Abstract
It is assumed that the spread of breast cancer cells via the lymphatic system might be influenced by inflammatory reactions and/or the application of chemotherapy or molecularly targeted therapy. Therefore, we analysed survival according to lymphatic vessel density (LVD), lymphovascular invasion (LVI) (both assessed using podoplanin as immunohistochemical marker of lymphatic endothelium) and well-established clinico-pathological features in a group of 358 patients with invasive ductal breast cancer: 139 chemotherapy-naïve (pT1-2/pN0/M0) and 219 treated with chemotherapy (pT1-4/pN1-3/M0). Univariate analysis revealed that high LVD was related to unfavourable disease-free survival (DFS) in pN0/chemotherapy/trastuzumab-naïve patients (P = 0.028). Conversely, in pN+/chemotherapy-treated individuals high LVD was related to favourable DFS (P = 0.019). LVI was a significant indicator of survival (P = 0.005) only in pN0/chemotherapy/trastuzumab-naïve patients. The following parameters were significant independent adverse prognostic factors for DFS: (i) in pN0/chemotherapy/trastuzumab-naïve patients: high LVD (LVD > 7 vessels/mm2; RR = 2.7, P = 0.039), LVI (RR = 3.3, P = 0.046) and high tumor grade (G3 vs. G1 + G2; RR = 2.6, P = 0.030); (ii) in pN+/chemotherapy/trastuzumab-treated patients: low LVD (RR = 1.8, P = 0.042), the number of involved lymph nodes (pN3 vs. pN1-2; RR = 2.3, P = 0.012) and the breast cancer subtype (expression of steroid receptors together with HER2 immunonegativity and high proliferation index vs. other breast cancer immunophenotypes; RR = 3.0, P < 0.001). High LVD may identify high progression risk in pN0/chemotherapy/trastuzumab-naïve patients, and low progression risk in pN+/chemotherapy-treated patients. This phenomenon might be explained by potential involvement of lymphangiogenesis in two processes related to cancer eradication: a chemotherapy-stimulated activity of the immune system against cancer cells, or increased tumour drainage influencing the efficacy of cytotoxic drugs.
Keywords: Breast cancer, podoplanin, lymphatic vessels, prognostic significance
Introduction
Breast cancer is a malignancy which is assumed to spread mainly via the lymphatic system, therefore, the number of tumour lymphatic vessels might correlate with the probability of patient survival [1]. The prognostic significance of lymphangiogenesis was found in breast [2-9], colorectal [10], head and neck cancer patients [11], as well as in individuals suffering from melanoma [12]. However, some researchers failed to find the relationship between the aforementioned parameters and survival [13-15]. Moreover, there is an urgent need for standardization and simplification of methods applied for the assessment of lymphatic vascularization and for future confirmatory studies on its prognostic/predictive role [2-9,13-16].
Hypothetically, the number of blood and lymphatic vessels in the tumour tissue is responsible for: (1) the spread of cancer cells leading to metastases, (2) the delivery of nutrients and oxygen to cancer cells, (3) the delivery of anti-cancer drugs, and (4) activation/delivery of immune cells, which are able to eradicate cancer cells. The first two processes may be pro-cancerous, while the other two may be related to better cancer curability. Theoretically, the probability of intravasation (the passage of cancer cells into the blood or lymphatic vessel) is particularly high in “hot spots” (microscopic fields with the highest number of lymphatics) [7,13,14]. There are two possible explanations of this assumption. The first is that “mechanisms responsible for direct and indirect relationships between the tumour and the endothelial cell populations are particularly active in these highly vascular regions” [17]. They may activate the motility of cancer cells and increase the probability of their intravasation. The second is that the probability of intravasation is increased in “hot spots” because the number of vessels is high [17]. Therefore, the “hot spot” method is recommended for the assessment of vascular density [7,13,14]. On the other hand, we may speculate that in carcinomas with a high number of vessels two processes associated with cancer cell killing might be particularly active: (1) activation of the immune response through lymphatic vessels-governed transport of cancer antigen-bearing macrophages and dendritic cells to regional lymph nodes, (2) better tumour drainage, which causes more effective circulation of cytotoxic drugs. Therefore, it might be expected that in chemotherapy-treated breast cancer patients (we assume the same or similar potential for lymphangiogenesis in primary tumour and metastasis) a high number of lymphatics might indicate favourable survival.
The above-mentioned reasons prompted us to study disease-free survival (DFS) and metastasis-free survival (MSF) in chemotherapy/trastuzumab-naïve and chemotherapy-treated patients with invasive ductal breast cancer, according to well-established clinicopathological factors, lymphatic vessel density assessed in one “hot-spot” (LVD), and lymphovascular invasion (LVI).
Material and methods
Patients
The retrospective analysis was performed in a group of 358 patients (353 females and 5 males) with locoregionally advanced invasive ductal breast cancer, for whom we obtained immunohistochemistry results (material analyzed in grants: N401 173 31/3808, NN 401 096 137, NN401 2344 33 financed by the Polish Ministry of Science and Higher Education, and DEC-2013/09/B/NZ5/00764 financed by the National Science Centre), and who underwent standard therapeutic procedures (surgery, chemotherapy, radiotherapy and targeted therapy) in clinical departments of Maria Sklodowska-Curie Memorial Cancer Centre and Institute of Oncology, Krakow Branch, Poland. Survival analysis was performed in the two subgroups, homogenous in terms of tumor stage and treatment schedules: (1) 139 chemotherapy-naïve patients (pT1-2, pN0, M0; 134 females and 5 males)-treated surgically between 1992 and 2006 according to previous treatment standards (in this archival group we were able to study patients’ natural history) (Table 1); (2) 219 women (pT1-4, pN1-3, M0) treated surgically between 2001 and 2013, who received chemotherapy or chemotherapy/trastuzumab in adjuvant setting (Table 1). We excluded all pN+ patients who did not receive adjuvant chemotherapy and all pN+/HER2-overexpressing/trastuzumab-naïve individuals.
Table 1.
