Abstract
Clusterin (CLU) is a ubiquitously expressed heterodimeric glycoprotein that is involved in a variety of functions like cell-cell interactions, apoptosis, epithelial-mesenchymal transition, carcinogenesis, and chaperone function. In the testis, CLU is strongly expressed especially in Sertoli cells but very little is known about its testicular function, regulation of secretion and most enigmatic, its receptor(s). In this study, we approached these questions with a special emphasis on the link between CLU and meiosis. In cultured seminiferous tubules, we found that secretion of CLU protein is upregulated by transforming growth factor-betas (TGF-β1-3) and observed inhibition of staurosporine-induced apoptosis by recombinant CLU. Clusterin signaling in testicular cells seems to be modulated by very low density lipoprotein receptor (VLDLR) and apolipoprotein E receptor 2 (ApoER2), because these members of the low density lipoprotein (LDL) receptor family are present in rat germ cells. Furthermore, inhibition of VLDLR/ApoER2 by a specific inhibitor abrogates CLU-mediated phosphorylation of Akt, which mediates VLDLR/ApoER2 signaling. We could also show in tubules treated with recombinant CLU a significant upregulation of several meiosis-associated proteins such as V-myb avian myeloblastosis viral oncogene homolog-like 1 (Mybl1), stimulated by retinoic acid gene 8 (Stra8), lactate dehydrogenase C (LDHC), cAMP response element-binding protein (CREB) and histone H3 (H3S10P). Collectively, our data show for the first time the involvement of CLU in upregulation of meiosis through VLDLR/ApoER2 in male germ cells.
Keywords: Clusterin, VLDLR, Akt, ApoER2, meiosis, male germ cells
Introduction
Clusterin was originally identified as an androgen-repressed gene in the prostate [1] and is also known as testosterone-repressed prostate message, glycoprotein-80, serum protein-40,40, dimeric acidic glycoprotein, sulfated glycoprotein-2, complement lysis inhibitor, and apolipoprotein J [2]. CLU is a secreted glycosylated heterodimeric glycoprotein found in almost all physiological fluids [2]. Ubiquitous expression of CLU suggests manifold biological functions such as cell-cell interactions [1,3], apoptosis/cytoprotection [4,5], protection against oxidative stress [6], inhibition of epithelial cell proliferation [7], carcinogenesis [2], promotion of epithelial-mesenchymal transition [8], and chaperone function e.g. in Alzheimer’s disease [9].
Reduced CLU protein levels in testis are linked to male fertility [10,11] and CLU is accumulated on abnormal human sperm [12]. However, CLU gene inactivation has shown only minor physiologic defects in mice such as a higher susceptibility to cardiac autoimmunity [13] or chemically induced skin tumorigenesis [7]. Additionally, CLU knockout mice showed an increase in heat-induced apoptosis of testicular germ cells and defects in spermiation [4]. Furthermore, CLU reduced heat stress-induced apoptosis in cultured Sertoli cells [14]. However, the exact mechanisms how CLU contributes to male fertility are still unknown. Experiments in lung and prostate suggest that CLU gene expression is differentially regulated by TGF-βs as well as androgens [1,13]. However, posttranscriptional regulation can inverse TGF-β effects [3]. In testis, putative effects of TGF-βs on CLU secretion are unknown.
Another enigma about CLU is its receptor. A multitude of possible receptors that bind CLU have been described in other organs. A recent study showed a high affinity interaction between the multi-ligand receptors ApoER2/VLDLR and CLU [16]. ApoER2 is a member of the low density lipoprotein receptor (LDLR) family and is expressed in several tissues including brain, placenta, ovaries, and epididymis [17]. Interestingly, ApoER2 knockout mice showed reduced male fertility [18], lower brain and testis selenium levels and severe neurological defects [19].
VLDLR is also a member of LDLR family that specifically binds apolipoprotein E and is highly expressed in adipose tissue, heart, skeletal muscles, monocytes and macrophages [20]. VLDLR is required for the normal lipoprotein lipase (LpL) regulation in vivo, and the disruption of VLDLR results in hypertriglyceridemia associated with decreased LpL activity [21]. VLDLR knockouts showed only a modest decrease in body weight, body mass index, and adipose tissue mass [22] and experience deficiencies in fibrin-dependent leukocyte transmigration [23]. However, no influence on male fertility was reported.
In this study, we aim to analyze CLU function in spermatogenesis, by (i) investigation of putative TGFβ-dependent regulation of CLU secretion, (ii) analysis of possible anti-apoptotic and pro-meiotic effects of CLU in testicular cells, and (iii) if CLU effects are mediated by signaling via the ApoER2/VLDLR receptors.
Materials and methods
Preparation of seminiferous tubules
All animal experiments were done according to the guidelines of the local committee for animal experimentation (Giessen, Germany). Adult male Sprague Dawley rats ((Crl:CD (SD)IGS; Charles River, Germany) weighing 150-200 g were anesthetized with 5% isoflurane (Abbott, Germany). Testes were washed in 70% ethanol and briefly rinsed in PBS. All subsequent steps were performed on ice. The tunica albuginea was removed and the tissue cut into 2-3 mm3 pieces in Petri dishes containing ice-cold tubule medium [DMEM supplemented with 2.5 mM L-glutamine, 1% penicillin/streptomycin (all from PAA, Austria) and 10% fetal calf serum (FCS, Gibco, USA)]. Five of these pieces were placed into a Medicon™ unit (50 μm; Becton Dickinson, Germany) plus 500 μl of ice-cold tubule medium and processed for 50 s in the Medimachine (Becton Dickinson). The fine slurry was recovered and filtered through a 500 μm Filcon (Becton Dickinson) into a sterile Falcon tube. After centrifugation (800×g, 5 min, room temperature) the tubules were resuspended in tubule medium. Approximately equal numbers of tubule were plated onto BioCoat™ Collagen I coated 24-well plates (Becton Dickinson) and maintained at 32°C in 5% CO2. The tubule fragments attached during 24-48 hrs to the coated wells. After three days, the germ cells in the tubules were still viable similar to De Gendt et al. [24].
Treatment of tubules and collection of samples
After 48 hrs at 32°C in 5% CO2 the tubule medium was replaced by starvation medium (tubule medium with 1% FCS) for another 24 hrs followed by addition of 200 nM recombinant rat receptor associated protein (RAP, MyBioSource, USA) for 2 hrs. Then, tubules were stimulated with 2 µg/ml of recombinant mouse CLU (R&D Systems, Germany) at 32°C for 24 hrs - 48 hrs.
