Abstract
A number of patients with diabetes suffer from retinopathy; the pathogenesis is to be further investigated. Recent reports indicate that micro RNA (miR) plays critical roles in the development of immune inflammation. This study test a hypothesis that miR-17-92 cluster is associated with the pathogenesis of diabetes retinopathy (DR). In this study, peripheral blood samples were collected from DR patients and healthy subjects. B cells were isolated from the blood samples to be analyzed the expression of interleukin (IL)-10. The results showed that lower levels of IL-10 were detected in peripheral B cells of DR patients as compared with healthy subjects. miR-19a was increased in B cells of DR patients, which was negatively correlated with the IL-10 expression. Exposure of naive B cells to IL-17 increased the expression of miR-19a and suppression of IL-10 expression in the B cells, in which histone deacetylase 11 (HDAC 11) played a critical role. In conclusion, the IL-17 suppresses IL-10 expression in peripheral B cells via enhancing miR-19a expression and HDAC activity in DR patients. The miR-19a and HDAC 11 may be novel therapeutic targets in the treatment of DR.
Keywords: Autoimmunity, interleukin 10, interleukin 17, B lymphocyte, retinopathy
Introduction
Diabetic retinopathy (DR) is when damage occurs to the retina due to diabetes. It may lead to blindness eventually [1]. About 90% diabetes is type 2 diabetes, which is associated with impaired insulin excretion by the pancreas and insulin resistance of body tissues [2]. Persons with obesity are predisposed to suffer diabetes [3]. About 10% patients belong to type I diabetes, which results from the pancreas’s failure to produce enough insulin [4]. A common complication of diabetes is the vascular disorders [5]; one of which is the retinopathy occurring in about 1/3 diabetic patients [6].
The pathological changes of DR include the abnormal growth of new retinal blood vessels, and diabetic macular edema, in which there are exudation and edema in the central part of the retina because of blood vessel leaking [1]. The mechanisms of DR include genetic and epigenetic factors [7], increased production of free radicals [8], advanced glycosylation end products [9], inflammatory factors [10] and vascular endothelial growth factor [11]. It is reported that IL-10 gene polymorphism is associated with DR susceptibility [12]. The underlying mechanism remains to be further investigated.
Inflammatory factors play a critical role in the pathogenesis of diabetes [13]. Autoimmunity is proposed to underlie the mechanism of diabetes, in which body T cells recognize islet cells to differentiate into the islet-specific T cells. The islet-specific T cells may be eliminated by the thymic negative selection, or evade the thymic negative selection somehow leading islet cell attacks. On the other hand, the attacks may not occur; it may be suppressed by the regulatory T cells, or regulatory B cells by releasing inhibitory cytokines such as TGF-β or IL-10 [14]. IL-10 can suppress the antigen presenting function by inhibiting the expression of MHC-II molecules on the surface of antigen presenting cells [15]. Previous studies show that low levels of IL-10 were observed in patients with diabetes [16]. Low IL-10 levels may be caused by micro RNA (miR) such as miR-19a [17]. Immune deregulation is an important factor in the pathogenesis of DR [18].
Based on the information above, we hypothesize that miR-19a may interfere with the expression in peripheral B cells of patients with diabetic retinopathy. To test the hypothesis, we performed this study. We observed that the peripheral B cells of DR patients expressed low levels of IL-10 and high levels of miR-19a. Exposure to IL-17 increased the expression of miR-19a and suppressed the IL-10 expression in B cells, which could be inhibited by knocking down the gene of miR-19a.
Materials and methods
Study subjects
This study was carried out between January 2014 and January 2016 at the Department of Ophthalmology, Beijing Tongren Hospital. Patients with both retinopathy and diabetes were diagnosed by ophthalmologists and physicians in the Department of Internal Medicine. Healthy controls were recruited who did not have systemic diseases or ocular diseases (undergone routine ophthalmic examination). Patients with retinopathy were undergone full ophthalmologic examination. The study was carried out in accordance with the Declaration of Helsinki. An informed consent was obtained from each participant after approval from the Institutional Review Board. The study protocol was approved by the Human Ethics Committee of Capital Medical University. Exclusion criteria: Cardiovascular diseases, hypertension, severe anemia (hematocrit below 38%), post-stroke or chronic organ diseases, such as renal failure.
