculated to assess relationships between the expression of TGF-β/MAPK pathway-related proteins and mast cell number, levels of inflammatory cytokines, and inhibition rate of cell growth. P-values < 0.05 were considered statistically significant.
Results
BMSCs characterized by flow cytometry
Flow cytometry was used to characterize third-passage BMSCs. We found that 95.24%, 98.17%, and 99.05% of BMSCs expressed CD44, CD90, and CD105, respectively. Only 3.45% and 1.53% of BMCs expressed CD34 and CD45, respectively (Figure 1).
Figure 1.
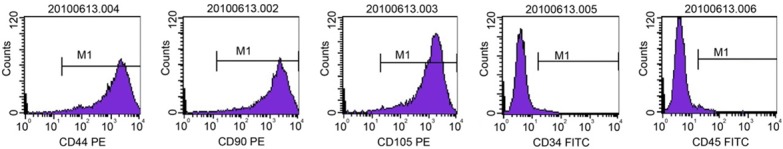
Characterization of third-passage BMSCs by flow cytometry. Note: BMSCs, bone marrow mesenchymal stem cells.
Cell growth assessed by IHC
IHC was used to assess BrdU-labeled BMSCs in the rat bladder, with positive cells stained brown or yellow. We found brown-labeled cells on the muscle layer of the bladder in the BMSCs group, suggesting that transplanted cells grew well in the bladder. By contrast, no positive cells were detected in the normal, NC, PS, or sp600125 groups, which were not transplanted with BMSCs (Figure 2).
Figure 2.

Cell growth as assessed by immunohistochemistry (200 × magnification). Note: BMSCs, bone marrow mesenchymal stem cells; NC, negative control; PS, protamine sulfate.
Bladder urinary excretion kinetics assessed by urodynamic testing
When compared with the normal group, no obvious changes in bladder urinary excretion kinetics were observed in the NC, BMSC, or sp600125 groups (all P > 0.05). However, the PS group exhibited decreased discharge volume, maximal micturition volume, contraction interval, and bladder capacity but increased residual urine volume, bladder pressure, and bladder peak pressure compared with the normal group (all P < 0.05). Compared with the PS group, discharge volume, maximal micturition volume, contraction interval, and bladder capacity were increased whereas residual urine volume, bladder pressure, and bladder peak pressure were decreased in the BMSCs and sp600125 groups (all P < 0.05) (Table 2).
Table 2.
Kinetics of bladder urinary excretion as assessed by urodynamic testing
| Normal group | NC group | PS group | BMSCs group | sp600125 group | |
|---|---|---|---|---|---|
| Discharge volume (mL) | 0.35 ± 0.03 | 0.31 ± 0.02# | 0.17 ± 0.02* | 0.33 ± 0.03# | 0.36 ± 0.04# |
| Residual urine volume (mL) | 0.26 ± 0.03 | 0.23 ± 0.03# | 0.48 ± 0.05* | 0.24 ± 0.02# | 0.27 ± 0.03# |
| Maximal micturition volume (mL) | 0.56 ± 0.07 | 0.52 ± 0.04# | 0.18 ± 0.02* | 0.54 ± 0.06# | 0.58 ± 0.06# |
| Bladder pressure (cm H2O) | 2.42 ± 0.25 | 2.53 ± 0.27# | 4.25 ± 0.43* | 2.39 ± 0.22# | 2.48 ± 0.21# |
| Bladder peak pressure (cm H2O) | 52.36 ± 5.74 | 53.37 ± 5.62# | 68.79 ± 7.32* | 57.48 ± 5.45# | 59.27 ± 5.31# |
| Contraction interval (s) | 162.8 ± 14.3 | 158.4 ± 12.5# | 78.6 ± 7.35* | 156 ± 13.2# | 167.2 ± 15.1# |
| Bladder capacity (mL) | 1.21 ± 0.13 | 1.18 ± 0.19# | 0.87 ± 0.09* | 1.17 ± 0.20# | 1.23 ± 0.11# |
Note: NC, negative control; PS, protamine sulfate; BMSCs, bone marrow mesenchymal stem cells;
P < 0.05 vs. normal group;
P < 0.05 vs. PS group.