Withdrawn supernatants were centrifuged (5000×g, 10 min, 4°C) and the protein concentration was determined with the precision Red Advance protein assay (Cytoskeleton, Denver, USA). After adding 1 mM PMSF and 1x protease inhibitor cocktail (PIC, Sigma-Aldrich, Germany) the supernatants were stored at -20°C until further use. The tubules were washed with PBS and lysed in 250 μl cell lysis buffer (Cell Signalling, Germany) containing 1 mM PMSF and 1× PIC on ice according to the protocol of the manufacturer. After 10 min, tubules were detached with a cell scraper and the mixture was sonicated 30 times for 1 s each with intervals of 1 s (Sonoplus mini 20, Bandelin, Germany) on ice. After centrifugation (13,000×g, 20 min, 4°C), protein concentrations of the supernatants were determined and samples were stored at -20°C until further use.
Treatment of Sertoli cells and sample collection
Approximately 3×105 cells/cm2 SCIT-C8 Sertoli cells [25] were grown in 6-well plates in a humidified incubator at 32°C in 5% CO2 until they reached subconfluence. Although the immortalized SCIT-C8 do not respond to androgens or FSH, they do secrete CLU, which is a typical marker for Sertoli cells [25]. Cells were washed once with PBS and incubated in starvation medium for 24 hrs. The culture medium was replaced with fresh starvation medium, and cells were incubated with 10 ng/ml TGF-β1, -β2 or -β3 (PromoCell, Germany) for another 48 hrs. Supernatants were collected, centrifuged at 5000×g for 10 min at 4°C and stored at -20°C after addition of 1 mM PMSF and 1× PIC until further use. The TβR1 inhibitor Ly364947 (10 µM, dissolved in DMSO, Sigma Aldrich) was added 2 hrs prior to stimulation with the distinct TGF-βs. An equal volume of DMSO was added to control cultures.
Measurement of apoptosis
Caspase 3/7 activity and phosphatidylserine apoptosis assay Kits (AAT Bioquest, USA) were employed according to the manufacturer’s instructions. Briefly, equal number of tubules were cultured in 96-well black plates (CellBIND surface; Corning, Germany) at 32°C in 5% CO2 for 48 hrs. After 24 hrs, medium was replaced with starvation medium and tubules were stimulated with 0.5 μM staurosporine (STS, Sigma-Aldrich) with or without 2 μg/ml recombinant CLU. After 24 hrs, 100 μl of caspase 3/7 or phosphatidylserine assay solution, respectively, were added to each well. The plate was incubated at room temperature for 1 hr, protected from light. The fluorescence intensity was measured at Ex/Em = 350/450 nm and at 540/590 nm respectively with the Benchmark Reader infinite M200.
Immunohistochemistry
Immunohistochemistry of 5 µm sections of Bouin-fixed, paraffin-embedded testes was performed as published [26]. Briefly, the Envision System from DAKO (Germany) combined with DAB staining was used according to the manufacturer’s instructions. Counterstaining was done with hematoxylin. The antibodies used in this study are listed in Table 1. Digital images were obtained with the inverse microscope FSX100 (Olympus) using the Olympus FSX-BSW software. Images were processed with Adobe Photoshop CS6.
Table 1.
Antibodies used in this study
| Protein | Source | Cat-No | Species | Clonality | Dilution |
|---|---|---|---|---|---|
| ApoER2 | Lifespan | LS-b5784 | Rabbit | polyclonal | 1:75 |
| CREB | Abcam | ab178322 | Mouse | monoclonal | 1:100 |
| H3S10P | Millipore | 06-570 | Rabbit | polyclonal | 1:100 |
| LDHC | Cloud-Clone | PAE131Ra01 | Rabbit | polyclonal | 1:100 |
| MYBL1 | Sigma | HPA008791 | Rabbit | polyclonal | 1:100 |
| Stra8 | Abcam | Ab49602 | Rabbit | polyclonal | 1:100 |
| VLDLR | Lifespan | LS-C180156 | Mouse | monoclonal | 1:100 |
| Anti-mouse IgG (Alexa555) | Thermo Fisher | A-31570 | Donkey | IgG | 1:100 |
| Anti-mouse | DAKO | K4000 | Goat | IgG | Ready |
| POD | |||||
| Anti-rabbit | DAKO | K4002 | Goat | IgG | Ready |
| POD |
Cat-No, catalog number; POD, peroxidase.
Enzyme-linked immunosorbent assay (ELISA)
Stimulated by retinoic acid gene 8 (Stra8) protein expression was quantitated by a commercially available sandwich ELISA kit (CUSABIO, China). V-myb avian myeloblastosis viral oncogene homolog-like 1 (MYBL1) and lactate dehydrogenase C (LDHC) ELISAs were from Cloud-Clone Corp (USA). Phosphorylation of cAMP response element-binding protein (CREB) at Ser133 was examined by Phospho-CREB (Ser133) Sandwich ELISA Kit and phosphorylation of Akt at Ser473 was detected using Phospho-Akt1 (Ser473) Sandwich ELISA Kit (Cell signalling, Germany). Levels of CLU secretion were quantitated by a CLU ELISA kit (BioVendor, Germany). Each ELISA was performed according to the manufacturer’s instructions, quantitated by a Benchmark Reader infinite M2000 (Tecan, Austria), and values were normalized to the total protein content of the corresponding samples.
Immunofluorescence
Tubules were plated into 8-well culture slides (BD Dickinson) and incubated for 48 hrs at 32°C and 5% CO2. After stimulation with recombinant CLU (2 µg/ml) for 24 hrs, tubules were rinsed with PBS and fixed with 4% paraformaldehyde for 20 min followed by permeabilization with 0.1% Triton X-100 for 10 min at room temperature. Then tubules were incubated in blocking solution (5% BSA + 5% FBS) at room temperature for 1 hr. The tubules were subsequently washed 3 times with PBS for 5 min each, and incubated with the primary antibody against H3S10P (Table 1) in blocking solution at 4°C overnight. The tubules were washed 3 times for 5 min each, and then incubated with goat anti-mouse conjugated with Alexa fluor-555 (Table 1) for 1 hr at room temperature. After washing 3 times, tubules were embedded in mounting medium (buffered glycerol pH 8.4) and put onto glass slides. Images were visualized using an inverse fluorescence microscope FSX 100 (Olympus, Germany).
