Abstract
To test direct and indirect effects of glomalin, mycorrhizal hyphae, and roots on aggregate stability, perspex pots separated by 37-μm nylon mesh in the middle were used to form root-free hyphae and root/hyphae chambers, where trifoliate orange (Poncirus trifoliata) seedlings were colonized by Funneliformis mosseae or Paraglomus occultum in the root/hyphae chamber. Both fungal species induced significantly higher plant growth, root total length, easily-extractable glomalin-related soil protein (EE-GRSP) and total GRSP (T-GRSP), and mean weight diameter (an aggregate stability indicator). The Pearson correlation showed that root colonization or soil hyphal length significantly positively correlated with EE-GRSP, difficultly-extractable GRSP (DE-GRSP), T-GRSP, and water-stable aggregates in 2.00–4.00, 0.50–1.00, and 0.25–0.50 mm size fractions. The path analysis indicated that in the root/hyphae chamber, aggregate stability derived from a direct effect of root colonization, EE-GRSP or DE-GRSP. Meanwhile, the direct effect was stronger by EE-GRSP or DE-GRSP than by mycorrhizal colonization. In the root-free hyphae chamber, mycorrhizal-mediated aggregate stability was due to total effect but not direct effect of soil hyphal length, EE-GRSP and T-GRSP. Our results suggest that GRSP among these tested factors may be the primary contributor to aggregate stability in the citrus rhizosphere.
Soil structure, the three-dimensional arrangement of organic/mineral aggregates and pore spaces in soil on scales1, has shown important roles in soil carbon (C) sequestration, nutrient and gas fluxes and water quality2. Soil macroaggregates (>0.25 mm size) establish the bulk soil structure, whilst the microaggregates (<0.25 mm size) constitute part of the sediment load3,4. Soil aggregation refers to soil microaggregates that are bound together by binding agents to form stable macroaggregates5. Meanwhile, water-stable aggregates (WSAs) in macroaggregates, which are stable to the action of repeated soil wetting and drying cycles, are widely used to evaluate aggregate stability by means of the wet-sieving analysis6. As an indicator of soil structure related to soil water regime, erodibility, and nutrient availability, aggregate stability is affected by soil physical, chemical, and/or microbial community properties, root systems, plant species and/or communities7,8,9,10.
Among soil microbial communities, fungi usually present a more profound functioning on stabilizing macroaggregates than bacteria11,12. The ubiquitous soil arbuscular mycorrhizal fungi (AMF) can contribute to soil aggregate stability directly by their extraradical fungal hyphae1,13,14,15 or indirectly by altering the biochemical and morphological properties of host plants2. Such direct and indirect contributions are often intertwined together16. For instance, a microcosm experiment showed that mean weight diameter (MWD, an indicator of aggregate stability) highly positively correlated with soil hyphal length but weakly with root volumes13. The contribution to the formation of 2–5 and 1–2 mm WSA was greater in mycorrhizal hyphae than in maize roots17. However, the AM fungus Glomus geosporum, G. mosseae (now Funneliformis mosseae), or G. intraradices did not, but the roots of Plantago lanceolata did, affect aggregate stability and aggregate size distribution in the rhizosphere of a sandy loam soil18. Recently a hierarchical aggregation model has shown that organic matter, roots, and AMF are required for soil aggregate stability, whilst the contribution of roots to soil structure is further stabilized by AMF19. In a plant-fungus symbiosis, aggregate stability significantly positively correlated with either root length or root mycorrhizal colonization, but the latter showed a stronger correlation9.
Glomalin, a fungal glycoprotein that has not been biochemically defined, but operationally quantified, from diverse soils as glomalin-related soil protein (GRSP)20,21, is only released by an AM fungus into soil during hyphal turnover and after the death of the fungus22. Glomalin generally contains 3–5% N, 36–59% C, 4–6% H, 33–49% O, 0.03–0.1% P, and 2–5% Fe23,24,25,26,27. As a soil particle binding agent, this insoluble, hydrophobic and heat-resistant GRSP strongly relates to the amount of WSA20,21,26,27,28 and aggregate stability in various soils15,17,20,29,30. Recently, GRSP was divided into fraction 1 and fraction 231. In general, the GRSP fraction 1, as a newly produced glomalin, is relatively more labile, corresponding to the former defined easily-extractable GRSP (EE-GRSP), whilst the GRSP fraction 2, as an older glomalin, is more difficultly-to-extract and recalcitrant in soils, defined as difficultly-extractable GRSP (DE-GRSP). However, the knowledge about their different contributions of these two GRSP fractions on soil aggregate stability is limited.
Meanwhile, the specific contribution strength of root AM colonization, mycorrhizal hyphae, GRSP, and root total length to aggregate stability, is not distinguished, directly or indirectly, in a plant-AMF-soil system, although the effect of AMF on aggregate stability has been already recognized by correlation and regression analyses. The path analysis, a statistical method of testing cause/effect relationships that is mainly used in genetics, is now also used to compare the strength of direct or indirect relationships among variables in plants, soil, and plant–soil relationships32,33,34. In general, the path analysis would permit the partitioning of correlation coefficients into components as path coefficients, to determine the direct or indirect effect35. As a result, the path analysis is able to test causal relationships among interacting biological factors. For instance, soil hyphal colonization showed larger direct and total (direct plus indirect) effects on soil water potential than did root hyphal colonization, GRSP or WSA32. Based on the path analysis, the direct effect of GRSP on soil aggregate stabilization was much stronger than that of mycorrhizal hyphae34. Therefore, the advantages of the path analysis are to examine the relative strength of causal factors involved in stabilizing aggregates32.
Citrus is one of the most important fruit trees with annually ~100 million tons of fruit production around the world. Citrus plants in the field show little or no root hairs and thus are highly dependent on mycorrhizal symbiosis to absorb water and nutrients. Our previous works showed that under potted conditions GRSP was significantly positively correlated with WSA at 2–4 and 1–2 mm size in the rhizosphere of potted trifoliate orange exposed to both well water and drought stress36. Under a citrus field GRSP was positively correlated with WSA at 0.25–0.50 mm size37. At present, the contribution strength of biological factors involving in the aggregate stabilization has not been recognized in a plant rhizosphere, no matter whether the root system is present or not. Using perspex pots separated by 37-μm nylon mesh in the middle to form individual root/hyphae and root-free hyphae chambers for the growth of Poncirus trifoliata, which was colonized by Funneliformis mosseae or Paraglomus occultum, the objectives of this study were to address: (a) effects of AMF on plant growth, root total length, soil hyphal length, distribution of WSA in 2.00–4.00, 1.00–2.00, 0.50–1.00, and 0.25–0.50 mm size, GRSP fractions, and MWD; (b) correlation coefficients to display the interrelationships between MWD and relevant biological factors including root total length, root AM colonization, soil hyphal length, and GRSP fractions by the regression analysis; and (c) their direct and indirect effects of these biological factors to aggregate stability determined by the path analysis.
