Abstract
Melanoma is the leading cause of death in patients with skin cancer. In the present study, we aimed to prove the functions and molecular mechanisms of lncRNA-GAS5 in melanoma. Herein, we found that the expression of GAS5 was down-regulated in melanoma tissues compared to adjacent normal tissues. GAS5 was significantly associated with distal metastasis and TNM stage in melanoma. Furthermore, we found that GAS5 suppressed melanoma cell proliferation, migration and invasion. Then, we found thatmiR-137 was decreased in melanoma tissues compared to adjacent normal tissues and was correlated with GAS5. Using a luciferase reporter gene assay, we also demonstrated that GAS5 positively regulated miR-137 transcription. Finally, we suggested that GAS5 inhibited the growth of melanoma through miR-137 in vivo. Therefore, our research demonstrated that the GAS5/miR-137 axis could be a potential therapeutic target for the treatment of melanoma.
Keywords: lncRNA-GAS5, miR-137, melanoma, proliferation, migration and invasion
Introduction
There are approximately 160,000 patients with melanoma, and approximately 48,000 patients die of malignant melanoma each year worldwide [1]. The incidence of melanoma is high in patients under 40 years of age [2]. The development of metastatic melanoma treatment is slow. Presently, interferons serve as an adjuvant drug that can improve overall survival [3]. For malignant melanoma, an early diagnosis is very important. It is reported that the 5-year survival rate is more than 90% for malignant melanoma diagnosed at an early stage after surgical excision. Nevertheless, when malignant melanoma is not diagnosed at an early stage, it may invade to the lymph nodes, and then the overall survival is less than 1 year [4]. Presently, systemic therapy is important for the treatment of malignant melanoma. However, there are many limitations. Because of a lack of effective biomarkers, the diagnosis technology for melanoma is limited. The molecular and functional mechanisms of melanoma still need to be further studied.
Currently, a large body of literature has emerged supporting the biological significance of noncoding RNAs (ncRNAs) in tumor progression. Increasingly, ncRNAs are especially important for the regulation of gene expression [5,6]. NcRNAs include microRNA (miRNA), long noncoding RNA (lncRNA), circular RNA (circRNA), transfer RNA, ribosomal RNA, and small nucleolar RNA. Among these, lncRNAs, with sizes larger than 200 nucleotides, account for the largest portion of RNA genes, which have important effects on diverse cellular maintenance functions, including protein scaffolding, chromatin looping, and the regulation of mRNA stability [7]. LncRNAs are regarded as a new class of non-coding RNAs that contribute to cancer development and progression [8]. However, understanding the function and mechanism of lncRNA-GAS5 in melanoma is just beginning. In this study, we demonstrated that the mRNA expression level of GAS5 was decreased in melanoma, and GAS5 inhibited the progression of melanoma cell proliferation, migration and invasion.
MiRNAs are also a major type of endogenous non-coding small RNAs of approximately 20 nucleotides. Studies indicate that miRNAs participate in post-transcriptional regulation to affect the biological processes by targeting the 3’-UTR of target genes [9-12] and playcrucial roles in the occurrence and development of various diseases, including the progression and tumorigenesis of human cancers [10,13,14]. Various miRNAs are related to the disease progression of melanoma, such as miR-137 [15], miR-214 [16], and miR-34a [17]. Thus, miRNAs can be used as biological targets for the diagnosis and treatment of melanoma. Using a dual luciferase reporter gene assay, our study indicated that GAS5 positively regulated miR-137 transcription in melanoma.
In our study, we demonstrated the effects of GAS5 in melanoma, the functional mechanisms between GAS5 and miR-137, and potential downstream genes regulating these processes. Our research also demonstrated that GAS5 could be a pivotal potential therapeutic target for the treatment of melanoma.
Materials and methods
Clinical specimens
In this study, we collected tissue samples from 94 patients with melanoma in The General Hospital of Jinan Military Command and Jinan Central Hospital Affiliated to Shandong University between 2014 and 2016. Informed consent was obtained from each patient. This study was approved by the Ethics committee of The General Hospital of Jinan Military Command and Jinan Central Hospital Affiliated to Shandong University. The histological diagnosis of melanoma was evaluated based on the World Health Organization (WHO). All of the tissue samples were stored at -80°C.
Cell lines
Human epidermal melanocytes (HEMn), melanoma cell lines (A2058, B16, M21, MM200, MEL-RM and A375), and HEK293T cells were purchased from the College of Life Science, Hunan Normal University, China. All cells were cultured in Dulbecco’s modified Eagle’s (DMEM) medium (Invitrogen, Carlsbad, CA, USA), including 10% fetal bovine serum (FBS; Invitrogen, Carlsbad, CA, USA), 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C in an appropriate incubator.
Lentiviral vector construction
Human GAS5 DNA was amplified by RT-PCR from A375 cells. Then, the PCR products were inserted into a lentiviral vector. Lentiviral vectors expressing EGFP were used as a control. Furthermore, we synthesized DNA fragments for shRNA and cloned the shRNA into the human U6 promoter-containing pBluescript SK (+) plasmid (pU6) after annealing. Then, wesubcloned the U6-shRNA cassettes into a lentiviral vector [18,19]. shLuc was used as the control. Lentiviral vectors expressing an shRNA targeting GAS5 were generated. Then, the lentivirus vectors were packaged into HEK293T cells by co-transfecting them with packaging vectors (pCMV-VSVG, pMDLg/pRRE and pRSV-REV). The lentivirus was ultracentrifuged, concentrated, and validated.
Transfection
A375 and A2058 cells (5 × 104 cells/well) were seeded into 24-well plates and were transduced with lentivirus supplemented with 8 µg/mL of polybrene (Sigma-Aldrich Chemie, The Netherlands). The A375 cells were transfected with control and Lenti-GAS5; The A2058 cells were transfected with control and Lenti-shGAS5. G418 (Life Technologies, 0.8 mg/mL) was used to select the stable expression cell lines. Then, the A375 cells transfected with Lenti-GAS5 were transfected with 200 μl of mature miR-137 mimics (100 nM), mock or inhibitors with Lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA) for 72 hrs according to the manufacturer’s protocol. Similarly, the A2058 cells transfected with Lenti-shGAS5 were transfected with 200 μl of mature miR-137 mimics (100 nM), mock or inhibitors with Lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA) for 72 hrs.