Collection of blood samples
Blood samples (20 ml per person) were collected from each subject by the ulnar vein puncture.
Isolation of B cells
Peripheral blood mononuclear cells (PBMC) were isolated from the blood samples by gradient density centrifugation. CD19+ B cells were isolated from the PBMCs by magnetic cell sorting using a B cell isolation kit (Miltenyi Biotech) following the manufacturer’s instructions. The purity of the isolated cells was 98% as checked by flow cytometry.
Cell culture
The isolated cells were cultured with RPMI1640 medium. The medium was supplemented with 10% fetal bovine serum, 100 U/ml penicillin, 0.1 mg/ml streptomycin and 2 mM L-glutamine. The cell viability was greater than 99% before using for further experiments as assessed by Trypan blue exclusion assay.
Assessment of miR-17-92 cluster and IL-10 mRNA by real time RT-PCR
Total RNAs were extracted from B cells using Trizol reagent (Invitrogen). The cDNA was synthesized using a reverse transcription Kit (Invitrogen). Real-time PCR was performed using SYBR Green qPCR Master Mix (Invitrogen) with miR-17-92 cluster primer set (Beijing Yijie Biotech; Beijing, China) according to the manufacturer’s instructions. All miRs were normalized to a small nucleolar RNA, RNU48, using the 2-∆∆Ct method, and presented as fold change. The sequence primers for PCR are, IL-10: gttctttggggagccaacag and gctccctggtttctcttcct; β-actin: cctctatgccaacacagtgc and cctgcttgctgatccacatc.
Detection of IL-10 protein by Western blotting
Total cellular proteins were extracted from isolated B cells. The proteins were separated on SDS-PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis) and transferred onto polyvinylidene fluoride membranes (Invitrogen). Immunoblotting was performed for IL-10 with antibodies (purchased from Santa Cruz Biotech). Immunoreactivity was revealed by incubation with peroxidase conjugated second antibodies (Santa Cruz Biotech) and followed by ECL (enhanced chemiluminescence) reaction. The results were photographed in an image station (UVI, Cambridge, UK).
Assessment of serum IL-17 by enzyme-linked immunosorbent assay (ELISA)
The sera were isolated from the blood samples by centrifugation. The levels of IL-17 in the sera were determined by ELISA with a commercial reagent kit (R&D Systems) following the manufacturer’s instructions.
Evaluation of the effects of IL-17 on miR-19a expression in B cells
CD19+ B cells were isolated from blood samples of healthy subjects. The B cells were cultured in the presence of recombinant IL-17 (rIL-17) at gradient concentrations for 48 h. The cells were collected and analyzed by RT-PCR for the miR-19a expression (Refer to the above procedures of RT-PCR).
Extracting cytosolic and nuclear proteins from B cells
B cells were treated with lysis buffer and centrifuged at 13000×g for 5 min at 4°C. The supernatant was collected as the cytosolic proteins. The nuclear extract buffer was added to the pellet and incubated for 10 min at 4°C, followed by centrifugation at 13,000×g for 5 min at 4°C. The supernatant was collected as the nuclear proteins. The protein concentrations were determined by the BCA method.
Chromatin immunoprecipitation assay (ChIP)
A commercial reagent kit (Sigma Aldrich) was used for ChIP following the manufacturer’s instructions. B cells were fixed with 1% formalin for 15 min, lysed with lysis buffer and sonicated to shear the DNA to 100-500 bp. The lysates were precleared by protein G-agarose beads for 2 h at 4°C. The supernatant was isolated by centrifugation and incubated with 2 μg of antibodies of interest or isotype IgG (Santa Cruz Biotech) overnight at 4°C. The antibody-chromatin complex was precipitated by protein G-agarose beads for 2 h at 4°C, and then washed and eluted in elution buffer. DNA was recovered from the precipitated samples by reverse crosslinking procedures at 65°C for 2 h and digested by proteinase K for 1 h at 45°C to remove proteins. The DNA was recovered by the phenol/chloroform extraction and ethanol precipitation. The DNA or input was analyzed by PCR. The primers of miR-19a promoter used in the present study are gcttacagtgcaggtagtga and ggagcacttagggcagtaga. The results were normalized to folds of input.
