Abstract
Normal interfollicular epidermis (IFE) homeostasis is maintained throughout the entire life by its own stem cells that self-renew and generate progeny that undergo terminal differentiation. However, the fine markers of the stem cells in interfollicular epidermis are not well defined yet. Here we found that TLR7 identified the existence of progenitors and interfollicular epidermal stem cells in murine skin. In vitro, TLR7-expressing cells comprised of two subpopulations that were competent to proliferate and exhibited distinct differentiation potentials. Three-dimensional (3D) organotypic culture and skin reconstitution assays showed that TLR7-expressing cells were able to reconstruct the interfollicular epidermis. Finally, TLR7-expressing cells maintained the intact interfollicular epidermal structures revealed in serial transplantation assays in vivo in mice. Taken together, our results suggest that TLR7-expressing cells comprise an interfollicular epidermal stem cell population.
The skin is the largest organ in the mammalian body and consists of the interfollicular epidermis (IFE) and associated appendages, including sebaceous glands (SG), sweat glands1,2, and hair follicles (HF) that undergo continuous cyclic phases of growth (anagen), regression (catagen), and rest (telogen).
Previously, the fraction of mouse epidermis was described on the basis of several different cell surface markers. α6 integrin is a described basal cell marker due to it expression on all undifferentiated epidermal cells and is employed as a valuable marker to isolate stem cells from keratinocytes in skin, although subsets in α6 integrin expressing population were not entirely clear yet3. In contrast, cell surface glycoprotein CD34 expression specifies mouse hair follicle stem cells that reside in the hair follicle bulge4.
The transmembrane protein Toll-like receptor 7 (TLR7) is a member of Toll-like receptor family that plays an important role in innate immune responses by recognizing pathogen-derived nucleotides in intracellular compartments. TLR7 is mainly detectable in intracellular compartments of plasmacytoid dendritic cells and B cells, as well as on the surface of chronic lymphocytic leukemia and some types of lymphoma cells. TLR7-deficient mice are viable and do not display any apparent abnormalities, with normal responses to a variety of Escherichia coli products5, indicating that TLR7 does not display important functions during embryonic development.
The TLR7 agonist imiquimod is the most frequently used TLR7 ligand in clinical practices, and is often used to treat primary skin tumors, cutaneous metastases, cutaneous warts, and actinic keratoses (AKs), which are premalignant lesions that appear as rough, dry patches on the skin6,7,8,9. The best understood mechanism of action for imiquimod involves the activation of immune cells via TLR7, leading to the production of a variety of inflammatory cytokines that mediate antitumor and antivirus immune responses in vivo. However, imiquimod has also been reported to induce antitumor and antiviral activities in immunosuppressed patients10,11. Furthermore, a 15-year-old adolescent girl with a seven year history of alopecia universal is experienced transient hair growth after topical application of imiquimod13. These reports suggest an alternative, non-inflammatory mechanism of action for imiquimod in the skin. Till now, the roles of imiquimod and its receptor TLR7 and the detailed underlying mechanisms are still remained to know.
Here, we examine TLR7 expression and function in the murine epidermis. Our results demonstrate expression of TLR7 on the surface of keratinocytes in IFE with the properties of stem cells and progenitors. The population of TLR7-positive cells from the dorsal skin of mice shows potent skin reconstitution ability. Notably, TLR7-positive cells undergo self-renewal and repopulation in serial transplantation to completely produce IFE, suggesting that TLR7 expression defines a new stem cell reservoir containing stem cells that can contribute to IFE.
Experimental procedures
Mice
All procedures involving the use of animals were conducted in compliance with the approved guidelines. The animal protocols were approved by the Animal Care and Use Committee of the Sichuan University. Both EGFP (enhanced green fluorescent protein) transgenic mice and C57 mice were obtained from Model Animal Research Center of Nanjing University.
Isolation of primary epidermal keratinocytes, flow cytometry and cell sorting
Dorsal skin from newborn mice was incubated horizontally in collagenase/dispase for 1 hour at 37°C. The dermis was gently separated from the epidermis, the epidermal layers were minced, and digestion was continued for another 2 hours. The suspension was gently mixed and filtered through a 70-μm cell strainer.