RNA reverse transcription
Total RNA was extracted with TRIzol reagent (Invitrogen, CA, USA) from the melanoma tissues, the matched adjacent noncancerous tissues and thetreated A375 and A2058 cells. According to the manufacturer’s protocols, cDNA was synthesized using the RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher).
Quantitative real-time reverse transcription PCR (qRT-PCR)
According to the manufacturer’s instruction, we analyzed the mRNA expression levels using a SYBR-Green PCR Master Mix Kit (Takara). The primer sequences for GAPDH were 5’-TGTTCGTCATGGGTGTGAAC-3’ (the forward primer) and 5’-ATGGCATGGACTGTGGTCAT-3’ (the reverse primer) (internal control). The primer sequences for GAS5 were 5’- TCT GAG CAG GAA TGG CAG TGT-3’ (the forward primer) and 5’- CAT CCT CCT TTG CCA CAG AAC T-3’ (the reverse primer). The primer sequences for U6 were 5’-CTCGCTTCGGCAGCACA-3’ (the forward primer) and 5’-AACGCTTCACGAATTTGCGT-3’ (the reverse primer). The primer sequences for hsa-miR-137 were 5’-TAT TGC TTA AGA ATA CGC GTA G-3’ (the forward primer) and 5’-AAC TCC AGC AGG ACC ATG TGA T-3’ (the reverse primer).
Dual luciferase reporter assay
GAS5 was constructed into a pGl3-basic luciferase reporter vector (Promega, Madison, WI). A375 cells were seeded at density of 5 × 104 cells/well in 24-well plates. A375 cells co-transfected with wild type GAS5 or mutant type GAS5, control or miR-137, and a renilla plasmid (RL-SV40) using Lipofectamine 3000. After 48 hrs, according to the manufacturer’s instructions, a Dual-Luciferase Reporter Assay System (Promega) was used to detect reporter gene activities.
Cell proliferation
An methylthiazoletetrazolium (MTT) assay was performed to determine the ability of cells to proliferate. The treated A375 and A2058 cells (3000 cells/well) were seeded into 96-well plates, and 20 mL of the MTT solution (5 mg/ml) was added to each well at 1, 2, 3, 4 and 5 days. After 4 hrs, 200 mL of dimethyl sulfoxide (DMSO) was added to dissolve the precipitates. A micro-plate reader (Bio Tek Instruments, Inc., Winooski, VT, USA) was used to detect the absorbance at 490 nm.
Migration and invasion assays
For the migration assay, the treated A375 and A2058 cells (1 × 105 cells/well), in serum-free medium, were added to the top of 24-well Millipore transwell chambers (Millipore Corporation, MA, USA), and 600 μl of DMEM complete medium was added to the lower chamber. The cells were incubated for 24 hrs, and the migratory cells were fixed using 4% paraformaldehyde and were stained using a 0.1% crystal violet solution. Then, the number of migratory cells was counted. For the invasion assay, the same procedures were followed, except we precoated diluted matrigel (BD Biosciences) on the upper well of the transwell chamber and incubated it for 1 hr at 37°C.
Nude mouse tumorigenicity assay
This study was approved by the Institutional Committee and was carried out based on the Institutional Animal Care and Use Committee. The treated A375 and A2058 cells (1 × 107 cells in 100 μl) were subcutaneously injected into athymic nude mice. At 10, 15, 20, 25, 30, 35, and 40 days, the mice were killed, and the tumor volumes were measured and calculated.
Statistical analysis
The data were analyzed using an independent t test (SPSS, USA) with Student’s t-test. P < 0.05 was considered statistically significant. All of the experiments were repeated 3 times. The data are presented as the mean ± SE.
Results
GAS5 is down-regulated in melanoma tissues
We separated out the melanoma tissues and randomly pairedmelanoma samples with the adjacent normal tissues from 94 patients. The mRNA expression level of GAS5 was analyzed by qRT-PCR. Our results showed that the mRNA expression level of GAS5 was down-regulated in melanoma tissues compared to the adjacent normal tissues (P < 0.0001) (Figure 1A). As shown in Table 1, the mRNA expression level of GAS5 was significantly related to distal metastasis (P < 0.05) and the TNM stage (P < 0.05) by a clinicopathological analysis in the melanoma tissues. The ROC curve serves as a comprehensive index and reflects the sensitivity and specificity of continuous variables. The incidence of melanoma was predicted by an ROC curve analysis using GAS5 between the 94 melanoma patients and controls. The area under the ROC curves was 0.886 (sensitivity = 0.904, specificity = 0.840, P < 0.001) (Figure 1B).
Figure 1.
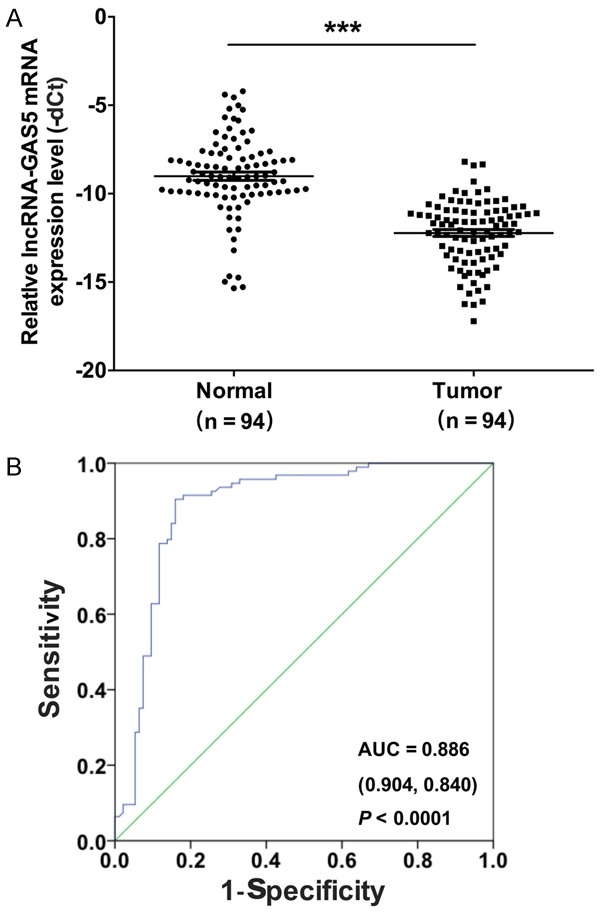
GAS5 is down-regulated in melanoma tissues. A. The mRNA expression level of GAS5 was detected by qRT-PCR in 94 melanoma tissues and their paired adjacent normal tissues (***P < 0.001). B. The receiver operating characteristic curve (ROC) was used to analyze the cut-off score of GAS5.
