Abstract
This study aims to observe expression of IL-27 on different cells in periapical tissues of different types of human chronic periapical diseases. Periapical tissue specimens of 60 donors, including healthy control (n=20), periapical granuloma group (n=20) and radicular cysts group (n=20), were fixed in 10% buffered formalin, stained with hematoxylin and eosin for histopathology. Then specimens were stained with double- immuno-fluorescence assay for identification of IL-27-tryptase (mast cells, MCs), IL-27-CD14 (mononuclear phagocyte cells, MPs) and IL-27-CD31 (endothelial cells, ECs) double-positive cells in periapical tissues. The results indicated that compared with healthy control, the densities (cells/mm2) of IL-27-tryptase, IL-27-CD14 and IL-27-CD31 double-positive cells were significantly increased in human chronic periapical diseases (periapical granuloma group and radicular cysts group) (P<0.001). The density of IL-27-tryptase double positive cells in radicular cysts group was significantly higher than those in periapical granuloma group (P<0.001). Densities of IL-27-CD14 and IL-27-CD31 double-positive cells in periapical granuloma group had no significant difference with those in radicular cysts group (P=0.170 and 0.138, respectively). IL-27-CD14 double positive cells density achieved to peak among three cell groups in radicular cysts groups. In conclusion, IL-27 expressed in MCs, MPs and ECs of human chronic periapical diseases with different degrees. IL-27-tryptase double-positive cells may participate in pathogenic mechanism of chronic periapical diseases, especially for formation of fibrous in periapical cysts. IL-27-CD14 and IL-27-CD31 double-positive cells may participate in immunologic response to resist periapical infection, and they may play an dual role in pathogenesis and localization of periapical diseases.
Keywords: Periapical diseases, interleukin-27, mast cells, mononuclear phagocyte cells, endothelial cells
Introduction
The bacterial infection of the dental pulps, usually as a sequel to dental caries, which could result in the chronic periapical diseases and the related lesions [1]. The chronic periapical diseases are often the immune reactions to the bacteria and by-products of the root canal system, which could ultimately cause the destroy of the perapical and perifurcal alveolar bone [2,3]. The previous study reported that the inflammatory lesions in the chronic periapical diseases is characterized by the appearance of immune cells, which could produce the inflammatory biomarkers, such the cytokines [4,5].
Interleukin-27 (IL-27) is a novel interleukin of the IL-6/IL-12 cytokine family, which is mainly produced by antigen-presenting cells (APCs) [6]. IL-27 can regulate the differentiation of the T helper cell (Th), promote the proliferation of the initial CD4+ cells, and facilitate the CD4+ cell’s differentiation to the Th1 cells. Meanwhile, IL-27 can also inhibit the growth of Th17 cells and Th2 cells [7,8]. The studies in recent years has shown that IL-27 was not only expressed in activated APCs, but also expressed in plasma cells, endothelial cells, microglia cells, trophoblastic cells and uterine NK cells, and also played a dual role by promoting and inhibiting the organism’s inflammation [6,7,9]. Presently, the IL-27 expression in the different immune cells and the pathogenic mechanisms of human periapical disease are yet unknown.
In this article, we observed the expression of IL-27 in different cells of the different human chronic periapical diseases, and investigated the specific role of IL-27 in the pathogenesis of chromic periapical diseases. The present study would provide the basis for the clinical application and drug development for the human chronic periapical diseases.
Materials and methods
Subjects
Patient selection
60 subjects (ages from 17 to 56), who had visited dental clinic of Guangdong armed police corps hospital between June 2013 and July 2014 were enrolled in this research, with 33 males (38 ± 18.32 years old), 27 females (31 ± 14.12 years old). There were no statistical significant differences in sex and age between groups in this study. We excluded the subjects who were pregnant, receiving oral contraceptive therapy, having systemic disease or had taken antibiotic therapy in the last three months. All subjects signed informed consent in this experiment (Appendix).
Trial grouping
According to the grouping criteria including the X-ray examination, operative exploration and histopathological findings, subjects were divided into 3 groups (20 cases per group).
Healthy control group
Samples were extracted from periodontal membrane of premolars extracted for orthodontics, and the premolars had healthy periapical tissue and complete root formation. Inclusive criteria: ① Vital teeth; ② No low density radiolucent shadow shown in the X-ray examination; ③ Periodontal ligament with smooth and continuous edges, and with no injuries during the extraction process; ④ No distinct capillary proliferation and inflammatory cell infiltration during the histological observation.
Periapical granuloma group
Samples were derived from periapical tissues with periapical diseases, which could not be cured with non-surgical treatment and required periapical curettage treatment. Inclusive criteria: ① Non-vital teeth; ② Round or ovoid shaped low-density radiolucent shadow less than 1 cm in size in the teeth root apex in X ray examination; ③ The periapical solid soft-tissue lesions which was found during operation; ④ Histological observations showed that the periapical lesions was granulation lumps with capillary proliferation and inflammatory cell infiltration.
