Abstract
Cisplatin is one of the most potent cytotoxic drug for the treatment of many types of cancer. However, the side effects on normal tissues, particularly on the kidney, greatly limited its use in clinic. Emerging evidence demonstrated that cisplatin could directly cause mesangial cell apoptosis, while the potential mechanism is still elusive. Here we examined the contribution of COX-2 in cisplatin-induced mesangial cell apoptosis. Firstly, we found cisplatin induced cell apoptosis in mesangial cells shown by increased number of apoptotic cells in parallel with the upregulation of Bax and the downregulation of Bcl-2. Interestingly, cisplatin-induced cell apoptosis was accompanied by an upregulation of COX-2 at both mRNA and protein levels in dose- and time-dependent manners. Importantly, inhibition of COX-2 via a specific COX-2 inhibitor celecoxib markedly blocked cisplatin-induced mesangial cell apoptosis as evidenced by the decreased number of apoptotic cells, blocked increments of cleaved caspase-3 and Bax, and reversed Bcl-2 downregulation. Meanwhile, cisplatin-induced PGE2 production was markedly blocked by the treatment of celecoxib. In conclusion, this study indicated that COX-2/PGE2 cascade activation mediated cisplatin-induced mesangial cell apoptosis. The findings not only offered new insights into the understanding of cisplatin nephrotoxicity but also provided the therapeutic potential by targeting COX-2/PGE2 cascade in treating cisplatin-induced kidney injury.
Keywords: Cisplatin, mesangial cell, apoptosis, COX-2, PGE2
Introduction
Cis-diamminedichloroplatinum (cisplatin), an inorganic molecule, is one of the most effective chemotherapeutic agents and is widely used in the treatment of a number of carcinomas [1-4]. The clinical use of cisplatin, however, is limited by myelotoxicity, nephrotoxicity, and intestinal toxicity. Nephrotoxicity is a frequent adverse effect incisplatin-treated patients shown by the decline of renal function and morphological damage [3,5].
In kidneys, cisplatin preferentially accumulates in renal tubular cells causing tubular cell injury and death, resulting in acute kidney injury (AKI) [6]. Cisplatin could trigger the inflammatory response in renal tubular cells. For example, the induction of inflammatory mediators including tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), cyclooxygenase-2 (COX-2) can be seen in renal tubular cells in vivo and in vitro [7,8]. In the previous studies, the most attention was paid to renal tubular cells but not other cell types of kidney. In consideration of a global toxicity of cisplatin, it is definitely worthwhile to keep an eye on the injury occurred in other kidney cells in cisplatin nephrotoxicity model.
Prostaglandin E2 (PGE2), a major product of arachidonic acid (AA) metabolism, has an established role in mediating pain and inflammatory response [9,10]. The biosynthesis of PGE2 requires three sequential steps: the release of AA from membrane glycerophospholipids by phospholipase A2, the conversion of AA to the unstable intermediate PGH2 by COX-1 or COX-2, and the isomerization of PGH2 to PGE2 by prostaglandin E synthase (PGES) [11,12]. COX-2, an inducible form of COX, plays important roles in the pathogenesis of inflammatory diseases [13]. Resent evidence showed that cisplatin-induced renal injury can be ameliorated by selective COX-2 inhibitors [13,14]. A study from our group also demonstrated that cisplatin nephrotoxicity could beat tenuated by genetic or pharmacologic blockade of prostaglandin synthesis [10]. However, the role of COX-2/PGE2 cascade activation in cisplatin-induced mesangial cell injury was not defined. In the present study, employing a special inhibitor of COX-2 and mesangial cells, we investigated the activation and contribution of COX-2/PGE2 cascade in mesangial cell apoptosis induced by cisplatin.
Materials and methods
Materials
Cisplatin was bought from Sigma-Aldrich. Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), penicillin-streptomycin, and trypsin solution (EDTA) were bought from Gibco (Invitrogen, Grand island, NY). COX-2 antibody (mouse, monoclonal) was bought from Cayman Chemicals (Ann Arbor, MI). The antibodies against caspase-3 (rabbit, monoclonal), cleaved caspase-3 (rabbit, monoclonal), Bax (rabbit, monoclonal), Bcl-2 (rabbit, monoclonal), and GAPDH antibody were purchased from Cell Signaling Technology (Danvers, MA). The PGE2 enzyme immunoassay kit was provided by Cayman Chemicals (Ann Arbor, MI). COX-2 inhibitor (celecoxib) was from Sigma-Aldrich.