Pathological changes in bladder tissue detected by HE staining
HE staining showed that the mucosa of bladder tissue was intact with few signs of inflammation in the normal and NC groups. In the PS group, rats showed severe epithelial injury, with a large amount of inflammatory cell infiltration and obvious edema in the mucosa and laminae propria. The integrity of epithelial tissue was largely maintained in the BMSCs and sp600125 groups, and inflammatory cell infiltration and edema were less pronounced than in the PS group (Figure 3).
Figure 3.
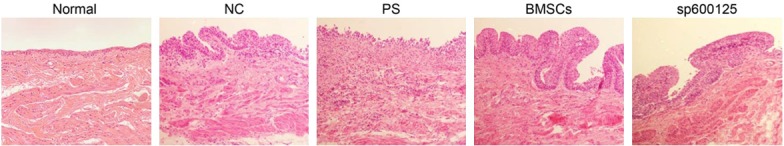
Pathological changes in bladder tissue as detected by HE staining (200 × magnification). Note: HE, hematoxylin-eosin; BMSCs, bone marrow mesenchymal stem cells; NC, negative control; PS, protamine sulfate.
Expression of TGF-β/MAPK pathway-related proteins assessed by western blotting
Western blotting showed that the expression of TGF-β/MAPK signaling pathway-related proteins was similar between the NC, BMSCs, and sp600125 groups and the normal group (all P > 0.05). The expression of TGF-β, p-/Smad2/3, CTGF, p-/JNK, p-/c-jun, p-/ERK1/2, and p-/p38 was up-regulated in bladder tissue of the PS group compared with that of the normal group (all P < 0.05). Compared with the PS group, the expression of TGF-β, p-/Smad2/3, CTGF, p-/JNK, p-/c-jun, p-/ERK1/2, and p-/p38 were down-regulated in the NC, BMSCs, and sp600125 groups, indicating the inhibition of the TGF-β/MAPK signaling pathway (all P < 0.05) (Figure 4).
Figure 4.
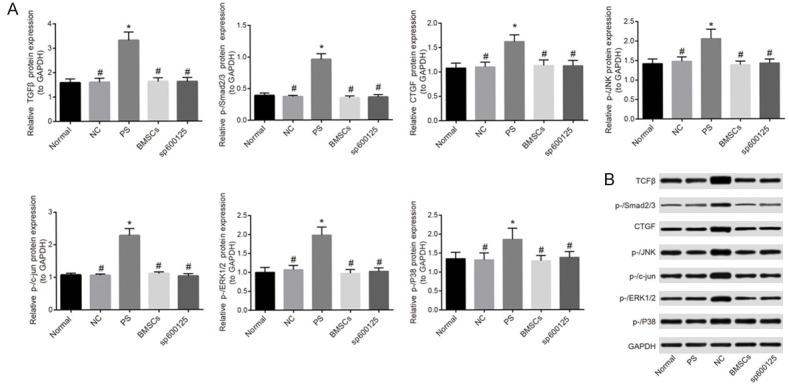
Expression of TGF-β/MAPK signaling pathway-related proteins as assessed by Western blotting. A: Quantification of TGF-β/MAPK signaling pathway-related protein expression. B: Western blotting images. Note: BMSCs, bone marrow mesenchymal stem cells; NC, negative control; PS, protamine sulfate; *P < 0.05 vs. normal group; #P < 0.05 vs. PS group.
Inflammatory cytokine levels assessed by ELISA
No significant differences were found in the levels of inflammatory cytokines (IL-2, IL-4, IL-10, TNF-α, and IFN-γ) in the NC, BMSCs, and sp600125 groups compared with the normal group (all P > 0.05). The PS group showed increased levels of IL-2, IL-4, IL-10, TNF-α, and IFN-γ compared with the normal group (all P < 0.05). The NC, BMSCs, and sp600125 groups showed decreased levels of IL-2, IL-4, IL-10, TNF-α, and IFN-γ than the PS group (all P < 0.05) (Figure 5).