Statistical analysis
All experiments were repeated independently at least three times in duplicate. Values from all experiments were used for calculation of the means and their respective standard errors of the mean (SEM). The comparison of the means between groups was performed by one-way analysis of variance (ANOVA) followed by Tukey’s post hoc-test using GraphPad prism software (Version 5.0, Graphpad Inc. La Jolla, CV, USA). P values of less than 0.05 were considered significant.
Results
Rat seminiferous tubules and Sertoli cells secrete CLU in vitro
Cultured seminiferous tubules secreted moderate amounts of CLU under basal conditions (Figure 1A). Treatment of Sertoli cells with recombinant TGF-βs, especially TGF-β2, significantly increased CLU secretion (Figure 1B). This effect was completely abrogated by the TGF-beta receptor-1 (TBR1) kinase inhibitor LY364947 (Figure 1B). Similar results were found with two different Sertoli cell lines (data not shown).
Figure 1.
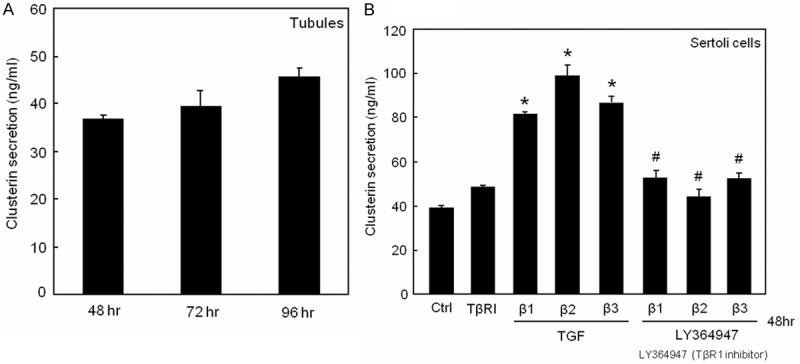
Effect of TGF-βs on CLU secretion in vitro. (A) CLU secretion by tubule cultures showed a time-dependent increase. (B) Sertoli cells were treated with 10 ng/ml of TGF-β1, -β2, -β3 or LY364947 (10 µM), respectively, or in combinations. TGF-β-increased CLU secretion was completely blocked by the TBR1 kinase inhibitor LY364947. The means ± SEMs of 4 independent experiments are shown (A, B). *P < 0.05 or #P < 0.05 are significantly different from control or from TGF-βs, respectively.
Clusterin protects against staurosporine-induced apoptosis
Induction of caspase3/7 activity in rat tubule cultures by STS was found to be concentration-dependent with a maximum at 0.5 µM (Figure 2A). Pre-treatment of tubules with 2 µg/ml recombinant CLU for 2 hrs followed by treatment with 0.5 µM STS for 24 hrs completely blocked caspase3/7 activity in contrast to STS alone (Figure 2B). Similar results were obtained with detection of apoptosis with a phosphatidylserine assay (Figure 2C) which was also completely blocked by pre-treatment with CLU (Figure 2D).
Figure 2.
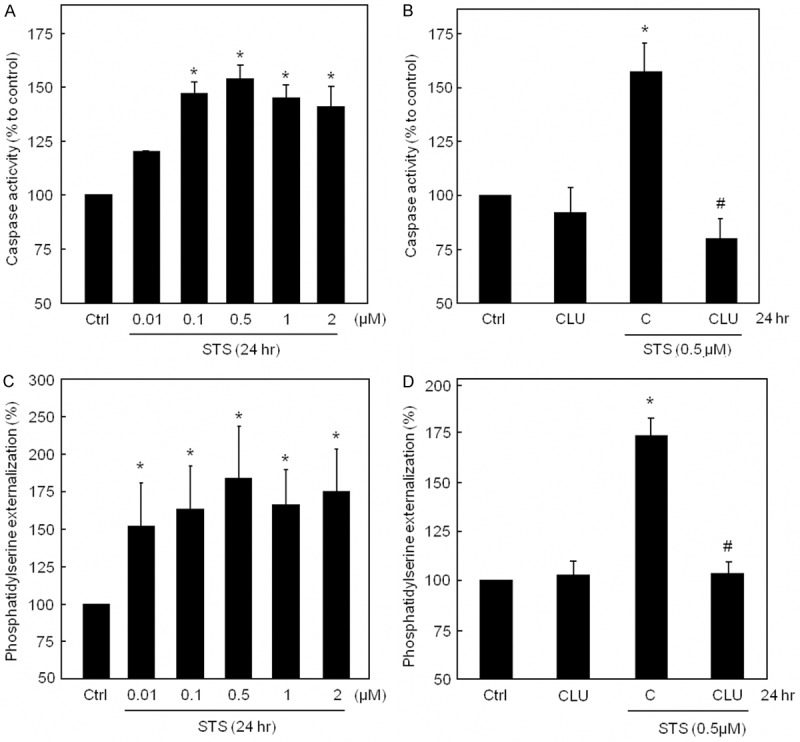
Clusterin protects against STS-induced apoptosis in tubule cultures. (A) Induction of caspase3/7 activity by STS was concentration-dependent with a maximum at 0.5 µM. (B) Pre-treatment with recombinant CLU followed by treatment with 0.5 µM STS for 24 hrs completely blocked caspase3/7 activity in contrast to STS alone. (C) Increasing concentrations of STS showed an increased phosphatidylserine externalization. (D) CLU nearly completely blocked STS-induced phosphatidylserine externalization. (A-D) *P < 0.05 significantly different from control; #P < 0.05 significantly different from STS alone. In (A-D) results are expressed as percentage of control (Ctrl = DMSO-treated tubules) and the means ± SEMs of 4 independent experiments are shown (A-D).
Localization of putative CLU receptors and signalling via Akt in rat testis
Because CLU was suggested to act via ApoER2 and VLDLR receptors [16], we investigated localization of both receptors by immunohistochemistry. ApoER2 protein is localized modestly in Sertoli cells and pachytene spermatocytes, but was found in high amounts in Leydig cells (Figure 3A, 3B). In contrast, VLDLR was strongly present in pachytene spermatocytes and Leydig cells (Figure 3C, 3D).
Figure 3.