MC culture
A mouse mesangial cell (MC) line HBZY-1 was obtained from the China Center for Type Culture Collection (CCTCC Wuhan, China). Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco) supplemented with 10% fetal bovine serum (FBS; Gibco), penicillin (100 U/ml) and streptomycin (100 μg/ml), and maintained at 37°C in a humidified 5% CO2 atmosphere.
Reverse transcription and quantitative real-time PCR (qRT-PCR)
Total RNA was isolated from MCs following the manufacturer’s instruction. In brief, 1 μg of RNA was reverse-transcribed in a 20 μL system using Revert Aid TM First Strand cDNA Synthesis Kit (Takara, DaLian). Subsequently, 1 μL complementary DNA was used as the template and qRT-PCR was performed using SYBR Green master mix (Vazyme) and 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). The relative mRNA expression levels were calculated using a ΔΔCt method. The amount of mRNA for each gene was standardized with the internal control GAPDH. Each treatment group was compared with the control group to show the relative mRNA level. The sequences of primers were as follows: GAPDH forward 5’-TCATGGATGACCTTGGCCAG-3’ and reverse 5’-GTCTTCACTACCATGGAGAAGG-3’; COX-1 forward 5’-CATTGCACATCCATCCACTC-3’ and reverse 5’-CCAAAGCGGACACAGACAC-3’; COX-2 forward 5’-AGGACTCTGCTCACGAAGGA-3’; and reverse 5’-TGACATGGATTGGAACAGCA-3’.
Western blotting analysis
MCs were lysed using the protein lysis buffer containing 50 mM Tris, 150 mM NaCl, 10 mM EDTA, 1% Triton X-100, 200 mM sodium fluoride, and 4 mM sodium orthovanadate, as a protease inhibitor (pH 7.5). Immunoblotting was then performed using primary antibodies against COX-2 (1:1000), Caspase-3 (1:1000), Cleaved caspase-3 (1:1000), Bax (1:1000), Bcl-2 (1:500), and GAPDH (1:1000), followed by the addition of HRP-labeled secondary antibodies. Protein bands were visualized using ECL Plus Western blotting detection reagents (Millipore, Bedford, MA, USA). Each blot was a representative of three independent experiments and band intensity was measured using Image J software (NIH, Bethesda, MD, USA).
Annexin V/PI double staining
Cells were incubated for 24 h with cisplatin, celecoxib separately or in combination. Apoptotic cells were identified by the AnnexinV-FITC Apoptosis Detection kit (Vazyme) in accordance with the manufacturer’s instructions. Flow cytometric analysis was performed immediately after supravitally staining.
Enzyme immunoassay (EIA)
The cell culture medium was centrifuged for 5 minutes at 10,000 rpm. The concentration of PGE2 was determined by enzyme immunoassay according to manufacturer’s instructions (Cayman Chemicals).
Statistical analysis
Data are presented as means ± SD. Statistical analysis was performed using ANOVA analysis followed by a Bonferroni posttest. P<0.05 was considered statistically significant.
Results
Cisplatin enhanced the expression of COX-2 in MCs
It had been reported that cisplatin could upregulate the expression of COX-2 in renal tubular cells [15,16]. However, whether cisplatin could enhance the expression of COX-2 in MCs is still unknown. Here we examined the expression of COX-1 and COX-2 in MCs challenged with cisplatin. As shown in Figure 1C, 1D, cisplatin treatment strikingly elevated COX-2 mRNA expression in dose- and time-dependent manners. However, the expression of COX-1 was unaltered following cisplatin treatment (Figure 1A, 1B). Next, we further examined COX-2 protein expression by Western blotting. Similarly, COX-2 protein was also significantly upregulated by cisplatin in dose- and time-dependent manners (Figure 2A-D). These results indicated that COX-2 could be directly induced by cisplatin in MCs.
Figure 1.