Treatment schedules for 358 invasive ductal breast cancer patients
| Parameter | Category | Total (N) | 139 Cht/Tr-naïve patients (N) | 219 ChT/Tr-treated patients (N) |
|---|---|---|---|---|
| Local therapy | Breast conserving surgery | 24 | 12 | 12 |
| Patey/Madden mastectomy | 323 | 118 | 205 | |
| Halsted mastectomy | 11 | 9 | 2 | |
| ChT | Not administered | 139 | 139 | 0 |
| TAC | 17 | 0 | 17 | |
| AC-T | 67 | 0 | 67 | |
| AC | 93 | 0 | 93 | |
| FAC | 35 | 0 | 35 | |
| CMF | 4 | 0 | 4 | |
| Taxanes | 3 | 0 | 3 | |
| RT | Not administered | 168 | 122 | 46 |
| Administered | 186 | 17 | 169 | |
| HT | Not administered | 102 | 29 | 73 |
| Tamoxifen | 193 | 103 | 90 | |
| GnRH analogue | 27 | 4 | 23 | |
| Tamoxifen + GnRH analogue | 14 | 2 | 12 | |
| Aromatase inhibitor | 4 | 1 | 3 | |
| Tamoxifen + Aromatase inhibitor | 17 | 0 | 17 | |
| Tamoxifen + Aromatase inhibitor + GnRH analogue | 1 | 0 | 1 | |
| Anti-HER2 therapy* | Not administered | 287 | 139 | 148 |
| Administered | 71 | 0 | 71 |
In Poland, trastuzumab in the adjuvant setting was included for use in standard treatment in 2008.
ChT: chemotherapy; HT: hormonal therapy; RT: radiotherapy; Tr: trastuzumab; AC: doxorubicin and cyclophosphamide; FAC: 5-fluorouracil, doxorubicin, and cyclophosphamide; TAC: docetaxel, doxorubicin, and cyclophosphamide; AC-T: doxorubicin, cyclophosphamide and paclitaxel; CMF: cyclophosphamide, methotrexate and 5-fluorouracil.
Radiotherapy (RT) was performed in 186 patients (52% of all the analyzed), in all 24 patients after breast conserving surgery, and in 162 patients (with more than 3 lymph nodes involved) after mastectomy, who constituted 48.5%. RT was applied after chemotherapy and before trastuzumab treatment. Trastuzumab in the adjuvant setting was given to all patients with tumors presenting HER2 overexpression. It is worth mentioning that in Poland, trastuzumab in the adjuvant setting was included for use as standard treatment in 2008.
The study was approved by Ethics Committee at the Regional Medical Chamber in Krakow although no specific consent was needed; this was a retrospective study performed on archived tissues, with no direct patient contact, no modification of diagnostic or treatment procedures and no personal patients’ data was revealed (No. 11KBL/OIL/2009 and 12KBL/OIL/2009, in the case of DEC-2013/09/B/NZ5/00764, decision of 4 December 2013).
Material
All archival material from tumour specimens was re-examined independently by two pathologists (J.R., A.A.) in order to confirm the histological subtype (invasive breast carcinoma of no special type, according to the current WHO classification) [18] and tumour grade (Bloom-Richardson scale in Elston-Ellis modification) [19].
Immunohistochemistry (IHC)
Immunohistochemistry was performed on formalin-fixed, paraffin-embedded sections, cut at 4 µm, mounted on SuperFrostPlus (Menzel-Gläser, Braunschweig, Germany) slides, and then deparaffinized in xylene and rehydrated through a series of alcohols.
After antigen retrieval (Target Retrieval Solution-TRS, pH = 6.0, Agilent Technologies Dako Denmark A/S, Glostrup, Denmark: 50 min. water bath at temperature 96°C), blocking of non-specific binding of antibodies (UltraVision Protein Block, Thermo Fisher Scientific, Waltham, MA USA) and quenching the activity of endogenous peroxidases (0.3% H2O2 in 100% methanol for 30 min), slides were incubated with primary antibodies (overnight at 4°C). We applied the following antibodies: (i) podoplanin, clone: D2-40, dilution: 1:100 (Cell Marque, Rocklin, California, USA); (ii) CD34, clone: QBEnd 10, dilution: 1:50, (iii) HER2, polyclonal, dilution: 1:250; (iv) Ki-67, clone: MIB-1, dilution: 1:75 (Agilent Technologies Dako Denmark A/S, Glostrup, Denmark); (v) ERα, clone: 6F11, dilution: 1:100; (vi) PR, clone: PGR/2, dilution: 1:200 (Leica Biosystems Newcastle Ltd, Newcastle, UK). Finally, the slides were incubated with BrightVision (ImmunoLogic, Duiven)-30 min. at room temperature and 3,3’-diaminobenzidine (DAB, Vector Laboratories, Burlingame, USA)-5 min. room temperature. In the case of 136 (T1-T2, N0, M0) carcinomas treated between 1992 and 2000, for visualization of ER, PR, HER2 and MIB-1, DAKO EnVision system (Agilent Technologies Dako Denmark A/S, Glostrup) was applied. For patients treated after 2007, the data on ER, PR and HER2 status were retrieved from the patients’ files.
Visualization of podoplanin and CD34 was performed on the basis of double-staining procedure with the application of VIP (violet colour) and DAB (brown colour) as peroxidase substrates (Vector Laboratories, Burlingame, USA) (Figure 1A-I). Finally, the slides were counterstained with Mayer’s hematoxylin.
Figure 1.
