Abstract
Drug resistance is an obstacle to the treatment of epithelial ovarian cancer. Recently, research has suggested that miRNAs (microRNAs) are involved in cancer development, and speculation has been made about their possible involvement in drug resistance. Thus, we attempted to identify selected miRNAs involved in the development of chemo-resistance in epithelial ovarian cancer. Using miRNA profiles of a panel of cisplatin-resistant (SKOV3/DDP) cells, we validated data using quantitative real time-PCR (QRT-PCR), and studied the effects of miR-429 on cancer cell chemo-sensitivity, using gain- and loss-of-function studies. Data show that SKOV3/DDP expressed less miR-429 compared with parental SKOV3 cells and lower miR-429 expression conferred shorter overall survival (OS) and less progression-free survival (PFS) than the patients with more miR-429 expression (P < 0.01). Upregulation of miR-429 increases cisplatin sensitivity in epithelial ovarian cancer cells. Studies have confirmed that the zinc finger E-box binding homeobox1 (ZEB1) is a direct and functional target of miR-429 and that over-expression of miR-429 reduces autophagy-related protein anti-ATG7, anti-LC3A/B (P < 0.05). Thus, overexpression of miR-429 may suppress ZEB1, and may be a potential sensitizer to cisplatin treatment that may have therapeutic implications.
Keywords: Epithelial ovarian cancer miR-429 cisplatin resistance ZEB1
Introduction
Epithelial ovarian cancer (EOC) has the highest mortality worldwide and is the fourth most common cause of death due to cancer among women [1]. Early-stage malignancy is frequently asymptomatic and difficult to detect; thus, by the time of diagnosis, most women have advanced disease [2]. EOC is most frequently treated with platinum- and taxane-based chemotherapy. Although initially, 80-90% of patients will have a complete clinical response to this treatment [3], most of these women will then develop platinum-resistance to a wide range of chemotherapeutic agents and develop recurrent or persistent disease [4]. Thus, the 5-year overall survival (OS) of patients with advanced-stage epithelial ovarian cancer (EOC) is only 30% [5,6]. A significant impediment to improving response rates and subsequent patient outcomes is an incomplete understanding of the molecular underpinnings of EOC cell chemo-sensitivity.
MiRNAs (microRNAs) are a class of small, non-coding RNAs, about 19 to 25-nucleotides in length that regulate the expression of thousands of genes by either translation suppression or degradation of mRNA [7-9]. MiRNAs are known to play important roles in most physiological processes-normal development, cell growth, differentiation, and apoptosis in mammals [10]. Until now 1,048 human miRNAs have been annotated in miRNA registries (miRBase, www.miRBase.org) and this number is increasing [11]. Several studies have focused on circulating miRNAs in the blood as a means of non-invasive diagnosis and early detection in EOC [12]. MiRNAs regulate cancer cell biology, including multiple drug resistances by binding to imperfect complementary sites in the 3’-untranslated region of their target mRNA transcripts [13-15], leading to the development of microRNAs as early indicators of disease and to their potential therapeutic use. An appreciation of the effects of these repressors may provide a more complete understanding of the indirect effects of miRNA dysregulation in diseases such as cancer, and to their successful clinical application. Hence, miRNA expression analysis has a diagnostic value and is a promising therapeutic approach.
MiR-429 belong to the miR-200 family, which consists of five members localized in two genomic clusters (miR-200b/a, miR-429 on chromosome 1, and miR-200c, miR-141 on chromosome 12) [16]. The miR-200b/c/429 cluster may contribute to the development of MDR in both gastric and lung cancer cell lines, at least in part by modulation of apoptosis via targeting BCL2 and XIAP [17]. Proteasome inhibitor-resistant cells cause EMT-induction via suppression of E-cadherin by miR-200s and zinc finger E-box binding homeobox1 (ZEB1) [18]. Therefore, our goal was to determine the role of miR-429 in EOC.
Material and methods
Patients and samples
Surgical specimens from 72 EOC patients were obtained postoperatively from 2007 to 2011 from the Department of Gynecologic Oncology, Affiliated Tumor Hospital of Guangxi Medical University. The study was endorsed by the Ethics Committee of the Guangxi Medical University. All patients gave signed, informed consent for their tissues to be used for scientific research. Ethical approval for the study was obtained from the local institutional review board. Tissues were obtained and stored at -80°C prior to qRT-PCR assay. The accuracy of drug resistance prediction was evaluated by receiver-operating characteristics (ROC) curves (AUC).
Cell lines and cell culture
The human EOc cell line SKOV3 was stored in our laboratory, and routinely maintained in DMEM (Corning, city, state) supplemented with 10% fetal bovine serum (FBS) (Corning) and 2 μM/L L-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C in a humidified atmosphere of 5% CO2. A stable cisplatin-resistant cell line (SKOV3/DDP) was established from SKOV3 cells as previously described [19,20].
cDNA synthesis and quantitative real time-PCR to detect miR-429
Total RNA was prepared from cells using an RNeasy® Mini Kit (QIAGEN, Germany) according to the manufacturer’s instructions. RNA integrity was verified using a NanoDrop2000 (1.8-2.0; NanoDrop2000, Thermo, city, state). RNA (1 µg) was reverse transcribed using a miScript II RT (Qiagen, Germany) as detailed in the manufacturer’s instructions. A quantitative real-time PCR (qRT-PCR) assay, used to measure miR-429 in RNA extracted from EOC cell lines and ovarian tumors, was performed with FastStart Universal SYBR Green PCR Master (ROX) in a total volume of 10 µL on an MxPro 3000p (Agilent, Palo Alto, USA). The conditions were as follows: 40 cycles of three-step PCR (95°C for 15 sec, 55°C for 30 sec and 72°C for 30 sec) following initial denaturation at 95°C for 10 min. All primers were supplied by BGI (The Beijing Genomics Institute). Primer sequences were as follows: 5’-CGTAATACTGTCTGGTAAAACCGT-3’ for miR-429; 5’-CAAGGA TGACACGCAAATTCG-3’ for U6; U6 was used as internal standards to normalize the expression of miR-429.
Cell viability assay and cell growth assay
Proliferation assay
Cell proliferation assays were performed using a Cell Counting Kit-8 (CCK8). Cells were seeded at a density of 1.0 × 103 cells per well in 96-well plates, and were incubated at 37°C for 1, 2, 3, and 4 days, with fresh media containing either drug and/or vehicle being replaced every day where appropriate.