Table 1.
The relationship between GAS5 mRNA expression levels (dCt) and the clinicopathological factors in melanoma tissues
| Characteristics | No. of patients (%) | GAS5 | |
|---|---|---|---|
|
|
|||
| Mean ± SEM | P value | ||
| Total no. of patients | 94 | ||
| Age (years) | |||
| > 60 | 42 (44.7) | 10.97 ± 2.75 | 0.85 |
| ≤ 60 | 52 (55.3) | 11.58 ± 1.75 | |
| Gender | |||
| Male | 36 (38.3) | 11.95 ± 2.53 | 0.91 |
| Female | 58 (61.7) | 11.47 ± 2.86 | |
| Lymphatic metastasis | |||
| N0 | 41 (43.6) | 9.96 ± 2.63 | 0.54 |
| N1-N2 | 53 (56.4) | 11.85 ± 1.74 | |
| Distal metastasis | |||
| M0 | 57 (60.6) | 9.08 ± 1.27 | 0.04* |
| M1 | 37 (39.4) | 12.83 ± 1.04 | |
| TNM stage | |||
| 0, I & II | 39 (41.5) | 9.25 ± 0.67 | 0.02* |
| III & IV | 55 (58.5) | 12.65 ± 1.05 | |
Indicated statistical significance (P < 0.05).
GAS5 suppresses the ability of melanoma cell proliferation, migration and invasion
We further analyzed the expression effect of GAS5 in human epidermal melanocytes (HEMn) and melanoma cell lines (A2058, B16, M21, MM200, MEL-RM and A375). qRT-PCR was performed to detect the mRNA expression level of GAS5 in the cell lines mentioned above. We found that GAS5 was dramatically decreased in the melanoma cells compared to the HEMn cells (P < 0.0001). Among the melanoma cells, the expression level of GAS5 was lowest in the A375 cells and highest in the A2058 cells (Figure 2A). Thus, we chose the A375 and A2058 cells as target cells.
Figure 2.
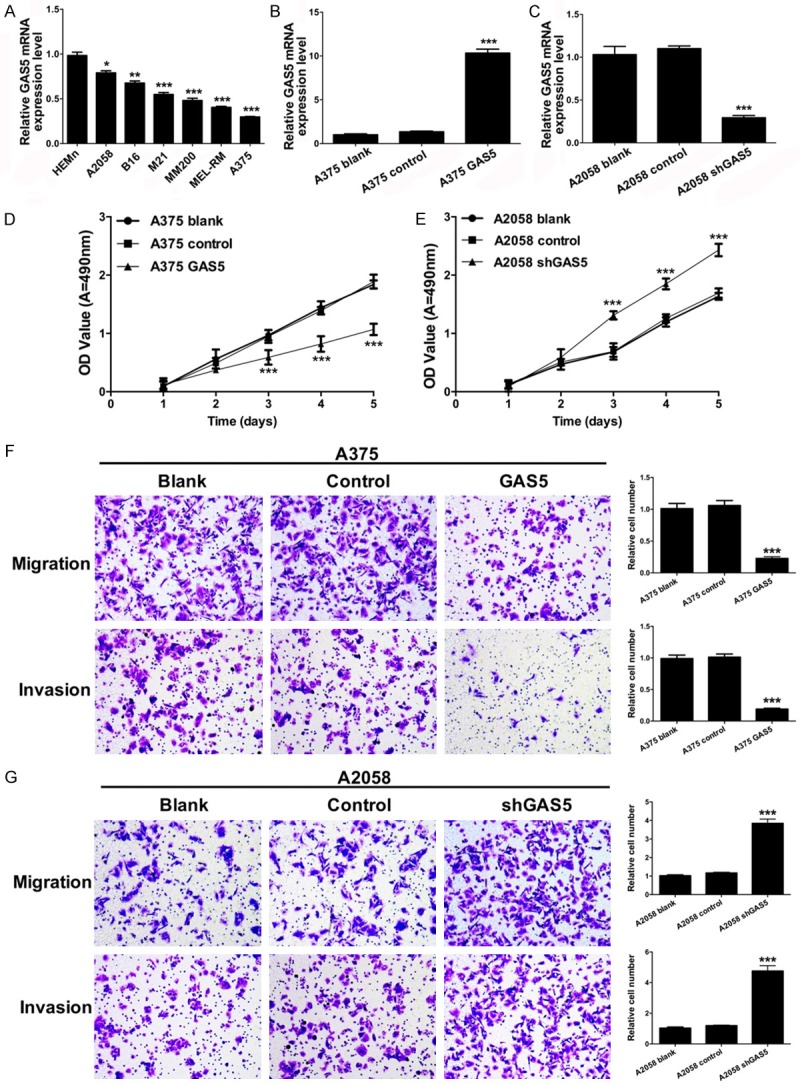
GAS5 suppresses melanoma cell proliferation, migration and invasion. A. qRT-PCR was used to analyze the mRNA expression level of GAS5 in human epidermal melanocytes (HEMn) and melanoma cell lines (A2058, B16, M21, MM200, MEL-RM and A375) (*P < 0.05, **P < 0.01, ***P < 0.001). B. The mRNA expression level of GAS5 was measured by qRT-PCR in the A375 cells transfected with control and GAS5 (***P < 0.001). C. The mRNA expression level of GAS5 was measured by qRT-PCR in the A2058 cells transfected with control and shGAS5 (***P < 0.001). D. An MTT assay was performed to detect the proliferative ability of the A375 cells transfected with control and GAS5 (***P < 0.001). E. An MTT assay was performed to detect the proliferative ability of the A2058 cells transfected with control and shGAS5 (***P < 0.001). F. Migration and invasion were measured by a Transwell assay in the A375 cells transfected with control and GAS5 (***P < 0.001). G. Migration and invasion were measured by a Transwell assay in the A2058 cells transfected with control and shGAS5 (***P < 0.001).