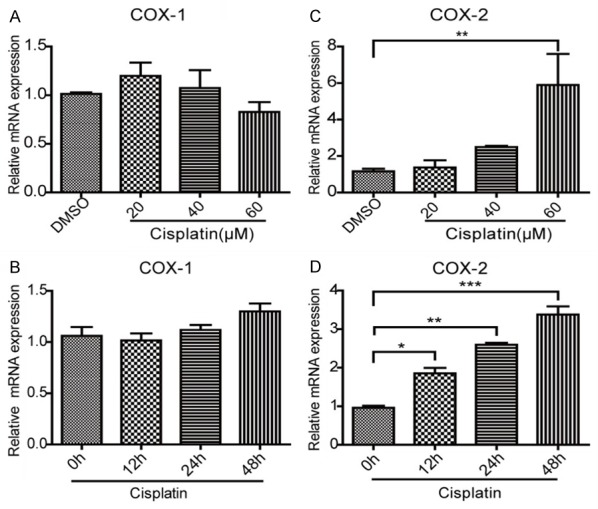
The effect of cisplatin on the mRNA expression of COX-1 and COX-2 in mesangial cells. A, B. qRT-PCR was used to measure the mRNA expression of COX-1 in mesangial cells after cisplatin treatment. The dose- and time-dependent experiments were performed, respectively. C, D. qRT-PCR was used to determine the mRNA level of COX-2 in mesangial cells following cisplatin treatment. The dose- and time-dependent experiments were performed, respectively. In time-dependent studies, the MCs were treated with cisplatin at a dose of 40 μM. N=3-4 for each group. *P<0.05 vs. control; **P<0.01 vs. control; ***P<0.001 vs. control.
Figure 2.
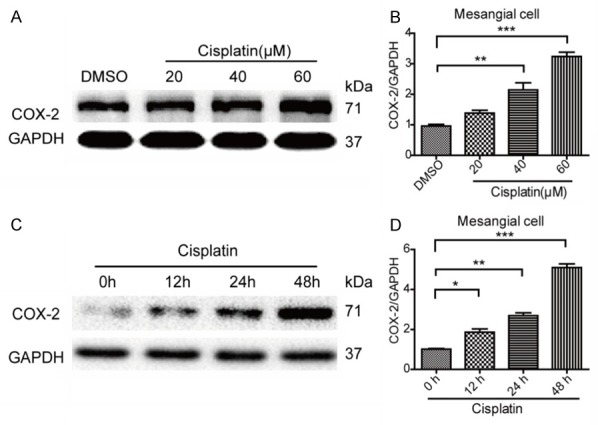
The effects of cisplatin on the protein levels of COX-1 and COX-2 in mesangial cells. A, B. Cisplatin treatment increased the protein expression of COX-2 in a dose-dependent manner in mesangial cells determined by Western blotting. C, D. Cisplatin treatment increased the protein expression of COX-2 in a time-dependent manner in mesangial cells determined by Western blotting. N=3-4 for each group. *P<0.05 vs. control; **P<0.01 vs. control; ***P<0.001 vs. control.
Cisplatin induced MC apoptosis
To investigate whether cisplatin could induce MC apoptosis, we treated MCs with cisplatin at different doses. The apoptotic response was assessed by the ratio of apoptotic cells (flow cytometry) and the expression of Bax and Bcl-2. As expected, cisplatin treatment at 40 μM for 24 h significantly increased cell apoptosis (Figure 3A, 3B). Consistent with this result, the expression of Bax in cisplatin-treated MCs was significantly elevated contrasting to a reduction of Bcl-2 (Figure 3C, 3D). These results indicated that cisplatin could directly trigger apoptotic response in MCs.
Figure 3.