IC50 assay
Cell viability and IC50 were measured with a Cell Counting Kit-8 (CCK-8; Dojindo Laboratories, Kumamoto, Japan). Cells were seeded in 96-well plates 12 h before treatment at a density of 5,000 cells/well. Cells were treated with increasing concentrations of cisplatin (5, 10, 20, and 40 μM up to 80 μM) dissolved in 100 μL DMEM. DMEM (100 μL) without cisplatin was added to the cells as a negative control and 48 h of incubation, DMEM was changed with RPMI-1640 for 100 μl/well. RPMI-1640 (100 μL) without cells was used as a blank control. CCK8 (10 μL) was added and incubated for an additional 2 h. The absorbance at 450 nm was read using a microplate MULTISKAN GO (Thermo, city, state). In vitro cytotoxicity was measured using percent cell survival expressed as {((ODcontrol-ODblank)-(ODtreated-ODblank))/(ODcontrol-ODblank)} × 100. All experiments were repeated in triplicate and mean values reported.
Lentiviral infection
Lentivirus packaging containing an miR-429 expression plasmid and its negative control (NC), LV-3 (pGLVH1/GFP+Puro), followed by a green fluorescent protein (GFP) reporter were designed and synthesized by GenePharma Inc (Shanghai, China). The resultant lentivirus containing the miR-429 (LV3-miR-429) and the NC sequences (LV3-NC), were used to infect SK/DDP cells to establish stably overexpressed cell lines. Lentivirus was added to 1.5 × 105 SK/DDP cells (final titration of 109) for 24 h, and then replaced with fresh DMEM with 10% FBS. GFP expression in infected cells (SK/DDP+miR-429 or SK/DDP-NC) was measured using fluorescent microscopy and more than 85% of cells were found to express GFP 72 h later. After Puro selection, stable pool cells were obtained, and miR-429 expression was confirmed by qRT-PCR.
A microOFFTM miR-429 inhibitor (Ribobio, Guangzhou, China) was transfected using Lipofectamine 2000 transfection agent (Invitrogen) according to the manufacturer’s instructions (final concentration 150 nM). Cells were incubated for 6 h in this reduced serum environment to optimize transfection, washed with PBS, and then incubated at 37°C at 5% CO2 for 48 h after adding fresh DMEM to the wells. Cell culture experiments were carried out using at least two-three independent biological replicates.
Colony formation assay
Cells were cultured in six-well plates and 24 h later were treated with 0, 0.6, or 1.2 μM cisplatin in media and incubated at 37°C for 11-14 days. Colonies were fixed by methanol solution and stained with Giemsa stain and colonies of more than 50 cells were counted. Plating efficiency (PE) was calculated as: PE = (colony number/plating cell number) × 100%, in triplicate.
Apoptosis analysis
Cells were cultured in six-well plates and were treated with 0, 1, or 2 μM WHAT, and incubated at 37°C and 5% CO2 for 48 h. All samples were washed in PBS and resuspended in 100 µl binding buffer. Next, 5 µl Annexin V-PE and 5 µl 7-AAD were added and the cell suspension was incubated in a dark chamber at room temperature for 15 min. Apoptosis was measured using a FACS Calibur flow cytometer (BD Biosciences, city, state) and data were analyzed using Cell Quest software (BD Biosciences).
MicroRNA target prediction
Target sites for animal miRNAs are not evenly distributed within 3’UTR but rather grouped at both ends of 3’UTR [21]. The miRNA sequence was analyzed using miRBase (http://microrna.san ger.ac.uk/sequences/), and the target gene information of miR-429 were selected from the intersection of the target prediction results by Targetscan (http://www.targetscan.org/) [22], miRBase (http://www.mirbase. org/) [11], miRanda (http://www.microrna.org/microrna/home.do) [23], PicTar (http://pictar.mdcberlin.de/) [24]. miRecords (http://miRecords.um n.edu/miRecords.) [25].
Western blot analysis
Cells were washed twice with cold PBS and lysed in RIPA lysis buffer (Beyotime Inst Biotech, China) supplemented with protease inhibitors (2 μg/ml PMSF) for 30 min on ice. Cell lysates were then clarified by centrifugation (14,000 rpm) at 4°C for 30 min, and the supernatant was collected for experiments. Protein concentration of lysates was measured with a BCA Protein Assay Kit (Thermo Fisher Scientific, city, state). A total of 80 µg protein lysate was separated by 10% SDS-PAGE, and proteins were transferred onto PVDF membranes and blocked with blocking solution (5% skim milk in PBST). Membranes were incubated overnight with the primary antibody: rabbit anti-human ZEB1 (diluted 1:250, Cell Signaling Technology, city, state) and rabbit anti-human GAPHD (diluted 1:1000, Cell Signaling Technology, city, state) followed by the incubation with appropriate HRP-conjugated secondary antibody (diluted 1:1000, DAKO, Denmark). Western blot densitometry results were obtained with ImageJ software [26]. We also measured autophagy protein using an autophagy sampler kit (4445S, Cell Signaling Technology, city, state).
Luciferase assay
The ZEB1 3’-UTR containing putative binding sites for miR-429 were cloned into pmirGLO vector (Promega, city, state), which was based on Promega dual-luciferase technology, with firefly luciferase (luc2) as the primary reporter gene and Renilla luciferase (hRluc-neo) as a control reporter gene. Two binding site in the 3’UTR of ZEB1 and its mutated version were cloned into luciferase reporter plasmids. MiR-429 mimics (40 pM, GenePharma Co, Ltd, Shanghai, China) and the reporter plasmids were co-transfected into HEK293 cells. Cells were harvested 24 h post-transfection and luciferase activities were assayed with Dual-Luciferase Reporter Assay System (Promega, E1960) according to the manufacturer’s instructions. Then the ratio of luc2 activity to hRluc-neo activity in the miR-429 treated group was calculated and compared with ratios in the miR-NC group, which was arbitrarily defined as 100%. Each experiment was performed in triplicate.
Statistical analysis
All experiments were repeated at least three times and the data are expressed as the means ± standard deviation. Statistical analysis was conducted with SPSS 16.0 statistical analysis package. Differences between two groups of miR-429 were analyzed by two tailed student’s t-test, and three groups were by One-way ANOVA; the value of miR-429 for the diagnosis chemo-resistant of EOC was analyzed by the ROC analysis; the survival curves were plotted by the Kaplan-Meier method, to construct a model for the prediction of survival, univariate and multivariate Cox regression model was performed. P < 0.05 was considered to indicate a statistically significant difference.