Figure 5.
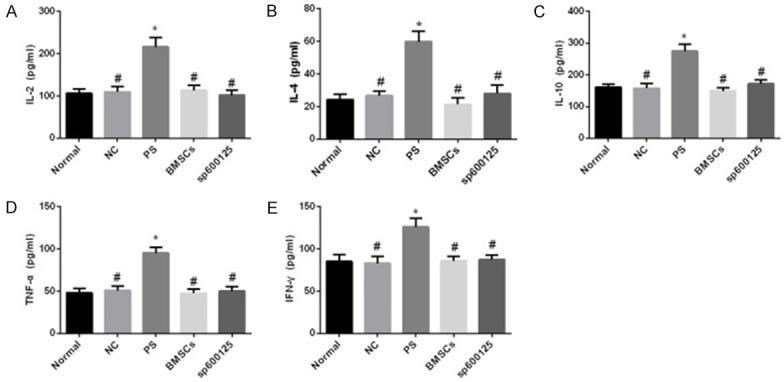
Serum inflammatory cytokine levels as assessed by ELISA. A: IL-2. B: IL-4. C: IL-10. D: TNF-α. E: IFN-γ. Note: BMSCs, bone marrow mesenchymal stem cells; NC, negative control; PS, protamine sulfate; ELISA, enzyme-linked immunosorbent assay; *P < 0.05 vs. normal group; #P < 0.05 vs. PS group.
Inhibitory effect of APF on cell growth assessed by MTT assay
MTT assay was used to analyze the inhibitory effect of APF on cell proliferation. The rate of inhibition of cell growth was similar between the NC, BMSCs, and sp600125 groups and the normal group (all P > 0.05). The inhibition rate was increased in the PS group compared with the normal group (all P < 0.05). The inhibition rate was decreased in the NC, BMSCs, and sp600125 groups compared with the PS group (all P < 0.05) (Figure 6).
Figure 6.
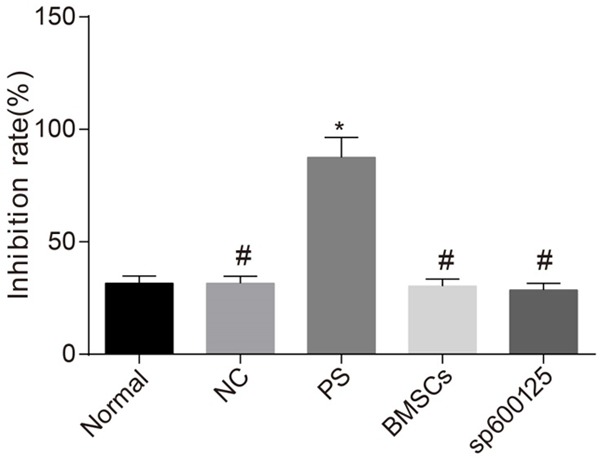
Inhibitory effect of APF on cell growth as assessed by MTT assay. Note: BMSCs, bone marrow mesenchymal stem cells; NC, negative control; PS, protamine sulfate; APF, anti-proliferation factor; HBE, human bladder epithelium; MTT, methyl thiazolyl tetrazolium; *P < 0.05 vs. normal group; #P < 0.05 vs. PS group.
Correlations between the expression of TGF-β/MAPK pathway-related proteins and mast cell number, levels of inflammatory cytokines, and inhibition of cell growth
We found significant positive correlations between the number of mast cells, levels of inflammatory cytokines, and inhibition rate of cell growth and the expression of TGF-β, CTGF, p-/Smad2/3, p-/JNK, p-/c-jun, p-/ERK1/2, and p-/p38 (all r > 0.80, P < 0.05) (Table 3).