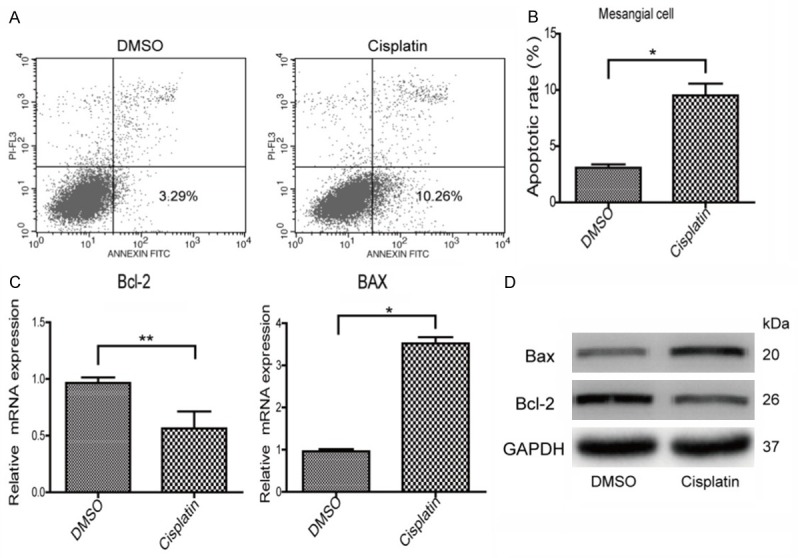
Cisplatin induced apoptotic response in mesangial cells. A, B. The apoptosis was assessed by Annexin-V/PI staining in mesangial cells. C, D. The mRNA and protein levels of Bax and Bcl-2 were assessed by qRT-PCR and Western blotting, respectively. N=3-4 for each group. *P<0.05 vs. control; **P<0.01 vs. control.
Inhibiting COX-2 blocked cisplatin-induced MC apoptosis
To define the role of COX-2 in cisplatin-induced MC apoptosis, a specific COX-2 inhibitor celecoxib was applied to the MCs before cisplatin administration. As shown by Figure 4A, 4B, COX-2 inhibition strikingly ameliorated cell apoptosis induced by cisplatin. Meanwhile, COX-2 inhibition blocked the upregulation of Bax and revered the downregulation of Bcl-2 at both mRNA and protein levels following cisplatin treatment (Figure 4C-E). Consistently, cisplatin-induced activation of caspase-3 was also ameliorated by COX-2 inhibition (Figure 4C). Moreover, the upregulation of COX-2 induced by cisplatin was suppressed by COX-2 inhibitor celecoxib at both mRNA and protein levels (Figure 5A, 5B). These data suggested that COX-2 played an important role in mediating cisplatin-induced MC apoptosis.
Figure 4.
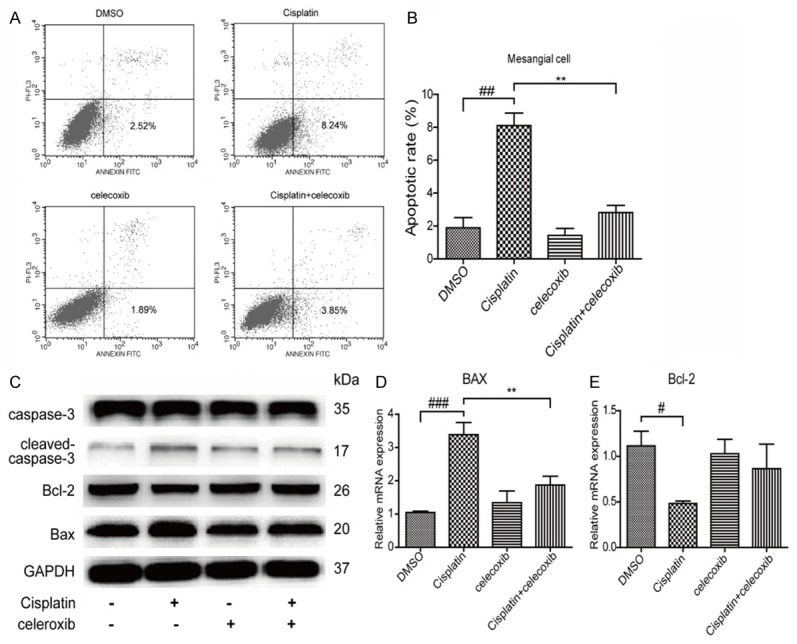
Inhibition of COX-2 attenuated cisplatin-induced MC apoptosis. (A, B) The MC apoptosis was assessed by Annexin-V/PI staining. (C) The protein levels of caspase-3, cleaved caspase-3, Bax, and Bcl-2 were examined by Western blotting in cisplatin-treated mesangial cells with or without COX-2 inhibition. (D, E) The mRNA levels of Bax (D) and Bcl-2 (E) were detected by qRT-PCR in cisplatin-treated mesangial cells with or without COX-2 inhibition. N=3-4 for each group. **P<0.01 vs. indicated group; ##P<0.01 vs. indicated group; ###P<0.001 vs. indicated group.