Results
Expression of miR-429 is associated with development cisplatin resistance
To explore the role of miR-429 involved in cisplatin resistance, total RNA was extracted from SKOV3 and SKOV3/DDP cells and qRT-PCR revealed that miR-429 was significantly downregulated in SKOV3/DDP cells compared with parental SKOV3 cells (Figure 1A), data that are consistent with previous publications [27]. We measured cisplatin sensitivity in SKOV3/DDP cells exposed to various concentrations of cisplatin for 48 h. IC50 for cisplatin in SKOV3/DDP cells was 2.5-fold greater than parental SKOV3 cells (Figure 1B). Next, after manipulating miR-429 expression in SKOV3/DDP and SKOV3 cells using LV3-miR-429 and miR-429 inhibitors, respectively, we measured changes in drug sensitivity. As shown in Figure 1D, miR-429 was increased significantly in cells transfected with LV3-miR-429 compared with negative controls (LV3-miR-NC), and lowered IC50 values for cisplatin compared with the NC (Figure 1E). Conversely, the miR-429 inhibitor increased IC50 values for cisplatin in SKOV3 cells compared with miR-NC (Figure 1F). Thus, downregulation of miR-429 is implicated in the development of cisplatin resistance in SKOV3/DDP cells, and its over-expression can increase drug sensitivity.
Figure 1.
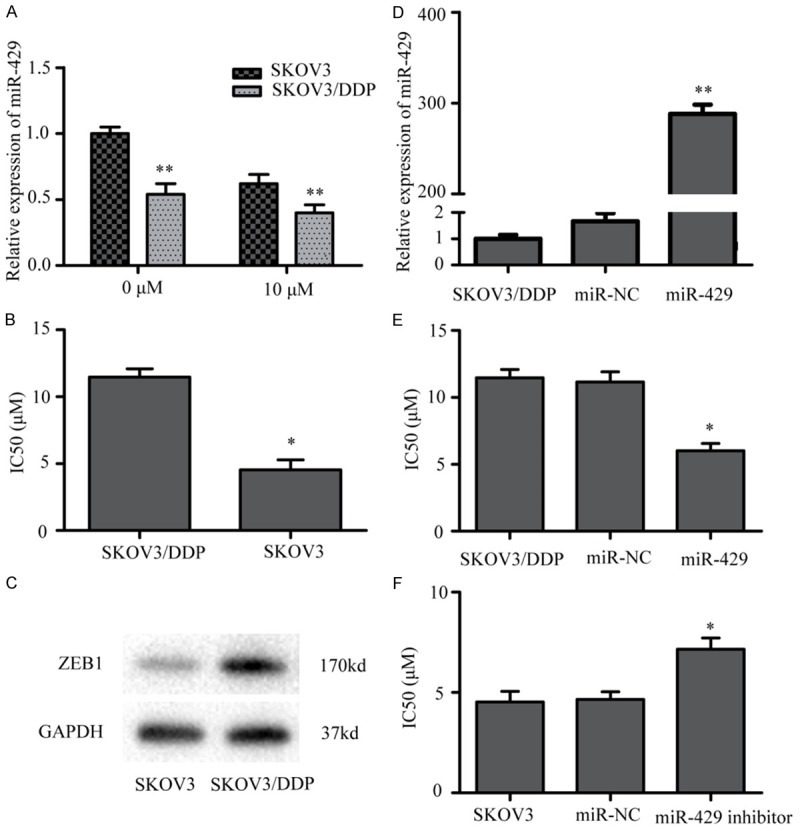
The expression of miR-429 in ovarian cancer cell lines. A. MiR-429 expression was measured by QRT-PCR in parental SKOV3 cells compared with that in cisplatin-resistant SKOV3/DDP cells. B. SKOV3/DDP and SKOV3 cells were treated with various concentrations of cisplatin for 48 h, and the 50% inhibitory concentration (IC50) of cisplatin was measured by the CCK8 assay. C. ZEB1 expression was up-regulated in SKOV3/DDP cells compared with in the parental A2780 cells. D. Displayed that the expressions of miR-429 were significantly increased in SKOV3/DDP cells infected by LV3-miR-429 compared to the blank groups. E. SKOV3/DDP cells were transfected with LV3-miR-429 or LV3-miR-NC and were exposed to a range of cisplatin concentrations. The IC50 of cisplatin was shown. F. SKOV3 cells were transient transfected with miR-429 inhibitor or miR-NC and were exposed to a range of cisplatin concentrations. The IC50 of cisplatin was shown (*P < 0.05,**P < 0.01).
Over-expression of miR-429 increased cisplatin sensitivity by inhibiting proliferation, inducing apoptosis and reducing autophagy of SKOV3/DDP cells
MiR-429-induced changes in OC cell viability were measured and miR-429 reduced both proliferation and colony-forming ability of SKOV3/DDP cells. A cell survival assay showed that LV3-miR-429 significantly inhibited cell growth compared with controls at 48 h (Figure 2A; P < 0.05). Colony formation assays confirmed that miR-429 inhibited cell growth. Colony formation was reduced in SKOV3/DDP cells transfected with miR-429 compared with the miR-NC in the presence of all concentrations of cisplatin (Figure 2B, 2C). Thus, miR-429 inhibits SKOV3/DDP cell growth.
Figure 2.