Periapical cyst group (radicular cysts)
Samples were extracted from the periapical tissue with periapical lesions, which could not be cured with non-operative treatment and required the periapical curettage treatment. Inclusion criteria: ① Non-vital teeth; ② The teeth root apex with white-plagued lines and round or ovoid shape low-density radiolucent shadow less than 1 cm in size in X ray examination; ③ Periapical solid soft-tissue lesions containing liquids or semi-solid cystic tissues which were found during operation; ④ Histological observations show the periapical tissues containing large amounts of collagen fibers, which were cysts or tissues fully or partially covered with non-keratinized stratified squamous epithelium [10].
Sample treatment and immuno-fluorescent double staining assay
The periapical tissues were extracted from patients and fixed in 10% buffered formalin for 24 to 48 h, then made into 5 μm thick serial slices and staining the periapical tissues with hematoxylin and eosin. We observed the histologic changes under the light microscopes.
The immuno-fluorescent double staining of IL-27-tryptase, IL-27-CD14, IL-27-CD31 was performed in this study. Firstly, the samples were incubated with the mouse anti-human IL-27 monoclonal antibody (Santa Cruz Biotechnology, United States), Mouse anti-human mast cell Tryptase monoclonal antibody (Abcam, United Kingdom), Mouse anti-huamn CD14 monoclonal antibody (Wuhan Boster, China), mouse-human anti-PECAM1/CD31 monoclonal antibody (Wuhan Boster, China) for 2 h under dark condition. Then, the samples were washed with the PBST solution for 5 min and 3 times. The samples were subsequently incubated with the rabbit anti-mouse Fluor®555 conjugate polyclonal antibody (Cell Signaling technology, United States), rabbit anti-mouse Alexa Fluor®488 conjugate polyclonal body (Cell Signaling technology, United States) under dark condition, and washed for 5 min and 3 times. Then, the tissue slices were immediately observed under a fluorescence microscope.
In the immuno-fluorescent double staining assay, the IL-27+ signal was stained as red and the tryptase+, CD14+ and CD31+ were stained as green. All the signals were located in the cell membrane or cytoplasm. The over-lapping of the red and green fluorescence in the same visual field introduced the orange fluorescence [11]. The DAPI nuclear-stained nuclei appeared as blue fluorescence under a fluorescence microscope. Tissue slices were blindly observed by 2 pathologists under the fluorescence microscope, and based on the counting method of Batista, et al. [12]. Ten representative conductive visual field (0.0725 mm2/field) were opted for the counting of the DAPI positive and the IL-27-tryptase, IL-27-CD14, IL-27-CD31 double positive cells. Then, the double-positive cell density (cells/mm2) was obtained and averaged subsequently.
Statistical analysis
Data were represented with mean ± standard error (mean ± SEM) and analyzed by using SPSS 13.0. The one-way ANOVA of the completely randomized design was applied in comparison in the average density of IL-27-tryptase, IL-27-CD14 and IL-27-CD31 double positive cells. The significant level was double side α=0.05, and P<0.05 was considered to be significantly different.
Results
Histological observation of periodontal tissues
Healthy control group
Apical periodontal membrane with abundant fibrous tissues was structurally integrated, and no explicit infiltration of inflammatory cells was observed (Figure 1A, 1B).
Figure 1.
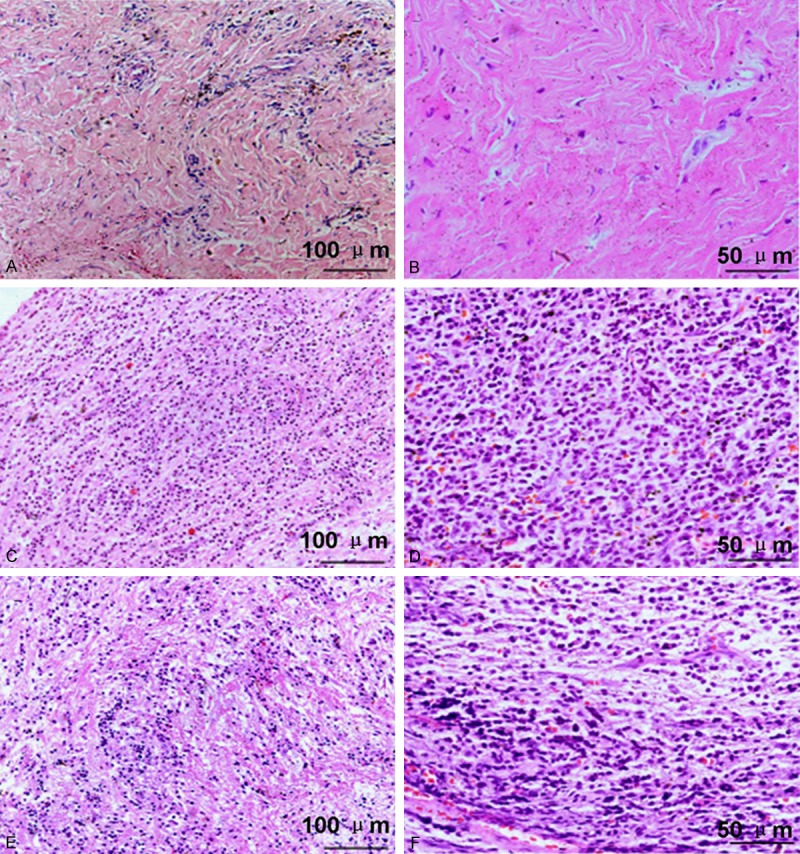
Histological observation of periodontal tissues by using HE staining. (A and B) Healthy control; (B and C) Periapical granulomas. (E and F) Radicular cysts. The scale bars have been illustrated in the images (A, C, E) with the original magnification, ×200. The scale bars have been illustrated in the images (D-F) with the original magnification, ×400.