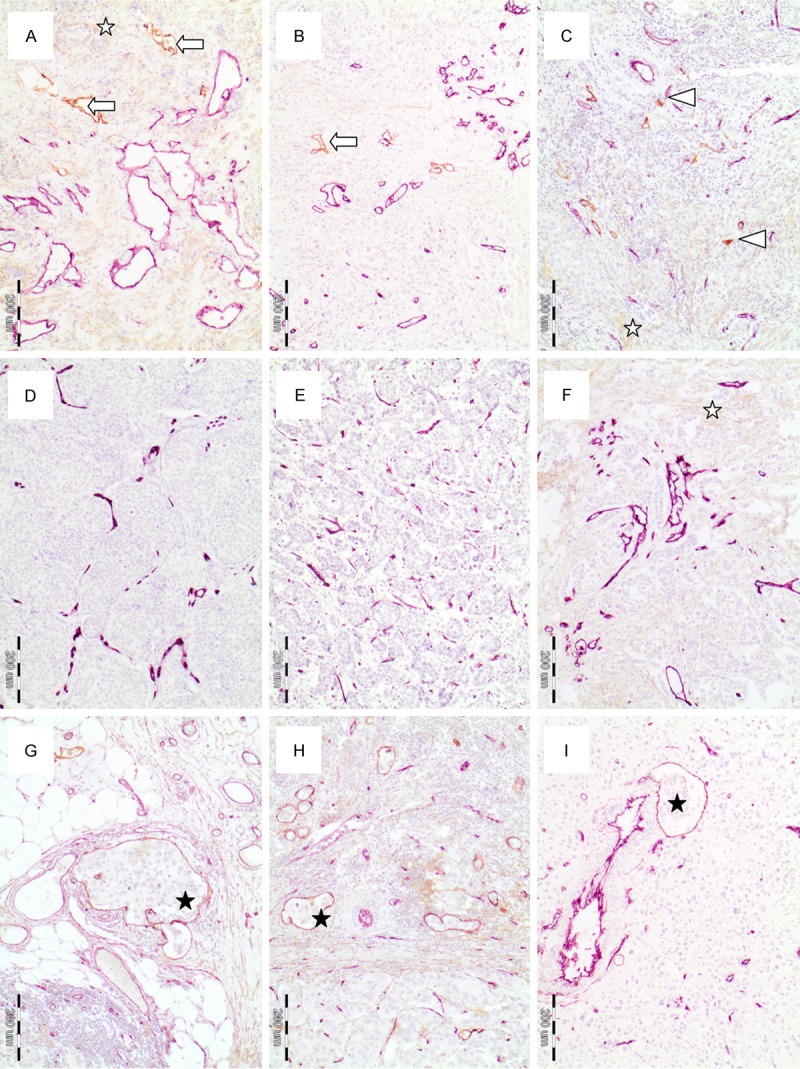
Tumour slides stained immunohistochemically against endothelial cells of blood vessels (anti-CD34 antibody; violet colour) and podoplanin as a marker of lymphatic vessels (brown colour): (A-C) The tumor sections with podoplanin-stained lymphatic vessels (brown colour) and CD34-stained blood vessels (violet colour): (A, B) Podoplanin-stained lymphatic vessels with lumen (white arrows), (C) podoplanin-stained lymphatic vessels without lumen (white arrowhead); (D-F) The tumor sections with CD34-stained blood vessels (violet colour) and without podoplanin-stained lymphatic vessels; (G-I) The tumor sections with lymphatic vessel invasion (black asterisks); (A, F) White asterisks indicate cancer associated fibroblasts stained for podoplanin, Microphotographs were taken at 10 × (objective) magnification.
IHC evaluation
All evaluations were performed blinded to the study endpoint. Podoplanin-stained vessels with visible lumen (Figure 1A, 1B arrow) or without lumen (Figure 1C, arrowhead) with lymphatic vessel characteristics, clearly distinguishable from podoplanin-positive cancer associated fibroblasts (Figure 1A, 1C, 1F, white asterisk), and other tissue structures and cells were included into lymphatic vessel density assessment. Lymphatics from intratumoral, peripheral and peritumoral areas of the tumour were included in LVD assessment. In the peritumoral area, only vessels located at a distance of about 900 μm from the tumour tissue were included in the vessel count. Podoplanin immunopositivity of myoepithelial cells was an internal positive control.
LVD was assessed with digital image analysis using BX 41 microscope, DP71 camera and Cell D software (Olympus Europa GmbH, Hamburg, Germany). The whole tumour section was scanned (4 × objective), and about 20 fields with a high number of lymphatics were selected (10 × objective = area 1.46 mm2 of the microscopic field) for counting. In each of the 20 selected fields podoplanin-positive vessels were marked manually and then counted automatically. Out of these 20 fields, the one with the highest number of lymphatics was selected. The number of vessels in the aforementioned high-power field (“hot spot”) was recognized as LVD and expressed as the number of vessels per 1 mm2 [17].
Lymphatic vascular invasion (LVI) was considered evident if at least one tumour cell cluster was clearly visible inside the podoplanin-stained vascular space. This parameter was assessed in intratumoral, peripheral and peritumoral areas (Figure 1G-I, black asterisk).
Nuclear expression of estrogen receptor α (ER) and progesterone receptor (PR) were considered positive if > 1% of tumour cells showed immunopositivity [20]. HER2 overexpression was defined as IHC score 3+ or IHC score 2+ confirmed by FISH [21].
MIB-1 labelling index (MIB-1LI) was calculated as a percentage of cancer cells with nuclear Ki-67 expression. For each slide, between 500 and 1000 cells (at 400 × magnification) were counted in 5-10 high-power fields. MIB-1LI was assessed twice by two observers and the mean value of the two measurements was calculated.
On the basis of ER/PR/HER2 expression and MIB-1LI, four breast cancer immunophenotypes were distinguished: (1) luminal A (LA): ER+ and/or PR+, HER2- and MIB-1LI ≤ 28%, (2) luminal B: (i) ER+ and/or PR+ and HER2+ (LBHER2) or (ii) ER+ and/or PR+, HER2- and MIB-1LI > 28%, (LBhighMIB) (3) HER2-overexpressing (HER2): ER- and PR- and HER2+, and (4) triple-negative phenotype (TNP): ER- and PR- and HER2-. We decided to choose 28% as a cut-off value for MIB-1LI, which is a little higher than 20% recommended by St. Gallen experts [22] because it was the most significant cut-off point in survival analysis in all the subgroups we studied.
Statistical analysis
The STATISTICA v.12 software (StatSoft, Inc. Tulsa, OK, USA) was used for all calculations. P value < 0.05 was considered significant. The relationship between LVD and pN or tumour grade, or breast cancer subtypes was studied using one-way ANOVA. For the assessment of independence between two categorical variables like LVI and pN or tumour grade, or breast cancer subtypes Pearson χ2 test was used (more than two columns).
Disease-free survival (DFS) was defined as the time (number of months) from surgery to the occurrence of distant metastasis or local recurrence, while metastasis-free survival (MFS) as the time from surgery to the occurrence of distant metastasis. Cumulative survival probabilities were calculated using the Kaplan-Meier method. The minimum P-value method of the log-rank test was applied for the selection of cut-off points for LVD. The differences between survival rates according to LVD, LVI, breast cancer subtype, tumor grade and nodal involvement (all categorized into two subgroups) were evaluated with the log-rank test.
All variables significant in the univariate analysis were entered into Cox multivariate analysis. The joint effect of the remaining covariates was analysed using Cox proportional hazard model with the stepwise regression procedure.
Results
The relationship between clinico-pathological parameters and LVD
Podoplanin-stained lymphatic vessels were found in 311/358 (86.9%) breast carcinomas, while in 47/358 (13.1%) such vessels were not visible in the tumour section (Figure 1D-F). The mean value of LVD was 9.0 ± 0.6 (SE) vessels/mm2 (median value 5.9, range 0-160.2).
LVD was significantly lower in patients with: (i) pN0 tumour stage, (ii) luminal A breast cancer subtype and (iii) carcinomas without LVI (Table 2, P < 0.001).
Table 2.