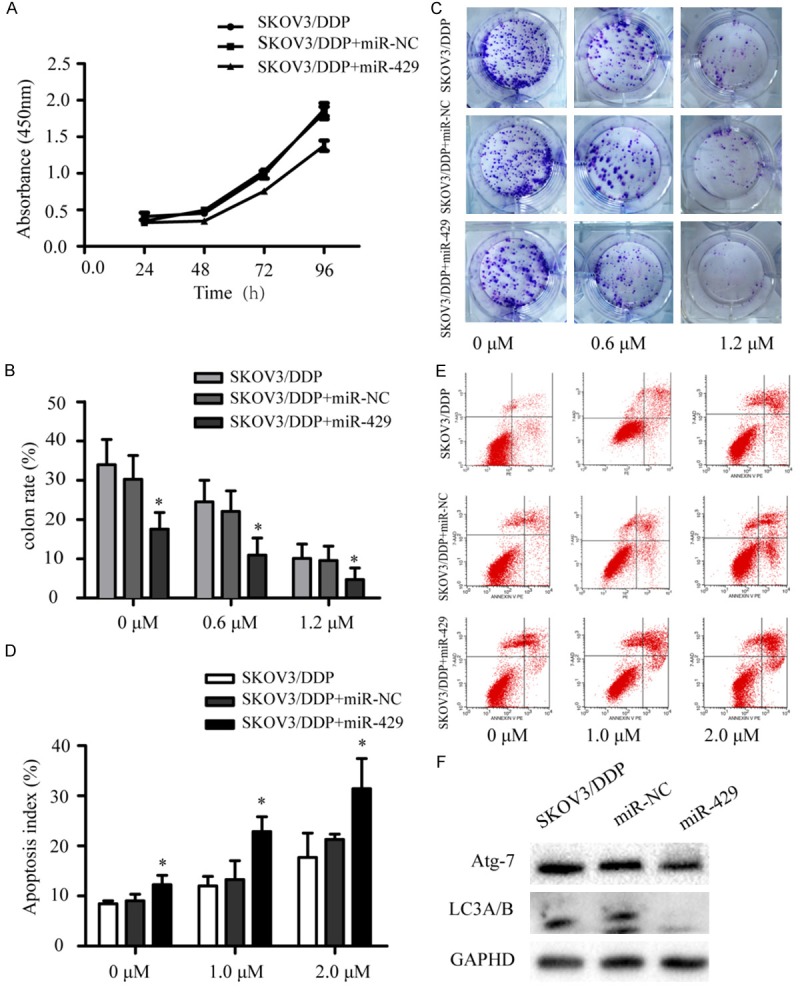
MiR-429 overexpression increased cisplatin sensitivity by inhibiting cell growth and promoting apoptosis. A. CCK8 assay data for SKOV3/DDP cells transfected with LV3-miR-429 or miR-NC. B, C. Colony formation data for SKOV3/DDP cells transfected with LV3-miR-429 or miR-NC, and then treated with cisplatin for 11-14 days. D, E. Apoptosis of SKOV3/DDP cells transfected with LV3-miR-429 or miR-NC and treated with cisplatin for 48 h. F. Protein after LV3-miR-429 transfection of SKOV3/DDP cells. *P < 0.05.
Many chemotherapeutic agents exert anticancer activity by inducing apoptosis. Therefore, we studied the role of miR-429 in cisplatin-induced apoptosis. SKOV3/DDP cells were transfected with miR-429, control after cisplatin treatment for 48 h. Cells were stained and flow cytometry analysis indicated that apoptosis was greater in the miR-429 group compared to controls (Figure 2D, 2E).
Previous studies indicate that autophagy is associated with tumor drug resistance and OC drug resistance [28]. Therefore, we measured miR-429 overexpression and found that miR-429 over-expression reduced Anti-ATG7 and Anti-LC3A/B (Figure 2F).
MiR-429 promoted SKOV3/DDP cell sensitivity to cisplatin via directly targeting ZEB1
As shown in Figure 1C, ZEB1 expression was high in the SKOV3/DDP cell line, but low in the parental SKOV3 cell line. The bioinformatics algorithm predicted that ZEB1 was a potential target gene of miR-429 (Figure 3A). Using the Expression Omnibus database (GEO), we found a microarray analyses (GSE56967) in HEY OC cells that were transiently transfected either with miR-429 oligos or negative control. High miR-429 significantly inhibited ZEB1 expression (~1.8-fold) compared with controls (Figure 3B). Western blots indicated ZEB1 expression was inhibited by miR-429 as ZEB1 protein was significantly reduced after transfection with LV3-miR-429 compared with controls in SKOV3/DDP cells (Figure 3C). In contrast, transfection of SKOV3 cells with miR-429 inhibitor ZEB1 compared with controls (Figure 3D).
Figure 3.
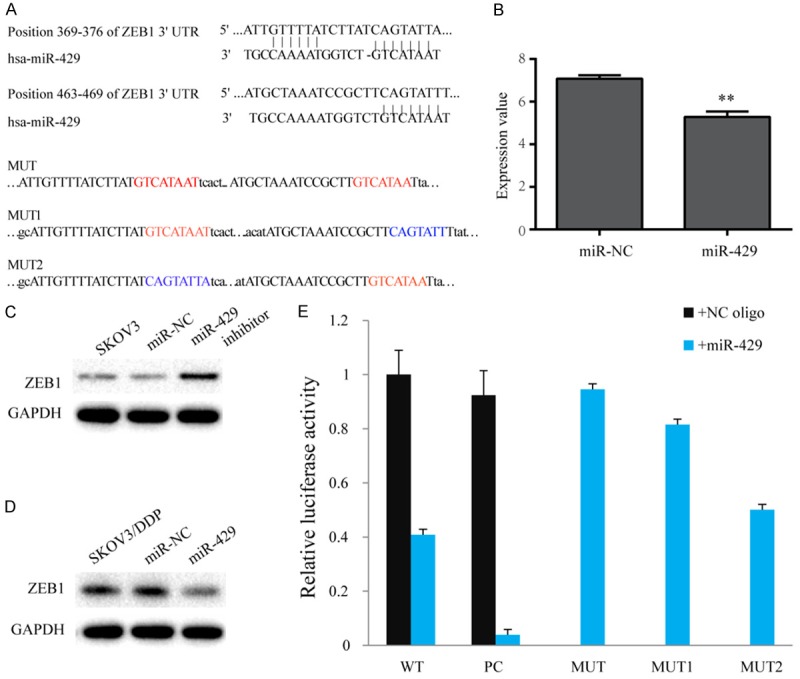
ZEB1 targeted by miR-429. A. ZEB1 was predicted to be a potential target of miR-429 and mutated sites of miR-429 are shown. B. Expression of ZEB1 in the microarray dataset GSE56967. C. ZEB1 protein after LV3-miR-429 transfection in SKOV3/DDP cells. D. ZEB1 protein after miR-429 inhibitor transfection in SKOV3 cells. E. Luciferase assay. HEK293T cells co-transfected with pmirGLO-ZEB1-wt or pmirGLO-ZEB1-mut and miR-429 mimics or NC. Relative luciferase activity. All data are shown as means ± sd of three separate experiments. **P < 0.01.
We investigated whether ZEB1 is a direct target of miR-429 by studying the interaction between miR-429 and the 3’UTR of ZEB1 using a luciferase assays in HEK293T cells. Compared to negative control, cells co-transfected miR-429 suppressed luciferase gene expression, which was located upstream of the wild type 3’UTR of ZEB1. Mutation of both predicted recognition sites within the 3’UTR of ZEB1 abolished the inhibitory effect of miR-429. Thus, the inhibitory function of miR-429 on the target gene requires interaction with the predicted binding sites (Figure 3E).