Figure 5.
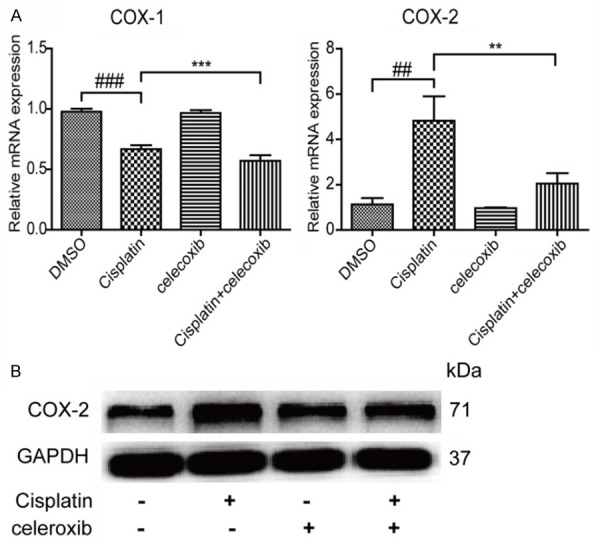
COX-2 inhibitor decreased COX-2 expression in MCs with cisplatin treatment. A. COX-1 and COX-2 mRNA levels were examined by qRT-PCR in mesangial cells treated with COX-2 inhibitor. B. The COX-2 protein level was examined by Western blotting in mesangial cells treated with COX-2 inhibitor. N=3-4 for each group. **P<0.01 vs. indicated group; ***P<0.001 vs. indicated group; ##P<0.01 vs. indicated group; ###P<0.001 vs. indicated group.
Inhibiting COX-2 significantly blocked cisplatin-induced PGE2 production
To further examine the efficacy of COX-2 inhibition in this experimental setting, we measured PGE2 production in cell culture medium. As shown in Figure 6, cisplatin treatment increased PGE2 level by 2 folds, which was almost entirely abolished by COX-2 inhibition. The result demonstrated a COX-2-dependent induction of PGE2 in response to cisplatin treatment in MCs.
Figure 6.
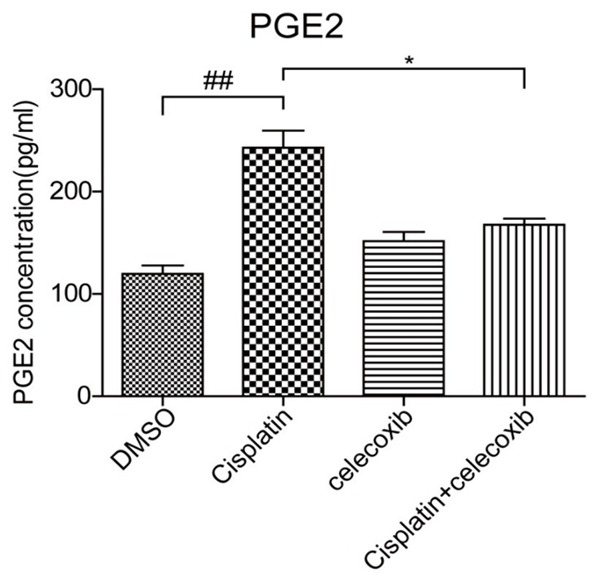
The effect COX-2 inhibitor on PGE2 production in MCs with cisplatin treatment. The PGE2 concentration in cell culture medium was determined by a EIA kit. N=3-4 for each group. *P<0.05 vs. indicated group; ##P<0.01 vs. indicated group.