Furthermore, we investigated the effects of GAS5 on melanoma cell proliferation, migration, and invasion. A375 cells were transfected with control and lenti-GAS5, and A2058 cells were transfected with control and lenti-shGAS5. The expression effects of GAS5 were measured by qRT-PCR. We found that GAS5 mRNA was markedly overexpressed in the A375 cells transfected with lenti-GAS5 (P < 0.0001) (Figure 2B), and the mRNA expression of GAS5 was significantly suppressed in the A2058 cells transfected with lenti-shGAS5 (P < 0.0001) (Figure 2C).
We then detected the effect of GAS5 on melanoma cell growth using an MTT assay. As shown in Figure 2D, the proliferative ability of the A375 cells transfected with GAS5 was significantly decreased compared to the control group (P < 0.0001). Similarly, the proliferative ability of the A2058 cells transfected with shGAS5 was significantly increased compared to the control group (P < 0.0001) (Figure 2E).
The effects of GAS5 on migration and invasion of melanoma cells were analyzed using the Transwell assays. As shown in Figure 2F, the migration and invasion abilities were significantly inhibited in the A375 cells transfected with GAS5 compared with the control groups (P < 0.0001). Simultaneously, the migration and invasion abilities were significantly promoted in the A2058 cells transfected with shGAS5 compared with the control groups (P < 0.0001) (Figure 2G).
miR-137 is down-regulated in melanoma tissues
To evaluate the mRNA expression level of miR-137 in melanoma, qRT-PCR was used to measure the miR-137 expression in the melanoma tissues (n = 94) and the paired adjacent normal tissues (n = 94). The results indicated that the mRNA expression level of miR-137 was significantly lower in the melanoma tissues compared to paired the adjacent normal tissues (Figure 3A). Furthermore, the mRNA expression level of miR-137 was significantly related to distal metastasis (P < 0.05) and the TNM stage (P < 0.05) by a clinicopathological analysis in the melanoma tissues (Table 2). We then analyzed the incidence of melanoma with a ROC curve analysis of GAS5 between the 94 melanoma patients and controls. The area under the ROC curves was 0.886 (sensitivity = 0.894, specificity = 0.851, P < 0.001) (Figure 3B). Then, we analyzed the mRNA expression relationship between GAS5 and miR-137 by qRT-PCR. We found that GAS5 mRNA expression positively correlated with miR-137 mRNA expression (R2 = 0.2122, P < 0.001, Figure 3C).
Figure 3.
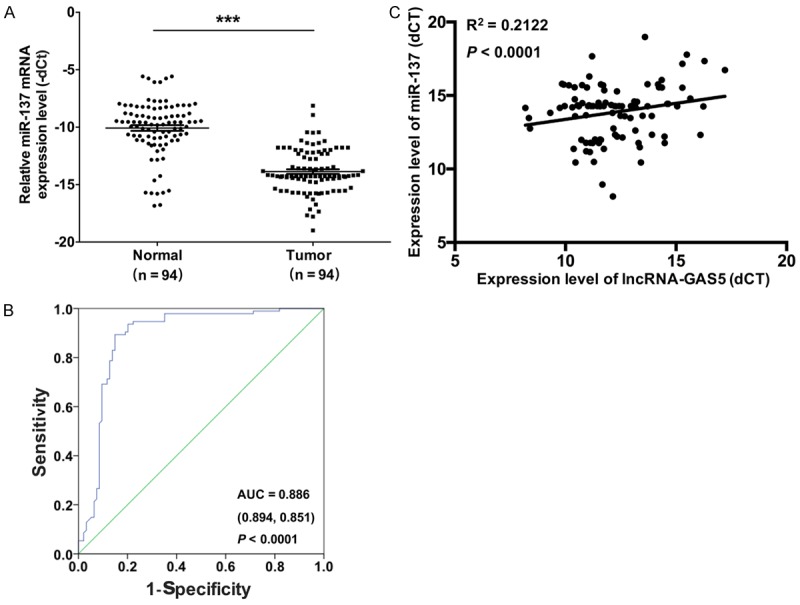
miR-137 is down-regulated in melanoma tissues. A. The mRNA expression level of miR-137 was detected by qRT-PCR in 94 melanoma tissues and their paired adjacent normal tissues (***P < 0.001). B. The receiver operating characteristic curve (ROC) was used to analyze the cut-off score of miR-137. C. The correlation between GAS5 and miR-137 expression was analyzed (R2 = 0.2122, P < 0.001).
Table 2.
The relationship between miR-137 mRNA expression level (dCt) and the clinicopathological factors in melanoma tissues
| Characteristics | No. of patients (%) | miR-137 | |
|---|---|---|---|
|
|
|||
| Mean ± SE | P value | ||
| Total no. of patients | 94 | ||
| Age (years) | |||
| > 60 | 42 (44.7) | 12.11 ± 1.37 | 0.83 |
| ≤ 60 | 52 (55.3) | 11.64 ± 1.69 | |
| Gender | |||
| Male | 36 (38.3) | 12.96 ± 1.97 | 0.63 |
| Female | 58 (61.7) | 11.82 ± 1.38 | |
| Lymphatic metastasis | |||
| N0 | 41 (43.6) | 11.07 ± 1.24 | 0.20 |
| N1-N2 | 53 (56.4) | 13.55 ± 1.41 | |
| Distal metastasis | |||
| M0 | 57 (60.6) | 10.17 ± 0.95 | 0.02* |
| M1 | 37 (39.4) | 13.47 ± 1.01 | |
| TNM stage | |||
| 0, I & II | 39 (41.5) | 10.27 ± 0.94 | 0.03* |
| III & IV | 55 (58.5) | 13.75 ± 1.11 | |
Indicated statistical significance (P < 0.05).