Periapical granuloma group
The structure of the periodontal tissues were destructed, and the new capillaries fibroblasts and inflammatory cells infiltration were abundant. The linear epithelial cells could be identified in some slices (Figure 1C, 1D).
Periapical cyst group
The structure of the periodontal tissues were destructed, and the capillaries were dilated. Microscopically, the periapical cyst was covered with the epithelial layer, which was intercellular edematous, and infiltrate with a large number chronic inflammatory cells. In the fibrous layer dominated with collagen fibers, no explicit infiltration of inflammatory cells could be identified. In part of the cyst wall, there existed spindle-shaped intervals rich in hemosiderin and cholesterol Crystal deposition (Figure 1E, 1F).
Staining results of IL-27-tryptase double-positive cells
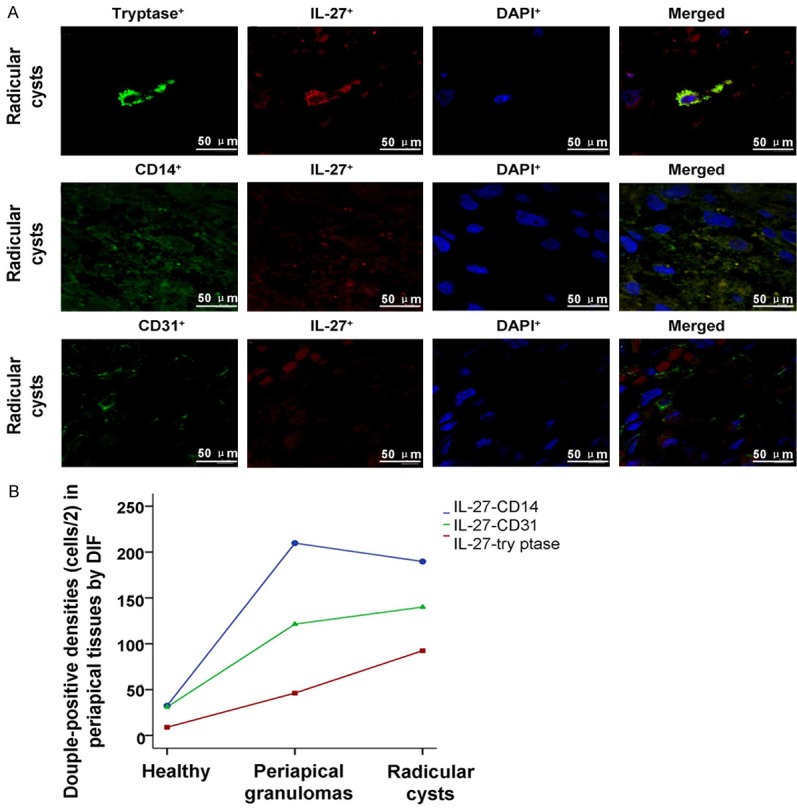
In order to investigate the interaction between IL-27 and tryptase, IL-27 and CD14, IL-27 and CD31, the cells were stained by using the immuno-fluorescent assay. From the Figure 2, we found that there were only a few double-positive cells distributed in the periapical tissues of the healthy control group. However, a abundant infiltration of double-positive cells and mature erythrocyte and epithelial structures was observed in the Periapical cyst group.
Figure 2.