RNA interference (RNAi) of miR-19a
B cells were treated with a shRNA miR-19a reagent kit (GeneChem; Shanghai, China) following the manufacturer’s instructions. The sequence of miR-19a shRNA was AGTTGCACTACAAGAAGAATG. The RNAi effect on B cells was assessed by RT-PCR.
Statistical analysis
Data were presented as mean ± SD. Differences between two groups were evaluated with the Student t test or using one-way ANOVA in more than two groups. Bonferroni test was used as a post hoc test after one-way ANOVA. The criterion of significance was set at P<0.05.
Results
Low IL-10 levels are detected in peripheral B cells of DR patients
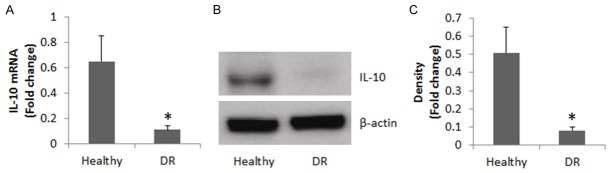
We collected peripheral blood samples from 20 DR patients (10 male and 10 female; age: 36.44 ± 6.73 years old) and 20 healthy subjects (10 male and 10 female; age: 35.84 ± 6.14 years old). B cells were isolated from the blood samples and analyzed. The results showed the IL-10 levels were significantly lower in the DR group than in the healthy group at both mRNA levels and protein levels (Figure 1).
Figure 1.
IL-10 levels in peripheral B cells. A. The bars indicate the IL-10 mRNA levels in peripheral B cells. B. The immune blots indicate the IL-10 protein levels in peripheral B cells. C. The bars indicate the image density of the immune blots of B. Healthy group = 20 subjects. DR group = 20 patients. Samples from individual subjects were analyzed separately. The data represent 20 independent experiments. Data of bars are presented as mean ± SD. *, P<0.01, compared with the healthy group.
miR-19a levels are negatively correlated with IL-10 levels in peripheral B cells of DR patients
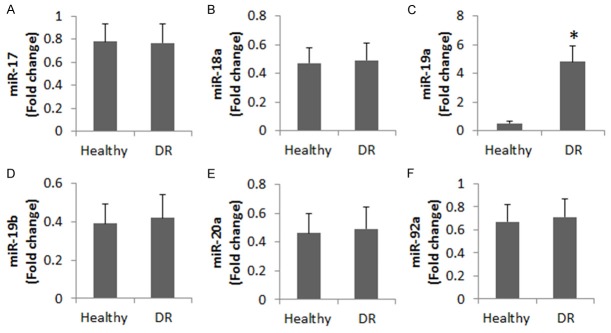
It is reported that miR-17-92 cluster is associated with the pathogenesis of retinopathy [19]. We wondered if miR-17-92 was involved in the IL-10 expression in peripheral B cells of DR patients. To test this, we analyzed IL-10 expression in the B cells collected from DR patients and healthy subjects. The results showed that in the 6 subtypes of miR-17-92 cluster, the miR-19a levels were significantly higher in the DR group than that in healthy group (Figure 2). A correlation assay was performed with the data of miR-19a and IL-10 mRNA in the B cells. A negative correlation was identified between miR-19a and IL-10 (r = -0.7739, P<0.01).
Figure 2.
Levels of miR-17-92 cluster in peripheral B cells of DR patients. The bars indicate the 6 members of miR-17-92 cluster family in peripheral B cells of 20 DR patients and 20 healthy subjects. Samples from individual subjects were analyzed separately. The data represent 20 independent experiments. Data of bars are presented as mean ± SD. *, P<0.01, compared with the healthy group.