Relationship between LVD or LVI and clinicopathological parameters in 358 patients with invasive breast cancer
| Parameter | Category | N | LVD (vessels/mm2) Mean ± SD | ***P | LVI | ****P | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Absent N | Present N | ||||||
| All cases | 358 | 9.0 ± 12.1 | 300 | 58 | |||
| pN | 0 | 139 | 5.0 ± 14.2 | 131 | 8 | ||
| 1** | 124 | 11.3 ± 8.9 | 102 | 22 | |||
| 2** | 62 | 12.3 ± 11.4 | 42 | 20 | |||
| 3** | 33 | 10.7 ± 9.6 | < 0.001 | 25 | 8 | < 0.001 | |
| Grade* | 1 | 46 | 8.4 ± 23.7 | 45 | 1 | ||
| 2 | 139 | 7.7 ± 9.4 | 131 | 8 | |||
| 3 | 170 | 10.2 ± 9.2 | 0.180 | 121 | 49 | < 0.001 | |
| Breast cancer subtype | LA | 160 | 6.3 ± 8.7 | 150 | 10 | ||
| LBhighMIB | 49 | 13.6 ± 22.8 | 41 | 8 | |||
| LBHER2 | 49 | 7.4 ± 6.8 | 40 | 9 | |||
| HER2 | 41 | 12.8 ± 10.8 | 28 | 13 | |||
| TNP | 59 | 10.8 ± 9.7 | < 0.001 | 41 | 18 | < 0.001 | |
| LVI | Absent | 300 | 7.9 ± 12.2 | - | - | ||
| Present | 58 | 14.3 ± 10.1 | < 0.001 | - | - | - | |
G was not assessed in 2 cases;
chemotherapy-treated patients;
from ANOVA test;
Pearson χ2 test (for more than two rows);
LA (luminal A): ER+ and/or PR+, HER2- and MIB-1LI ≤ 28%, LBHER2 (HER2-positive luminal B): (i) ER+ and/or PR+ and HER2+; LBhighMIB (HER2-negative luminal B with high MIB-1LI): ER+ and/or PR+, HER2- and MIB-1LI > 28%; HER2 (HER2-overexpressing): ER- and PR- and HER2+, and TNP (triple-negative phenotype): ER- and PR- and HER2- ; LVD, lymphatic vessel density; LVI, lymphovascular invasion.
Low-grade tumours, pN0 carcinomas, and luminal A cancers less frequently presented lymphatic vascular invasion (Table 2, P < 0.001).
Survival analysis
In a group of 139 patients (pT1-2, pN0, M0 treated between 1993 and 2006), 114 patients survived without progression, 16 developed distant metastases, 4 suffered from local recurrence, while in 5 cases both distant metastases and local recurrence were diagnosed. In this group, the follow-up time ranged between 1 and 238.2 months with the median value 133.7. Twelve patients were followed-up for ≤ 60 months, 40 for 61-120 months, 66 for 121-180 months, while 21 individuals for more than 180 months. On the other hand, in a group of 215 patients (out of 219 included; pT1-4, pN1-3, M0 treated between 1984 and 2011), 166 patients survived without progression, in 39 distant metastases occurred, 3 developed local recurrence, while in 7 cases both distant metastases and local recurrence were diagnosed. In this group, the follow-up time ranged between 9.4 and 130 months with median value 77.3. Nineteen patients were followed-up for ≤ 36 months, 46 patients for 37-60 months, 150 for over 60 months.
In chemotherapy-naïve patients, significant (with P-value < 0.05) differences in the survival rate between the groups were found with cut-off points (found using the minimum p-value method of the log-rank test) ranging from 5.0 to 8.5 vessels/mm2. In chemotherapy/trastuzumab-treated patients, significant differences in the survival rate between the groups were found with cut-off points ranging from 7.0 to 17 vessels/mm2. Finally, the value of 7.0 vessels/mm2 (7.0 is the 60th percentile of LVD) was established as the cut-off point for both groups.
Univariate analysis
The probability of DFS according to LVD, breast cancer subtype, tumour grade and nodal involvement is shown in Figure 2A-H. In chemotherapy/trastuzumab-naïve patients, high LVD (> 7 vessels/mm2), LVI, breast cancer subtype other than LA + LBhighMIB and high grade were related to unfavourable DFS and MFS (for DFS: P = 0.026, P = 0.005, P = 0.013, P = 0.005 respectively, Figure 2A-D; for MFS: P = 0.009, P = 0.045, P = 0.013, P = 0.004 respectively).
Figure 2.
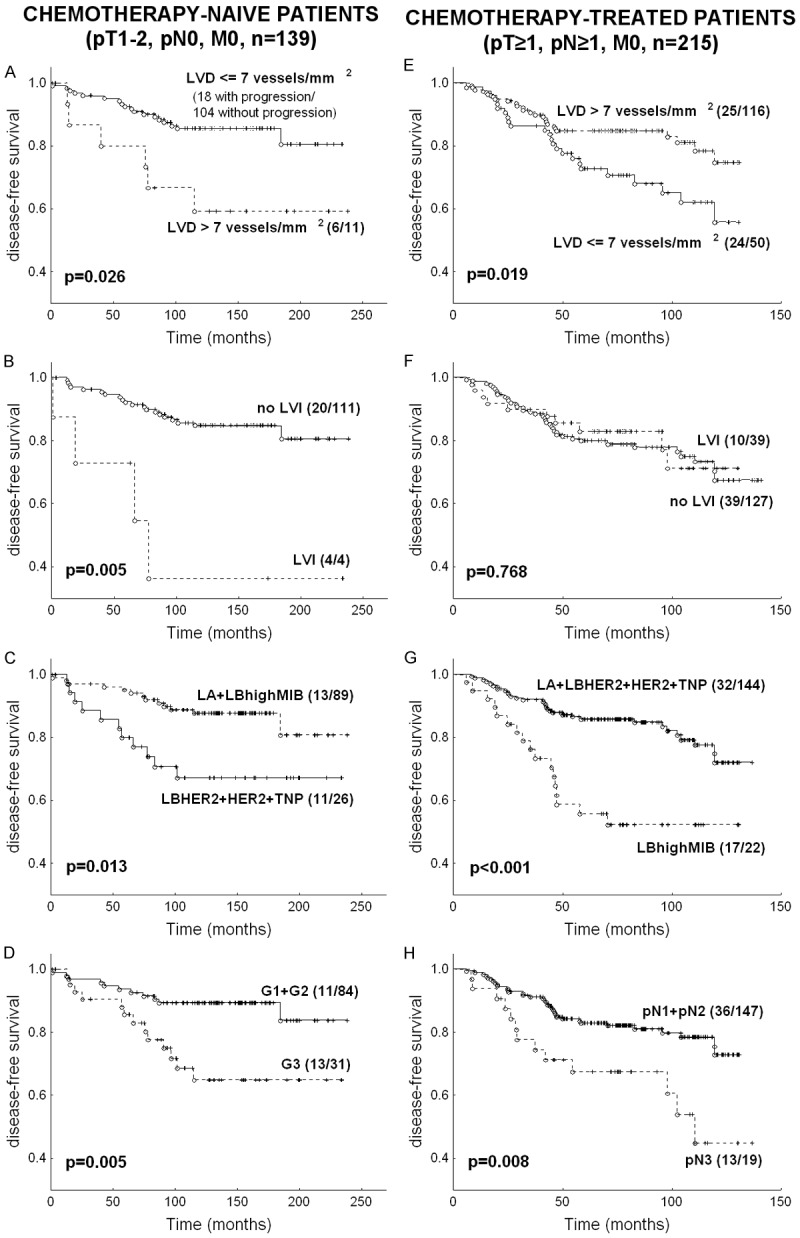
Disease-free survival in chemotherapy/trastuzumab-naïve subgroup and chemotherapy-treated subgroup according to: (1) lymphatic vessel density (A, E); (2) lymphatic vessel invasion (B, F); (3) breast cancer subtype: luminal A (LA): ER+ and/or PR+, HER2- and MIB-1LI ≤ 28%; HER2-positive luminal B (LBHER2): ER+ and/or PR+ and HER2+, HER2-negative luminal B with high MIBLI (LBhighMIB): ER+ and/or PR+, HER2- and MIB-1LI > 28%; HER2-overexpressing (HER2): ER- and PR- and HER2+, and triple negative phenotype (TNP): ER- and PR- and HER2- (C, G); (4) tumour grade (D); (5) pN (H). The number of patients with metastases or recurrence as compared to the number of patients without progression is presented in brackets.