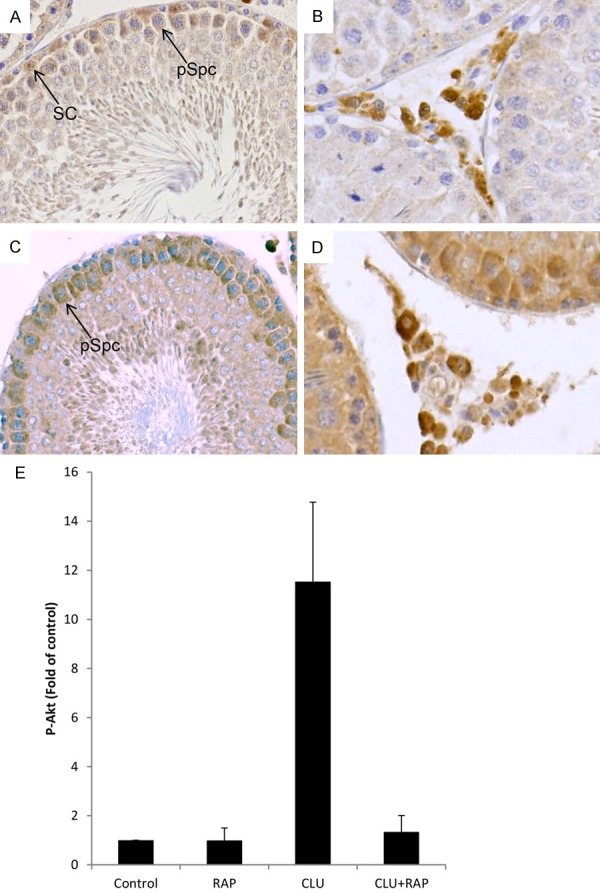
Localization of ApoER2/VLDLR and signalling via Akt in rat testis. (A) ApoER2 can be found modestly in Sertoli cells (arrow, SC) and pachytene spermatocytes (arrow, pSpc), but strongly in Leydig cells (B). (C) VLDLR is localized in pachytene spermatocytes (arrow, pSpc) and strongly in Leydig cells (D). Magnification ×40. An example for a negative control is shown in Figure 4F. (E) Tubules were stimulated with CLU (2 µg/ml), RAP (200 nM), RAP+CLU or vehicle alone (Ctrl, control) for 24 hrs and Akt phosphorylation (P-Akt) was quantified. RAP completely inhibited CLU-induced Akt phosphorylation in the tubules. Results are expressed as x-fold of control. The means ± SEMs of 3 independent experiments in duplicates are shown. *P < 0.05 significantly different from control; #P < 0.05 significantly different from CLU alone.
Since binding of CLU to ApoER2/VLDLR triggers Akt phosphorylation in other cell types [16], we therefore investigated whether CLU is also able to signal via the Akt pathway in testis. As shown in Figure 3E, tubules respond to CLU with a strong phosphorylation of Akt. Interestingly, the level of CLU-induced Akt phosphorylation was found to be significantly reduced in the presence of RAP, suggesting that CLU signals through ApoER2/VLDLR and Akt phosphorylation in testis.
Localization of meiosis-specific proteins in rat testis
Because we hypothesized that CLU might modulate male germ cell meiosis, we first analyzed the localization of the meiosis-specific proteins Stra8, MYBL1, CREB and LDHC in rat testis. The immunohistochemical staining clearly demonstrates that Stra8 is localized in the nuclei of spermatogonia (Figure 4A). However, remarkably, we found also a strong cytoplasmic staining in round and elongated spermatids (Figure 4A). In order to show the specificity of the antibody, we also used mouse testis and found Stra8 only in spermatogonia as expected (Figure 4B). MYBL1 was found in primary spermatocytes (Figure 4C). CREB was detectable strongly in spermatogonia, round spermatids and peritubular cells, but only weakly in Sertoli cells (Figure 4D). A moderate to weak expression of LDHC protein was found in Leydig cells and pachytene spermatocytes, respectively, whereas it was abundantly detectable in round spermatids and spermatozoa (Figure 4E).
Figure 4.
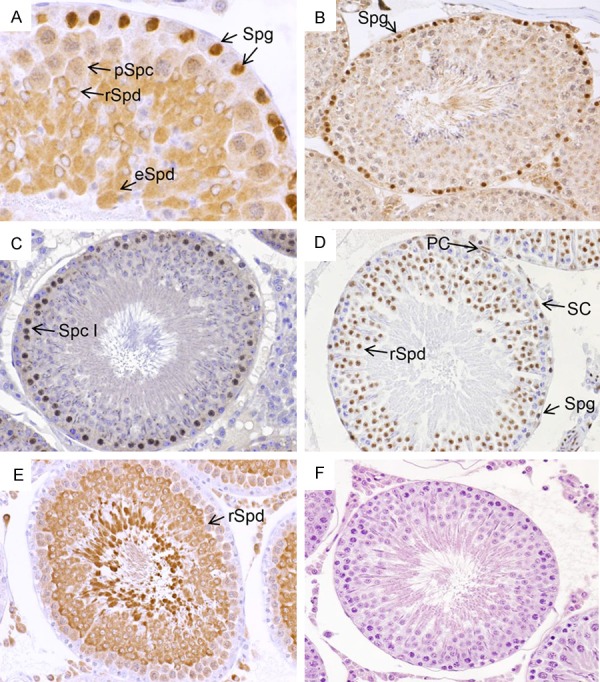
Localization of meiosis-specific proteins in adult rat testis. A. Stra8 was detected strongly in spermatogonia (Spg, arrows), round (rSpd, arrow) and elongated spermatids (eSpd, arrow). In pachytene spermatocytes (pSpc) Stra8 staining was weak. B. In mouse testis, Stra8 was found strongly in spermatogonia (Spg, arrow). C. MYBL1 was localized in primary spermatocytes (Spc I, arrow). D. CREB was detected faintly in Sertoli cells (SC, arrow) and pachytene spermatocytes, but strongly in round spermatids (rSpd, arrow), and peritubular cells (PC, arrow). E. LDHC protein was found in Leydig cells, only very weak in pachytene spermatocytes, but abundantly in round spermatids (rSpd, arrow) and spermatozoa. F. One negative control without secondary antibody is exemplarily shown. Magnification A, ×40; C-F, ×25.