miR-19a mediates the effect of IL-17 on suppression of IL-10 in B cells
Published data indicate that IL-17 is involved in the pathogenesis of diabetes [20]. We wondered if there was a link between serum IL-17, miR-19a and IL-10 in peripheral B cells in DR patients. Thus, we measured the serum levels of IL-17 in DR patients. The results showed that significantly higher IL-17 levels in the sera of DR patients than that in healthy subjects. We then performed a correlation assay with the data of serum IL-17 and miR-19a in peripheral B cells (Figure 3A). A positive correlation was identified between serum IL-17 and miR-19a in peripheral B cells (r = 0.8332, P<0.01). The results implicate that IL-17 may be able to up regulate the expression of miR-19a in B cells. To test this, we stimulated B cells (from healthy subjects) with IL-17 in the culture for 48 h. Indeed, the exposure to IL-17 increased the expression of miR-19a in B cells in an IL-17 dose-dependent manner (Figure 3B). The results implicate that IL-17 may inhibit IL-10 expression in B cells via up regulating expression of miR-19a. To test this, we stimulated B cells with LPS, which markedly increased the expression of miR-19a in B cells. The LPS-induced IL-10 expression was abolished by the presence of IL-17 in the culture, which was inhibited by the knockdown of miR-19a in B cells.
Figure 3.
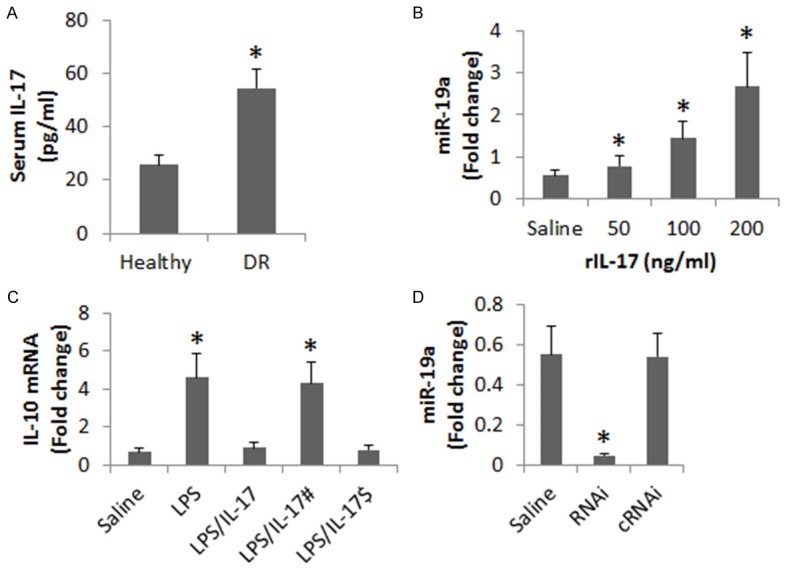
IL-17 up regulates miR-19a to suppress IL-10 expression in B cells. A. The bars indicate serum IL-17 levels of DR patients (n = 20) and healthy subjects (n = 20). B. The bars indicate the miR-19a levels in B cells after exposure to rIL-17 in the culture for 24 h (the concentrations of rIL-17 are denoted on the X axis). C. The bars indicate the IL-10 mRNA levels in the B cells after treatment with the conditions on the X axis in the culture for 24 h. LPS = 1 µg/ml. IL-17 = 200 ng/ml. #, miR-19a-deficient B cells. $, B cells were treated with controls shRNA. D. The bars indicate the miR-19a levels in B cells after treating with the conditions denoted on the X axis. Saline: B cells were treated with saline. RNAi: B cells were treated with miR-19a shRNA. cRNAi: B cells were treated with control shRNA. Data in B and C were summarized from 3 independent experiments.