On the other hand, in chemotherapy-treated patients we noted significantly unfavourable DFS and MFS in patients with tumours presenting LVD ≤ 7 vessels/mm2 as compared to patients with tumour LVD > 7 vessels/mm2 (for DFS: P = 0.019, Figure 2E, for MFS: P = 0.014). Moreover, in chemotherapy-treated patients, poor DFS and MFS were observed for LBhighMIB vs. LA + LBHER2 + HER2 + TNP and for pN3 vs. pN1-2 (for DFS: P < 0.001 and P = 0.008 respectively, Figure 2G, 2H; for MFS: P < 0.001 and p = 0.003 respectively). In the aforementioned group, LVI (Figure 2F, P = 0.768) and tumour grade (P = 0.610) were not significant.
Multivariate analysis
In chemotherapy/trastuzumab-naïve patients, the following parameters were significant independent adverse factors for high progression risk: a. For DFS: (i) LVD > 7 vessels/mm2 (RR = 2.7, 1.0 < 95% confidence interval (CI) < 6.8, P = 0.039); (ii) LVI (RR = 3.3, 1.0 < 95% CI < 10.4, P = 0.046); (iii) G3 (RR = 2.6, 1.1 < 95% CI < 6.1, P = 0.030). b. For MFS: (i) LVD > 7 vessels/mm2 (RR = 3.7, 1.4 < 95% CI < 9.4, P = 0.007); (ii) G3 (RR = 3.5, 1.5 < 95% CI < 8.4, P = 0.004).
In chemotherapy/trastuzumab-treated patients, the following parameters were significant independent adverse factors for high progression risk: a. For DFS: (i) LVD ≤ 7 vessels/mm2 (RR = 1.8, 1.0 < 95% CI < 3.2, P = 0.042); (ii) pN3 (RR = 2.3, 1.2 < 95% CI < 4.3, P = 0.012); (iii) LBhighMIB (RR = 3.0, 1.7 < 95% CI < 5.5, P < 0.001). b. For MFS: (i) LVD ≤ 7 vessels/mm2 (RR = 1.9, 1.1 < 95% CI < 3.4, P = 0.029); (ii) pN3 (RR = 2.5, 1.3 < 95% CI < 4.8, P = 0.005); (iii) LBhighMIB (RR = 3.0, 1.6 < 95% CI < 5.5, P < 0.001).
Discussion
In the present study, a high number of lymphatic vessels in the most vascularized intratumoral “hot-spot” (high LVD), as well as LVI were adverse prognostic factors for DFS in a group of pN0/chemotherapy/trastuzumab-naïve patients. Our results confirm the findings of other authors who reported an unfavourable outcome in individuals with tumours presenting high LVD [3-9] or LVI [4 (significant only in young patients), 13-15]. The relationship between high LVD and unfavourable DFS in chemotherapy/trastuzumab-naïve patients confirms that the presence of highly vascularized “hot spots” might identify the increased risk of cancer cell intravasation in areas with an intensive “dialog” between cancer cells and endothelial cells, and hence, a higher risk of metastasis formation [17,23].
On the other hand, in chemotherapy/trastuzumab-treated patients with synchronous lymph node metastases, contrary to the above-discussed group, we found a low risk of cancer spread in patients with tumours presenting high LVD.
The relationship between favourable survival and a high number of lymphatics in N+/chemotherapy-treated patients has previously been found [24], however, in the great majority of studies such relationship has not been reported (Table 3). This might result from the fact that, to the best of our knowledge, none of the researchers (Table 3) have analysed survival separately for pN+/chemotherapy/trastuzumab-treated and pN0/chemotherapy/trastuzumab-naïve patients. Moreover, we analysed prognostic significance of LVD and LVI in one of the largest groups of patients which was homogeneous in terms of treatment (Table 3). Additionally, we included only tumours with known steroid receptor status, HER2 and MIB-1LI.
Table 3.