Table 3.
Correlations between the expression of TGF-β/MAPK signaling pathway-related proteins and mast cell number, levels of inflammatory cytokines, and inhibition of cell growth
| Mast cell number | IL-2 | IL-4 | IL-10 | TNF-α | IFN-γ | Inhibition rate | |
|---|---|---|---|---|---|---|---|
| TGFβ | 0.991* | 0.994* | 0.970* | 0.975* | 0.935* | 0.978* | 0.991* |
| CTGF | 0.940* | 0.964* | 0.916* | 0.913* | 0.950* | 0.959* | 0.940* |
| p-/Smad2/3 | 0.949* | 0.918* | 0.922* | 0.922* | 0.806* | 0.882* | 0.949* |
| p-/JNK | 0.966* | 0.976* | 0.954* | 0.956* | 0.930* | 0.968* | 0.966* |
| p-/c-jun | 0.991* | 0.981* | 0.965* | 0.969* | 0.915* | 0.958* | 0.991* |
| p-/ERK1/2 | 0.980* | 0.989* | 0.968* | 0.969* | 0.949* | 0.979* | 0.980* |
| p-/p38 | 0.877* | 0.925* | 0.890* | 0.886* | 0.934* | 0.938* | 0.877* |
Note: IL, interleukin; TNF-α, tumor necrosis factor-α; IFN-γ, interferon-γ; TGF-β, transforming growth factor-β; CTGF, connective tissue growth factor; JNK, c-Jun N-terminal kinase; ERK, extracellular signal-regulated kinase;
P < 0.05.
Discussion
We examined the role of the TGF-β/MAPK signaling pathway in the effects of BMSC transplantation on urinary control and IC. We found that the TGF-β/MAPK signaling pathway mediates the beneficial effects of BMSCs on urinary control and IC, suggesting that this pathway can be targeted for treating IC.
The expression of TGF-β/MAPK signaling pathway-related proteins was positively correlated with mast cell number and levels of inflammatory cytokines. As a pleiotropic cytokine, TGF-β regulates a large variety of cellular actions and pathophysiological processes with spatiotemporal precision [17]. The MAPK signaling pathway is composed of a four-kinase cascade module (i.e., MAPK, MAP2K, MAP3K, and MAP4K) that can negatively or positively control the expression of various functional genes through different transcription factors [18]. The MAPK signaling pathway is connected with many cellular processes including differentiation, homeostasis, proliferation, and cellular survival [19]. TGF-β can induce rapidphosphorylation of MAPKs and subfamilies such as ERKs, JNKs, and p38, suggesting that TGF-β is positively related to p-/ERKs, p-/JNKs, and p-/p38 [11]. Mast cells are considered to be involved in allergic reactions via the release of chemokines, cytokines, proteases, and bioactive polyamines, and the activation of mast cells can lead to their distribution and inflammatory reactions, which are characteristics of IC [20,21]. Cytokines, including IL-2, IL-4, IL-10, IFN-γ, and TNF-α, are soluble proteins synthesized by diverse cells within the immune system [22]. On one hand, activated mast cells can increase cytokine expression and production of TGF-β, and MAPK can contribute to cytokine expression, suggesting positive relationships among TGF-β/MAPK proteins, mast cells, cytokines, and TGF-β. On the other hand, the pathogenesis of IC may be closely correlated with the production of inflammatory cytokines, thus activation of the TGF-β/MAPK signaling pathway may be related to IC pathogenesis via the expression of cytokines [23,24]. A previous study shows that intense pulsed light may promote skin rejuvenation via the MAPK-mediated secretion of TGF-β1 [25]. The TGF-β/MAPK signaling pathway is activated in IC, and IC patients have larger numbers of mast cells, providing further evidence of a positive association between expression of TGF-β/MAPK signaling pathway-related proteins and mast cells [21]. Furthermore, Corcoran et al. proposed that cytokines and chemokines such as IL-2, IL-6, IL-8, and TNF-α are up-regulated in urine and/or bladder tissue from IC patients [24], which indirectly supports our finding that activation of the TGF-β/MAPK signaling pathway is associated with higher levels of cytokines. Taken together, the present and previous studies suggest that the TGF-β/MAPK signaling pathway is positively correlated with mast cell numbers and levels of inflammatory cytokines.