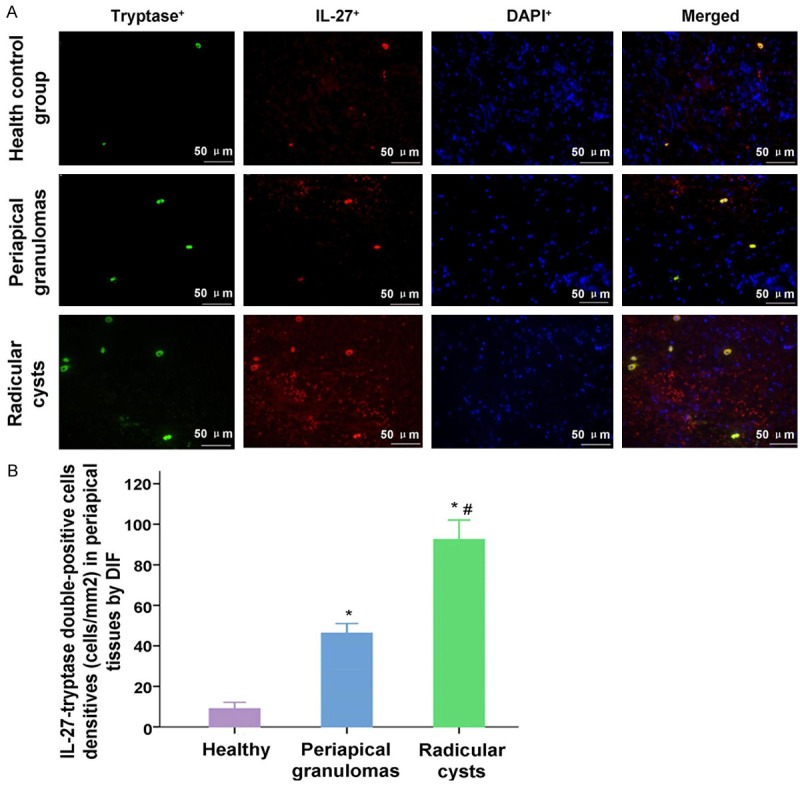
Observation for the IL-27+-tryptase+ double-positive staining cells in health control group, periapical granulomas group and Radicular cysts group. A. The IL-27+, tryptase+ and DAPI+ positive stained cells, and the merged stained cells were illustrated in the images. B. Statistical analysis of the densities of IL-27+-tryptase+ double-positive cells in every group. *P<0.05 represents the values in periapical granulomas group and radicular group compared to health control group. #P<0.05 represents the value in radicular group compared to periapical granulomas group. The scale bars have been illustrated in the images with the original magnification, ×400.
The results indicated that the IL-27-tryptase double staining cells were more significantly in Periapical granulomas group and Radicular cysts group compared to the Health control group (Figure 2, both P<0.001). Meanwhile, IL-27-tryptase double staining cells in Radicular cysts group was also more significantly compared to the Periapical granulomas group (Figure 2, P<0.001).
Staining results of IL-27-CD14 double-positive cells
The results indicated that IL-27-CD14 double staining cells were more significantly in Periapical granulomas group and Radicular cysts group compared to the Health control group (Figure 3, both P<0.001). However, the densities of IL-27-CD14 positive cells between the periapical granuloma group and the periapical cyst group had no significant difference (Figure 3, P=0.170).
Figure 3.
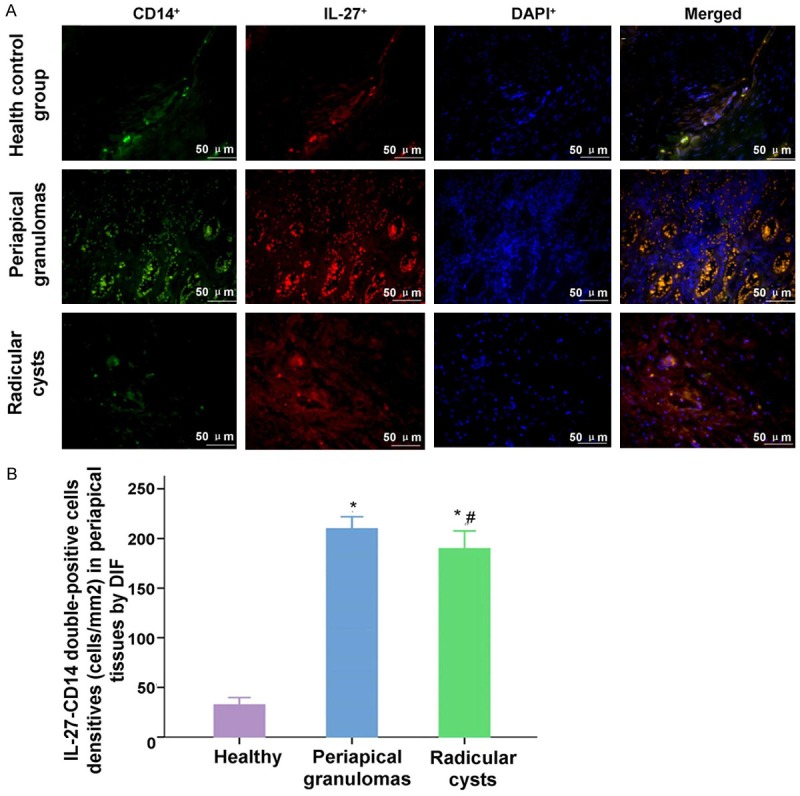
Observation for the IL-27+-CD14+ double-positive staining cells in health control group, periapical granulomas group and Radicular cysts group. A. The IL-27+, CD14+ and DAPI+ positive stained cells, and the merged stained cells were illustrated in the images. B. Statistical analysis of the densities of IL-27+-CD14+ double-positive cells in every group. *P<0.05 represents the values in periapical granulomas group and radicular group compared to health control group. #P<0.05 represents the value in radicular group compared to periapical granulomas group. The scale bars have been illustrated in the images with the original magnification, ×400.