Results
Root colonization and plant growth performance
Root colonization of 5-month-old trifoliate orange was significantly higher when associated with Paraglomus occultum (43.7%) than with Funneliformis mosseae (34.7%) (Table 1). No root AMF colonization was observed in the non-AMF seedlings. AMF colonization significantly increased plant height, stem diameter, leaf number, shoot and root dry weight, and root total length, irrespective of AMF species. Meanwhile, in general significantly greater growth responses of trifoliate orange to AMF colonization were with F. mosseae than with P. occultum (Table 1).
Table 1. Effects of Funneliformis mosseae and Paraglomus occultum on root colonization, plant growth performance, and root total length of 5-month-old trifoliate orange seedlings grown in 37 μm nylon-mesh separated root/hyphae chambers.
| Dry weight (g) | |||||||
|---|---|---|---|---|---|---|---|
| Treatment | Root colonization (%) | Plant height (cm) | Stem diameter (cm) | Leaf number per plant | Root total length (cm) | Shoot | Root |
| Non-AMF | 0.0 ± 0.0c | 9.6 ± 1.5c | 0.28 ± 0.03b | 12 ± 2c | 242 ± 18b | 0.89 ± 0.19c | 1.03 ± 0.11c |
| F. mosseae | 34.7 ± 4.5b | 27.7 ± 3.9a | 0.37 ± 0.03a | 25 ± 2a | 374 ± 42a | 2.57 ± 0.43a | 1.86 ± 0.14a |
| P. occultum | 43.7 ± 3.4a | 21.4 ± 3.6b | 0.33 ± 0.03a | 21 ± 3b | 320 ± 39a | 1.82 ± 0.24b | 1.29 ± 0.15b |
Note: Data (means ± SE, n = 4) followed by different letters indicate significant differences (P < 0.05) among mycorrhizal treatments.
Hyphal length and GRSPs in root/hyphae or root-free hyphae chambers
Significantly higher hyphal length was in the root/hyphae chamber (ranged 0.26 to 0.88 m g−1) than in the root-free hyphae chamber (0.20 to 0.60 m g−1), irrespective of AMF species; or under P. occultum than under F. mossae, irrespective of the chamber (Table 2). No AM hyphae were found in the chambers of all non-AMF control treatments.
Table 2. Effects of Funneliformis mosseae and Paraglomus occultum on soil hyphal length and GRSP in 37 μm nylon-mesh separated root/hyphae and root-free hyphae chambers.
| GRSP (mg g−1 DW) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hyphal length (m g−1 FW) | EE-GRSP | DE-GRSP | T-GRSP | Ratio of EE-GRSP/DE-GRSP | ||||||
| Treatment | Root/hyphae chamber | Hyphae only chamber | Root/hyphae chamber | Hyphae only chamber | Root/hyphae chamber | Hyphae only chamber | Root/hyphae chamber | Hyphae only chamber | Root/hyphae chamber | Hyphae only chamber |
| Non-AMF | NA | NA | 0.34 ± 0.03b,x | 0.30 ± 0.02b,y | 0.53 ± 0.03b,x | 0.53 ± 0.02b,x | 0.87 ± 0.01c,x | 0.82 ± 0.01c,y | 0.63 ± 0.09b,x | 0.56 ± 0.05b,x |
| F. mosseae | 0.26 ± 0.02b,x | 0.20 ± 0.01b,y | 0.44 ± 0.03a,x | 0.35 ± 0.02a,y | 0.53 ± 0.03b,x | 0.52 ± 0.02b,x | 0.97 ± 0.04b,x | 0.88 ± 0.04b,y | 0.83 ± 0.05a,x | 0.68 ± 0.04a,y |
| P. occultum | 0.88 ± 0.06a,x | 0.60 ± 0.03a,y | 0.44 ± 0.03a,x | 0.36 ± 0.02a,y | 0.64 ± 0.02a,x | 0.64 ± 0.03a,x | 1.08 ± 0.03a,x | 1.00 ± 0.03a,y | 0.70 ± 0.04b,x | 0.56 ± 0.05b,y |
Note: Trifoliate orange seedlings were 5-month-old and grown in 37 μm nylon-mesh separated two-chambered pots. Data (means ± SE, n = 4) followed by different letters indicate significant differences (P < 0.05) between mycorrhizal treatments for the same chamber (a, b, c) or between root chamber and root-free hyphal chamber for the same mycorrhizal treatment (x, y). Abbreviations: DE-GRSP, difficultly-extractable glomalin-related soil protein (DE-GRSP); EE-GRSP, easily-extractable glomalin-related soil protein; NA, not available; T-GRSP, total (EE-GRSP + DE-GRSP) glomalin-related soil protein.
The EE-GRSP and T-GRSP, but not the DE-GRSP, were significantly higher in the root/hyphae chamber than in the root-free hyphae chamber, irrespective of AMF species (Table 2). Compared to the non-AMF control, mycorrhization with P. occultum significantly increased the concentrations of all these three GRSP fractions, whereas F. mosseae significantly increased the EE-GRSP and T-GRSP only, irrespective of the chambers. In addition, the EE-GRSP/DE-GRSP ratio was significantly higher under mycorrhization with F. mosseae than with P. occultum.
Distribution of WSA fractions and MWD in root/hyphae or root-free hyphae chambers
In both the root/hyphae and root-free hyphae chamber, the WSA2.00–4.00 mm fraction was significantly increased by both F. mosseae and P. occultum, whereas the WSA1.00–2.00 mm fraction only by F. mosseae, and both the WSA0.50–1.00 mm and WSA0.25–0.50 mm fraction only by P. occultum (Table 3). In general, the WSA2.00–4.00 mm fraction was higher in the root-free hyphae chamber than in the root/hyphae chamber, whereas the opposite was true for both the WSA0.50–1.00 mm and WSA0.25–0.50 mm fractions. In addition, mycorrhization significantly increased the MWD values by 89–134% or 78–81% in the root/hyphae or root-free hyphae chamber, respectively (Table 3). However, the MWD values were similar between the root/hyphae and root-free hyphae chamber, no matter whether the soils were under non-mycorrhization or mycorrhization.