Discussion
Nephrotoxicity is one of the main side effects of anticancer drug cisplatin [4,16,17]. The signaling mechanisms responsible for cisplatin-induced cytotoxicity appear to be multifactorial, involving the inflammation and oxidative stress [5]. In this study, the hypothesis is that cisplatin activates COX-2/PGE2 cascade to initiate apoptosis of glomerular mesangial cells, which might play a role in the pathogenesis of cisplatin nephrotoxicity to some extent.
Apoptosis was the predominant mode of cell death caused by cisplatin in renal cells [18]. Caspases are cysteine proteases and activated by the cleavage after the aspartic acid residues [6,19]. Many studies have shown that cisplatin induced apoptosis in renal cells by activating caspase-3 [20]. In agreement with these evidence, we showed that cisplatin induced MC apoptosis in line with the activation of caspase-3 and the increase of Bax which is apro-apoptotic protein, demonstrating a direct effect of cisplatin on promoting MC apoptosis as previously reported by Ju SM. et al [21].
A number of studies indicated that COX-2/PGE2 cascade activation is of importance in the pathogenesis of many diseases. In cancer tissues, high expression of COX-2 can inhibit cancer cell apoptosis and promote cancer progression. In most normal tissues, COX-2 has a low baseline expression but can be induced to mediate inflammation and cell injury [22]. Thus, in theory, tumor chemotherapy drugs combined with specific COX-2 inhibitors not only can increase the anti-cancer effect but also may help to avoid some drug-related side effects in normal tissues [23].
Recently, accumulating evidence indicated that COX-2 could accelerate renal disease in the remnant kidney and cyclosporine nephropathy [13,24,25]. What’s more, COX metabolites also served as mediators of inflammatory injury in renal diseases [26,27]. In the kidney, this cascade is of importance in regulating fluid metabolism, blood pressure, and renal hemodynamics [28,29]. In a recent study from our group, cisplatin has been shown to induce renal tubular injury through COX-2/PGE2 pathway [10]. However, whether this pathway contributes to the cisplatin effect on mesangial cell apoptosis was unclear. The cisplatin-related mesangial cell apoptosis might participate in the glomerular damage, which could play a role in the development and progression of cisplatin nephrotoxicity to some extent [30]. A recent report demonstrated that cisplatin could induce MC apoptosis via an oxidative stress-related mechanism [21]. However, oxidative stress mostly served as a non-specific contributor in various diseases. Thus, the detailed molecular mechanisms still need to be explored. In this study, we firstly examined the regulation of COX-2 in MCs following cisplatin treatment. As expected, COX-2 was remarkably elevated by cisplatin in time- and dose-dependent manners. In order to define the role of COX-2 in cisplatin-induced MC apoptosis, a specific COX-2 inhibitor was applied to the cells before cisplatin administration. Notably, COX-2 inhibition strikingly blocked cisplatin-induced MC apoptosis in line with a blockade of PGE2 production. These data demonstrated a detrimental role of COX-2/PGE2 cascade in promoting MC apoptosis in response to cisplatin treatment.
In summary, in mouse MCs, we identified an activation of COX-2/PGE2 cascade in response to cisplatin challenge. Moreover, employing a specific COX-2 inhibitor, we proved that COX-2/PGE2 cascade activation played a detrimental role in mediating cisplatin-induced MC apoptosis, which might serve as an important contributor in cisplatin nephrotoxicity.
Acknowledgements
This work was supported by Grants from the National Natural Science Foundation of China (nos. 81600557, 81600532, 81600352, 81370802, 81300591, 81670647, and 81570616), the National Key Research and Development Program (no. 2016YFC0906103), and the Natural Science Foundation of Jiangsu Province (no. BK2012001, BK20160136, BK20160137).
Disclosure of conflict of interest
None.