Staining results of IL-27-CD31 double-positive cells
Similar to the IL-27-tryptasse and IL-27-CD14 double staining results, the data results of IL-27-CD31 double positive cells in Periapical granulomas group and Radicular cysts group were also higher significantly compared to the Health control group (Figure 4, P<0.001). The densities of IL-27-CD31 positive cells in periapical granuloma group had no significant difference compared with those in periapical cyst group (Figure 4, P=0.138).
Figure 4.
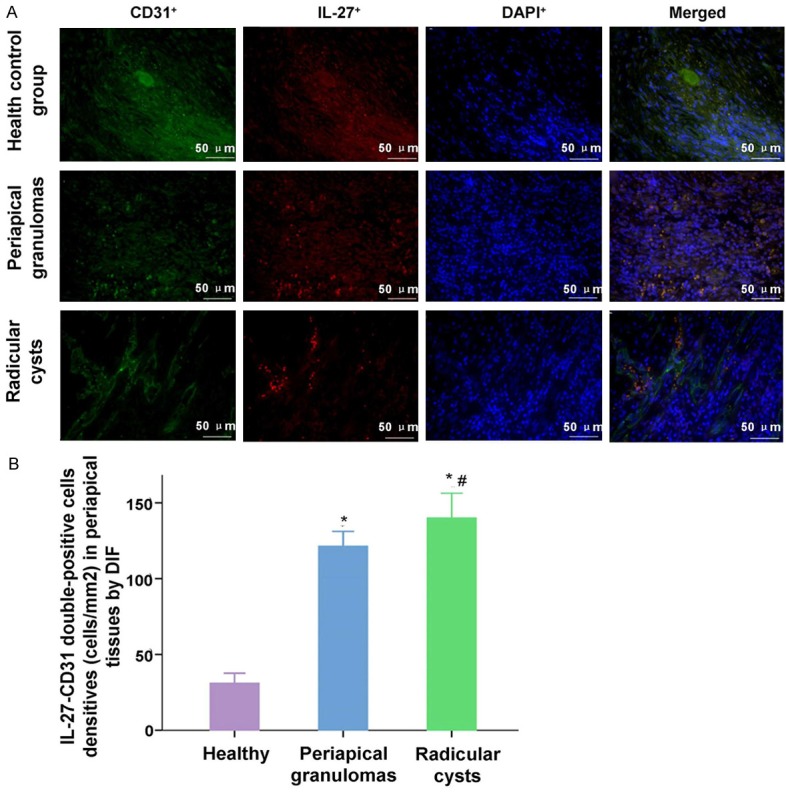
Observation for the IL-27+-CD31+ double-positive staining cells in health control group, periapical granulomas group and Radicular cysts group. A. The IL-27+, CD31+ and DAPI+ positive stained cells, and the merged stained cells were illustrated in the images. B. Statistical analysis of the densities of IL-27+-CD31+ double-positive cells in every group. *P<0.05 represents the values in periapical granulomas group and radicular group compared to health control group. #P<0.05 represents the value in radicular group compared to periapical granulomas group. The scale bars have been illustrated in the images with the original magnification, ×400.