Table 3. Effects of Funneliformis mosseae and Paraglomus occultum on water-stable aggregate (WSA) size distribution and mean weight diameter (MWD) in 37 μm nylon-mesh separated root/hyphae and root-free hyphae chambers.
| Distribution of WSA fraction (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2.00–4.00 mm | 1.00–2.00 mm | 0.50–1.00 mm | 0.25–0.50 mm | MWD (mm) | ||||||
| Treatment | Root/hyphae chamber | Hyphae only chamber | Root/hyphae chamber | Hyphae only chamber | Root/hyphae chamber | Hyphae only chamber | Root/hyphae chamber | Hyphae only chamber | Root/hyphae chamber | Hyphae only chamber |
| Non-AMF | 1.77 ± 0.31b,y | 2.67 ± 0.31b,x | 1.43 ± 0.18b,x | 0.95 ± 0.21b,y | 2.51 ± 0.56b,x | 1.72 ± 0.22b,y | 3.71 ± 0.33b, x | 2.43 ± 0.38b,y | 0.07 ± 0.01c,x | 0.08 ± 0.01b,x |
| F. mosseae | 4.52 ± 0.08a,y | 4.80 ± 0.21a,x | 2.01 ± 0.30a,y | 2.69 ± 0.33a,x | 2.83 ± 0.45b,x | 2.06 ± 0.39b,y | 4.06 ± 0.17b,x | 5.15 ± 0.83a,y | 0.14 ± 0.00b,x | 0.14 ± 0.01a,x |
| P. occultum | 4.47 ± 0.34a,x | 4.94 ± 0.55a,x | 1.55 ± 0.29b,x | 1.02 ± 0.08b,y | 10.80 ± 0.87a,x | 3.84 ± 1.53a,y | 5.34 ± 0.78a,x | 5.42 ± 0.34a,x | 0.17 ± 0.02a,x | 0.14 ± 0.02a,y |
Note: Trifoliate orange seedlings were 5-month-old and grown in 37 μm nylon-mesh separated two-chambered-root perspex pots. Data (means ± SE, n = 4) followed by different letters indicate significant differences (P < 0.05) between mycorrhizal treatments for the same chamber (a, b, c) or between root chamber and root-free hyphal chamber for the same mycorrhizal treatment (x, y).
Correlation analyses
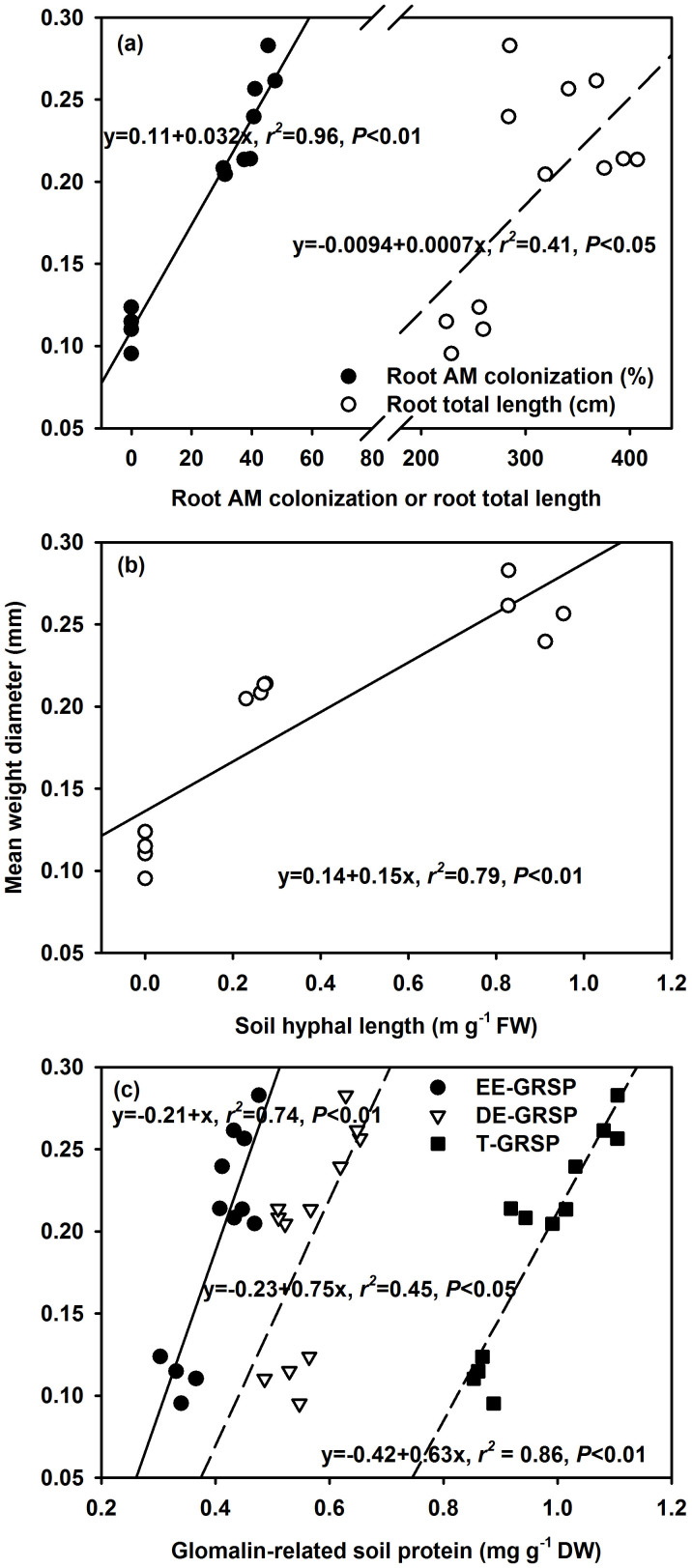
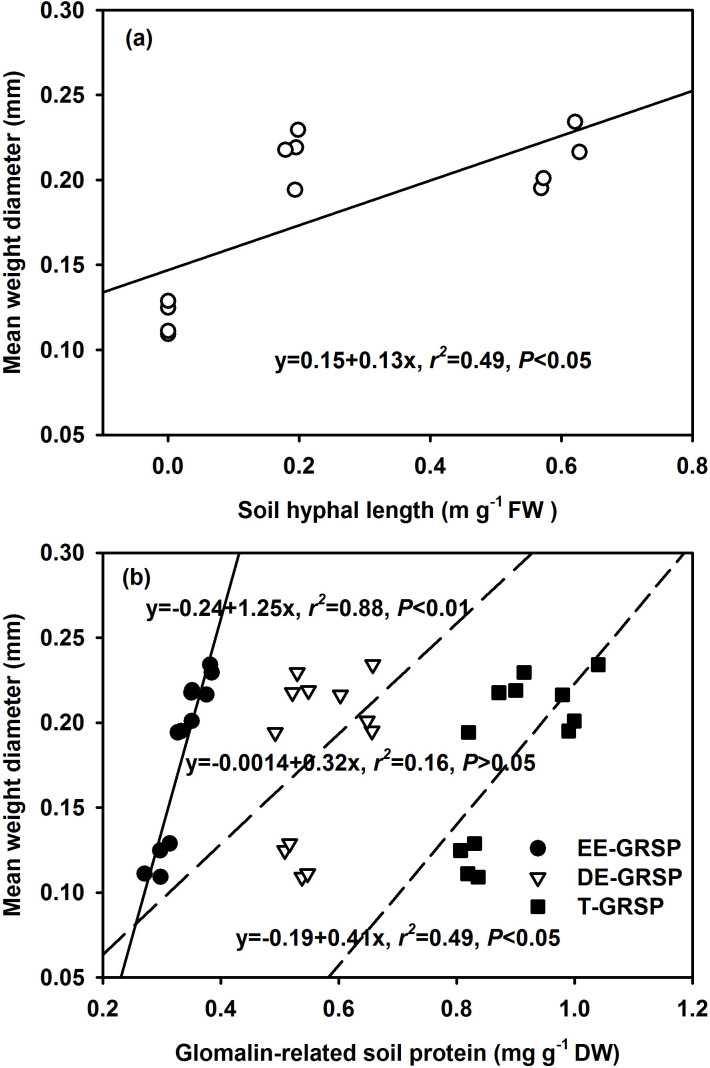
Pearson correlation analyses indicated that root AM colonization in the root/hyphae chamber positively correlated with root total length, hyphal length, EE-GRSP, DE-GRSP, and T-GRSP (Table 4). The hyphal length in both the root/hyphae and root-free hyphae chamber positively correlated with these three GRSP fractions. The WSA2.00–4.00 mm, WSA0.50–1.00 mm, and WSA0.25–0.50 mm fraction in both the root/hyphae and root-free hyphae chamber generally positively correlated with root colonization, hyphal length, EE-GRSP, DE-GRSP and T-GRSP, except EE-GRSP for WSA0.50–1.00 mm and DE-GRSP for WSA2.00–4.00 mm (Table 4). Interestingly, the WSA1.00–2.00 mm fraction did not correlate with any tested variable, and almost all WSAs did not correlate with root total length, except the WSA2.00–4.00 mm fraction. In addition, MWD significantly positively correlated with both the root mycorrhizal colonization and root total length (Fig. 1), and also with hyphal length (Fig. 2a), and EE-GRSP, DE-GRSP and T-GRSP (Fig. 2b).