miR-19a enhances HDAC11 to repress IL-10 gene transcription
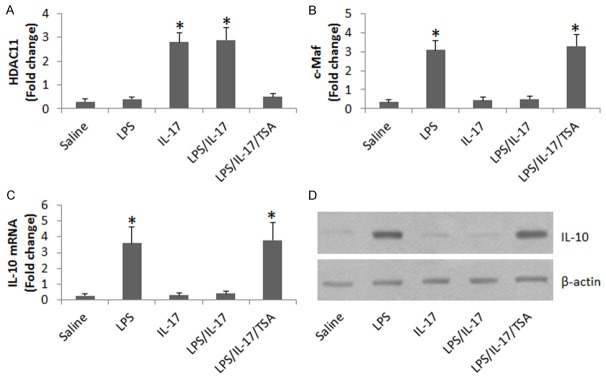
Since HDAC11 suppress IL-10 expression [17], we wondered if HDAC11 was involved in the IL-17-suppressed IL-10 expression in B cells. To test this, we stimulated LPS-primed B cells with both LPS and IL-17 for 48 h. The B cells were analyzed by ChIP. The results showed that the levels of HDAC11 were markedly increased (Figure 4A), and the levels of the IL-10 transcription factor, c-Maf, were markedly decreased (Figure 4B) at the IL-10 promoter locus. The results implicate that HDAC11 may play an important role in the IL-17-suppressed IL-10 expression in B cells. To test this, we treated B cells with LPS to increase the expression of IL-10; the expression of IL-10 could be suppressed by the presence of IL-17, which was abolished by adding Trichostatin A, a HDAC11 inhibitor, to the culture (Figure 4C, 4D).
Figure 4.
Evaluation of the effect of HDAC11 on IL-17-suppressed IL-10 expression in B cells. A, B. The bars indicate the levels of HDAC11 and c-Maf at the IL-10 promoter locus. C. The bars indicate the IL-10 mRNA levels in the B cells. D. The immune blots indicate the IL-10 protein levels in the B cells. The treatments of B cells in the culture are denoted on the X axis. LPS: 1 µg/ml. IL-17: 200 ng/ml. TSA: Trichostatin A, 1 μM. Data of bars are presented as mean ± SD. *, P<0.01, compared with the saline group. The data are representatives of 3 independent experiments.
Discussion
DR is one of the important causative factors in vision loss. Current therapeutic remedies for the DR-related vision loss are not satisfactory [21]. Thus, to identify the pathogenesis of DR is of significance. The present study reveals a previously unknown phenomenon that the peripheral B cells of DR patients express low levels of IL-10 and high levels of miR-19a; the latter mediates the effects of IL-17 on suppression of IL-10 in B cells, which can be blocked by knocking down the expression of miR-19. HDAC11 is involved in mediating the repression of IL-10 in B cells. The data contribute to the further understanding of the pathogenesis of diabetic retinopathy.
IL-10-producing B cells are one of the immune regulatory cells. The major function of immune regulatory cells is to suppress the abnormal immune response by other immune cells. Inflammation is one of the pathological changes in the DR. Deregulation of immune regulation is recognized in DR [22]. The present data add new information to this pathogenic spot by showing that the peripheral B cells of DR patients have low IL-10 expression. Although this datum is new to the DR study, it has been observed in other diseases; such as low frequency of IL-10-producing regulatory B cell was found in patients with food allergy [23]. Animal studies of intestinal allergy also found that low frequency of IL-10-producing B cells in the intestinal mucosa of food allergy mice [24]. Our data are in line with these reports.
The present data expand the previous finding by showing that peripheral B cells have low levels of IL-10 in DR patients, implicating that these B cells are incompetent to produce IL-10. A possible explanation of this phenomenon may be because the miR-19a levels also increase in the B cells. A negative correlation was identified between the expression of IL-10 and miR-19a in the B cells, which is supported by previous studies; Simpson et al also found that miR-19a inhibited the expression of IL-10 in asthma patients [17]. This phenomenon also exists in other environment; Akhtar et al indicate that endothelial HIF-1α promoted atherosclerosis by triggering miR-19a-mediated CXCL1 expression and monocyte adhesion. The investigators propose that inhibition of the endothelial HIF-1α/miR-19a pathway may be a therapeutic option against atherosclerosis [25].
HDAC11 is involved in the IL-10 expression in B cells [26]. The present study also found that HDAC11 was increased at the IL-10 promoter locus, together with low levels of c-Maf, the gene transcription factor of IL-10. The data suggest that HDAC11 mediates the effects of IL-17 on repressing IL-10 gene transcription. The inference is supported by subsequent data that showed that inhibiting HDAC11 by an inhibitor abolished the IL-17-suppressed IL-10 expression in B cells.
In summary, the present data show that peripheral B cells from DR patients show low levels of IL-10 and high levels of miR-19a. miR-19a and HDAC11 mediate the IL-17-suppressed IL-10 expression in B cells. Inhibition of miR-19a and HDAC11 may be a new target in the treatment of DR.