Main characteristics, methodological aspects and results of studies evaluating lymphatic vessel density (LVD)
| First author, year of publication | No. of patients included into the study [Percentage of: N+; G3; ER+; PR+; HER2+; ductal] | aCHT | LVD assessment method | Correlation between high LVD and | Lack of correlation between LVD and | Cut-off point | OS | DFS | Cox |
|---|---|---|---|---|---|---|---|---|---|
| Schoppmann, 2004 [13] | 374 [43.3; 40.4; 77.0; ?; ?; 87.4] | 123 | 1 “hot spot”/field | Age, ↑G, LVI, menopausal status | N, pT, histology, ER | ? | NS | NS | NS |
| Nakamura, 2005 [3] | 113, M0 [49.6; 33.6; 59.3; 55.8; 28.3; 92.0] | 113 | 5 “hot spots”/field | T, ↑N, ↓ER, HER2, ↑LVI, VEGF-C | Age, histology, PR, p53, G | LVD = 10 | S* | S* | NS |
| van der Schaft, 2007 [4] | 121 [42.1; 16.5; 53.7; 43.8; ?; 100.0] | ? | 4 randomly selected fields/mm2, in IT and ST area | None | Both ST and IT: age, ER, PR, T, G, N | Median | ST LVD: NS (in Tu and LNM) | ST LVD: NS (in Tu and LNM) | - |
| IT LVD: NS (in Tu), S* (in LNM) | IT LVD: NS (in Tu), S* (in LNM) | ||||||||
| Mylona, 2007 [5] | 146, LVD was assessed only in 111, without preCHT, preRT [59.6; 24.3; 55.5; 48.2; ?; 80.0] | 85 | 5 “hot spots”/mm2 | None | TNM, G, T, N, ER, PR, topoIIa, VEGF-C, VEGF-D | LVD = 15 (upper quartile) | S* only in ER- | S* only in ER- | NS |
| El-Gohary, 2008 [14] | 48, without preCT, preRT [50.0; 18.8; 70.8; 70.8; 29.2; 56.3] | ? | 1 “hot spot” assessed in IT and PT area | IT and PT LVD: N, ↑T, TNM, ↑G, LVI, ↑BVI | IT and PT LVD: histology, ER, PR, VI | ? | NS | NS | - |
| Perit LVD: HER2, | |||||||||
| Zhang, 2008 [6] | 70, unilateral, M0, without preCHT, preRT [45.7; 41.4; 77.1; 65.7; 18.6; 84.3] | ? | 5 “hot spot”/field | VEGF-C and COX-2 | Other correlation no studied | ? | - | - | DFS: S* |
| OS:S* | |||||||||
| Gu, 2008 [7] | 61, M0, without preCHT, preRT [52.5; 9.8; 59.0; 65.6; 37.7; 85.2] | 61 aCHT and/or HT | 1 “hot spot”/field | N, ↑TNM | Histology, age, ER, PR, HER2 | LVD = 6 (median) | S* | S* | - |
| Mohammed, 2009 [8] | 177, without preCHT, preRT [29.0; 29.4; 66.7; 56.4; ?; ?] | 132 aCHT and/or HT | All vessels in whole section/mm2 in IT, PT and PP | PTNM (IT, PP, PT, tot), ↓ER (IT, PP, tot), ↓PR (IT), ↑G (IT), ↑N (IT, PP, PT, tot), LVI (tot) | Age, T, histology, MVD | totLVD = 1.37 (median) | In 132 with aCHT (totLVD)S* | In 132 with aCHT (totLVD)S* | NS |
| Tsutsui, 2010 [9] | 242, M0, invasive ductal [43.3; ?; 43.8; ?; ?; 242] | 208 | 3 “hot spots” field | N, ↑MIB-1, VEGF-A, MVD, ↑Akt | T, ER | LVD = 10.67 (55th percentile) | - | S* | NS |
| Mohammed, 2011 [15] | 397 including: (i) 197 basal (CK5/6+ and/or CK14+), < 1.5 cm, N0 and 200 non-basal, N0 (ii) 99/397: TNP [0; 50.3; 54.4; 41.6; ?; ?] | ? | All vessels in whole section/mm2 | None | Basal, T, G, ER, PR (in basal and TNP) | LVD = 1.43 (median) | NS (in basal and TNP) | NS (in basal and TNP) | NS |
N: nodal status; G: grade; T: tumour size; TNM: stage; ER: estrogen receptor; PR: progesterone receptor; HER2: human epidermal growth factor receptor 2; TNP: triple-negative phenotype (ER-/PR-/HER2-); ?: unknown data; LVD: lymphatic vessel density; LVI: lymphatic vessel invasion, BVI: blood vessel invasion, VI: vessel invasion in H&E staining; Tu: tumour; LNM: lymph node metastases; ST: stromal area; IT: intratumoral area; PT: peritumoral area; PP: peripheral area; tot: IT+PT+PP area; aCHT: adjuvant chemotherapy; preCHT: pre-operative chemotherapy; HT: hormonal therapy; preRT: preoperative radiotherapy; OS: overall survival; DFS: disease-free survival; ↓: negative correlation; ↑: positive correlation; +: presence of a particular feature; -: absence of a particular feature, NS: not significant; S: significant;
high LVD related to poor patients survival.
We can speculate that a high number of intratumoral lymphatic vessels (identifying favourable DFS in chemotherapy-treated patients) might be related to chemotherapy/trastuzumab-induced activation of the immune system against cancer cells [25-28]. Lymphatic vessels involvement in anticancer activity of immune cells (and favourable survival as a consequence) might be pursued by: (i) recruitment of leukocytes (macrophages and dendritic cells) from blood vessels by chemokines secreted by lymphatic endothelium and (ii) transport of cancer antigen-bearing macrophages and dendritic cells to regional lymph nodes in order to present them to lymphocytes [25]. This activation might be enhanced by the application of chemotherapy, particularly chemotherapy together with trastuzumab [28]. To summarize, the success of anti-cancer action of adjuvant chemotherapy may depend on pre-existing immune response, and may be greater in patients with carcinomas presenting a high number of tumour-infiltrating lymphocytes (TILs) and/or high LVD. In our series, the assessment of TILs and other parameters related to anti-cancer activity of immune cells is in progress. Another explanation of low risk of cancer spread in patients with tumours presenting high LVD is better tumour drainage, which improves the efficacy of cytotoxic drugs in killing cancer cells.
In our study, in pN0/chemotherapy/trastuzumab-naïve patients LVI was a significant indicator of poor survival, while in pN+/chemotherapy/trastuzumab-treated patients it was insignificant. This confirms our previous suggestion that LVI is particularly important in early-stage breast cancer [29,30].
The same parameters (except LVI in chemotherapy-naïve patients) influenced MFS and DFS, because in the whole group (354 patients) there were only 7 patients who developed only local recurrence (without distant metastases - for details, see Survival analysis).