Table 4. Pearson correlations (r) between variables in 37 μm nylon-mesh separated root/hyphae and root-free hyphae chambers.
| Root colonization | Hyphal length | WSA2.00–4.00 mm | WSA1.00–2.00 mm | WSA0.50–1.00 mm | WSA0.25–0.50 mm | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Independent variable | Root/hyphae chamber | Hyphae only chamber | Root/hyphae chamber | Hyphae only chamber | Root/hyphae chamber | Hyphae only chamber | Root/hyphae chamber | Hyphae only chamber | Root/hyphae chamber | Hyphae only chamber | Root/hyphae chamber | Hyphae only chamber |
| Root colonization | 1.00 | NA | 0.83** | NA | 0.96** | NA | 0.41 | NA | 0.67* | NA | 0.70* | NA |
| Root total length | 0.74** | NA | 0.34 | NA | 0.78** | NA | 0.56 | NA | 0.13 | NA | 0.25 | NA |
| Hyphal length | 0.83** | NA | 1.00 | 1.00 | 0.69* | 0.75** | −0.03 | −0.16 | 0.96** | 0.72** | 0.84** | 0.74** |
| EE-GRSP | 0.88** | NA | 0.67* | 0.70* | 0.90** | 0.91** | 0.45 | 0.37 | 0.46 | 0.40 | 0.64* | 0.76** |
| DE-GRSP | 0.58* | NA | 0.86** | 0.87** | 0.41 | 0.45 | −0.23 | −0.51 | 0.90** | 0.67* | 0.74** | 0.51 |
| T-GRSP | 0.88** | NA | 0.92** | 0.95** | 0.79** | 0.73** | 0.13 | −0.22 | 0.82** | 0.67* | 0.83** | 0.71** |
Note: Trifoliate orange seedlings were 5-month-old and colonized with Funneliformis mosseae and Paraglomus occultum and grown in 37 μm nylon-mesh separated two-chambered perspex pots. *, P < 0.05; **, P < 0.01. Abbreviations: DE-GRSP, difficulty-extractable glomalin-related soil protein (DE-GRSP); EE-GRSP, easily-extractable glomalin-related soil protein; NA, not available; T-GRSP, total (EE-GRSP + DE-GRSP) glomalin-related soil protein; WSA, water-stable aggregate.
Figure 1. Relationships between root arbuscular mycorrhizal (AM) colonization or root total length (a), hyphal length (b), or glomalin-related soil protein (c) and mean weight diameter (MWD, an indicator of aggregate stability) in the root/hyphae chamber of 5-month-old trifoliate orange seedlings colonized by Funneliformis mosseae and Paraglomus occultum (n = 12).
Figure 2. Relationships between soil arbuscular mycorrhizal hyphal length (a) or glomalin-related soil protein fractions (b) and mean weight diameter (MWD, an indicator of aggregate stability) in the root-free hyphae chamber of 5-month-old trifoliate orange seedlings colonized by Funneliformis mosseae and Paraglomus occultum (n = 12).
Path analyses
Six independent variables (root AM colonization, root total length, hyphal length, and EE-GRSP, DE-GRSP, and T-GRSP) were considered in the path analysis (Table 5). In the root/hyphae chamber, either root AM colonization, EE-GRSP or DE-GRSP showed a significantly direct and total (direct plus indirect) effect on MWD, and the direct contribution of these three variables ranked as DE-GRSP ≈ EE-GRSP > root AM colonization (Table 5). On the other hand, although root total length, soil hyphal length, or T-GRSP did not show a direct effect on MWD in the root/hyphae chamber, these three independent variables still showed a strongly total effect on MWD.
Table 5. Path analyses between mean weight diameter (MWD) and arbuscular mycorrhizal (AM) colonization, root total length, hyphal length, or soil GRSP fractions in 37 μm nylon-mesh separated root/hyphae and root-free hyphae chambers.
| Direct effect | Indirect effect | Total effect | ||||
|---|---|---|---|---|---|---|
| Independent variable | Root/hyphae chamber | Hyphae only chamber | Root/hyphae chamber | Hyphae only chamber | Root/hyphae chamber | Hyphae only chamber |
| AM colonization | 0.63* | NA | 0.35 | NA | 0.98** | NA |
| Root total length | 0.08 | NA | 0.56 | NA | 0.64* | NA |
| Hyphal length | 0.16 | 0.30 | 0.73 | 0.40 | 0.89** | 0.70* |
| EE-GRSP | 13.41* | 7.70 | −12.55 | −6.76 | 0.86** | 0.94** |
| DE-GRSP | 13.60* | 11.29 | −12.93 | −10.89 | 0.67* | 0.40 |
| T-GRSP | −22.21 | −15.69 | 23.14 | 16.39 | 0.93** | 0.70* |
Note: Trifoliate orange seedlings were 5-month-old and colonized with Funneliformis mosseae and Paraglomus occultum and grown in 37 μm nylon-mesh separated two-chambered perspex pots. *, P < 0.05; **, P < 0.01. Abbreviations: DE-GRSP, difficultly-extractable glomalin-related soil protein (DE-GRSP); EE-GRSP, easily-extractable glomalin-related soil protein; NA, not available; T-GRSP, total (EE-GRSP + DE-GRSP) glomalin-related soil protein.
In the root-free hyphae chamber, the following four independent variables, hyphal length, EE-GRSP, DE-GRSP and T-GRSP showed no direct effects on MWD, whilst the hyphal length, EE-GRSP and T-GRSP exhibited a significantly total effect on MWD (Table 5).