IL-27-CD14 double staining cells illustrates the peak density
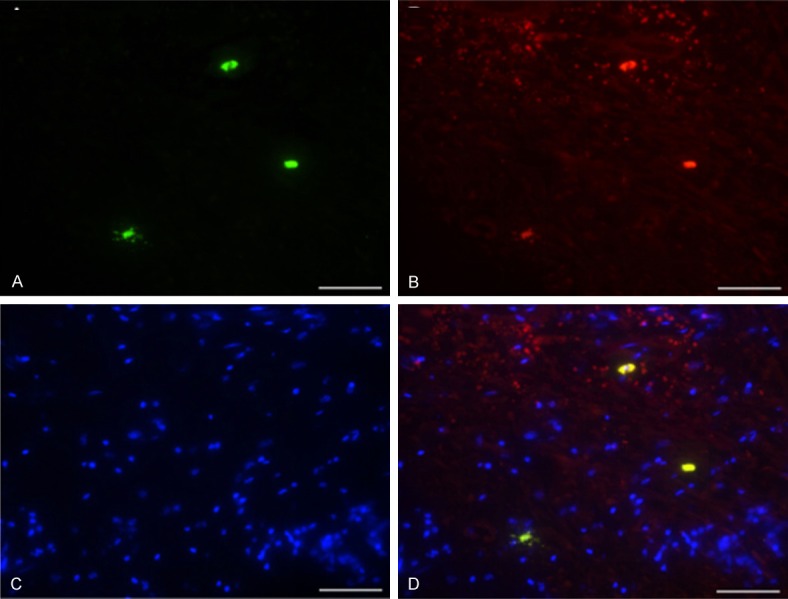
According to the double staining results from Figures 2, 3 and 4, we found that the there were more IL-27-tryptase, IL-27-CD14 and IL-27-CD31 double staining cells in Periapical cyst group. In order to compare the best biomarker (tryptase, CD14 and CD31) interacted with IL-27, we also analyzed the double staining results in periapical cyst group (Figure 5). The results indicated that the IL-27-CD14 double positive cells density achieved to the peak among the three cell groups, following with the IL-27-CD31 double positive cells, and the IL-27-tryptase double positive cells (Figure 5).
Figure 5.
Observation for the IL-27+-tryptase+, IL-27+-CD14+ and IL-27+-CD31+ double staining results in periapical cyst group by using laser scanning confocal microscope. The scale bars have been illustrated in the images with the original magnification, ×1260.
Discussion
IL-27, a new member of the IL-12 family, has a dual immuno-modulatory activity, in neoplasms, autoimmune diseases, and infectious diseases. Previous study has validated that IL-27 mainly generated by the activated MPs and DCs [13] and played an important role in the Th1’s initial immune responses and meanwhile it also had general inhibitory effects on the activity of Th1, Th2, Th17 and regulatory T cells [14]. In the observation with IL-27 expression on human T1-associated granulomatous diseases (tuberculosis disease, sarcoidosis and Crohn’s disease), Larousserie ect. [15] found an elevated expression of IL-27 in the granulomatous and T1 associated diseases, suggesting that IL-27 might play an important role in the pathogenesis.
MPs including the monocytes in blood and macrophages in tissues, which are responsible for 4% to 50% of the inflammatory cells in periapical diseases and plays an important role in periapical diseases. The MPs protect body by phagocytizing and killing pathogens, and also participate in the tissue cell injuries, contributing to the disease progressing and the long-term inflammatory response [16]. By exposing the Molar pulp cavity of rats to oral microorganisms, we introduced the experimental periapical Granuloma. The results showed that macrophages had already entered the periapical tissues in the early stages, suggesting that macrophages were the first line of the local immune defense in eliminating the harmful stimuli and pretecting the marrow periapical tissues from the infections [17]. Vascular endothelial growth factor (VEGF) regulates the proliferation, differentiation and migration of the vascular ECs, and exerts significant stimulation on the development of granulation tissues and the expansion of cysts [18,19]. MCs expression in the damaged areas can facilitate the expression of angiogenetic growth factors and stimulate local micro-vascular and also can increase the micro-vessel density (MVD). Moreover, the MCs could also change the vascular activity, increase the vascular permeability, help the plasma protein enter the periapical cysts, and increase the cyst osmotic pressure and gradually expand the cysts. Drazic et al. [20] observed that the degranulated MCs were adjacent to fibroblasts, which suggests that MCs may play an important role in the formation of fibrous tissues. Our results showed that the density of cells with MCs expression in the periapical cyst group was higher than in Periapical Granuloma group. MCs’s impact with granulation tissue was preliminary on the wound repair. The tryptase and PGs released during the MCs degranulation participated in the bone resorption of the bone cyst-contact area and promoted the expansion of the cyst, suggesting that MCs may play a role in reconstruction in the cyst development, which resulted in the jaw cyst expansion [21].
Chronic Periapical diseases was characterized with inflammatory destruction. The inflammatory bone re-absorption was introduced by the CKs secreted by immuno-competent cells or immuno-competent cells. The previous studies indicated that the genesis, development and prognosis of periapical diseases were closely associated with immune responses of periapical tissues [17-21]. Cells associated with the immune responses of Periapical diseases included lymphocytes, plasmocytes, neutrophils, MCs, MPs, and etc. The APCs, including MPs, DCs and neutrophils, could dispose the pathogenic stimulus. Meanwhile, they can inflict damages to the normal tissues, which results in the periapical tissue destruction and the alveolar bone absorption [22].