Clinical significance of miR-429 expression in human OC tissues
To determine whether miR-429 expression was related to patient outcome, expression of miR-429 in cancer tissues from 72 EOC specimens were examined via qRT-PCR assay. miR-429 was down-regulated in chemo-resistant patients (n = 30) compared with chemo-sensitive tissues (n = 42), with an average 0.35-fold reduction (P < 0.001, Figure 4A). As shown in Table 1, miR-429 was significantly associated with chemotherapeutic response (P < 0.05), but was not associated with age, FIGO stage, differentiation or residual tumor (P > 0.05). And the ROC analysis showed that Thus, miR-429 is a valuable biomarker for the diagnosis chemo-resistant of EOC (Table 2). The area under the curve (AUC) was 0.744 (95% CI: 0.63-0.86, P = 0.057). Next, we studied the prognostic significance of miR-429 and (Figure 4B, 4C; Table 3) patients with less miR-429 expression had significantly shorter OS and PFS than those with high miR-429 expression (HR = 0.398, 95% CI = 0.201-0.709, P = 0.006 and HR = 0.491, 95% CI = 0.290-0.832, P = 0.041) (P < 0.01). Low miR-429-expressing patients had poorer prognoses [29]. Only the response to treatment was an independent prognostic factor for PFS or OS according to a multivariate Cox regression model (Table 3).
Figure 4.
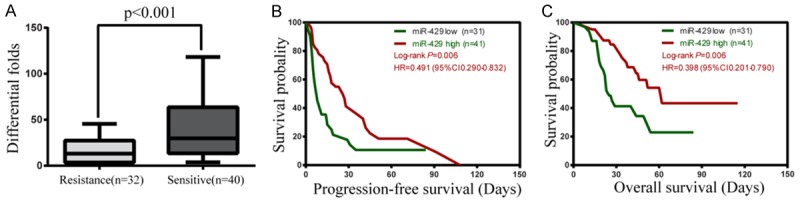
Expression and prognostic value of miR-429 in epithelial OC tissues. (A) MiR-429 expression in cisplatin-sensitive (n = 40) and -resistant (n = 32) human EOC tissues. (B, C) Kaplan-Meier plots estimated according to miR-429 for overall survival (B) and PFS (C) of EOC patients.
Table 1.
Correlation between miR-429 expression and clinicopathologic characteristics of 72 OC patients
| Factor | Cases | T value | P | |
|---|---|---|---|---|
| Age | ≤ 50 | 38 | -0.139 | 0.890 |
| > 50 | 34 | |||
| FIGO stage | I-II | 14 | 0.165 | 0.869 |
| III-IV | 58 | |||
| Histologicalsubtype | Serous | 40 | 0.391 | 0.697 |
| Mucinous | 15 | |||
| Grade | G1 | 60 | -0.626 | 0.534 |
| G2 | 6 | |||
| OS | Dead | 34 | -1.734 | 0.087 |
| Survival | 38 | |||
| PFS | Recurrence | 59 | -1.541 | 0.128 |
| Unrecurrence | 13 | |||
| Response to treatment | Clinical remission | 51 | 2.934 | 0.005 |
| No clinical remission | 21 |
Table 2.
Sensitivity of miR-429 to identify the EOC with drug resistance
| AUC | 95% CI | Sensitivity | Specificity | Youden | Cutoff | P | |
|---|---|---|---|---|---|---|---|
| MiR-429 | 0.744 | 0.63-0.86 | 0.633 | 0.714 | 0.348 | 0.532 | P < 0.05 |
Table 3.
Cox proportional hazards regression analyses of clinical parameters and miR-429 signature in relation to disease outcomes
| Variable | Progression-free survival | Overall survival | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||
|
|
|
|||||||
| HR (95% CI) | p | HR (95% CI) | P | HR (95% CI) | p | HR (95% CI) | P | |
| Age | 1.234 (0.732-2.079) | 0.431 | 0.881 (0.475-1.636) | 0.689 | 1.155 (0.587-2.272) | 0.676 | 0.807 (0.367-1.778) | 0.595 |
| FIGO | 1.717 (0.839-3.517) | 0.139 | 1.421 (0.653-3.091) | 0.376 | 2.337 (0.821-6.652) | 0.112 | 1.882 (0.621-5.702) | 0.264 |
| Grade | 0.872 (0.610-1.246) | 0.451 | 0.916 (0.605-1.388) | 0.680 | 0.921 (0.572-1.482) | 0.734 | 1.035 (0.603-1.775) | 0.902 |
| Histological subtype | 1.000 (0.727-1.376) | 0.999 | 1.213 (0.844-1.741) | 0.296 | 1.208 (0.814-1.794) | 0.348 | 1.755 (1.119-2.753) | 0.014 |
| Residual lesions | 1.661 (0.928-2.974) | 0.088 | 1.060 (0.564-1.992) | 0.857 | 1.877 (0.926-3.803) | 0.081 | 0.904 (0.402-2.032) | 0.806 |
| Responseto treatment | 0.200 (0.111-0.358) | 0.000 | 0.211 (0.106-0.420) | 0.000 | 0.085 (0.04-0.179) | 0.000 | 0.055 (0.021-0.145) | 0.000 |
| MiR-429 | 0.661 (0.478-0.915) | 0.012 | 0.710 (0.504-1.001) | 0.05 | 0.641 (0.412-0.996) | 0.048 | 0.763 (0.458-1.270) | 0.298 |
Discussion
EOC is the leading cause of death of all gynecologic malignancies, and the average 5-year survival is approximately 35-40%, but patients with advanced-stage disease have a survival rate of only 10-20% due to less effective chemotherapy, and drug resistance [4]. Cellular mechanisms of drug resistance are various [30] and microRNAs may be chief contributors to this resistance [31].
Recent studies suggest that during tumorigenesis, miRNAs can act either as oncogenic miRNAs (oncomiRs) or tumor suppressor miRNAs (tumor suppressor miRs) depending on the cellular context and the expression of the miRNA targets in the particular malignant tissues [32]. Therefore, miRNAs have been studied for associations with chemo-sensitivity [33]. For example, upregulation of miR-21 reduced cell growth, proliferation and invasion via the JNK-1/c-Jun pathway [34]. Furthermore, miR-136 expression in human EOC can induce chemo-resistance at least in part by downregulating apoptosis and promoting the repair of cisplatin-induced DNA damage [35].