GAS5 positively regulates miR-137 transcription
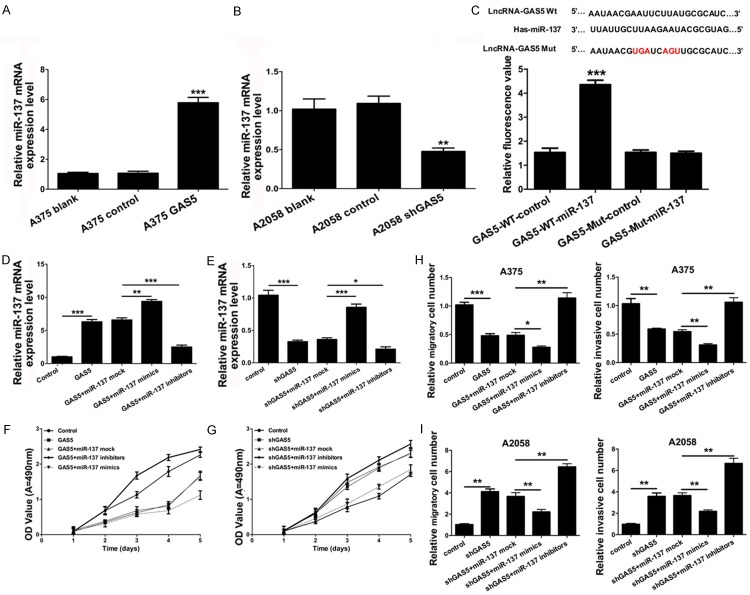
According to the positive correlation between GAS5 mRNA expression and miR-137 mRNA expression, we further investigated that miR-137 may be the downstream effect or gene of GAS5. Our results demonstrated that miR-137 was upregulated in the A375 cells transfected with GAS5 relative to the control group (P < 0.001) (Figure 4A). Meanwhile, miR-137 was down-regulated in the A2058 cells transfected with shGAS5 relative to the control group (P < 0.001) (Figure 4B). Therefore, the qRT-PCR confirmed that the over-expression of GAS5 increased the mRNA expression level of miR-137, whereas silencing GAS5 decreased the mRNA expression of miR-137. To determine whether GAS5 interacts with miR-137 in melanoma cells, we speculated that GAS5 may directly regulate the expression of miR-137. We cloned the wild type GAS5 or a mutant type GAS5 into a pGL3-basic luciferase reporter vector. The luciferase reporter gene assays indicated that GAS5 increased the fluorescence activity (Figure 4C).
Figure 4.
GAS5 positively regulates miR-137 transcription and inhibits the progression of cell proliferation, migration and invasion via miR-137 in melanoma cells. A. qRT-PCR was used to analyze the mRNA expression level of miR-137 in the A375 cells transfected with control and GAS5 (***P < 0.001). B. The mRNA expression level of miR-137 was measured by qRT-PCR in the A2058 cells transfected with control and shGAS5 (***P < 0.001). C. The relative fluorescence value was detected by the luciferase reporter gene assay in theA375 cells co-transfected with wild type GAS5 or a mutant type GAS5 and control or miR-137 (***P < 0.001). D. qRT-PCR was used to analyze the mRNA expression level of miR-137 in the A375 cells transfected with control, GAS5, GAS5 and miR-137 control, GAS5 and miR-137 mimics, or GAS5 and miR-137 inhibitors (**P < 0.01, ***P < 0.001). E. qRT-PCR was used to analyze the mRNA expression level of miR-137 in the A2058 cells transfected with control, shGAS5, shGAS5 and miR-137 control, shGAS5 and miR-137 mimics, or shGAS5 and miR-137 inhibitors (*P < 0.05, ***P < 0.001). F. An MTT assay was performed to measure cell proliferation in thetreated A375 cells as described in D. G. An MTT assay was performed to measure cell proliferation in thetreated A2058 cells as described in E. H. Migration and invasion were detected in thetreated A375 cells as described in D by a Transwell assay (*P < 0.05, **P < 0.01, ***P < 0.001). I. Migration and invasion were detected in the A2058 cells treated as described E by a Transwell assay (**P < 0.01).
GAS5 inhibits cell proliferation, migration and invasion via miR-137 in melanoma cells
Due to the interaction relationship between GAS5 and miR-137, we sought to determine whether GAS5 inhibits the progression of cell proliferation via miR-137 in melanoma cells. First, A375 cells were transfected with control, GAS5, GAS5 and miR-137 control, GAS5 and miR-137 mimics, or GAS5 and miR-137 inhibitors; A2058 cells were transfected with control, shGAS5, shGAS5 and miR-137 control, shGAS5 and miR-137 mimics, or shGAS5 and miR-137 inhibitors. Second, qRT-PCR was used to analyze the mRNA expression level of miR-137. We found that GAS5 markedly increased the mRNA expression of miR-137 in the A375 cells transfected with GAS5 (P < 0.001). The mRNA expression of miR-137 was upregulated in the A375 cells transfected with GAS5 and miR-137 mimics compared to GAS5 and the miR-137 control (P < 0.001). The mRNA expression of miR-137 was down-regulated in the A375 cells transfected with GAS5 and miR-137 inhibitors compared to GAS5 and the miR-137 control (P < 0.001) (Figure 4D). Similarly, we found that GAS5 markedly decreased the mRNA expression of miR-137 in the A2058 cells transfected with shGAS5 (P < 0.001). The mRNA expression of miR-137 was upregulated in the A2058 cells transfected with GAS5 and the miR-137 mimics compared to GAS5 and the miR-137 control (P < 0.001). The mRNA expression of miR-137 was down-regulated in the A2058 cells transfected with GAS5 and the miR-137 inhibitors compared to GAS5 and the miR-137 control (P < 0.001) (Figure 4E). Furthermore, our results indicated that GAS5 suppressed the progression of cell proliferation via miR-137 in the A375 cells (Figure 4F); Silencing GAS5 promoted the progression of cell proliferation through miR-137 in the A2058 cells (Figure 4G). As shown in Figure 4H, GAS5 inhibited the progression of cell migration and invasion via miR-137 in the A375 cells. Similarly, silencing GAS5 promoted the progression of cell migration and invasion through miR-137 in the A2058 cells (Figure 4I).
GAS5 inhibits the growth of melanoma through miR-137 in vivo
To further assess the effect of GAS5 on tumorigenesis in vivo, A375 cells transfected with control and GAS5 were subcutaneously implanted into nude mice, and A2058 cells transfected with control and shGAS5 were also subcutaneously implanted into nude mice. The mice were killed at 10, 15, 20, 25, 30, 35 and 40 days. The tumor volume was measured and calculated. As shown in Figure 5A, GAS5 inhibited the growth of melanoma. Silencing GAS5 promoted the growth of melanoma (Figure 5B). We then extracted total RNA from the nude mice. qRT-PCR was used to detect the mRNA expression level of miR-137. We found that miR-137 was increased in the A375 cells that were transfected with GAS5 compared to the control group (P < 0.001) (Figure 5C), and miR-137 was decreased in the A2058 cells that were transfected with shGAS5 compared to the control group (P < 0.001) (Figure 5C).