Discussion
Our study showed a significantly positive Pearson correlation between root AMF colonization and WSA2.00–4.00 mm, WSA0.50–1.00 mm, WSA0.25–0.50 mm, or MWD in the root/hyphae chamber (Table 4; Fig. 1a). These results agreed with a previous study in amaranth, Bermuda grass, maize, and sunflower plants38. Moreover, the contribution of root AMF colonization to MWD was direct (Table 5), implying that root colonization, as a direct component, could confer upon a primary role in aggregate stability. However, in both the salt-stressed Lactuca sative and the drought-stressed Phaseolus vulgaris plants, AMF colonization did not correlate with aggregate stability32,39. It seemed that abiotic stresses might interfere with such a direct contribution of root colonization.
Mycorrhizal hyphae have been confirmed primarily to stabilize macroaggregates in various soils by enmeshing soil particles and binding microaggregates into macroaggregates13,14,15,40,41. Our study also showed that soil hyphal length was significantly positively correlated with WSA2. 00–4.00 mm, WSA0. 50–1.00 mm, and WSA0. 25–0.50 mm (Table 4), as well as MWD (Fig. 1b, 2a), in both the root/hyphae and root-free hyphae chamber. These results indicated that mycorrhizal hyphae involved in the distribution of WSA size in 2.00–4.00, 0.50–1.00, and 0.25–0.50 mm and aggregate stability. This is in accordance with a strong relationship between soil hyphal length and MWD in F. mosseae and G. intraradices-infected Medicago sativa11. However, the path analysis indicated that the Pearson correlation between soil hyphal length and MWD was not from a direct effect but from a total effect (Table 5). Here we do not argue that soil mycorrhizal hyphae could physically entangle primary soil particles, enmesh and bind microaggregates and small macroaggregates into larger aggregates40. However, a 0.20–0.88 m g−1 hyphal length (Table 2) was obviously lower than 4.0–6.2 m g−1 from soil growing Lolium rigidum with Scutellospora calospora14. A shorter length of hyphae in the present study might confer a weaker enmeshment between soil WSAs, and the slaking forces during the wet-sieving process might have disrupted the enmeshing role of mycorrhizal hyphae in aggregates. Such shorter hyphal length was only easier to stabilize sandy soil with a smaller specific surface area40. On the other hand, the hyphae also released glomalin into soils as GRSP22, which could give a direct effect on MWD (Table 5). Even so, mycorrhizal hyphae in lower length still showed a significantly Pearson correlation with MWD (Fig. 1b, 2a) through a total effect. Moreover, Tisdall et al.41 found that besides hyphal length, hyphal surface area might also determine the role of hyphae-mediated aggregate stability. Graf and Frei9 further proposed that mycorrhizal hyphae might act as “flexible string bags” due to their tensile strength and release of GRSP, thereby exhibiting a certain plasticity to stabilize aggregates. Mycorrhizal hyphae might thus play a key role in aggregate stability, which would depend on hyphal length, plant and/or mycorrhizal species.
Besides mycorrhizal hyphae, soil aggregate stability also involves physical entanglement of fine roots of the host plants9,13,42,43. In the present study, AMF inoculation significantly increased root total length, irrespective of AMF source (Table 1), which might modulate the distribution of WSA2.00–4.00 mm, thereby positively stabilizing WSA (e.g., MWD) (Table 4; Fig. 1a). However, the direct effect of root total length on aggregate stability was not significant on the basis of the path analysis in the root/hyphae chamber (Table 5), which is in agreement with the results of Willer and Jastrow44. The Pearson correlation between root total length and MWD (Fig. 1a) was due to an indirect effect of EE-GRSP and/or T-GRSP, but not a direct effect, according to the path analysis in our study (Table 5).
In addition, the root total length exhibited lower direct and total effects on MWD than did the hyphae in the root/hyphae chamber (Table 5), which is in agreement with previous studies13,17. Possibly, in a relatively smaller root/hyphae chamber, the development of hyphal network throughout the whole soil was obviously faster than root growth11. Greater root systems could provide more chances to be colonized by AMF, thereby increasing the production of hyphae, finally more GRSP production. On the other hand, a better root system could also release more root exudates into the rhizosphere16, which could directly affect aggregate stability. However, no information is available if a direct effect of roots on aggregate stability in a larger growth container could be stronger than that of mycorrhizal hyphae.
Our results showed that except a similar concentration of DE-GRSP between non-AMF and P. occultum treatment, F. mosseas or P. occultum colonization significantly increased the concentrations of EE-GRSP, DE-GRSP, and T-GRSP, irrespective of the root/hyphae or root-free hyphae chamber, which is in coincidence with previous studies36,45. Moreover, the inoculation with AMF also increased the ratio of EE-GRSP versus DE-GRSP in both the root/hyphae and root-free hyphae chamber (Table 3), but the significant differences occurred only between non-AMF and F. mosseae. Since EE-GRSP is recently produced, and DE-GRSP is relatively older and also from EE-GRSP31, a higher ratio of EE-GRSP versus DE-GRSP in the mycorrhizosphere might suggest that AMF colonization induced more new GRSP production, and EE-GRSP had also partly given rise to DE-GRSP. In addition, root colonization and soil hyphal length highly positively correlated with these three GRSP fractions (Table 4), suggesting that GRSP production depended on mycorrhizal hyphae production37,46,47, since GRSP originated from AM hyphae and spore walls22.
Our results also indicated that EE-GRSP significantly positively correlated with WSA2.00–4.00 mm, WSA0.25–0.50 mm, and MWD, whilst DE-GRSP significantly positively correlated with WSA0.50–1.00 mm, and T-GRSP with WSA2.00–4.00 mm, WSA0.50–1.00 mm, and WSA0.25–0.50 mm, regardless of the presence of roots or not (Table 4). The positive effect of GRSP thereby, stabilized the aggregates, resulting in a positive Pearson correlation between EE-GRSP or T-GRSP and MWD in both the root/hyphae and root-free hyphae chamber, though there was a positive Pearson correlation between the DE-GRSP and MWD in the root/hyphae chamber (Fig. 1c, 2b). Meanwhile, EE-GRSP and DE-GRSP showed a significantly direct effect on MWD under the presence of both roots and hyphae, whilst the direct effect of DE-GRSP was slightly greater than that of the EE-GRSP (Table 5). Peng et al.45 also found that GRSP presented a direct effect on MWD in a neutral purple soil. These results suggested that although the two GRSP fractions could give direct effect on MWD, new glomalin, e.g. the EE-GRSP, could characterize relatively more labile, and the older glomalin, e.g., the DE-GRSP, could be more stable, thereby contributing more to stabilize aggregates than the labile EE-GRSP. As reported by Daynes et al.19, formation and stabilization of the macroaggregates (>0.25 mm) mainly depended on diverse factors including fine roots and AMF. The results in the present study confirmed that macroaggregate stability in root/hyphae chamber of trifoliate orange was directly due to mycorrhizal colonization, EE-GRSP and DE-GRSP, whilst GRSP played a primary role in aggregate stability. Using the fluorescently labeled lectin, Caesar-Tonthat48 revealed that fungal-derived materials such as fucosyl residues played a vital role in soil aggregration. It is possible that GRSP as an important AM-released material has the key functioning on aggregration stabilization under the presence of root and hyphae.