Data from previous studies indicate that miR-429 is of the miR-200 family [36], and members of this family have been observed to be downregulated in several types of cancers and upregulated in others, so they may be correlated with chemo-resistance to paclitaxel and cisplatin in a panel of ovarian adenocarcinoma cell lines with inherent or acquired drug-resistance [36-38]. MiR-429 is reported to inhibit cell growth and invasion in colorectal carcinoma [39]. miR-200c expression may increase sensitivity to microtubule-targeting drugs by repressing the tubulin molecule TUBB3-high expression of which is linked with resistance against microtubule-directed agents [40].
Our results implicate miR-429 as a phenotypic regulator of chemo-resistance and our work is focused on the role of miR-429 as a regulator of chemotherapy response in EOC tumors. We found showed significantly lower miR-429 in cisplatin-resistant SKOV3/DDP cells compared with primary cultures of parent cells. Overexpression of miR-429 enhanced cisplatin chemo-sensitivity in SKOV3/DDP cells, but in SKOV3 cells, when transfected with miR-429 inhibitor, IC50 increased, data that are consistent with Wang’s study [41]. These findings suggest that miR-429 can regulate cisplatin chemo-sensitivity in SKOV3 cells and that miR-429 inhibited viability and proliferation of SKOV3/DDP cells compared with SKOV3/DDP and miR-NC groups.
Although many factors contribute to cisplatin-resistance of tumor cells, apoptosis has been shown to contribute to the development of drug resistance [42], and increased tumor cell autophagy after cisplatin treatment is key to this process [43]. MicroRNA-30a sensitizes tumor cells to cisplatin via suppressing Beclin 1-mediated autophagy [28]. Downregulation of ATG14 by EGR1-MIR152 sensitizes ovarian cancer cells to cisplatin-induced apoptosis by inhibiting cyto-protective autophagy [44]. Our data show that tumor cell apoptosis in the miR-429 group was higher (P < 0.05), data that are consistent with published work [45], and extraneous expression of miR-429 significantly decreased autophagy related protein 7 (Atg-7) and LC3A/B expression, indicating that miR-429 expression may increase OC cell sensitivity to cisplatin by inducing apoptosis and inhibiting autophagy.
ZEB1, also known as TCF8 or delta EF1, is a transcriptional repressor of E-cadherin and polarity factor genes, and is a crucial EMT activator in human colon and breast cancers [46]. Epithelial to mesenchymal transition (EMT) is associated with overall OS or PFS, suggesting that tumor EMT is associated with chemo-resistance [47]. The miR-200 family is known as the main suppressor of EMT, which is a reversible embryonic program aberrantly activated in tumor progression and metastasis [48]. miR-429 is reported to regulate EMT-related marker genes by targeting Onecut2 in colorectal carcinoma [39]. Collectively, these data show that the miR-200 family is a powerful regulator of EMT/MET by targeting ZEB1 and ZEB2, which control E-cadherin expression. Indeed, inhibiting ZEB1 and ZEB2, ectopic expression of miR-200s causes upregulation of E-cadherin and reduces cell motility [49]. Our data also show that endogenous ZEB1 expression was specifically downregulated by miR-429 at the post-transcriptional level, and was significantly elevated in SKOV3 cells after treatment with inhibitors of miR-429 as compared with controls. ZEB1 therefore may be a target of miR-429 and overexpression of miR-429 may decrease ZEB1 function. These findings provide new insights into the molecular functions of miR-429 as well as the role of ZEB1 in EOC chemotherapeutic resistance.
Previous work suggests that low miR-429 expression predicts poor PFS in advanced stage EOC patients [38]. We observed that, in 72 epithelial EOC samples miR-429 expression was decreased in drug-resistant samples, data that are similar to published reports [36]. Moreover, down-regulation of miR-429 was correlated with cisplatin resistance (P < 0.05), and poor prognosis for EOC patients, the same conclusion drawn for patients with osteosarcoma [26].
In summary, we demonstrated that miR-429 is deregulated in drug-resistant cell lines, targeting the expression of the resistant factor ZEB1. Furthermore, data suggest a possible role for miR-429 both as a prognostic factor and a marker of treatment failure in EOC. This discovery lays a foundation for new opportunities for EOC research and treatment.
Acknowledgements
This study was supported by a grant from the National Natural Science Foundation of China (No. 81572579) and Guangxi scientific research and technological development program topics (No. 14124004) and the Research Fund for the Doctoral Program of Higher Education of China (No. 20124503110003) Youth Science Foundation of Guangxi Medical University (No. GXMUYSF201402).