Figure 5.
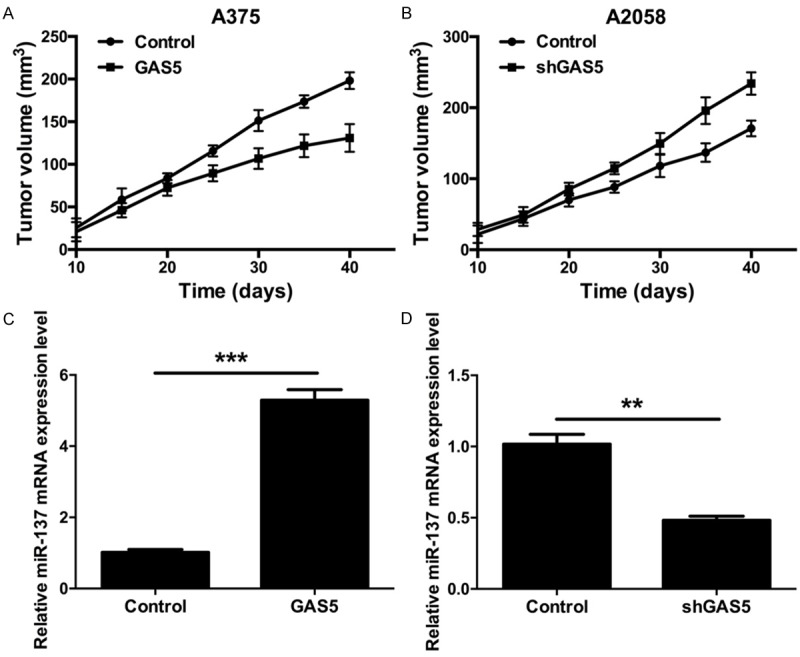
GAS5 inhibits the growth of melanoma in vivo. A. GAS5 expression reduced the volume of melanoma in nude mice. Athymic mice were injected with the A375 cells transfected with control or GAS5. Tumor volume was detected at particular time points. B. GAS5 expression reduced the volume of melanoma in nude mice. Athymic mice were injected with the A2058 cells transfected with control or shGAS5. Tumor volume was detected at particular time points. C. The mRNA expression level of miR-137 was detected by qRT-PCR in nude mice injected with the A375 cells that were transfected with control and GAS5 (***P < 0.001). D. The mRNA expression level of miR-137 was detected by qRT-PCR in nude mice injected with the A2058 cells that were transfected with control or shGAS5 (**P < 0.01).
Discussion
LncRNAs are long non-coding RNAs long greater than 200 nucleotides [5,20] which are non-protein coding transcripts transcribed by RNA polymerase II [21]. LncRNAs play important roles in chromatin structure [22], including epigenetic, transcriptional, posttranscriptional, and translational mechanisms [23,24], promoting numerous biological functions. LncRNAs participate in various biological processes, such as proliferation [25], differentiation [26], and carcinogenesis [27], and numerous human diseases [28-30]. For example, SLNCR1 regulates melanoma invasion through the SRA1-like region [31]; the UCA1-miR-507-FOXM1 axis participates in melanoma cell proliferation, invasion and cell cycle arrest [32]; and HOTAIR is related to the motility, invasion, and metastasis of metastatic melanoma [33]. Studies indicate that GAS5 is involved in various cancers. Currently, accumulating studies demonstrate that a class of lncRNAs are dysregulated in hepatocellular carcinoma (HCC) and are closely related with tumorigenesis, metastasis, prognosis or diagnosis [34]. The down-regulation of GAS5 is associated with the prognosis of HCC, and GAS5 suppresses the migration and invasion of HCC cells through miR-21 [35]. Furthermore, there is a critical role for GAS5 in the proliferation and apoptosis abilities of non-small-cell lung cancer [36]. GAS5 also inhibits bladder cancer cell proliferation [37]. In our study, we found that the mRNA expression level of GAS5 was down-regulated in melanoma tissues compared to adjacent normal tissues. GAS5 was significantly associated with distal metastasis and the TNM stage, and there was a good prognosis effect of GAS5 in melanoma. Furthermore, we demonstrated that GAS5 suppressed melanoma cell proliferation, migration and invasion. Therefore, GAS5 inhibits the developmental process of melanoma.
MiRNAs are small, noncoding RNAs 19-22 nucleotides in length with important functions in development, cell differentiation, and the regulation of the cell cycle and apoptosis [38]. A growing body of research suggests that miRNAs are dysregulated in various tumor types, and the detection of their levels promises to be a new indicator for cancer diagnosis. miRNAs can serve as oncogenes or tumor suppressor genes to regulate mRNA expression [39]. Currently, miR-137 acts as an important tumor suppressor and plays an important role in tumor growth, migration and invasion. For example, miR-137 was shown to inhibit the invasion ability of melanoma cells by down-regulating oncogenes [40], and miR-137 serves as a tumor suppressor in colorectal cancer [41]. MiR-137 suppresses the proliferative ability of lung cancer cells by modulating the expression of Cdc42 and Cdk6 [42] and serves as a tumor suppressor in neuroblastoma by regulating KDM1A [43]. In our study, we found that miR-137 was down-regulated in melanoma tissues compared to adjacent normal tissues. MiR-137 was significantly associated with distal metastasis and the TNM stage, and there was a good prognostic effect of miR-137 in melanoma. Furthermore, we found that there was a positive correlation between miR-137 and GAS5 in melanoma tissues and that GAS5 positively regulated miR-137 transcription. At the same time, we indicated that GAS5 inhibited cell proliferation, migration and invasion through miR-137 in melanoma cells, and GAS5 inhibited the growth of melanoma through miR-137 in vivo.
In summary, we demonstrated that the mRNA expression levels of both GAS5 and miR-137 were down-regulated in melanoma tissues compared to adjacent normal tissues. Both GAS5 and miR-137 were significantly associated with distal metastasis and the TNM stage, and there were good prognostic effects for both GAS5 and miR-137 in melanoma. Furthermore, we found that there was a positive correlation between miR-137 and GAS5 in melanoma tissues, and GAS5 positively regulated miR-137 transcription. We also indicated that GAS5 inhibited the progression of cell proliferation, migration and invasion through miR-137 in melanoma cells. Finally, we proved that GAS5 inhibited the growth of melanoma through miR-137 in vivo. Therefore, our research indicates that GAS5 could be a potential therapeutic target for the treatment of melanoma.