However, under the root-free hyphae condition, the two GRSP fractions of EE-GRSP and DE-GRSP did not show a direct effect on MWD, but the EE-GRSP showed a significantly total effect (Table 5). These results thus suggested that under a root-free hyphae condition, an active EE-GRSP, T-GRSP, and hyphal length might undertake a total effect, but not a direct effect, on aggregation. The distinct function of EE-GRSP and DE-GRSP on aggregate stability between the root/hyphae and root-free hyphae chamber might relate with the presence or absence of roots. In the presence of roots, AM would have already formed and more AMF spores were produced, leading to a steady GRSP production. In contrast, in the absence of roots, the GRSP production is exclusively dependent on extraradical hyphae49. In the present study, the root-free hyphae chamber possessed lower hyphal length, indicating a less GRSP production. It is reasonable that the functioning of GRSP on aggregate stability might thus be greater in the root/hyphae chamber than in the root-free chamber. As a result, the respective functioning of EE-GRSP or DE-GRSP might be ascertained if the exact component of these two GRSP fractions could be distinguished.
In short, under the root plus mycorrhizal hyphae condition, aggregate stability mainly depended on the direct effect of root colonization, EE-GRSP and DE-GRSP, combining with the total effect of T-GRSP, hyphal length and root total length. On the other hand, under the root-free hyphae conditions, aggregate stability mainly relied on the total effect of soil hyphal length, EE-GRSP and T-GRSP.
Methods
Experimental design
The experiment had three inoculations or treatments: (1) Funneliformis mosseae, (2) Paraglomus occultum, and (3) non-AMF (control). Each treatment had four replicates, for a total of 12 experimental perspex pots in a completely randomized arrangement.
Mycorrhizal inocula
The fungal isolates, Funneliformis mosseae (Nicol. & Gerd.) Schüßler & Walker and Paraglomus occultum (Walker) Morton & Redecker, were from the rhizosphere of Incarvillea younghusbandii in Dangxiong (90°45′E and 29°31′N, 4,300 m above the sea level), Tibet, and of Prunus persica in Pinggu (116°55′E and 40°02′N, ~700 m above the sea level), Beijing, China, respectively. Through identified fungal spores, the mycorrhizal inocula, propagated with white clover (Trifolium repens) for 16 weeks, were a mixture of sands, fine root segments and spores (23 or 28 spores g−1 for F. mosseae or P. occultum).
Experimental pots and plant growth conditions
Perspex pots (20 × 10 × 18 cm = length × width × height) were separated in the middle by 37-μm nylon mesh to form two equal sized chambers (a root/hyphae chamber and a root-free hyphae chamber). The nylon mesh allows AM hyphae, but not plant roots, to penetrate from one chamber to another and to grow over there. The root/hyphae chamber was for root/hyphae growth (with AM inoculation), while the root-free hyphae chamber was for hyphae (passed through the nylon mesh) growth only.
Seeds of trifoliate orange [Poncirus trifoliata (L.) Raf.] from a Citrus Orchard of Yangtze University campus were surface-sterilized with 70% ethanol for 10 min, rinsed thoroughly with distilled water, and germinated in a plastic box containing autoclaved sands at 28°C for 7 days at dark. Two three-leaf-old (under sterilization) seedlings were transplanted into the central region of the root/hyphae chamber. Each chamber contained 1,500 g autoclaved soil (Xanthi-Udic Ferralsols, FAO system), which was collected at 0–15 cm depth from a Citrus Orchard at Yangtze University campus in February 2012. This soil had a pH of 6.2, 9.4 g kg−1 organic carbon, 120.3 mg kg−1 available nitrogen, 16.2 mg kg−1 Oslen-P, and 22.7 mg kg−1 available potassium. Soils were air-dried, sieved by 4 mm mesh screen, and then autoclaved (121°C, 0.11 Mpa, 2 h) before use.
For the AM treatments 100 g AM inocula were applied in the root/hyphae chamber. The non-AM treatments in the root/hyphae chamber received 100 g autoclaved (121°C, 0.11 Mpa, 2 h) inocula plus 2 mL filtrate (25 μm) of mycorrhizal inoculum to maintain similar other microorganisms.
Seedlings were grown in an environment controlled plastic greenhouse on the Yangtze University campus from March 25 to August 3, 2012, where had 768 μmol m−2 s−1 photosynthetic photon flux density, 28/21°C day/night temperature, and 85% relative humidity. Seedlings were watered with distilled water at an interval of three days to avoid waterlogging at the chamber bottom and fortnightly with 100 mL standard Hoagland solution.
Variable determinations
After 134 days of AMF inoculation, shoots and roots were separately harvested from the root/hyphae chamber, and plant height, stem diameter, and leaf numbers were recorded. The root systems were carefully washed with distilled water and scanned with an Epson Perfection V700 Photo Dual Lens System (J221A, Indonesia). The root images were analyzed by a WinRHIZO professional 2007b software (Regent Instruments Inc., Quebec, Canada), and root total length was then automatically obtained. Soils from the two chambers were also separately collected and air-dried.
A total of fifty 1.0-cm-long root segments from two seedling in each pot were cleared with 10% KOH at 95°C for 1.5 h and stained with 0.05% trypan blue in lactoglycerol for 10 min50. Root mycorrhizal colonization was expressed as the percentage of colonized root length against observed root length. Determination of soil hyphal length was based on Bethlenfalvay and Ames51. Soil organic carbon (SOC) was determined by the dichromate oxidation spectrophotometric method52.
Determination of GRSP concentration was based on the protocol described by Koide and Peoples31. Briefly, 1.0 g air-dried soil sample was extracted with 8 mL 20 mM citrate (pH 7.0) at 121°C for 30 min under 0.11 Mpa and centrifuged at 10,000 g for 3 min. The supernatants were used for the analysis of EE-GRSP. The remaining residues of EE-GRSP extraction were subsequently autoclaved with 8 mL 50 mM citrate (pH 8.0) for 60 min at 121°C under 0.11 Mpa and centrifuged at 10,000 g for 3 min, and the supernatants were used for the determination of DE-GRSP. These two supernatants were analyzed for GRSP with bovine serum albumin as a standard according to the Bradford assay53. T-GRSP was the sum of the EE-GRSP and DE-GRSP.
The water-stable aggregate (WSA) distribution at 2.00–4.00, 1.00–2.00, 0.50–1.00, and 0.25–0.50 mm size was determined using the wet-sieving method5. Determination of mean weight diameter (MWD, an indicator of aggregate stability) was as follows: 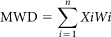 , where Xi is the diameter of the i sieve opening (mm), Wi is the proportion of the i size fraction in the total sample mass, and n is the number of size fractions5.