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Shah MM, Landen CN. Ovarian cancer stem cells: are they real and why are they important? Gynecol Oncol. 2014;132:483–489. doi: 10.1016/j.ygyno.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bast RC Jr, Hennessy B, Mills GB. The biology of ovarian cancer: new opportunities for translation. Nat Rev Cancer. 2009;9:415–428. doi: 10.1038/nrc2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gou Q, Liu L, Wang C, Wu Q, Sun L, Yang X, Xie Y, Li P, Gong C. Polymeric nanoassemblies entrapping curcumin overcome multidrug resistance in ovarian cancer. Colloids Surf B Biointerfaces. 2015;126:26–34. doi: 10.1016/j.colsurfb.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 5.Banerjee S, Kaye SB. New strategies in the treatment of ovarian cancer: current clinical perspectives and future potential. Clin Cancer Res. 2013;19:961–968. doi: 10.1158/1078-0432.CCR-12-2243. [DOI] [PubMed] [Google Scholar]
- 6.Vaughan S, Coward JI, Bast RC Jr, Berchuck A, Berek JS, Brenton JD, Coukos G, Crum CC, Drapkin R, Etemadmoghadam D, Friedlander M, Gabra H, Kaye SB, Lord CJ, Lengyel E, Levine DA, McNeish IA, Menon U, Mills GB, Nephew KP, Oza AM, Sood AK, Stronach EA, Walczak H, Bowtell DD, Balkwill FR. Rethinking ovarian cancer: recommendations for improving outcomes. Nat Rev Cancer. 2011;11:719–725. doi: 10.1038/nrc3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T. Identification of novel genes coding for small expressed RNAs. Science. 2001;294:853–858. doi: 10.1126/science.1064921. [DOI] [PubMed] [Google Scholar]
- 8.Lee RC, Ambros V. An extensive class of small RNAs in caenorhabditis elegans. Science. 2001;294:862–864. doi: 10.1126/science.1065329. [DOI] [PubMed] [Google Scholar]
- 9.Benes V, Castoldi M. Expression profiling of microRNA using real-time quantitative PCR, how to use it and what is available. Methods. 2010;50:244–249. doi: 10.1016/j.ymeth.2010.01.026. [DOI] [PubMed] [Google Scholar]
- 10.Costa PM, Pedroso de Lima MC. MicroRNAs as molecular targets for cancer therapy: on the modulation of microRNA expression. Pharmaceuticals (Basel) 2013;6:1195–1220. doi: 10.3390/ph6101195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griffiths-Jones S, Saini HK, van Dongen S, Enright AJ. miRBase: tools for microRNA genomics. Nucleic Acids Res. 2008;36:D154–158. doi: 10.1093/nar/gkm952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng H, Zhang L, Zhao Y, Yang D, Song F, Wen Y, Hao Q, Hu Z, Zhang W, Chen K. Plasma miRNAs as diagnostic and prognostic biomarkers for ovarian cancer. PLoS One. 2013;8:e77853. doi: 10.1371/journal.pone.0077853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blower PE, Chung JH, Verducci JS, Lin S, Park JK, Dai Z, Liu CG, Schmittgen TD, Reinhold WC, Croce CM, Weinstein JN, Sadee W. MicroRNAs modulate the chemosensitivity of tumor cells. Mol Cancer Ther. 2008;7:1–9. doi: 10.1158/1535-7163.MCT-07-0573. [DOI] [PubMed] [Google Scholar]
- 14.Song H, Liu Y, Pan J, Zhao ST. Expression profile analysis reveals putative prostate cancer-related microRNAs. Genet Mol Res. 2013;12:4934–4943. doi: 10.4238/2013.October.24.4. [DOI] [PubMed] [Google Scholar]
- 15.Xie L, Jing R, Qi J, Lin Z, Ju S. Drug resistance-related microRNAs in hematological malignancies: translating basic evidence into therapeutic strategies. Blood Rev. 2015;29:33–44. doi: 10.1016/j.blre.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Korpal M, Kang Y. The emerging role of miR-200 family of microRNAs in epithelial-mesenchymal transition and cancer metastasis. RNA Biol. 2008;5:115–119. doi: 10.4161/rna.5.3.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu W, Xu H, Zhu D, Zhi H, Wang T, Wang J, Jiang B, Shu Y, Liu P. miR-200bc/429 cluster modulates multidrug resistance of human cancer cell lines by targeting BCL2 and XIAP. Cancer Chemother Pharmacol. 2012;69:723–731. doi: 10.1007/s00280-011-1752-3. [DOI] [PubMed] [Google Scholar]
- 18.Asakura T, Yamaguchi N, Ohkawa K, Yoshida K. Proteasome inhibitor-resistant cells cause EMT-induction via suppression of E-cadherin by miR-200 and ZEB1. Int J Oncol. 2015;46:2251–2260. doi: 10.3892/ijo.2015.2916. [DOI] [PubMed] [Google Scholar]
- 19.Li L, Luan Y, Wang G, Tang B, Li D, Zhang W, Li X, Zhao J, Ding H, Reed E, Li QQ. Development and characterization of five cell models for chemoresistance studies of human ovarian carcinoma. Int J Mol Med. 2004;14:257–264. [PubMed] [Google Scholar]
- 20.Shi L, Yu H, Zhang W, Li L, Wang Q. Establishment and biological characteristics of a platinum-resistance nude mouse model in epithelial ovarian cancer. Chin J Obstet Gynecol. 2014;49:523–530. [PubMed] [Google Scholar]
- 21.Hon LS, Zhang Z. The roles of binding site arrangement and combinatorial targeting in microRNA repression of gene expression. Genome Biol. 2007;8:R166. doi: 10.1186/gb-2007-8-8-r166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 23.John B, Enright AJ, Aravin A, Tuschl T, Sander C, Marks DS. Human microRNA targets. PLoS Biol. 2004;2:e363. doi: 10.1371/journal.pbio.0020363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krek A, Grun D, Poy MN, Wolf R, Rosenberg L, Epstein EJ, MacMenamin P, da Piedade I, Gunsalus KC, Stoffel M, Rajewsky N. Combinatorial microRNA target predictions. Nat Genet. 2005;37:495–500. doi: 10.1038/ng1536. [DOI] [PubMed] [Google Scholar]
- 25.Xiao F, Zuo Z, Cai G, Kang S, Gao X, Li T. miRecords: an integrated resource for microRNA-target interactions. Nucleic Acids Res. 2009;37:D105–110. doi: 10.1093/nar/gkn851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu X, Liu Y, Wu S, Shi X, Li L, Zhao J, Xu H. Tumor-suppressing effects of miR-429 on human osteosarcoma. Cell Biochem Biophys. 2014;70:215–224. doi: 10.1007/s12013-014-9885-8. [DOI] [PubMed] [Google Scholar]
- 27.Liu L, Zou J, Wang Q, Yin FQ, Zhang W, Li L. Novel microRNAs expression of patients with chemotherapy drug-resistant and chemotherapy-sensitive epithelial ovarian cancer. Tumour Biol. 2014;35:7713–7717. doi: 10.1007/s13277-014-1970-5. [DOI] [PubMed] [Google Scholar]