The correlation between LVD or LVI and nodal involvement, found in the present study, additionally reaffirms the suggestion of lymphatic vessel-related spread of cancer cells. The relationship between high LVD or LVI and lymph node metastases was also reported by other researchers [for LVD: 3, 7-9, 14, for LVI: 13, 14], of whom the majority [3,7-9,14] used the “hot-spot” method (Table 3). The results of our study confirm the relationship between a high number of lymphatic vessels and ER/PR negativity found by other researchers [3,8,31]. Moreover, in HER2 and TNP carcinomas we noted higher LVD and found LVI more frequently (Table 2). El-Gohary and co-workers [14] found a similar negative correlation between ER/PR status and LVI, as well as a positive correlation between HER2 overexpression and LVI. However, in most studies the relationship between LVD and breast cancer immunophenotype was not found [4,5,7,9,13-15] (Table 3). Some authors found a statistically significant relationship between a high number of lymphatics and tumour grade (in the present study the difference was statistically insignificant) [8,13,14] (Tables 2, 3), while others did not [3-5,15] (Table 3). In our and other authors’ studies LVI was observed more frequently in high-grade tumours [13,14]. These discrepancies may result from different methodology applied for LVD assessment (Table 3) and different clinico-pathological characteristics of the study groups.
In chemotherapy/trastuzumab-naïve patients we found ER/PR immunopositivity and HER2 negativity as significant indicators of better survival, which warrants current treatment recommendations [22]. It is worth pointing out that in chemotherapy/trastuzumab-treated individuals, LBhighMIB subtype (as compared to other subtypes) was an indicator of high risk of cancer spread. Taking into account the fact that all patients from the study group received optimal (according to current standards) chemotherapy and targeted therapy, the result suggests the need for new therapies for patients with tumours presenting ER/PR positivity and high proliferative potential. To verify the hypothesis about the relationship between high LVD and favourable survival in pN+/chemotherapy-treated patients, we plan to extend the study group and assess the presence of TILs and the pattern of their distribution, as well as other parameters related to anti-cancer activity of immune cells.
Conclusion
The results obtained from our present study concerning pN0/chemotherapy-naïve patients confirm the hypothesis that a high number of lymphatics or the presence of lymphovascular invasion may indicate high risk of metastasis formation. On the other hand, in pN+/chemotherapy-treated patients, we found high LVD as an indicator of favourable survival. This relationship might be explained by potential involvement of lymphangiogenesis in two processes related to cancer eradication: the activation of the immune system against cancer cells or increased tumour drainage, which improves the efficacy of cytotoxic drugs. It might be particularly important for planning new anti-cancer strategies such as anti-angiogenic/-lymphangiogenic targeted therapies or immunotherapy.
Acknowledgements
The study was financed by the Polish Ministry of Science and Higher Education (grant number NN 401 096137, NN 401 2344 33 and NN 401 173 31/3808) and National Science Centre (decision No. DEC-2013/09/B/NZ5/00764). The authors have no conflict of interest to declare.
Disclosure of conflict of interest
None.
References
- 1.Cunnick GH, Jiang WG, Gomez KF, Mansel RE. Lymphangiogenesis and breast cancer metastasis. Histol Histopathol. 2002;17:863–870. doi: 10.14670/HH-17.863. [DOI] [PubMed] [Google Scholar]
- 2.Wang J, Guo Y, Wang B, Bi J, Li K, Liang X, Chu H, Jiang H. Lymphatic microvessel density and vascular endothelial growth factor-C and -D as prognostic factors in breast cancer: a systematic review and meta-analysis of the literature. Mol Biol Rep. 2012;39:11153–11165. doi: 10.1007/s11033-012-2024-y. [DOI] [PubMed] [Google Scholar]
- 3.Nakamura Y, Yasuoka H, Tsujimoto M, Imabun S, Nakahara M, Nakao K, Nakamura M, Mori I, Kakudo K. Lymph vessel density correlates with nodal status, VEGF-C expression, and prognosis in breast cancer. Breast Cancer Res Treat. 2005;91:125–132. doi: 10.1007/s10549-004-5783-x. [DOI] [PubMed] [Google Scholar]
- 4.van der Schaft DW, Pauwels P, Hulsmans S, Zimmermann M, van de Poll-Franse LV, Griffioen AW. Absence of lymphangiogenesis in ductal breast cancer at the primary tumor site. Cancer Lett. 2007;254:128–136. doi: 10.1016/j.canlet.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Mylona E, Nomikos A, Alexandrou P, Giannopoulou I, Keramopoulos A, Nakopoulou L. Lymphatic and blood vessel morphometry in invasive breast carcinomas: relation with proliferation and VEGF-C and -D proteins expression. Histol Histopathol. 2007;22:825–835. doi: 10.14670/HH-22.825. [DOI] [PubMed] [Google Scholar]
- 6.Zhang XH, Huang DP, Guo GL, Chen GR, Zhang HX, Wan L, Chen SY. Coexpression of VEGF-C and COX-2 and its association with lymphangiogenesis in human breast cancer. BMC Cancer. 2008;8:4. doi: 10.1186/1471-2407-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gu Y, Qi X, Guo S. Lymphangiogenesis induced by VEGF-C and VEGF-D promotes metastasis and a poor outcome in breast carcinoma: a retrospective study of 61 cases. Clin Exp Metastasis. 2008;25:717–725. doi: 10.1007/s10585-008-9180-4. [DOI] [PubMed] [Google Scholar]
- 8.Mohammed RA, Ellis IO, Elsheikh S, Paish EC, Martin SG. Lymphatic and angiogenic characteristics in breast cancer: morphometric analysis and prognostic implications. Breast Cancer Res Treat. 2009;113:261–273. doi: 10.1007/s10549-008-9936-1. [DOI] [PubMed] [Google Scholar]
- 9.Tsutsui S, Matsuyama A, Yamamoto M, Takeuchi H, Oshiro Y, Ishida T, Maehara Y. The Akt expression correlates with the VEGF-A and -C expression as well as the microvessel and lymphatic vessel density in breast cancer. Oncol Rep. 2010;23:621–630. doi: 10.3892/or_00000677. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Yan J, Wang Z, Yu S, Yuan Z, Yang C, Zheng Q. A meta-analysis of the relationship between lymphatic microvessel density and the survival of patient with colorectal cancer. Lymphology. 2013;46:42–51. [PubMed] [Google Scholar]