Disclosure of conflict of interest
None.
Authors’ contribution
CW, QY, XC and HG performed experiments, analyzed data and reviewed the manuscript. XP designed the project, supervised experiments and wrote the manuscript.
References
- 1.Jotheeswaran AT, Lovakanth N, Nadiga S, Anchala R, Murthy GV, Gilbert CE. Estimating the proportion of persons with diabetes developing diabetic retinopathy in India: A systematic review and meta-analysis. Indian J Endocrinol Metab. 2016;20:S51–58. doi: 10.4103/2230-8210.179774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nigro E, Scudiero O, Monaco ML, Palmieri A, Mazzarella G, Costagliola C, Bianco A, Daniele A. New insight into adiponectin role in obesity and obesity-related diseases. Biomed Res Int. 2014;2014:658913. doi: 10.1155/2014/658913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ostenson CG. The pathophysiology of type 2 diabetes mellitus: an overview. Acta Physiol Scand. 2001;171:241–247. doi: 10.1046/j.1365-201x.2001.00826.x. [DOI] [PubMed] [Google Scholar]
- 4.Knip M, Siljander H. The role of the intestinal microbiota in type 1 diabetes mellitus. Nat Rev Endocrinol. 2016;12:154–167. doi: 10.1038/nrendo.2015.218. [DOI] [PubMed] [Google Scholar]
- 5.Harcourt BE, Penfold SA, Forbes JM. Coming full circle in diabetes mellitus: from complications to initiation. Nat Rev Endocrinol. 2013;9:113–123. doi: 10.1038/nrendo.2012.236. [DOI] [PubMed] [Google Scholar]
- 6.Wong TY, Cheung CM, Larsen M, Sharma S, Simo R. Diabetic retinopathy. Nat Rev Dis Primers. 2016;2:16012. doi: 10.1038/nrdp.2016.12. [DOI] [PubMed] [Google Scholar]
- 7.Kowluru RA, Mishra M. Contribution of epigenetics in diabetic retinopathy. Sci China Life Sci. 2015;58:556–563. doi: 10.1007/s11427-015-4853-0. [DOI] [PubMed] [Google Scholar]
- 8.Kowluru RA, Kowluru A, Mishra M, Kumar B. Oxidative stress and epigenetic modifications in the pathogenesis of diabetic retinopathy. Prog Retin Eye Res. 2015;48:40–61. doi: 10.1016/j.preteyeres.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eshaq RS, Wright WS, Harris NR. Oxygen delivery, consumption, and conversion to reactive oxygen species in experimental models of diabetic retinopathy. Redox Biol. 2014;2:661–666. doi: 10.1016/j.redox.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu Y, Chen H, Su SB. Neuroinflammatory responses in diabetic retinopathy. J Neuroinflammation. 2015;12:141. doi: 10.1186/s12974-015-0368-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ajlan RS, Silva PS, Sun JK. Vascular Endothelial Growth Factor and Diabetic Retinal Disease. Semin Ophthalmol. 2016;31:40–48. doi: 10.3109/08820538.2015.1114833. [DOI] [PubMed] [Google Scholar]
- 12.Dong H, Li Q, Wang M, Wan G. Association Between IL-10 Gene Polymorphism and Diabetic Retinopathy. Med Sci Monit. 2015;21:3203–3208. doi: 10.12659/MSM.894371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Velloso LA, Eizirik DL, Cnop M. Type 2 diabetes mellitus--an autoimmune disease? Nat Rev Endocrinol. 2013;9:750–755. doi: 10.1038/nrendo.2013.131. [DOI] [PubMed] [Google Scholar]