Our observations of the kinetics of bladder urinary excretion suggest that BMSC intervention therapy could improve the urinary control of rats after tissue transplantation. BMSCs possess the ability to self-renew and differentiate into multiple cell types, which can provide an alternative cell source for tissue engineering and cell-based therapies [26]. As a hollow organ, the bladder is composed of tissues from the mesoderm (also called smooth muscle cells (SMCs)) and endoderm (also called urothelium), and the interaction between SMCs and urothelium is critical for the development and maintenance of bladder structural integrity and contractile function. The application of BMSCs to urological cell-based tissue engineering can help reconstruct bladder function by inducing stem cell differentiation into both urothelium and SMCs, suggesting that BMSCs may promote urinary control by maintaining urothelium structure and bladder contractile function [27]. Previous studies show that stem cell transplantation is an effective option for treating bladder dysfunction, as rat MSCs are capable of differentiating into both striated and smooth muscles, and the periurethral injection of MSCs in an animal model of stress urinary incontinence can help restore the damaged external urethral sphincter and thereby improve the urinary control of rats after tissue transplantation [28,29].
In summary, we found that the TGF-β/MAPK signaling pathway mediates the beneficial effects of BMSC transplantation on urinary control and IC by maintaining urothelium structure and bladder contractile function. However, the precise mechanism by which this signaling pathway mediates the effects of BMSCs remains unknown and requires further study.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 81170693; Grant No. 81570675) and the Chongqing Municipal Science and Technology Commission (project approval No: cstc2013jcyjA10127). We thank everyone who contributed to editing the manuscript.
Disclosure of conflict of interest
None.
References
- 1.Humphrey L, Arbuckle R, Moldwin R, Nordling J, van de Merwe JP, Meunier J, Crook T, Abraham L. The bladder pain/interstitial cystitis symptom score: development, validation, and identification of a cut score. Eur Urol. 2012;61:271–279. doi: 10.1016/j.eururo.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Park CS, Bochner BS. Potential targeting of siglecs, mast cell inhibitory receptors, in interstitial cystitis. Int Neurourol J. 2011;15:61–63. doi: 10.5213/inj.2011.15.2.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berry SH, Elliott MN, Suttorp M, Bogart LM, Stoto MA, Eggers P, Nyberg L, Clemens JQ. Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the United States. J Urol. 2011;186:540–544. doi: 10.1016/j.juro.2011.03.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsieh CH, Chang WC, Huang MC, Su TH, Li YT, Chiang HS. Treatment of interstitial cystitis in women. Taiwan J Obstet Gynecol. 2012;51:526–532. doi: 10.1016/j.tjog.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Hanno PM, Burks DA, Clemens JQ, Dmochowski RR, Erickson D, Fitzgerald MP, Forrest JB, Gordon B, Gray M, Mayer RD, Newman D, Nyberg L Jr, Payne CK, Wesselmann U, Faraday MM Interstitial Cystitis Guidelines Panel of the American Urological Association Education and Research, Inc. AUA guideline for the diagnosis and treatment of interstitial cystitis/bladder pain syndrome. J Urol. 2011;185:2162–2170. doi: 10.1016/j.juro.2011.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi WW, Freire MP, Soukup JR, Yin L, Lipsitz SR, Carvas F, Williams SB, Hu JC. Nerve-sparing technique and urinary control after robot-assisted laparoscopic prostatectomy. World J Urol. 2011;29:21–27. doi: 10.1007/s00345-010-0601-z. [DOI] [PubMed] [Google Scholar]