Clusterin upregulates meiosis-specific proteins
In vitro, entry of spermatogonia into meiosis is blocked and depends upon the structural support of the seminiferous epithelium [27]. Thus, we examined the effect of CLU on meiosis-associated proteins. Stimulation of tubules with recombinant CLU increased expression of Stra8 protein, which was almost completely blocked by the VLDLR/ApoER2 antagonist RAP (Figure 5A). These results strongly suggest that CLU might be involved in meiotic initiation by modulating Stra8 protein expression in male germ cells via VLDLR/ApoER2. Furthermore, the effect of CLU was also assessed on expression of MYBL1, a spermatocyte protein which is a male-specific master regulator of meiosis. Stimulation of tubules with CLU increased MYBL1 protein expression compared to controls; however, this effect was also abrogated in the presence of RAP, again confirming that VLDLR/ApoER2 receptors are required for CLU signalling in testes (Figure 5B). Since MYBL1 regulates the expression of multiple testis-specific genes in spermatocytes like LDHC through CREB whose activity is critical for germ cell survival, we next determined whether CREB phosphorylation and hence activity could be modulated by CLU. Treatment of tubules with recombinant CLU significantly increased CREB phosphorylation compared to controls, which was prevented by co-incubation with RAP (Figure 5C). In contrast, no transient effects of recombinant CLU on CREB phosphorylation was detected at any time point between 1-3 hrs (data not shown). Figure 5D clearly shows that CLU increased expression of the LDHC protein that was blocked by RAP, indicating once more the importance of CLU in spermatogenesis. Furthermore, treatment of cultured tubules with recombinant CLU increased phosphorylation of histone H3 on Ser-10 in tubule cultures compared with untreated tubules (Figure 5E).
Figure 5.
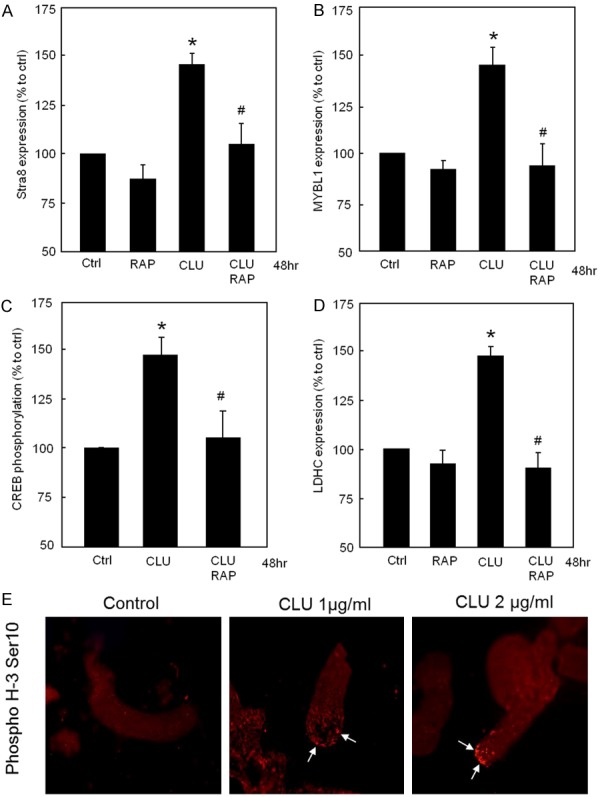
Clusterin upregulates meiosis-associated proteins via VLDLR/ApoER2 in tubule cultures. (A) RAP completely inhibited CLU-induced Stra8 protein expression in tubules. (B) RAP completely inhibited CLU-induced MYBL1 protein expression in tubules. (C) Tubules were treated with CLU in the presence and absence of RAP. RAP completely inhibited CLU-induced CREB phosphorylation in tubules. (D) RAP completely inhibited CLU-induced LDHC protein expression. (A-D) *P < 0.05 significantly different from control; #P < 0.05 significantly different from CLU alone. In (A-D) results are expressed as percentage of control (Ctrl). Each column represents the means of at least three independent experiments (A-C n=4; D n=3). (E) Tubules treated with and without CLU were stained with mouse anti-H3S10) followed by anti-mouse IgG-Alexa555 (red). Histone H3 phosphorylation (arrows) was clearly stronger in CLU-treated tubules compared to the untreated control.
Discussion
The major findings of this study are: (i) TGF-βs increase CLU secretion by testicular cells in a TBR1-dependent fashion, (ii) recombinant CLU up-regulates meiosis-associated proteins in testicular tubules, (iii) CLU acts via ApoER2/VLDLR receptors and the Akt pathway in testicular cells, and (iiii) CLU protects against STS-induced apoptosis in testicular cells. Our results clearly showed that CLU strongly decreased apoptosis indicated by reduced STS-induced activity of caspases. Comparable cytoprotective and anti-apoptotic effects of CLU have been also demonstrated in cardiac, epithelial, tumour, and endothelial cells [2,28].
We found that CLU secretion is up-regulated by TGF-βs in Sertoli cells. Previous studies in other tissues suggested that TGF-βs directly regulate CLU transcription, even though the effect of TGF-βs on CLU protein levels depends on various cell-specific factors [3]. Cytoplasmic CLU protein was demonstrated to bind to the intracellular domain of TBR2, potentially modulating the receptor activity [29]. A later study showed that CLU increased Smad protein levels and TGF-β-mediated Smad2/3 phosphorylation, suggesting that CLU stabilizes TGF-β signalling [30].
Experiments in chicken and mice showed that CLU is a ligand for VLDLR and ApoER2 [31,32]. In vitro analysis revealed a high affinity of CLU to VLDLR and ApoER2 and downstream signalling via the Akt pathway [16]. Interestingly, CLU was previously reported to induce Akt signaling in cardiomyocytes, human retinal pigment epithelial cells and neuronal cells [16,33,34]. In concordance with these findings, we observed strong Akt phosphorylation after CLU stimulation, suggesting that Akt is involved in the regulation of CLU-induced events in testicular cells. Moreover, our experiments showed that CLU-induced Akt phosphorylation depends on ApoER/VLDLR since the stimulatory effect was inhibited by pre-treatment with RAP.
Our experiments demonstrated that in seminiferous tubules treatment with recombinant CLU increased the expression or phosphorylation of meiosis-associated proteins (Stra8, MYBL1, CREB, LDHC and H3S10P), indicating a supportive effect of CLU on meiosis induction and maintenance. We found a clear upregulation of Stra8 protein expression by CLU. In the mouse, Stra8 protein expression was found only in pre-meiotic germ cells [35]. Thus, Stra8 was considered as a “gatekeeper” of meiosis and its upregulation suggested to be associated with meiotic entry [36]. However, our data of Stra8 localization in adult rat testis clearly shows besides the nuclear localization in spermatogonia, also a prominent cytoplasmic localization in round spermatids. Of note, Stra8 promoter activity was found to activate the overexpression of c-FLIPL in round and elongated spermatids [37]. Furthermore, Stra8 localization in both nucleus and cytoplasm of the germ cell line GC-1 could be demonstrated [38]. The heterogenous distribution of Stra8 in germ cells—nuclear in pre-meiotic and cytoplasmic in post-meiotic germ cells—suggests a different biological function of Stra8 in male germ cells in the rat.