, where Xi is the diameter of the i sieve opening (mm), Wi is the proportion of the i size fraction in the total sample mass, and n is the number of size fractions5.
Statistical analyses
Root AM colonization and distribution of WSA at 2.00–4.00, 1.00–2.00, 0.50–1.00, and 0.25–0.50 mm size were arcsine transformed prior to variance analyses. One-factor analysis of variance with four replicates was performed using the SAS software (v8.1). Comparison of means between treatments was accomplished with the Duncan's Multiple Range Test at 0.05 level. The Pearson's correlation coefficients between variables were performed using the Proc Corr's procedure54.
The direct or indirect effects of mycorrhization on MWD were calculated with the path analysis without considering the potential effects of multicollinearity. The path analysis was also used to analyze the relationships among MWD and other relevant variables involved. Information about the path analysis can explain the ultimate contribution of variables to MWD. Meanwhile, the dependent variable was MWD, and the independent variables were root AM colonization, root total length, soil hyphal length, and EE-GRSP, DE-GRSP and T-GRSP in the root/hyphae chamber, while soil hyphal length, and EE-GRSP, DE-GRSP and T-GRSP in the root-free hyphae chamber, respectively.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (31372017), the Key Project of Chinese Ministry of Education (211107), the Key Project of Natural Science Foundation of Hubei Province (2012FFA001), the Science-Technology Research Project for the Excellent Middle-aged and Young Talents of Hubei Provincial Department of Education (Q20111301), and the Excellent Young Teacher Research Support Program of Yangtze University (cyq201324).
Footnotes
The authors declare no competing financial interests.
Author Contributions Q.S.W. planned the study, conducted statistical analyses and wrote the manuscript. M.Q.C. conducted greenhouse and laboratory work. Y.N.Z. planned the study and made all tables and figures. X.H.H. planned the study and wrote the manuscript. All authors approved the manuscript submission.
References
- Rillig M. C. & Mummey D. L. Mycorrhizas and soil structure. New Phytol. 171, 41–53 (2006). [DOI] [PubMed] [Google Scholar]
- Borie F., Rubio R. & Morales A. Arbuscular mycorrhizal fungi and soil aggregation. J. Soil Sci. Plant Nutr. 8, 9–18 (2008). [Google Scholar]
- Amézketa E. Soil aggregate stability: A review. J Sustain Agric 14, 83–151 (1999). [Google Scholar]
- Williams N. D. & Petticrew E. L. Aggregate stability in organically and conventionally farmed soils. Soil Use Manag. 25, 284–292 (2009). [Google Scholar]
- Kemper W. D. & Rosenau K. [Size distribution of aggregates]. Methods of Soil Analysis, Part 1 [Klute, A. (ed.)] [425–442] (ASA, Madison, 1986). [Google Scholar]
- Sun T. et al. A novel soil wetting technique for measuring wet stable aggregates. Soil Till. Res. 141, 19–24 (2014). [Google Scholar]
- Zadorova T., Jaksik O., Kodesova K. & Penizek V. Influence of terrain attributes and soil properties on soil aggregate stability. Soil Water Res. 6, 111–119 (2011). [Google Scholar]
- Tanaka H., Katsuta A., Toyota K. & Sawada K. [Soil fertility and soil microorganisms]. Research Approaches to Sustainable Biomass Systems [Tojo, S. (ed.)] [107–142] (Academic Press, New York, 2014). [Google Scholar]
- Graf F. & Frei M. Soil aggregate stability related to soil density, root length, and mycorrhiza using site-specific Alnus incana and Melanogaster variegatus s.l. Ecol. Eng. 57, 314–323 (2013). [Google Scholar]
- Pérès G. et al. Mechanisms linking plant community properties to soil aggregate stability in an experimental grassland plant diversity gradient. Plant Soil 373, 285–299 (2013). [Google Scholar]
- Leifheit E. F., Veresoglou S. D., Lehmann A., Morris E. K. & Rillig M. C. Multiple factors influence the role of arbuscular mycorrhizal fungi in soil aggregation–a meta-analysis. Plant Soil 374, 523–537 (2014). [Google Scholar]
- Tang J., Mo Y. H., Zhang J. Y. & Zhang R. D. Influence of biological aggregating agents associated with microbial population on soil aggregate stability. Appl. Soil Ecol. 47, 153–159 (2011). [Google Scholar]
- Bedini S. et al. Changes in soil aggregation and glomalin-related soil protein content as affected by the arbuscular mycorrhizal fungal species Glomus mosseae and Glomus intraradices. Soil Biol. Biochem. 41, 1491–1496 (2009). [Google Scholar]
- Degens B. P., Sparling G. P. & Abbott L. K. Increasing the length of hyphae in a sandy soil increases the amount of water-stable aggregates. Appl. Soil Ecol. 3, 149–159 (1996). [Google Scholar]
- Peng S. L., Guo T. & Liu G. C. The effects of arbuscular mycorrhizal hyphal networks on soil aggregations of purple soil in southwest China. Soil Biol Biochem 57, 411–417 (2013). [Google Scholar]
- Kohler-Milleret R., Le Bayon R. C., Chenu C., Gobat J. M. & Boivin P. Impact of two root systems, earthworms and mycorrhizae on the physical properties of an unstable silt loam Luvisol and plant production. Plant Soil 370, 251–265 (2013). [Google Scholar]
- Feng G., Zhang Y. F. & Li X. L. Effect of external hyphae of arbuscular mycorrhizal plant on water-stable aggregates in sandy soil. J Soil Water Conserv. 15, 99–102 (2001) (in Chinese with English abstract). [Google Scholar]
- Martin S. L., Mooney S. J., Dickinson M. J. & West H. M. The effects of simultaneous root colonisation by three Glomus species on soil pore characteristics. Soil Biol. Biochem. 49, 167–173 (2012). [Google Scholar]
- Daynes C. N., Field D. J., Saleeba J. A., Cole M. A. & McGee P. A. Development and stabilisation of soil structure via interactions between organic matter, arbuscular mycorrhizal fungi and plant roots. Soil Biol. Biochem. 57, 683–694 (2013). [Google Scholar]
- Rillig M. C. Arbuscular mycorrhizae, glomalin, and soil aggregation. Can. J. Soil Sci. 84, 355–363 (2004). [Google Scholar]
- Wright S. F. & Upadhyaya A. Extraction of an abundant and unusual protein from soil and comparison with hyphal protein of arbuscular mycorrhizal fungi. Soil Sci. 161, 575–586 (1996). [Google Scholar]
- Driver J. D., Holben W. E. & Rillig M. C. Characterization of glomalin as a hyphal wall component of arbuscular mycorrhizal fungi. Soil Biol. Biochem. 37, 101–105 (2005). [Google Scholar]