Cell suspensions were stained with primary antibodies for 30 min, with shaking every 10 min. After washing with 2% FBS/PBS, cells were incubated for 30 min with secondary antibodies, with shaking every 10 min. The primary antibody used was TLR7 antibody (sc-16245, Santa Cruz). Dead cells were excluded with 7AAD (BD Biosciences), and Mouse Lineage Panel (BD Biosciences) was used to exclude Lin−. AlexaFluor 488 donkey anti-goat IgG (Invitrogen) was used as secondary antibody.
Flow cytometry was performed on FACS Aria (BD Biosciences) with FACS DiVa software (BD Biosciences), as previously described14.
Isolation of dermal cells from C57 mice
Dorsal skin from newborn C57 mice was incubated horizontally in collagenase/dispase for 1 hour at 37°C. The epidermis was gently separated from the dermis. The dermis was minced in 3 mg/ml collagenase and incubated for 2 hours at 37°C. The suspension was pipetting and filtered through a 70-μm cell strainer. Count the dermal cells and plate them at 2 × 106 cells per T75 culture flask in high glucose DMEM. Culture dermal cells at passage 2–3 were used for three-dimensional organotypic cultures and epidermal reconstitution assay.
Magnetic cell sorting
FACS usually decreased cells vitality in our previous experiments. For acquiring cells with higher viability to perform three-dimensional organotypic cultures in vitro and epidermal reconstitution assay in vivo, we used magnetic cell sorting. Cells from newborn mice were incubated with primary antibodies (TLR7 antibody, sc-16245, Santa Cruz) binding to extracellular domain of TLR7 for 30 min, followed by incubation with biotinylated antibody at the appropriate concentration for 30 min. After washing with an excess volume of 1 × BD IMAG™ buffer, cells were incubated with BD IMAG™ streptavidin particles for 30 min. The labeling volume was increased to 2 × 107 cells/ml with 1 × BD IMAG™ buffer, and the tube was transferred onto the BD IMagnet™ for magnetic cell sorting, following the manufacturer's guidelines. TLR7-positive cells were collected and subjected to flow cytometric analysis for the quantification of TLR7-positive cell enrichment. The positive fraction was labeled with TLR7 antibody (IMG-581A, Imgenex) binding to intracellular domain of TLR7 and AlexFluor 488 donkey anti-rabbit IgG (Invitrogen). After staining with 7AAD, the cells were analyzed by FACS (BD, FACS Aria).
Mouse keratinocyte culture and treatment
Mouse keratinocytes were isolated and cultured on type I collagen coated cover slips in 6-well plates. 3000 to 20000 cells were plated per well and maintained in proliferation medium containing low concentrations Ca2+ (CnT-07 medium, CELLnTEC). Unless specifically stated, media and compounds were replaced on an alternate day. For proliferation assay, cells were treated with imiquimod. Cells treated with equivalent volume of DMSO and cells untreated served as control. Imiquimod was dissolved in 100% DMSO to 4‰ and this solution was further diluted to a final concentration of 1 μg/ml and 10 μg/ml, respectively. Experiment treating cells with DMSO diluted in medium revealed no effect on growth with these concentrations of DMSO. Barrat et al. identified synthetic oligodeoxynucleotides IRS661 as TLR7 specific antagonist, which shows inhibitory effect on TLR7 signaling but not on TLR912. IRS661 is usually used to oppose the effects of imiquimod on skin. To inhibit the proliferation induced by imiquimod, we used endotoxin-free oligodeoxyribonucleotides (ODN) IRS661. IRS661 was prepared in sterile water and was utilized at 0.03 mg/ml. Cells treated with IRS661 alone served as control. All treatments were performed for 96 hours. To mark replicating cells, EdU was added to medium at a final concentration of 10 μM 4 hours prior to fixation. To induce differentiation, proliferation medium was replaced with differentiation medium containing high concentrations Ca2+ (CnT-02 medium, CELLnTEC). At least 3 wells/treatment were counted. Experiments were conducted three-five times.
Epidermal reconstitution assay
Freshly isolated TLR7-positive epidermal keratinocytes from EGFP mouse pups sorted by magnetic cell sorting or unsorted cells from the primary graft (for secondary graft) were combined with equal numbers of newborn dermal fibroblasts at 104/μl, and 40 μl were transferred into a silicone chamber implanted onto the back of an anesthetized nude mouse. After one week, the top of the silicon chamber was removed with scissors. Another week later, chambers were removed15. Hair growth typically appeared 3–4 weeks after the transplantation. The primary and secondary grafts were harvested six weeks after implantation for analysis. The results shown were representative of six experiments.