References
- 1.Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov. 2005;4:307–320. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- 2.Cohen SM, Lippard SJ. Cisplatin: from DNA damage to cancer chemotherapy. Prog Nucleic Acid Res Mol Biol. 2001;67:93–130. doi: 10.1016/s0079-6603(01)67026-0. [DOI] [PubMed] [Google Scholar]
- 3.Arany I, Safirstein RL. Cisplatin nephrotoxicity. Semin Nephrol. 2003;23:460–464. doi: 10.1016/s0270-9295(03)00089-5. [DOI] [PubMed] [Google Scholar]
- 4.Hoek J, Bloemendal KM, van der Velden LA, van Diessen JN, van Werkhoven E, Klop WM, Tesselaar ME. Nephrotoxicity as a dose-limiting factor in a high-dose cisplatin-based chemoradiotherapy regimen for head and neck carcinomas. Cancers (Basel) 2016:8. doi: 10.3390/cancers8020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joy J, Nair CK. Amelioration of cisplatin induced nephrotoxicity in Swiss albino mice by Rubia cordifolia extract. J Cancer Res Ther. 2008;4:111–115. doi: 10.4103/0973-1482.43139. [DOI] [PubMed] [Google Scholar]
- 6.Zhu S, Pabla N, Tang C, He L, Dong Z. DNA damage response in cisplatin-induced nephrotoxicity. Arch Toxicol. 2015;89:2197–2205. doi: 10.1007/s00204-015-1633-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teng ZY, Cheng XL, Cai XT, Yang Y, Sun XY, Xu JD, Lu WG, Chen J, Hu CP, Zhou Q, Wang XN, Li SL, Cao P. Ancient Chinese Formula Qiong-Yu-Gao protects against cisplatin-induced nephrotoxicity without reducing anti-tumor activity. Sci Rep. 2015;5:15592. doi: 10.1038/srep15592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Domitrovic R, Cvijanovic O, Pugel EP, Zagorac GB, Mahmutefendic H, Skoda M. Luteolin ameliorates cisplatin-induced nephrotoxicity in mice through inhibition of platinum accumulation, inflammation and apoptosis in the kidney. Toxicology. 2013;310:115–123. doi: 10.1016/j.tox.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 9.Xu Y, Wang J, Pan T, Chen X, Xu X, Jiang D, Yin J. Role of the ER stress in prostaglandin E2/E-prostanoid 2 receptor involved TGF-beta1-induced mice mesangial cell injury. Mol Cell Biochem. 2016;411:43–55. doi: 10.1007/s11010-015-2567-z. [DOI] [PubMed] [Google Scholar]
- 10.Jia Z, Wang N, Aoyagi T, Wang H, Liu H, Yang T. Amelioration of cisplatin nephrotoxicity by genetic or pharmacologic blockade of prostaglandin synthesis. Kidney Int. 2011;79:77–88. doi: 10.1038/ki.2010.331. [DOI] [PubMed] [Google Scholar]
- 11.Norregaard R, Kwon TH, Frokiaer J. Physiology and pathophysiology of cyclooxygenase-2 and prostaglandin E2 in the kidney. Kidney Res Clin Pract. 2015;34:194–200. doi: 10.1016/j.krcp.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nasrallah R, Hassouneh R, Hebert RL. PGE2, kidney disease, and cardiovascular risk: beyond hypertension and diabetes. J Am Soc Nephrol. 2016;27:666–676. doi: 10.1681/ASN.2015050528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamamato E, Izawa T, Sawamoto O, Juniantito V, Kuwamura M, Yamate J. Amelioration of cisplatin-induced rat renal lesions by a cyclooxygenase (COX)-2 selective inhibitor. Exp Toxicol Pathol. 2012;64:625–631. doi: 10.1016/j.etp.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Honma S, Takahashi N, Shinohara M, Nakamura K, Mitazaki S, Abe S, Yoshida M. Amelioration of cisplatin-induced mouse renal lesions by a cyclooxygenase (COX)-2 selective inhibitor. Eur J Pharmacol. 2013;715:181–188. doi: 10.1016/j.ejphar.2013.05.023. [DOI] [PubMed] [Google Scholar]