- 28.Zou Z, Wu L, Ding H, Wang Y, Zhang Y, Chen X, Chen X, Zhang CY, Zhang Q, Zen K. MicroRNA-30a sensitizes tumor cells to cis-platinum via suppressing beclin 1-mediated autophagy. J Biol Chem. 2012;287:4148–4156. doi: 10.1074/jbc.M111.307405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu X, Macdonald DM, Huettner PC, Feng Z, El Naqa IM, Schwarz JK, Mutch DG, Grigsby PW, Powell SN, Wang X. A miR-200 microRNA cluster as prognostic marker in advanced ovarian cancer. Gynecol Oncol. 2009;114:457–464. doi: 10.1016/j.ygyno.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 30.Cohen K, Emmanuel R, Kisin-Finfer E, Shabat D, Peer D. Modulation of drug resistance in ovarian adenocarcinoma using chemotherapy entrapped in hyaluronan-grafted nanoparticle clusters. ACS Nano. 2014;8:2183–2195. doi: 10.1021/nn500205b. [DOI] [PubMed] [Google Scholar]
- 31.Allen KE, Weiss GJ. Resistance may not be futile: microRNA biomarkers for chemoresistance and potential therapeutics. Mol Cancer Ther. 2010;9:3126–3136. doi: 10.1158/1535-7163.MCT-10-0397. [DOI] [PubMed] [Google Scholar]
- 32.Avci CB, Baran Y. Use of microRNAs in personalized medicine. Methods Mol Biol. 2014;1107:311–325. doi: 10.1007/978-1-62703-748-8_19. [DOI] [PubMed] [Google Scholar]
- 33.Di Leva G, Croce CM. The role of microRNAs in the tumorigenesis of ovarian cancer. Front Oncol. 2013;3:153. doi: 10.3389/fonc.2013.00153. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Echevarria-Vargas IM, Valiyeva F, Vivas-Mejia PE. Upregulation of miR-21 in cisplatin resistant ovarian cancer via JNK-1/c-Jun pathway. PLoS One. 2014;9:e97094. doi: 10.1371/journal.pone.0097094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao H, Liu S, Wang G, Wu X, Ding Y, Guo G, Jiang J, Cui S. Expression of miR-136 is associated with the primary cisplatin resistance of human epithelial ovarian cancer. Oncol Rep. 2015;33:591–598. doi: 10.3892/or.2014.3640. [DOI] [PubMed] [Google Scholar]
- 36.Zidar N, Bostjancic E, Gale N, Kojc N, Poljak M, Glavac D, Cardesa A. Down-regulation of microRNAs of the miR-200 family and miR-205, and an altered expression of classic and desmosomal cadherins in spindle cell carcinoma of the head and neck-hallmark of epithelial-mesenchymal transition. Hum Pathol. 2011;42:482–488. doi: 10.1016/j.humpath.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 37.Li J, Du L, Yang Y, Wang C, Liu H, Wang L, Zhang X, Li W, Zheng G, Dong Z. MiR-429 is an independent prognostic factor in colorectal cancer and exerts its anti-apoptotic function by targeting SOX2. Cancer Lett. 2013;329:84–90. doi: 10.1016/j.canlet.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 38.Leskela S, Leandro-Garcia LJ, Mendiola M, Barriuso J, Inglada-Perez L, Munoz I, Martinez-Delgado B, Redondo A, de Santiago J, Robledo M, Hardisson D, Rodriguez-Antona C. The miR-200 family controls beta-tubulin III expression and is associated with paclitaxel-based treatment response and progression-free survival in ovarian cancer patients. Endocr Relat Cancer. 2011;18:85–95. doi: 10.1677/ERC-10-0148. [DOI] [PubMed] [Google Scholar]
- 39.Sun Y, Shen S, Liu X, Tang H, Wang Z, Yu Z, Li X, Wu M. MiR-429 inhibits cells growth and invasion and regulates EMT-related marker genes by targeting Onecut2 in colorectal carcinoma. Mol Cell Biochem. 2014;390:19–30. doi: 10.1007/s11010-013-1950-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cochrane DR, Spoelstra NS, Howe EN, Nordeen SK, Richer JK. MicroRNA-200c mitigates invasiveness and restores sensitivity to microtubule-targeting chemotherapeutic agents. Mol Cancer Ther. 2009;8:1055–1066. doi: 10.1158/1535-7163.MCT-08-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang L, Mezencev R, Svajdler M, Benigno BB, McDonald JF. Ectopic over-expression of miR-429 induces mesenchymal-to-epithelial transition (MET) and increased drug sensitivity in metastasizing ovarian cancer cells. Gynecol Oncol. 2014;134:96–103. doi: 10.1016/j.ygyno.2014.04.055. [DOI] [PubMed] [Google Scholar]
- 42.Chan JK, Blansit K, Kiet T, Sherman A, Wong G, Earle C, Bourguignon LY. The inhibition of miR-21 promotes apoptosis and chemosensitivity in ovarian cancer. Gynecol Oncol. 2014;132:739–744. doi: 10.1016/j.ygyno.2014.01.034. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y, Cheng Y, Ren X, Zhang L, Yap KL, Wu H, Patel R, Liu D, Qin ZH, Shih IM, Yang JM. NAC1 modulates sensitivity of ovarian cancer cells to cisplatin by altering the HMGB1-mediated autophagic response. Oncogene. 2012;31:1055–1064. doi: 10.1038/onc.2011.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.He J, Yu JJ, Xu Q, Wang L, Zheng JZ, Liu LZ, Jiang BH. Downregulation of ATG14 by EGR1-MIR152 sensitizes ovarian cancer cells to cisplatin-induced apoptosis by inhibiting cyto-protective autophagy. Autophagy. 2015;11:373–384. doi: 10.1080/15548627.2015.1009781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu D, Xia P, Diao D, Cheng Y, Zhang H, Yuan D, Huang C, Dang C. MiRNA-429 suppresses the growth of gastric cancer cells in vitro. J Biomed Res. 2012;26:389–393. doi: 10.7555/JBR.26.20120029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burk U, Schubert J, Wellner U, Schmalhofer O, Vincan E, Spaderna S, Brabletz T. A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep. 2008;9:582–589. doi: 10.1038/embor.2008.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marchini S, Fruscio R, Clivio L, Beltrame L, Porcu L, Fuso Nerini I, Cavalieri D, Chiorino G, Cattoretti G, Mangioni C, Milani R, Torri V, Romualdi C, Zambelli A, Romano M, Signorelli M, di Giandomenico S, D’Incalci M. Resistance to platinum-based chemotherapy is associated with epithelial to mesenchymal transition in epithelial ovarian cancer. Eur J Cancer. 2013;49:520–530. doi: 10.1016/j.ejca.2012.06.026. [DOI] [PubMed] [Google Scholar]
- 48.Brabletz S, Bajdak K, Meidhof S, Burk U, Niedermann G, Firat E, Wellner U, Dimmler A, Faller G, Schubert J, Brabletz T. The ZEB1/miR-200 feedback loop controls Notch signalling in cancer cells. EMBO J. 2011;30:770–782. doi: 10.1038/emboj.2010.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wiklund ED, Bramsen JB, Hulf T, Dyrskjot L, Ramanathan R, Hansen TB, Villadsen SB, Gao S, Ostenfeld MS, Borre M, Peter ME, Orntoft TF, Kjems J, Clark SJ. Coordinated epigenetic repression of the miR-200 family and miR-205 in invasive bladder cancer. Int J Cancer. 2011;128:1327–1334. doi: 10.1002/ijc.25461. [DOI] [PubMed] [Google Scholar]