Moreover, CLU also increased MYBL1 protein expression in testicular tubules. MYBL1 is a spermatocyte-specific protein and a master regulator of genes encoding proteins required for cell-cycle progression through the pachytene stage of meiosis [39]. Male mice homozygous for a germ line mutation in MYBL1 demonstrate arrested pachytene spermatocytes resulting in male infertility besides other severe growth defects [40]. MYBL1 stimulates the promoter activity of multiple testis-specific genes including LDHC through CREB [41]. We found both upregulated by CLU, putatively in a MYBL1-dependent manner. Additionally, we observed increased histone H3 phosphorylation at Ser-10, an established marker of chromosome condensation during meiosis [42], in CLU-treated tubules compared to the control. Interestingly, the effect of CLU on Stra8, MYBL1, LDHC expression and on CREB phosphorylation was abrogated by the application of the VLDLR/ApoER2 antagonist RAP, suggesting that the effect of CLU on meiosis is transmitted via the receptors VLDLR/ApoER2 and Akt signalling.
Previous studies have shown a clear link between enhanced Akt signalling and resumption of meiosis in both male and female germ cells [43,44]. Although the link between Akt and CREB in testicular cells remains to be investigated, in other organs phosphorylation of CREB by Akt was demonstrated [45]. In the rat testis, phosphorylated Akt is mainly found in spermatogonia, spermatocytes and round spermatids [46]. Of note, Akt knockout mice exhibit reduced tubule diameters, more apoptotic sperm and sperm with a markedly reduced fertilization rate compared to wild-type animals resulting in decreased spermatogenesis and fertility [47].
In summary, our study shows that CLU confers protection against apoptosis and appears to increase expression of meiosis-specific proteins in rat seminiferous tubules. The TGF-β-increased CLU secretion, which in turn, might support meiosis, is possibly the missing link between TGF-βs and meiosis/spermatocyte differentiation and migration, an interaction that has been proposed previously, especially in pachytene spermatocytes [48,49]. Interestingly, the biological effects of CLU seem to be mediated through the VLDLR/ApoER2 receptors and the Akt pathway in male germ cells. Thus, our findings revealed a novel role of CLU in testis that might contribute to better understand the causes of male fertility problems.
Acknowledgements
The help of Cornelia Hof and Kai-Hui Chan is acknowledged. The study was funded by the DFG KFO 181 to L. Konrad.
Disclosure of conflict of interest
None.
References
- 1.Fritz IB, Burdzy K, Setchell B, Blaschuk O. Ram rete testis fluid contains a protein (clusterin) which influences cell-cell interactions in vitro. Biol Reprod. 1983;28:1173–1188. doi: 10.1095/biolreprod28.5.1173. [DOI] [PubMed] [Google Scholar]
- 2.Shannan B, Seifert M, Leskov K, Willis J, Boothman D, Tilgen W, Reichrath J. Challenge and promise: roles for clusterin in pathogenesis, progression and therapy of cancer. Cell Death Differ. 2006;13:12–19. doi: 10.1038/sj.cdd.4401779. [DOI] [PubMed] [Google Scholar]
- 3.Park S, Mathis KW, Lee IK. The physiological roles of apolipoprotein J/clusterin in metabolic and cardiovascular diseases. Rev Endocr Metab Disord. 2014;15:45–53. doi: 10.1007/s11154-013-9275-3. [DOI] [PubMed] [Google Scholar]
- 4.Bailey RW, Aronow B, Harmony JA, Griswold MD. Heat shock-initiated apoptosis is accelerated and removal of damaged cells is delayed in the testis of clusterin/ApoJ knock-out mice. Biol Reprod. 2002;66:1042–1053. doi: 10.1095/biolreprod66.4.1042. [DOI] [PubMed] [Google Scholar]
- 5.Trougakos IP, Gotthardt M, Hiesberger T, Shelton J, Stockinger W, Nimpf J, Hammer RE, Richardson JA, Herz J. Silencing expression of the clusterin/apolipoprotein j gene in human cancer cells using small interfering RNA induces spontaneous apoptosis, reduced growth ability, and cell sensitization to genotoxic and oxidative stress. Cancer Res. 2004;64:1834–1842. doi: 10.1158/0008-5472.can-03-2664. [DOI] [PubMed] [Google Scholar]
- 6.Mishima K, Inoue H, Nishiyama T, Mabuchi Y, Amano Y, Ide F, Matsui M, Yamada H, Yamamoto G, Tanaka J, Yasuhara R, Sakurai T, Lee MC, Chiba K, Sumimoto H, Kawakami Y, Matsuzaki Y, Tsubota K, Saito I. Transplantation of side population cells restore the function of damaged exocrine glands through clusterin. Stem Cells. 2012;30:1925–1937. doi: 10.1002/stem.1173. [DOI] [PubMed] [Google Scholar]
- 7.Thomas-Tikhonenko A, Viard-Leveugle I, Dews M, Wehrli P, Sevignani C, Yu D, Ricci S, el-Deiry W, Aronow M, Kava G, Saurat JH, French LE. Myc-transformed epithelial cells down-regulate clusterin, which inhibits their growth in vitro and carcinogenesis in vivo. Cancer Res. 2004;64:3126–3136. doi: 10.1158/0008-5472.can-03-1953. [DOI] [PubMed] [Google Scholar]
- 8.Lenferink AE, Cantin C, Nantel A, Wang E, Durocher Y, Banville M, Paul-Roc B, Marcil A, Wilson MR, O’Connor-McCourt MD. Transcriptome profiling of a TGF-β-induced epithelial-to-mesenchymal transition reveals extracellular clusterin as a target for therapeutic antibodies. Oncogene. 2010;29:831–844. doi: 10.1038/onc.2009.399. [DOI] [PubMed] [Google Scholar]