- 7.Wang W, Li B, Yang J, Xin L, Li Y, Yin H, Qi Y, Jiang Y, Ouyang H, Gao C. The restoration of full-thickness cartilage defects with BMSCs and TGF-beta 1 loaded PLGA/fibrin gel constructs. Biomaterials. 2010;31:8964–8973. doi: 10.1016/j.biomaterials.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe Y, Itoh S, Goto T, Ohnishi E, Inamitsu M, Itoh F, Satoh K, Wiercinska E, Yang W, Shi L, Tanaka A, Nakano N, Mommaas AM, Shibuya H, Ten Dijke P, Kato M. TMEPAI, a transmembrane TGF-beta-inducible protein, sequesters Smad proteins from active participation in TGF-beta signaling. Mol Cell. 2010;37:123–134. doi: 10.1016/j.molcel.2009.10.028. [DOI] [PubMed] [Google Scholar]
- 9.Yang H, Cao C, Wu C, Yuan C, Gu Q, Shi Q, Zou J. TGF-betal suppresses inflammation in cell therapy for intervertebral disc degeneration. Sci Rep. 2015;5:13254. doi: 10.1038/srep13254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tyagi P, Tyagi V, Yoshimura N, Witteemer E, Barclay D, Loughran PA, Zamora R, Vodovotz Y. Gender-based reciprocal expression of transforming growth factor-beta1 and the inducible nitric oxide synthase in a rat model of cyclophosphamide-induced cystitis. J Inflamm (Lond) 2009;6:23. doi: 10.1186/1476-9255-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang P, Han J, Hui L. MAPK signaling in inflammation-associated cancer development. Protein Cell. 2010;1:218–226. doi: 10.1007/s13238-010-0019-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kyriakis JM, Avruch J. Mammalian MAPK signal transduction pathways activated by stress and inflammation: a 10-year update. Physiol Rev. 2012;92:689–737. doi: 10.1152/physrev.00028.2011. [DOI] [PubMed] [Google Scholar]
- 13.Wisler JA, Afshari C, Fielden M, Zimmermann C, Taylor S, Carnahan J, Vonderfecht S. Raf inhibition causes extensive multiple tissue hyperplasia and urinary bladder neoplasia in the rat. Toxicol Pathol. 2011;39:809–822. doi: 10.1177/0192623311410442. [DOI] [PubMed] [Google Scholar]
- 14.Lu T, Yang C, Sun H, Lv J, Zhang F, Dong XJ. FGF4 and HGF promote differentiation of mouse bone marrow mesenchymal stem cells into hepatocytes via the MAPK pathway. Genet Mol Res. 2014;13:415–424. doi: 10.4238/2014.January.21.9. [DOI] [PubMed] [Google Scholar]
- 15.Li ZL, Shi Y, Le G, Ding Y, Zhao Q. 24-Week exposure to oxidized tyrosine induces hepatic fibrosis involving activation of the MAPK/TGF-beta1 signaling pathway in sprague-dawley rats model. Oxid Med Cell Longev. 2016;2016:3123294. doi: 10.1155/2016/3123294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orlans FB. Ethical decision making about animal experiments. Ethics Behav. 1997;7:163–171. doi: 10.1207/s15327019eb0702_7. [DOI] [PubMed] [Google Scholar]