- 14.Serr I, Fürst RW, Achenbach P, Scherm MG, Gökmen F, Haupt F, Sedlmeier EM, Knopff A, Shultz L, Willis RA, Ziegler AG, Daniel C. Type 1 diabetes vaccine candidates promote human Foxp3(+)Treg induction in humanized mice. Nat Commun. 2016;7:10991. doi: 10.1038/ncomms10991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Waal Malefyt R, Haanen J, Spits H, Roncarolo MG, te Velde A, Figdor C, Johnson K, Kastelein R, Yssel H, de Vries JE. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991;174:915–924. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaas A, Pfleger C, Kharagjitsingh AV, Schloot NC, Hansen L, Buschard K, Koeleman BP, Roep BO, Mortensen HB, Alizadeh BZ. Association between age, IL-10, IFNgamma, stimulated C-peptide and disease progression in children with newly diagnosed Type 1 diabetes. Diabet Med. 2012;29:734–741. doi: 10.1111/j.1464-5491.2011.03544.x. [DOI] [PubMed] [Google Scholar]
- 17.Simpson LJ, Patel S, Bhakta NR, Choy DF, Brightbill HD, Ren X, Wang Y, Pua HH, Baumjohann D. A microRNA upregulated in asthma airway T cells promotes TH2 cytokine production. Nat Immunol. 2014;15:1162–1170. doi: 10.1038/ni.3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takeuchi M, Sato T, Tanaka A, Muraoka T, Taguchi M, Sakurai Y, Karasawa Y, Ito M. Elevated Levels of Cytokines Associated with Th2 and Th17 Cells in Vitreous Fluid of Proliferative Diabetic Retinopathy Patients. PLoS One. 2015;10:e0137358. doi: 10.1371/journal.pone.0137358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nittner D, Lambertz I, Clermont F, Mestdagh P, Kohler C, Nielsen SJ, Jochemsen A, Speleman F, Vandesompele J, Dyer MA, Schramm A, Schulte JH, Marine JC. Synthetic lethality between Rb, p53 and Dicer or miR-17-92 in retinal progenitors suppresses retinoblastoma formation. Nat Cell Biol. 2012;14:958–965. doi: 10.1038/ncb2556. [DOI] [PubMed] [Google Scholar]
- 20.Kumar P, Subramaniyam G. Molecular underpinnings of Th17 immune-regulation and their implications in autoimmune diabetes. Cytokine. 2015;71:366–376. doi: 10.1016/j.cyto.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 21.Hernandez C, Dal Monte M. Neuroprotection as a Therapeutic Target for Diabetic Retinopathy. J Diabetes Res. 2016;2016:9508541. doi: 10.1155/2016/9508541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kastelan S, Zjacic-Rotkvic V, Kastelan Z. Could diabetic retinopathy be an autoimmune disease? Med Hypotheses. 2007;68:1016–1018. doi: 10.1016/j.mehy.2006.05.073. [DOI] [PubMed] [Google Scholar]
- 23.Noh J, Lee JH, Noh G, Bang SY, Kim HS, Choi WS, Cho S, Lee SS. Characterisation of allergen-specific responses of IL-10-producing regulatory B cells (Br1) in Cow Milk Allergy. Cell Immunol. 2010;264:143–149. doi: 10.1016/j.cellimm.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 24.Shi Y, Xu LZ, Peng K, Wu W, Wu R, Liu ZQ, Yang G, Geng XR, Liu J, Liu ZG, Liu Z, Yang PC. Specific immunotherapy in combination with Clostridium butyricum inhibits allergic inflammation in the mouse intestine. Sci Rep. 2015;5:17651. doi: 10.1038/srep17651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akhtar S, Hartmann P, Karshovska E, Rinderknecht FA, Subramanian P, Gremse F, Grommes J, Jacobs M, Kiessling F, Weber C, Steffens S, Schober A. Endothelial Hypoxia-Inducible Factor-1alpha Promotes Atherosclerosis and Monocyte Recruitment by Upregulating MicroRNA-19a. Hypertension. 2015;66:1220–1226. doi: 10.1161/HYPERTENSIONAHA.115.05886. [DOI] [PubMed] [Google Scholar]
- 26.Villagra A, Cheng F, Wang HW, Suarez I, Glozak M, Maurin M, Nguyen D, Wright KL, Atadja PW, Bhalla K, Pinilla-Ibarz J, Seto E, Sotomayor EM. The histone deacetylase HDAC11 regulates the expression of interleukin 10 and immune tolerance. Nat Immunol. 2009;10:92–100. doi: 10.1038/ni.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]