Disclosure of conflict of interest
None.
References
- 1.Boyle P, Levin B International Agency for Research on Cancer; World Health Organization. World cancer report 2008. LyonGeneva: International Agency for Research on Cancer; Distributed by WHO Press; 2008. [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 3.Petrella T, Verma S, Spithoff K, Quirt I, McCready D Melanoma Disease Site Group. Adjuvant interferon therapy for patients at high risk for recurrent melanoma: an updated systematic review and practice guideline. Clin Oncol (R Coll Radiol) 2012;24:413–423. doi: 10.1016/j.clon.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Mashima E, Inoue A, Sakuragi Y, Yamaguchi T, Sasaki N, Hara Y, Omoto D, Ohmori S, Haruyama S, Sawada Y, Yoshioka M, Nishio D, Nakamura M. Nivolumab in the treatment of malignant melanoma: review of the literature. Onco Targets Ther. 2015;8:2045–2051. doi: 10.2147/OTT.S62102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell. 2011;43:904–914. doi: 10.1016/j.molcel.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mattick JS, Makunin IV. Non-coding RNA. Hum Mol Genet. 2006;15 Spec No 1:R17–29. doi: 10.1093/hmg/ddl046. [DOI] [PubMed] [Google Scholar]
- 7.Hayes EL, Lewis-Wambi JS. Mechanisms of endocrine resistance in breast cancer: an overview of the proposed roles of noncoding RNA. Breast Cancer Res. 2015;17:40. doi: 10.1186/s13058-015-0542-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li CH, Chen Y. Targeting long non-coding RNAs in cancers: progress and prospects. Int J Biochem Cell Biol. 2013;45:1895–1910. doi: 10.1016/j.biocel.2013.05.030. [DOI] [PubMed] [Google Scholar]
- 9.Iorio MV, Croce CM. MicroRNAs in cancer: small molecules with a huge impact. J. Clin. Oncol. 2009;27:5848–5856. doi: 10.1200/JCO.2009.24.0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farazi TA, Hoell JI, Morozov P, Tuschl T. MicroRNAs in human cancer. Adv Exp Med Biol. 2013;774:1–20. doi: 10.1007/978-94-007-5590-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Djuranovic S, Nahvi A, Green R. A parsimonious model for gene regulation by miRNAs. Science. 2011;331:550–553. doi: 10.1126/science.1191138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kasinski AL, Slack FJ. Epigenetics and genetics. MicroRNAs en route to the clinic: progress in validating and targeting microRNAs for cancer therapy. Nat Rev Cancer. 2011;11:849–864. doi: 10.1038/nrc3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pimentel AM, Kobayashi D, Kliemann LM, Franjdlich R, Capp E, Corleta HV. Transvaginal ultrasound ovarian diathermy: sheep as an experimental model. J Ovarian Res. 2012;5:1. doi: 10.1186/1757-2215-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Leva G, Croce CM. The role of microRNAs in the tumorigenesis of ovarian cancer. Front Oncol. 2013;3:153. doi: 10.3389/fonc.2013.00153. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Bemis LT, Chen R, Amato CM, Classen EH, Robinson SE, Coffey DG, Erickson PF, Shellman YG, Robinson WA. MicroRNA-137 targets microphthalmia-associated transcription factor in melanoma cell lines. Cancer Res. 2008;68:1362–1368. doi: 10.1158/0008-5472.CAN-07-2912. [DOI] [PubMed] [Google Scholar]
- 16.Penna E, Orso F, Cimino D, Tenaglia E, Lembo A, Quaglino E, Poliseno L, Haimovic A, Osella-Abate S, De Pitta C, Pinatel E, Stadler MB, Provero P, Bernengo MG, Osman I, Taverna D. microRNA-214 contributes to melanoma tumour progression through suppression of TFAP2C. EMBO J. 2011;30:1990–2007. doi: 10.1038/emboj.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yan D, Zhou X, Chen X, Hu DN, Dong XD, Wang J, Lu F, Tu L, Qu J. MicroRNA-34a inhibits uveal melanoma cell proliferation and migration through downregulation of c-Met. Invest Ophthalmol Vis Sci. 2009;50:1559–1565. doi: 10.1167/iovs.08-2681. [DOI] [PubMed] [Google Scholar]
- 18.Chen Y, Lin MC, Yao H, Wang H, Zhang AQ, Yu J, Hui CK, Lau GK, He ML, Sung J, Kung HF. Lentivirus-mediated RNA interference targeting enhancer of zeste homolog 2 inhibits hepatocellular carcinoma growth through down-regulation of stathmin. Hepatology. 2007;46:200–208. doi: 10.1002/hep.21668. [DOI] [PubMed] [Google Scholar]
- 19.Jiang L, Lai YK, Zhang J, Wang H, Lin MC, He ML, Kung HF. Targeting S100P inhibits colon cancer growth and metastasis by Lentivirus-mediated RNA interference and proteomic analysis. Mol Med. 2011;17:709–716. doi: 10.2119/molmed.2011.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kong L, Zhang Y, Ye ZQ, Liu XQ, Zhao SQ, Wei L, Gao G. CPC: assess the protein-coding potential of transcripts using sequence features and support vector machine. Nucleic Acids Res. 2007;35:W345–349. doi: 10.1093/nar/gkm391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guttman M, Amit I, Garber M, French C, Lin MF, Feldser D, Huarte M, Zuk O, Carey BW, Cassady JP, Cabili MN, Jaenisch R, Mikkelsen TS, Jacks T, Hacohen N, Bernstein BE, Kellis M, Regev A, Rinn JL, Lander ES. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature. 2009;458:223–227. doi: 10.1038/nature07672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rinn JL, Kertesz M, Wang JK, Squazzo SL, Xu X, Brugmann SA, Goodnough LH, Helms JA, Farnham PJ, Segal E, Chang HY. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell. 2007;129:1311–1323. doi: 10.1016/j.cell.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qiu MT, Hu JW, Yin R, Xu L. Long noncoding RNA: an emerging paradigm of cancer research. Tumour Biol. 2013;34:613–620. doi: 10.1007/s13277-013-0658-6. [DOI] [PubMed] [Google Scholar]