- 17.Yan X, Liao H, Cheng M, Shi X, Lin X, Feng XH, Chen YG. Smad7 protein interacts with receptor-regulated smads (R-Smads) to inhibit transforming growth factor-beta (TGF-beta)/Smad signaling. J Biol Chem. 2016;291:382–392. doi: 10.1074/jbc.M115.694281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo Z, Kang S, Chen D, Wu Q, Wang S, Xie W, Zhu X, Baxter SW, Zhou X, Jurat-Fuentes JL, Zhang Y. MAPK signaling pathway alters expression of midgut ALP and ABCC genes and causes resistance to Bacillus thuringiensis Cry1Ac toxin in diamondback moth. PLoS Genet. 2015;11:e1005124. doi: 10.1371/journal.pgen.1005124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimizu T, Kasamatsu A, Yamamoto A, Koike K, Ishige S, Takatori H, Sakamoto Y, Ogawara K, Shiiba M, Tanzawa H, Uzawa K. Annexin A10 in human oral cancer: biomarker for tumoral growth via G1/S transition by targeting MAPK signaling pathways. PLoS One. 2012;7:e45510. doi: 10.1371/journal.pone.0045510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shea-Donohue T, Stiltz J, Zhao A, Notari L. Mast cells. Curr Gastroenterol Rep. 2010;12:349–357. doi: 10.1007/s11894-010-0132-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gamper M, Regauer S, Welter J, Eberhard J, Viereck V. Are mast cells still good biomarkers for bladder pain syndrome/interstitial cystitis? J Urol. 2015;193:1994–2000. doi: 10.1016/j.juro.2015.01.036. [DOI] [PubMed] [Google Scholar]
- 22.Maciel GS, Uscategui RR, de Almeida VT, Oliveira ME, Feliciano MA, Vicente WR. Quantity of IL-2, IL-4, IL-10, INF-gamma, TNF-alpha and KC-like cytokines in serum of bitches with pyometra in different stages of oestrous cycle and pregnancy. Reprod Domest Anim. 2014;49:701–704. doi: 10.1111/rda.12360. [DOI] [PubMed] [Google Scholar]
- 23.Aceves SS, Chen D, Newbury RO, Dohil R, Bastian JF, Broide DH. Mast cells infiltrate the esophageal smooth muscle in patients with eosinophilic esophagitis, express TGF-beta1, and increase esophageal smooth muscle contraction. J Allergy Clin Immunol. 2010;126:1198–1204. e1194. doi: 10.1016/j.jaci.2010.08.050. [DOI] [PubMed] [Google Scholar]
- 24.Corcoran AT, Yoshimura N, Tyagi V, Jacobs B, Leng W, Tyagi P. Mapping the cytokine profile of painful bladder syndrome/interstitial cystitis in human bladder and urine specimens. World J Urol. 2013;31:241–246. doi: 10.1007/s00345-012-0852-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang J, Luo X, Lu J, Chen J, Zuo C, Xiang Y, Yang S, Tan L, Kang J, Bi Z. IPL irradiation rejuvenates skin collagen via the bidirectional regulation of MMP-1 and TGF-beta1 mediated by MAPKs in fibroblasts. Lasers Med Sci. 2011;26:381–387. doi: 10.1007/s10103-010-0870-1. [DOI] [PubMed] [Google Scholar]
- 26.Li Z, Han S, Wang X, Han F, Zhu X, Zheng Z, Wang H, Zhou Q, Wang Y, Su L, Shi J, Tang C, Hu D. Rho kinase inhibitor Y-27632 promotes the differentiation of human bone marrow mesenchymal stem cells into keratinocyte-like cells in xeno-free conditioned medium. Stem Cell Res Ther. 2015;6:17. doi: 10.1186/s13287-015-0008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tian H, Bharadwaj S, Liu Y, Ma PX, Atala A, Zhang Y. Differentiation of human bone marrow mesenchymal stem cells into bladder cells: potential for urological tissue engineering. Tissue Eng Part A. 2010;16:1769–1779. doi: 10.1089/ten.tea.2009.0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim JH, Lee HJ, Song YS. Treatment of bladder dysfunction using stem cell or tissue engineering technique. Korean J Urol. 2014;55:228–238. doi: 10.4111/kju.2014.55.4.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corcos J, Loutochin O, Campeau L, Eliopoulos N, Bouchentouf M, Blok B, Galipeau J. Bone marrow mesenchymal stromal cell therapy for external urethral sphincter restoration in a rat model of stress urinary incontinence. Neurourol Urodyn. 2011;30:447–455. doi: 10.1002/nau.20998. [DOI] [PubMed] [Google Scholar]