- 9.Nuutinen T, Suuronen T, Kauppinen A, Saliminen A. Clusterin: a forgotten player in Alzheimer’s disease. Brain Res Rev. 2009;61:89–104. doi: 10.1016/j.brainresrev.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Thacker S, Yadav SP, Sharma RK, Kashou A, Willard B, Zhang D. Evaluation of sperm proteins in infertile men: a proteomic approach. Fertil Steril. 2011;95:2745–2748. doi: 10.1016/j.fertnstert.2011.03.112. [DOI] [PubMed] [Google Scholar]
- 11.Salehi M, Akbari H, Heidari MH. Correlation between human clusterin in seminal plasma with sperm protamine defi ciency and DNA fragmentation. Mol Reprod Dev. 2013;80:718–724. doi: 10.1002/mrd.22202. [DOI] [PubMed] [Google Scholar]
- 12.O’Bryan MK, Baker HW, Saunders JR. Human seminal clusterin (SP-40, 40). Isolation and characterization. J Clin Invest. 1990;85:1477–1486. doi: 10.1172/JCI114594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McLaughlin L, Zhu G, Mistry M, Ley-Ebert C, Stuart WD, Florio C, Groen PA, Witt SA, Kimball TR, Witte DP, Harmony AK, Aronow BJ. Apolipoprotein J/clusterin limits the severity of murine autoimmune myocarditis. J Clin Invest. 2000;106:1105–1113. doi: 10.1172/JCI9037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsushita K, Miyake H, Chiba K, Fujisawa M. Clusterin produced by Sertoli cells inhibits heat stress-induced apoptosis in the rat testis. Andrologia. 2016;48:11–19. doi: 10.1111/and.12404. [DOI] [PubMed] [Google Scholar]
- 15.Jin G, Howe PH. Regulation of clusterin gene expression by transforming growth factor β. J Biol Chem. 1997;272:26620–26626. doi: 10.1074/jbc.272.42.26620. [DOI] [PubMed] [Google Scholar]
- 16.Leeb C, Eresheim C, Nimpf J. Clusterin is a ligand for apolipoprotein E receptor 2 (ApoER2) and very low density lipoprotein receptor (VLDLR) and signals via the Reelin-signaling pathway. J Biol Chem. 2014;289:4161–4172. doi: 10.1074/jbc.M113.529271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andersen OM, Yeung CH, Vorum H, Wellner M, Andreassen TK, Erdmann B, Mueller EC, Herz J, Otto A, Cooper TG, Willnow TE. Essential role of the apolipoprotein E receptor-2 in sperm development. J Biol Chem. 2009;275:23989–23995. doi: 10.1074/jbc.M302157200. [DOI] [PubMed] [Google Scholar]
- 18.Trommsdorff M, Gotthardt M, Hiesberger T, Shelton J, Stockinger W, Nimpf J, Hammer RE, Richardson JA, Herz J. Reeler/disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell. 1999;97:689–701. doi: 10.1016/s0092-8674(00)80782-5. [DOI] [PubMed] [Google Scholar]
- 19.Burk RF, Hill KE, Olson GE, Weeber EJ, Motley AK, Winfrey VP, Austin LM. Deletion of apolipoprotein E receptor-2 in mice lowers brain selenium and causes severe neurological dysfunction and death when a low-selenium diet is fed. J Neurosci. 2007;27:6207–6211. doi: 10.1523/JNEUROSCI.1153-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takahashi S, Suzuki J, Kohno M, Oida K, Tamai T, Miyabo S, Yamamoto T, Nakai T. Enhancement of the binding of triglyceride-rich lipoproteins to the very low density lipoprotein receptor by apolipoprotein E and lipoprotein lipase. J Biol Chem. 1995;270:15747–15754. doi: 10.1074/jbc.270.26.15747. [DOI] [PubMed] [Google Scholar]
- 21.Yagyu H, Lutz EP, Kako Y, Marks S, Hu Y, Choi SY, Bensadoun A, Goldberg IJ. Very low density lipoprotein (VLDL) receptor-deficient mice have reduced lipoprotein lipase activity. Possible causes of hypertriglyceridemia and reduced body mass with VLDL receptor deficiency. J Biol Chem. 2002;277:10037–10043. doi: 10.1074/jbc.M109966200. [DOI] [PubMed] [Google Scholar]
- 22.Frykman PK, Brown MS, Yamamoto T, Goldstein JL, Herz J. Normal plasma lipoproteins and fertility in gene-targeted mice homozygous for a disruption in the gene encoding very low density lipoprotein receptor. Proc Natl Acad Sci U S A. 1995;92:8453–8457. doi: 10.1073/pnas.92.18.8453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yakovlev S, Mikhailenko I, Zhang L, Strickland DK, Medved L. Identification of VLDLR as a novel endothelial cell receptor for fibrin that modulates fibrin-dependent transendothelial migration of leukocytes. Blood. 2012;119:637–644. doi: 10.1182/blood-2011-09-382580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Gendt K, McKinnell C, Willems A, Saunders PT, Sharpe RM, Atanassova N, Swinnen JV, Verhoeven G. Organotypic cultures of prepubertal mouse testes: A method to study androgen action in Sertoli cells while preserving their natural environment. Biol Reprod. 2009;81:1083–1092. doi: 10.1095/biolreprod.109.078360. [DOI] [PubMed] [Google Scholar]
- 25.Konrad L, Keilani MM, Cordes A, Volck-Badouin E, Laible L, Albrecht M, Renneberg H, Aumuller G. Rat Sertoli cells express epithelial but also mesenchymal genes after immortalization with SV40. Biochim Biophys Acta. 2005;1722:6–14. doi: 10.1016/j.bbagen.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 26.Konrad L, Scheiber JA, Völck-Badouin E, Keilani MM, Laible L, Brandt H, Schmidt A, Aumüller G, Hofmann R. Alternative splicing of TGF-betas and their high-affinity receptor TbetaRI, TbetaRII and TbetaRIII (betaglycan) reveal new variants in human prostatic cells. BMC Genomics. 2007;11:318–330. doi: 10.1186/1471-2164-8-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huleihel M. Concise review: Spermatogenesis in an artificial three-dimensional system. Stem Cells. 2012;30:2355–2360. doi: 10.1002/stem.1238. [DOI] [PubMed] [Google Scholar]
- 28.Klock G, Baiersdörfer M, Koch-Brandt C. Chapter 7: Cell protective functions of secretory Clusterin (sCLU) Adv Cancer Res. 2009;104:115–138. doi: 10.1016/S0065-230X(09)04007-X. [DOI] [PubMed] [Google Scholar]