In this study, we adopted the immuno-fluorescence double staining method, and observed the distribution of MCs, MPs and ECs in the different periapical disease tissues. Then, we analyzed the IL-27 expression in these three diseases of cells. We found that the IL-27 positive cells abundantly existed below the cystic epithelium and inside the connective tissues and epithelium. Compared with the healthy control, the densities of IL-27-tryptase, IL-27-CD14 and IL-27-CD31 double-positive cells were significantly increased in human chronic periapical diseases. The density of IL-27-tryptase double positive cells in periapical cyst group was significantly higher than those in periapical granuloma. The densities of IL-27-CD14 and IL-27-CD31 double-positive cells in periapical granuloma group had no significant difference with those in periapical cyst group. The result showed that IL-27 expressed in MCs, MPs and ECs of human chronic periapical diseases with different degree. IL-27-tryptase double-positive cells may participate in the pathogenic mechanism of chronic periapical diseases, especially for the formation of fibrous in periapical cysts. IL-27-CD14 and IL-27-CD31 double-positive cells may participate in the immune regulation of chronic periapical diseases, and play an dual role in the disease pathogenesis. Our result was complied with the previous research results [23,24]. But how the IL-27 expression of the immune cells in the periapical tissues could affect the immuno-pathological regulation in periapical diseases and the mechanisms of the IL-27’s role in cell interaction require further investigation. And the in vitro experiments could be considered in the future follow-up studies. The study result had theoretical points and clinic guidance values in illuminating the IL-27’s concrete effects on the pathogenesis of periapical diseases. Therefore, inhibiting the IL-27 generation of immune cells could be a novel treatment target to prevent the disease progressing. This research provided a new direction for the periapical disease treatment.
Although we received some interesting and significant results, there were also a few limitations in this study. The present study mainly observed and described the role of IL-27 in the human chronic periapical diseases, but without specific mechanism investigation. In the following study, we would clarify the mechanism that involved in the role of the IL-27 in the human chronic periapical diseases.
In conclusion, IL-27 expressed in MCs, MPs and ECs of human chronic periapical diseases with different degrees. IL-27-tryptase double-positive cells may participate in pathogenic mechanism of chronic periapical diseases, especially for formation of fibrous in periapical cysts. IL-27-CD14 and IL-27-CD31 double-positive cells may participate in immunologic response to resist periapical infection, and they may play an dual role in pathogenesis and localization of periapical diseases.
Acknowledgements
This study was granted by the funding project in the social progress area of Science and Technology Program of Guangdong (Grant No: 2013B021800043 and 2014A020212212).
Appendix.
Periapical granulomas. A: Tryptase+; B: IL-27+; C: DAPI+; D: Merged. (DIF staining, scale bars = 50 μm, Original magnification, ×400).
Disclosure of conflict of interest
None.
References
- 1.Kangarlou Haghighi A, Davar M, Kazem M, Dianat O. Presence of leptin in chronic periapical lesions. Iran Endod J. 2010;5:147–150. [PMC free article] [PubMed] [Google Scholar]
- 2.Sattari M, Haghighi AK, Tamijani HD. The relationship of pulp polyp with the presence and concentration of immunoglobulin E, histamine, interleukin-4 and interleukin-12. Aust Endod J. 2009;35:164–168. doi: 10.1111/j.1747-4477.2009.00160.x. [DOI] [PubMed] [Google Scholar]
- 3.Sattari M, Ama-Ashari M, Hejazi M. Correlation between IgE and different states of dental pulps. Iran J Allergy Asthma Immunol. 2000;1:141–145. [Google Scholar]
- 4.Stashenko P, Teles R, D’Souza R. Periapical inflammatory response and their modulation. Crit Rev Oral Biol Med. 1998;9:498–521. doi: 10.1177/10454411980090040701. [DOI] [PubMed] [Google Scholar]
- 5.Sasaki H, Hirai K, Martins CM, Furusho H, Battaglino R, Hashimoto K. Interrelation between periapical lesion and systemic metabolic disorders. Curr Pharm Des. 2016;22:2204–2215. doi: 10.2174/1381612822666160216145107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sonobe Y, Yawata I, Kawanokuchi J, Takeuchi H, Mizuno T, Suzumura A. Production of IL-27 and other IL-12 family cytokines by microglia and their subpopulations. Brain Res. 2005;1040:202–207. doi: 10.1016/j.brainres.2005.01.100. [DOI] [PubMed] [Google Scholar]