Three-dimensional organotypic cultures
TLR7-positive cells were sorted by magnetic cell sorting from freshly isolated EGFP epidermal keratinocytes, and 2 × 105 cells were mixed with equal numbers of newborn dermal fibroblasts in 400 μl proliferation medium containing low concentrations Ca2+ (CnT-07 medium, CELLnTEC) per insert (Millipore). Medium levels inside and surrounding the insert were equal. The cells were then cultured in an incubator at 37°C and 5% CO2 until the monolayer was nearly confluent. The CnT-07 medium inside and surrounding the insert was replaced with differentiation medium containing high concentrations Ca2+ (CnT-02-3DP5 medium, CELLnTEC). After 15–16 hours in the incubator, aspirate out the medium inside the inserts to exposure tissues to the air-liquid interface, then initiate 3D culture. Cultures were harvested until the model was established and ready for analysis.
Histology and immunofluorescence
Cells on a coverslip were fixed for 20 min in 4% paraformaldehyde at room temperature. After three washes, the cells were incubated with 0.1% Triton X-100 for 15 min and blocked by 3% BSA for 1 hour. Cells were incubated with different primary antibodies overnight at 4°C. After three washes, cells were treated with the appropriate secondary antibodies for 1 hour. The primary antibodies were used as follows: anti-K1 (rabbit, 1:500, Abcam), anti-AE13 (mouse, 1:200, Abcam), anti-PPARγ (rabbit, 1:200, Abcam). All AlexaFluor-labeled secondary antibodies were from Invitrogen. Nuclei were labeled with DAPI.
All tissue specimens were embedded in OCT and stored at −80°C. Sections (4–5 μm) were then subjected to standard multiple-labeled immunofluorescence or immunohistochemistry staining. The following antibodies were used for analysis: anti-TLR7 (sc-16245, 1:50, Santa Cruz), anti-K1 (ab9286, 1:200, Abcam), anti-K17 (rabbit, 1:200 for immunofluorescence and 1:500 for immunohistochemistry, Abcam), anti-GFP (06-896, 1:200, Millipore), anti-PCNA (NA03, 1:800, Calbiochem), anti-H3S10p (06-570, 1:1500, Millipore), anti-ITGA6 (ab20142, 1:500, Abcam), anti-PPARγ (ab19481, 1:500, Abcam), anti-CD34 (sc-18917, 1:200, Santa Cruz), anti-AE13 (ab16113, 1:400, Abcam), normal chicken IgY (sc-2718, Santa Cruz), normal goat IgG (sc-2028, Santa Cruz) and AlexaFluor-labeled secondary antibodies (Invitrogen). Nuclei were stained with DAPI for immunofluorescence. Sections incubated goat IgG and chicken IgY instead of primary antibody as isotype control.
Treatment mice
The 6 weeks old of C57BL/6 mice were randomly divided into an untreatment group and six treatment groups: (A) imiquimod, (B) imiquimod + IRS661, (C) imiquimod + control ODN, (D) DMSO (E) DMSO + IRS661, (F) DMSO + control ODN. Imiquimod was dissolved in 100% DMSO to 4‰, Group D, E and F were used as vehicle control. All C57 mice were removed the dorsal hair by shaver. 4‰ imiquimod (Enzo) in DMSO or DMSO were applied onto the surface of dorsal skin shaved. Experiment revealed no effect on skin with 100% DMSO. The endotoxin-free oligodeoxyribonucleotides (ODN) IRS661 as the specific inhibitor for TLR7 and its control ODN were prepared in sterile water to 0.3 mg/ml and subcutaneously injected at a dose of 30 μg/per mice 30 min before TLR7 agonist treatment12,16. All treatments were performed on an alternate day till two weeks. The dorsal skin was harvested at 14 days. The samples were fixed overnight in 10% neutral-buffered formalin, embedded in paraffin and sectioned at 5 μm thickness. For assessment of the distribution of injected IRS661 or control ODN, 3′-cy3-labeled IRS661 or control ODN was subcutaneously injected into 6-wk-old C57BL/6 mice. The following phosphothioate ODN (Invitrogen) were used for in vivo studies: IRS661 5′-TGCTTGCAAGCTTGCAAGCA-3′; Control ODNs were 5′-TCCTGCAGGTTAAGT-3′. To mark replicating cells, 10 mg/kg EdU was injected intraperitoneally 1 hour prior to sacrifice. This experiment was repeated three times with similar results. The EdU labeled cells were visualized using EdU Imaging Kit (Life Technologies), according to the manufacturer's protocol.