- Lovelock C. E., Wright S. F., Clark D. A. & Ruess R. W. Soil stocks of glomalin produced by arbuscular mycorrhizal fungi across a tropical rain forest landscape. J. Ecol. 92, 278–287 (2004). [Google Scholar]
- Rillig M. C., Wright S. F., Nichols K. A., Schmidt W. F. & Torn M. S. Large contribution of arbuscular mycorrhizal fungi to soil carbon pools in tropical forest soils. Plant Soil 233, 167–177 (2001). [Google Scholar]
- Schindler F. V., Mercer E. R. & Rice J. A. Chemical characteristics of glomalin-related soil protein (GRSP) extracted from soils of varying organic matter content. Soil Biol. Biochem. 39, 320–329 (2007). [Google Scholar]
- Wright S. F. & Upadhyaya A. A survey of soils for aggregate stability and glomalin, a glycoprotein produced by hyphae of arbuscular mycorrhizal fungi. Plant Soil 198, 97–107 (1998). [Google Scholar]
- Wright S. F. & Anderson R. L. Aggregate stability and glomalin in alternative crop rotations for the central Great Plains. Biol. Fertil. Soils 31, 249–253 (2000). [Google Scholar]
- Wu Q. S., He X. H., Cao M. Q., Zou Y. N., Wang S. & Li Y. Relationships between glomalin-related soil protein in water-stable aggregate fractions and aggregate stability in citrus rhizosphere. Int. J. Agric. Biol. 15, 603–606 (2013). [Google Scholar]
- Fokom R. et al. Glomalin related soil protein, carbon, nitrogen and soil aggregate stability as affected by land use variation in the humid forest zone of south Cameroon. Soil Till. Res. 120, 69–75 (2012). [Google Scholar]
- Hontoria C., Velásquez R., Benito M., Almorox J. & Moliner A. Bradford-reactive soil proteins and aggregate stability under abandoned versus tilled olive groves in a semi-arid calcisol. Soil Biol. Biochem. 41, 1583–1585 (2009). [Google Scholar]
- Koide R. T. & Peoples M. S. Behavior of Bradford-reactive substances is consistent with predictions for glomalin. Appl. Soil Ecol. 63, 8–14 (2013). [Google Scholar]
- Augé R. M. et al. Relating foliar dehydration tolerance of mycorrhizal Phaseolus vulgaris to soil and root colonization by hyphae. J. Plant Physiol. 160, 1147–1156 (2003). [DOI] [PubMed] [Google Scholar]
- Ferrari T. J. Causal soil–plant relationships and path coefficients. Plant Soil 19, 81–96 (1963). [Google Scholar]
- Rillig M. C., Wright S. F. & Eviner V. T. The role of arbuscular mycorrhizal fungi and glomalin in soil aggregation: comparing effects of five plant species. Plant Soil 238, 325–333 (2002). [Google Scholar]
- Farhatullah, Farooq-E-Azam & Khalil I. F. Path analysis of the coefficients of sunflower (Helianthus annuus L.) hybrids. Int. J. Agric. Biol. 8, 621–625 (2006). [Google Scholar]
- Wu Q. S., Xia R. X. & Zou Y. N. Improved soil structure and citrus growth after inoculation with three arbuscular mycorrhizal fungi under drought stress. Eur. J. Soil Biol. 44, 122–128 (2008). [Google Scholar]
- Wu Q. S., He X. H., Zou Y. N., He K. P., Sun Y. H. & Cao M. Q. Spatial distribution of glomalin-related soil protein and its relationships with root mycorrhization, soil aggregates, carbohydrates, activity of protease and β-glucosidase in the rhizosphere of Citrus unshiu. Soil Biol. Biochem. 45, 181–183 (2012). [Google Scholar]
- Moreno-Espíndola I. P., Rivera-Becerril F., de Jesús Ferrara-Guerrero M. & León-González F. D. Role of root-hairs and hyphae in adhesion of sand particles. Soil Biol. Biochem. 39, 2520–2526 (2007). [Google Scholar]
- Kohler J., Caravaca F. & Roldán A. An AM fungus and a PGPR intensify the adverse effects of salinity on the stability of rhizosphere soil aggregates of Lactuca sativa. Soil Biol. Biochem. 42, 429–434 (2010). [Google Scholar]
- Bearden B. N. & Petersen L. Influence of arbuscular mycorrhizal fungi on soil structure and aggregate stability of a verticol. Plant Soil 218, 173–183 (2000). [Google Scholar]
- Tisdall J. M., Smith S. E. & Rengasamy P. Aggregation of soil by fungal hyphae. Soil Res. 35, 55–60 (1997). [Google Scholar]
- Tisdall J. M. & Oades J. M. Stabilization of soil aggregates by the root systems of ryegrass. Soil Res. 17, 429–441 (1979). [Google Scholar]
- Milleret R., Le Bayon R. C. & Gobat J. M. Root, mycorrhiza and earthworm interactions: their effects on soil structuring processes, plant and soil nutrient concentration and plant biomass. Plant Soil 316, 1–12 (2009). [Google Scholar]
- Willer R. M. & Jastrow J. D. Hierarchy or root and mycorrhizal fungal interactions with soil aggregation. Soil Biol. Biochem. 22, 579–584 (1990). [Google Scholar]
- Peng S. L., Shen H., Yuan J. J., Wei C. F. & Guo T. Impacts of arbuscular mycorrhizal fungi on soil aggregation dynamics of neutral purple soil. Acta Ecol. Sin. 31, 498–505 (2011) (in Chinese with English abstract). [Google Scholar]
- Curaqueo G., Acevedo E., Cornejo P., Seguel A., Rubio R. & Borie F. Tillage effect on soil organic matter, mycorrhizal hyphae and aggregates in a mediterranean agroecosystem. J. Soil Sci. Plant Nutr. 10, 12–21 (2010). [Google Scholar]
- Guo H. J., He X. L. & Li Y. P. Spatial distributuion of arbuscular mycorrhiza and glomalin in the rhizosphere of Caragana korshinskii Kom. in the Otindag sandy land, China. Afr. J. Microbiol. Res. 6, 5745–5753 (2012). [Google Scholar]
- Caesar-Tonthat T. C. Soil binding properties of mucilage produced by a basidiomycete fungus in a model system. Mycol. Res. 106, 930–937 (2002). [Google Scholar]
- Purin S. & Rillig M. C. Immuno-cytolocalization of gloamlin in the mycelium of the arbuscular mycorrhizal fungus Glomus intraradices. Soil Biol. Biochem. 40, 1000–1003 (2008). [Google Scholar]
- Phillips J. M. & Hayman D. S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 55, 158–161 (1970). [Google Scholar]
- Bethlenfalvay G. J. & Ames R. N. Comparison of two methods for quantifying extraradical mycelium of vesicular-arbuscular mycorrhizal fungi. Soil Sci. Soc. Am. J. 51, 834–837 (1987). [Google Scholar]
- Rowell D. L. Soil Science: Methods and Applications (Longman Group U.K. Ltd., London, 1994). [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–252 (1976). [DOI] [PubMed] [Google Scholar]
- SAS Institute, Inc. SAS User's Guide: Statistics. Version 8.1 (SAS Institute, Inc., Cary, 2001). [Google Scholar]