- 24.Eades G, Zhang YS, Li QL, Xia JX, Yao Y, Zhou Q. Long non-coding RNAs in stem cells and cancer. World J Clin Oncol. 2014;5:134–141. doi: 10.5306/wjco.v5.i2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136:629–641. doi: 10.1016/j.cell.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 26.Guttman M, Donaghey J, Carey BW, Garber M, Grenier JK, Munson G, Young G, Lucas AB, Ach R, Bruhn L, Yang X, Amit I, Meissner A, Regev A, Rinn JL, Root DE, Lander ES. LincRNAs act in the circuitry controlling pluripotency and differentiation. Nature. 2011;477:295–300. doi: 10.1038/nature10398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, Wang Y, Brzoska P, Kong B, Li R, West RB, van de Vijver MJ, Sukumar S, Chang HY. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–1076. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taft RJ, Pang KC, Mercer TR, Dinger M, Mattick JS. Non-coding RNAs: regulators of disease. J Pathol. 2010;220:126–139. doi: 10.1002/path.2638. [DOI] [PubMed] [Google Scholar]
- 29.Klattenhoff CA, Scheuermann JC, Surface LE, Bradley RK, Fields PA, Steinhauser ML, Ding H, Butty VL, Torrey L, Haas S, Abo R, Tabebordbar M, Lee RT, Burge CB, Boyer LA. Braveheart, a long noncoding RNA required for cardiovascular lineage commitment. Cell. 2013;152:570–583. doi: 10.1016/j.cell.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gutschner T, Hammerle M, Eissmann M, Hsu J, Kim Y, Hung G, Revenko A, Arun G, Stentrup M, Gross M, Zornig M, MacLeod AR, Spector DL, Diederichs S. The noncoding RNA MALAT1 is a critical regulator of the metastasis phenotype of lung cancer cells. Cancer Res. 2013;73:1180–1189. doi: 10.1158/0008-5472.CAN-12-2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmidt K, Joyce CE, Buquicchio F, Brown A, Ritz J, Distel RJ, Yoon CH, Novina CD. The lncRNA SLNCR1 mediates melanoma invasion through a conserved SRA1-like region. Cell Rep. 2016;15:2025–2037. doi: 10.1016/j.celrep.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei Y, Sun Q, Zhao L, Wu J, Chen X, Wang Y, Zang W, Zhao G. LncRNA UCA1-miR-507-FOXM1 axis is involved in cell proliferation, invasion and G0/G1 cell cycle arrest in melanoma. Med Oncol. 2016;33:88. doi: 10.1007/s12032-016-0804-2. [DOI] [PubMed] [Google Scholar]
- 33.Tang L, Zhang W, Su B, Yu B. Long noncoding RNA HOTAIR is associated with motility, invasion, and metastatic potential of metastatic melanoma. Biomed Res Int. 2013;2013:251098. doi: 10.1155/2013/251098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He Y, Meng XM, Huang C, Wu BM, Zhang L, Lv XW, Li J. Long noncoding RNAs: novel insights into hepatocelluar carcinoma. Cancer Lett. 2014;344:20–27. doi: 10.1016/j.canlet.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 35.Hu L, Ye H, Huang G, Luo F, Liu Y, Liu Y, Yang X, Shen J, Liu Q, Zhang J. Long noncoding RNA GAS5 suppresses the migration and invasion of hepatocellular carcinoma cells via miR-21. Tumour Biol. 2016;37:2691–2702. doi: 10.1007/s13277-015-4111-x. [DOI] [PubMed] [Google Scholar]
- 36.Shi X, Sun M, Liu H, Yao Y, Kong R, Chen F, Song Y. A critical role for the long non-coding RNA GAS5 in proliferation and apoptosis in non-small-cell lung cancer. Mol Carcinog. 2015;54(Suppl 1):E1–E12. doi: 10.1002/mc.22120. [DOI] [PubMed] [Google Scholar]
- 37.Liu Z, Wang W, Jiang J, Bao E, Xu D, Zeng Y, Tao L, Qiu J. Downregulation of GAS5 promotes bladder cancer cell proliferation, partly by regulating CDK6. PLoS One. 2013;8:e73991. doi: 10.1371/journal.pone.0073991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garzon R, Calin GA, Croce CM. MicroRNAs in cancer. Annu Rev Med. 2009;60:167–179. doi: 10.1146/annurev.med.59.053006.104707. [DOI] [PubMed] [Google Scholar]
- 39.Rupaimoole R, Calin GA, Lopez-Berestein G, Sood AK. miRNA deregulation in cancer cells and the tumor microenvironment. Cancer Discov. 2016;6:235–246. doi: 10.1158/2159-8290.CD-15-0893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luo C, Tetteh PW, Merz PR, Dickes E, Abukiwan A, Hotz-Wagenblatt A, Holland-Cunz S, Sinnberg T, Schittek B, Schadendorf D, Diederichs S, Eichmuller SB. miR-137 inhibits the invasion of melanoma cells through downregulation of multiple oncogenic target genes. J Invest Dermatol. 2013;133:768–775. doi: 10.1038/jid.2012.357. [DOI] [PubMed] [Google Scholar]
- 41.McFaline-Figueroa JL, Braun CJ, Stanciu M, Nagel ZD, Mazzucato P, Sangaraju D, Cerniauskas E, Barford K, Vargas A, Chen Y, Tretyakova N, Lees JA, Hemann MT, White FM, Samson LD. Minor changes in expression of the mismatch repair protein MSH2 exert a major impact on glioblastoma response to temozolomide. Cancer Res. 2015;75:3127–3138. doi: 10.1158/0008-5472.CAN-14-3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu X, Li Y, Shen H, Li H, Long L, Hui L, Xu W. miR-137 inhibits the proliferation of lung cancer cells by targeting Cdc42 and Cdk6. FEBS Lett. 2013;587:73–81. doi: 10.1016/j.febslet.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 43.Althoff K, Beckers A, Odersky A, Mestdagh P, Koster J, Bray IM, Bryan K, Vandesompele J, Speleman F, Stallings RL, Schramm A, Eggert A, Sprussel A, Schulte JH. MiR-137 functions as a tumor suppressor in neuroblastoma by downregulating KDM1A. Int J Cancer. 2013;133:1064–1073. doi: 10.1002/ijc.28091. [DOI] [PubMed] [Google Scholar]