- 11.Yu M, Liu L, Liang C, Li P, Ma X, Zhang Q, Wei Y. Intratumoral vessel density as prognostic factors in head and neck squamous cell carcinoma: a meta-analysis of literature. Head Neck. 2014;36:596–602. doi: 10.1002/hed.23301. [DOI] [PubMed] [Google Scholar]
- 12.Pastushenko I, Vermeulen PB, Carapeto FJ, Van den Eynden G, Rutten A, Ara M, Dirix LY, Van Laere S. Blood microvessel density, lymphatic microvessel density and lymphatic invasion in predicting melanoma metastases: systematic review and meta-analysis. Br J Dermatol. 2014;170:66–77. doi: 10.1111/bjd.12688. [DOI] [PubMed] [Google Scholar]
- 13.Schoppmann SF, Bayer G, Aumayr K, Taucher S, Geleff S, Rudas M, Kubista E, Hausmaninger H, Samonigg H, Gnant M, Jakesz R, Horvat R Austrian Breast and Colorectal Cancer Study Group. Prognostic value of lymphangiogenesis and lymphovascular invasion in invasive breast cancer. Ann Surg. 2004;240:306–312. doi: 10.1097/01.sla.0000133355.48672.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El-Gohary YM, Metwally G, Saad RS, Robinson MJ, Mesko T, Poppiti RJ. Prognostic significance of intratumoral and peritumoral lymphatic density and blood vessel density in invasive breast carcinomas. Am J Clin Pathol. 2008;129:578–586. doi: 10.1309/2HGNJ1GU57JMBJAQ. [DOI] [PubMed] [Google Scholar]
- 15.Mohammed RA, Ellis IO, Mahmmod AM, Hawkes EC, Green AR, Rakha EA, Martin SG. Lymphatic and blood vessels in basal and triple negative breast cancers: characteristics and prognostic significance. Mod Pathol. 2011;24:774–785. doi: 10.1038/modpathol.2011.4. [DOI] [PubMed] [Google Scholar]
- 16.Niemiec JA, Adamczyk A, Ambicka A, Mucha-Małecka A, Wysocki WM, Ryś J. Distribution of podoplanin-positive tumor vessels predicts disease-specific survival of node-positive breast cancer patients treated with anthracyclines and/or taxanes. Cancer Invest. 2014;32:168–177. doi: 10.3109/07357907.2014.889704. [DOI] [PubMed] [Google Scholar]
- 17.Vermeulen PB, Gasparini G, Fox SB, Toi M, Martin L, McCulloch P, Pezzella F, Viale G, Weidner N, Harris AL, Dirix LY. Quantification of angiogenesis in solid human tumours: an international consensus on the methodology and criteria of evaluation. Eur J Cancer. 1996;32A:2474–2484. doi: 10.1016/s0959-8049(96)00379-6. [DOI] [PubMed] [Google Scholar]
- 18.Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, van de Vijver MJ. WHO classification of tumours of the breast 4. Lyon: IARC Press; 2012. [Google Scholar]
- 19.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19:403–410. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 20.Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Osborne CK, Paik S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FC, Taube S, Torlakovic EE, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB, Wittliff JL, Wolff AC American Society of Clinical Oncology; College of American Pathologists. American society of clinical oncology/college of American pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version) Arch Pathol Lab Med. 2010;134:e48–72. doi: 10.5858/134.7.e48. [DOI] [PubMed] [Google Scholar]
- 21.Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, McShane LM, Paik S, Pegram MD, Perez EA, Press MF, Rhodes A, Sturgeon C, Taube SE, Tubbs R, Vance GH, van de Vijver M, Wheeler TM, Hayes DF American Society of Clinical Oncology/College of American Pathologists. American society of clinical oncology/college of American pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med. 2007;131:18–43. doi: 10.5858/2007-131-18-ASOCCO. [DOI] [PubMed] [Google Scholar]
- 22.Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thürlimann B, Senn HJ Panel Members. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen international expert consensus on the primary therapy of early breast cancer. Ann Oncol. 2013;24:2206–2223. doi: 10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weidner N. Current pathologic methods for measuring intratumoural microvessel density within breast carcinoma and other solid tumours. Breast Cancer Res Treat. 1995;36:169–180. doi: 10.1007/BF00666038. [DOI] [PubMed] [Google Scholar]
- 24.Niemiec JA, Adamczyk A, Ambicka A, Mucha-Małecka A, Wysocki WM, Ryś J. Distribution of podoplanin-positive tumor vessels predicts disease-specific survival of node-positive breast cancer patients treated with anthracyclines and/or taxanes. Cancer Invest. 2014;32:168–177. doi: 10.3109/07357907.2014.889704. [DOI] [PubMed] [Google Scholar]
- 25.Kim H, Kataru RP, Koh GY. Inflammation-associated lymphangiogenesis: a double-edged sword? J Clin Invest. 2014;124:936–942. doi: 10.1172/JCI71607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loi S, Michiels S, Salgado R, Sirtaine N, Jose V, Fumagalli D, Kellokumpu-Lehtinen PL, Bono P, Kataja V, Desmedt C, Piccart MJ, Loibl S, Denkert C, Smyth MJ, Joensuu H, Sotiriou C. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol. 2014;25:1544–1550. doi: 10.1093/annonc/mdu112. [DOI] [PubMed] [Google Scholar]
- 27.Kotoula V, Chatzopoulos K, Lakis S, Alexopoulou Z, Timotheadou E, Zagouri F, Pentheroudakis G, Gogas H, Galani E, Efstratiou I, Zaramboukas T, Koutras A, Aravantinos G, Samantas E, Psyrri A, Kourea H, Bobos M, Papakostas P, Kosmidis P, Pectasides D, Fountzilas G. Tumors with high-density tumor infiltrating lymphocytes constitute a favorable entity in breast cancer: a pooled analysis of four prospective adjuvant trials. Oncotarget. 2016;7:5074–5087. doi: 10.18632/oncotarget.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dushyanthen S, Beavis PA, Savas P, Teo ZL, Zhou C, Mansour M, Darcy PK, Loi S. Relevance of tumor-infiltrating lymphocytes in breast cancer. BMC Med. 2015;13:202. doi: 10.1186/s12916-015-0431-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arnaout-Alkarain A, Kahn HJ, Narod SA, Sun PA, Marks AN. Significance of lymph vessel invasion identified by the endothelial lymphatic marker D2-40 in node negative breast cancer. Mod Pathol. 2007;20:183–191. doi: 10.1038/modpathol.3800728. [DOI] [PubMed] [Google Scholar]
- 30.Roses DF, Bell DA, Flotte TJ, Taylor R, Ratech H, Dubin N. Pathologic predictors of recurrence in stage 1 (TINOMO) breast cancer. Am J Clin Pathol. 1982;78:817–820. doi: 10.1093/ajcp/78.6.817. [DOI] [PubMed] [Google Scholar]
- 31.Niemiec J, Adamczyk A, Ambicka A, Mucha-Małecka A, Wysocki W, Mituś J, Ryś J. Lymphangiogenesis assessed using three methods is related to tumour grade, breast cancer subtype and expression of basal marker. Pol J Pathol. 2012;63:165–171. doi: 10.5114/pjp.2012.31500. [DOI] [PubMed] [Google Scholar]