- 15.Domitrovic R, Cvijanovic O, Susnic V, Katalinic N. Renoprotective mechanisms of chlorogenic acid in cisplatin-induced kidney injury. Toxicology. 2014;324:98–107. doi: 10.1016/j.tox.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Chakraborty P, Roy SS, Sk UH, Bhattacharya S. Amelioration of cisplatin-induced nephrotoxicity in mice by oral administration of diphenylmethyl selenocyanate. Free Radic Res. 2011;45:177–187. doi: 10.3109/10715762.2010.521155. [DOI] [PubMed] [Google Scholar]
- 17.Hanigan MH, Devarajan P. Cisplatin nephrotoxicity: molecular mechanisms. Cancer Ther. 2003;1:47–61. [PMC free article] [PubMed] [Google Scholar]
- 18.Kintzel PE. Anticancer drug-induced kidney disorders. Drug Saf. 2001;24:19–38. doi: 10.2165/00002018-200124010-00003. [DOI] [PubMed] [Google Scholar]
- 19.Decker P, Muller S. Modulating poly (ADP-ribose) polymerase activity: potential for the prevention and therapy of pathogenic situations involving DNA damage and oxidative stress. Curr Pharm Biotechnol. 2002;3:275–283. doi: 10.2174/1389201023378265. [DOI] [PubMed] [Google Scholar]
- 20.Jin Z, El-Deiry WS. Overview of cell death signaling pathways. Cancer Biol Ther. 2005;4:139–163. doi: 10.4161/cbt.4.2.1508. [DOI] [PubMed] [Google Scholar]
- 21.Ju SM, Pae HO, Kim WS, Kang DG, Lee HS, Jeon BH. Role of reactive oxygen species in p53 activation during cisplatin-induced apoptosis of rat mesangial cells. Eur Rev Med Pharmacol Sci. 2014;18:1135–1141. [PubMed] [Google Scholar]
- 22.Greenhough A, Smartt HJ, Moore AE, Roberts HR, Williams AC, Paraskeva C, Kaidi A. The COX-2/PGE2 pathway: key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis. 2009;30:377–386. doi: 10.1093/carcin/bgp014. [DOI] [PubMed] [Google Scholar]
- 23.Senzaki M, Ishida S, Yada A, Hanai M, Fujiwara K, Inoue S, Kimura T, Kurakata S. CS-706, a novel cyclooxygenase-2 selective inhibitor, prolonged the survival of tumor-bearing mice when treated alone or in combination with anti-tumor chemotherapeutic agents. Int J Cancer. 2008;122:1384–1390. doi: 10.1002/ijc.23250. [DOI] [PubMed] [Google Scholar]
- 24.Jia Z, Sun Y, Liu S, Liu Y, Yang T. COX-2 but not mPGES-1 contributes to renal PGE2 induction and diabetic proteinuria in mice with type-1 diabetes. PLoS One. 2014;9:e93182. doi: 10.1371/journal.pone.0093182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang F, Lu X, Peng K, Zhou L, Li C, Wang W, Yu X, Kohan DE, Zhu SF, Yang T. COX-2 mediates angiotensin II-induced (pro)renin receptor expression in the rat renal medulla. Am J Physiol Renal Physiol. 2014;307:F25–32. doi: 10.1152/ajprenal.00548.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris RC. An update on cyclooxygenase-2 expression and metabolites in the kidney. Curr Opin Nephrol Hypertens. 2008;17:64–69. doi: 10.1097/MNH.0b013e3282f1bb7d. [DOI] [PubMed] [Google Scholar]
- 27.Harris RC, Zhang MZ. Cyclooxygenase metabolites in the kidney. Compr Physiol. 2011;1:1729–1758. doi: 10.1002/cphy.c100077. [DOI] [PubMed] [Google Scholar]
- 28.Jia Z, Zhang Y, Ding G, Heiney KM, Huang S, Zhang A. Role of COX-2/mPGES-1/prostaglandin E2 cascade in kidney injury. Mediators Inflamm. 2015;2015:147894. doi: 10.1155/2015/147894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nasrallah R, Hebert RL. Prostacyclin signaling in the kidney: implications for health and disease. Am J Physiol Renal Physiol. 2005;289:F235–246. doi: 10.1152/ajprenal.00454.2004. [DOI] [PubMed] [Google Scholar]
- 30.Martinez G, Costantino G, Clementi A, Puglia M, Clementi S, Cantarella G, De Meo L, Matera M. Cisplatin-induced kidney injury in the rat: L-carnitine modulates the relationship between MMP-9 and TIMP-3. Exp Toxicol Pathol. 2009;61:183–188. doi: 10.1016/j.etp.2008.07.004. [DOI] [PubMed] [Google Scholar]