- 7.Fitzgerald DC, Ciric B, Touil T, Harle H, Grammatikopolou J, Das Sarma J, Gran B, Zhang GX, Rostami A. Suppressive effect of IL-27 on encephalitogenic Th17 cells and the effector phase of experimental autoimmune encephalomyelitis. J Immunol. 2007;179:3268–3275. doi: 10.4049/jimmunol.179.5.3268. [DOI] [PubMed] [Google Scholar]
- 8.Villarino AV, Huang E, Hunter CA. Understanding the pro- and anti-inflammatory properties of IL-27. J Immunol. 2004;173:715–720. doi: 10.4049/jimmunol.173.2.715. [DOI] [PubMed] [Google Scholar]
- 9.Jung JY, Roberts LL, Robinson CM. The presence of interleukin-27 during monocyte-derived dendritic cell differentiation promotes improved antigen processing and stimulation of T cells. Immunology. 2015;144:649–660. doi: 10.1111/imm.12417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teixeira-Salum TB, Rodrigues DB, Gervásio AM, Souza CJ, Rodrigues V, Loyola AM. Distinct Th1, Th2 and Treg cytokines balance in chronic periapical granulomas and radicular cysts. J Oral Pathol Med. 2010;39:250–256. doi: 10.1111/j.1600-0714.2009.00863.x. [DOI] [PubMed] [Google Scholar]
- 11.Costa NL, Oton-Leite AF, Cheim-Júnior AP, Alencar Rde C, Bittar GO, Silva TA, Batista AC. Density and migration of mast cells in lip squamous cell carcinoma and actinic cheilitis. Histol Histopathol. 2009;24:457–465. doi: 10.14670/HH-24.457. [DOI] [PubMed] [Google Scholar]
- 12.Batista A, Rodini C, Lara V. Quantification of mast cells in different stages of human periodontal disease. Oral Dis. 2005;11:249–254. doi: 10.1111/j.1601-0825.2005.01113.x. [DOI] [PubMed] [Google Scholar]
- 13.Kastelein RA, Hunter CA, Cua DJ. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu Rev Immunol. 2007;25:221–242. doi: 10.1146/annurev.immunol.22.012703.104758. [DOI] [PubMed] [Google Scholar]
- 14.Stumhofer JS, Hunter CA. Advances in understanding the anti-inflammatory properties of IL-27. Immunol Lett. 2008;117:123–130. doi: 10.1016/j.imlet.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larousserie F, Pflanz S, Coulomb-L’Herminé A, Brousse N, Kastelein R, Devergne O. Expression of IL-27 in human Th1-associated granulomatous diseases. J Pathol. 2004;202:164–171. doi: 10.1002/path.1508. [DOI] [PubMed] [Google Scholar]
- 16.Kawashima N, Okiji T, Kosaka T, Suda H. Kinetics of macrophages and lymphoid cells during the development of experimentally induced periapical lesions in rat molars: a quantitative immunohistochemical study. J Endod. 1996;22:311–316. doi: 10.1016/S0099-2399(96)80266-4. [DOI] [PubMed] [Google Scholar]
- 17.Metzger Z. Macrophages in periapical lesions. Endod Dent Traumatol. 2000;16:1–8. doi: 10.1034/j.1600-9657.2000.016001001.x. [DOI] [PubMed] [Google Scholar]
- 18.Angelo LS, Kurzrock R. Vascular endothelial growth factor and its relationship to inflammatory mediators. Clin Cancer Res. 2007;13:2825–2830. doi: 10.1158/1078-0432.CCR-06-2416. [DOI] [PubMed] [Google Scholar]
- 19.Nagy JA, Benjamin L, Zeng H, Dvorak AM, Dvorak HF. Vascular permeability, vascular hyperpermeability and angiogenesis. Angiogenesis. 2008;11:109–119. doi: 10.1007/s10456-008-9099-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drazic R, Sopta J, Minic AJ. Mast cells in periapical lesions: potential role in their pathogenesis. J Oral Pathol Med. 2010;39:257–262. doi: 10.1111/j.1600-0714.2009.00870.x. [DOI] [PubMed] [Google Scholar]
- 21.Rodini CO, Batista AC, Lara VS. Comparative immunohistochemical study of the presence of mast cells in apical granulomas and periapical cysts: possible role of mast cells in the course of human periapical lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:59–63. doi: 10.1016/s1079-2104(03)00378-0. [DOI] [PubMed] [Google Scholar]
- 22.Stashenko P, Teles R, D’Souza R. Periapical inflammatory responses and their modulation. Crit Rev Oral Biol Med. 1998;9:498–521. doi: 10.1177/10454411980090040701. [DOI] [PubMed] [Google Scholar]
- 23.Rodini CO, Batista AC, Lara VS. Comparative immunohistochemical study of the presence of mast cells in apical granulomas and periapical cysts: possible role of mast cells in the course of human periapical lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:59–63. doi: 10.1016/s1079-2104(03)00378-0. [DOI] [PubMed] [Google Scholar]
- 24.Fonseca-Silva T, Santos CC, Alves LR, Dias LC, Brito M Jr, De Paula AM, Guimarães AL. Detection and quantification of mast cell, vascular endothelial growth factor, and microvessel density in human inflammatory periapical cysts and granulomas. Int Endod J. 2012;45:859–864. doi: 10.1111/j.1365-2591.2012.02043.x. [DOI] [PubMed] [Google Scholar]