- 29.Reddy KB, Karode MC, Harmony AK, Howe PH. Interaction of transforming growth factor beta receptors with apolipoprotein J/clusterin. Biochemistry. 1996;35:309–314. doi: 10.1021/bi951880a. [DOI] [PubMed] [Google Scholar]
- 30.Lee KB, Jeon JH, Choi I, Kwon OY, Yu K, You KH. Clusterin, a novel modulator of TGF-beta signaling, is involved in Smad2/3 stability. Biochem Biophys Res Commun. 2008;366:905–909. doi: 10.1016/j.bbrc.2007.12.033. [DOI] [PubMed] [Google Scholar]
- 31.Mahon MG, Lindstedt KA, Hermann M, Nimpf J, Schneider WJ. Multiple involvement of clusterin in chicken ovarian follicle development. Binding to two oocyte-specific members of the low density lipoprotein receptor gene family. J Biol Chem. 1999;274:4036–4044. doi: 10.1074/jbc.274.7.4036. [DOI] [PubMed] [Google Scholar]
- 32.Bajari TM, Strasser V, Nimpf J, Schneider WJ. A model for modulation of leptin activity by association with clusterin. FASEB J. 2003;17:1505–1507. doi: 10.1096/fj.02-1106fje. [DOI] [PubMed] [Google Scholar]
- 33.Kim JH, Kim JH, Jun HO, Yu YS, Min BH, Park KH, Kim KW. Protective effect of clusterin from oxidative stress-induced apoptosis in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2010;51:561–566. doi: 10.1167/iovs.09-3774. [DOI] [PubMed] [Google Scholar]
- 34.Jun OH, Kim DH, Lee SW, Lee HS, Seo JH, Kim JH, Kim JH, Min YS, Min BH, Kim KW. Clusterin protects H9c2 cardiomyocytes from oxidative stress-induced apoptosis via Akt/GSK-3β signaling pathway. Exp Mol Med. 2011;43:53–61. doi: 10.3858/emm.2011.43.1.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou Q, Nie R, Li Y, Friel P, Mitchell D, Hess RA, Small C, Griswold MD. Expression of stimulated by retinoic acid gene 8 (Stra8) in spermatogenic cells induced by retinoic acid: an in vitro study in vitamin A-sufficient postnatal murine testis. Biol Reprod. 2008;79:35–42. doi: 10.1095/biolreprod.107.066795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feng CW, Bowles J, Koopman P. Control of mammalian germ cell entry into meiosis. Mol Cell Endocrinol. 2014;382:488–497. doi: 10.1016/j.mce.2013.09.026. [DOI] [PubMed] [Google Scholar]
- 37.Antonangeli F, Petrungaro S, Coluccia P, Filippini A, Ziparo E, Giampietri C. Testis atrophy and reduced sperm motility in transgenic mice overexpressing c-FLIP(L) Fertil Steril. 2010;93:1407–1414. doi: 10.1016/j.fertnstert.2009.01.122. [DOI] [PubMed] [Google Scholar]
- 38.Tedesco M, La Sala G, Barbagallo F, De Felici M, Farini D. STRA8 shuttles between nucleus and cytoplasm and displays transcriptional activity. J Biol Chem. 2009;284:35781–35793. doi: 10.1074/jbc.M109.056481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bolcun-Filas E, Bannister LA, Barash A, Schimenti KJ, Hartford SA, Eppig JJ, Handel MA, Shen L, Schimenti JC. A-MYB (MYBL1) transcription factor is a master regulator of male meiosis. Development. 2011;138:3319–3330. doi: 10.1242/dev.067645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Toscani A, Mettus RV, Coupland R, Simpkins H, Litvin J, Orth J, Hatton KS, Reddy EP. Arrest of spermatogenesis and defective breast development in mice lacking A-myb. Nature. 1997;386:713–717. doi: 10.1038/386713a0. [DOI] [PubMed] [Google Scholar]
- 41.Tang H, Goldberg E. A-MYB (MYBL1) stimulates murine testis-specific Ldhc expression via the cAMP-responsive element (CRE) site. Biol Reprod. 2012;86:30. doi: 10.1095/biolreprod.111.095661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei Y, Yu L, Bowen J, Gorovsky MA, Allis CD. Phosphorylation of histone H3 is required for proper chromosome condensation and segregation. Cell. 1999;97:99–109. doi: 10.1016/s0092-8674(00)80718-7. [DOI] [PubMed] [Google Scholar]
- 43.Kalous J, Solc P, Baran V, Kubelka M, Schultz RM, Motlik J. PKB/AKT is involved in resumption of meiosis in mouse oocytes. Biol Cell. 2006;98:111–123. doi: 10.1042/BC20050020. [DOI] [PubMed] [Google Scholar]
- 44.Pellegrini M, Di Siena S, Claps G, Di Cesare S, Dolci S, Rossi P, Geremia R, Grimaldi P. Microgravity promotes differentiation and meiotic entry of postnatal mouse male germ cells. PLoS One. 2010;5:e9064. doi: 10.1371/journal.pone.0009064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mayr B, Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol. 2001;2:599–609. doi: 10.1038/35085068. [DOI] [PubMed] [Google Scholar]
- 46.Hixon ML, Boekelheide K. Expression and localization of total Akt1 and phosphorylated Akt1 in the rat seminiferous epithelium. J Androl. 2003;24:891–898. doi: 10.1002/j.1939-4640.2003.tb03141.x. [DOI] [PubMed] [Google Scholar]
- 47.Kim ST, Omurtag K, Moley KH. Decreased spermatogenesis, fertility, and altered Slc2A expression in Akt1-/- and Akt2-/- testes and sperm. Reprod Sci. 2012;19:31–42. doi: 10.1177/1933719111424449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lui WY, Lee WM, Cheng CY. Transforming growth factor b3 regulates the dynamics of Sertoli cell tight junctions via the p39 mitogen-activated protein kinase pathway. Biol Reprod. 2003;68:1597–1612. doi: 10.1095/biolreprod.102.011387. [DOI] [PubMed] [Google Scholar]
- 49.Damestoy A, Perrard MH, Vigier M, Sabido O, Durand P. Transforming growth factor beta-1 decreases the yield of the second meiotic division of rat pachytene spermatocytes in vitro. Reprod Biol Endocrinol. 2005;3:22. doi: 10.1186/1477-7827-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]