Results
TLR7 activation mediated by imiquimod stimulates epidermal cells proliferation
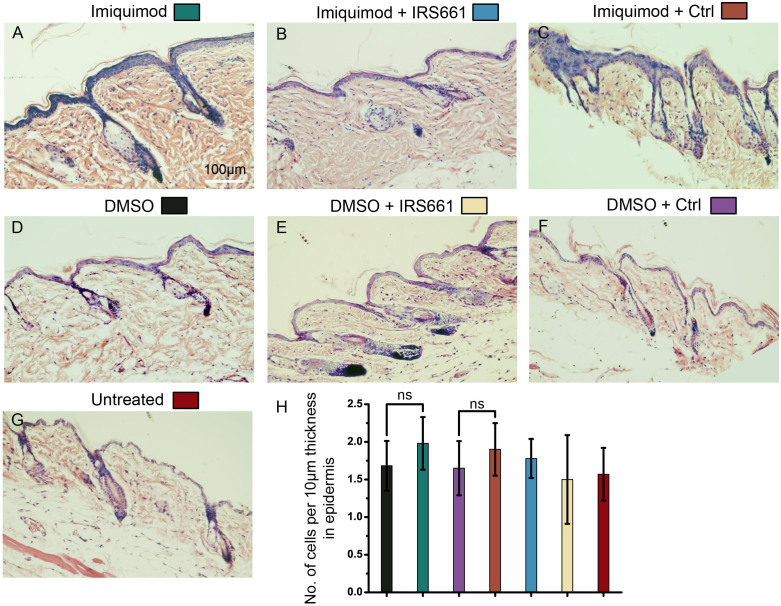
The facts that TLR7-deficient mice do not display any apparent abnormalities indicate that TLR7 does not display physiological functions or there are other proteins can overlap its functions in skin5. Previous data indicate that pharmaceutical activation of TLR7 by the TLR7 agonist can treat tumors but is able to induce abnormal changed in skins17. To visualize the pathological and histological alteration caused by the activation of TLR7 in the skin, we used the TLR7 agonist imiquimod (4‰ solution in DMSO) to treat the dorsally shaved skin of 6-week old C57BL/6 mice. The 6 weeks old of C57BL/6 mice were randomly divided into an untreated group and six treated groups: (A) imiquimod, (B) imiquimod + IRS661, (C) imiquimod + control ODN, (D) DMSO, (E) DMSO + IRS661, (F) DMSO + control ODN. Because imiquimod was dissolved in DMSO at a concentration of 4‰, group D, E and F were used as vehicle control. IRS661 as the specific inhibitor for TLR7 and its control ODN in group B, C, E and F were subcutaneously injected at a dose of 30 ug/per mice 30 min before TLR7 agonist treatment. After 2 weeks, the imiquimod-treated regions of the skin appeared abundantly flaking and rash, pointing to hyperkeratosis beneath the regions, as the same lesions are detected in skin of human patients after imiquimod treatment (Fig. S1A–C). In contrast, the phenotypes were not detected in untreated or vehicle control mice (Fig. S1G, D–F). The histological sections of the murine skin showed that the IFE of the imiquimod-treated mice was much thicker than untreated or DMSO-treated mice, and more cells resident in the thickening regions were observed (Fig. 1A, D and G). The murine skin treated with the TLR7 specific oligonucleotide-based inhibitor IRS661 showed that the IRS661 partly inhibited the scurf production (Fig. S1A–C) and dramatically inhibited the hyperplasia of epidermis induced by imiquimod (Fig. 1A–C). Vehicle control showed that DMSO, IRS661 and control ODN did not cause any abnormality (Fig. 1D–F and S1D–F).We counted the average nucleated cell number per 10 μm thickness of the IFE in each group and found no significant difference between imiquimod and DMSO-treatment (Fig. 1H). This result verified that the hyperplasia was directly caused by the increase of cell number, excluding the increase of cell size or intercellular space. The results indicate that pharmaceutical activation of TLR7 cause hyperplasia of murine skins.
Figure 1. Activation of TLR7 by imiquimod promoted epidermis thickening.
(A–G) Histological skin sections of the mice were used in hematoxylin-eosinstaining (HE). Imiquimod treated skin(A); Imiquimod and IRS661 treated skin (B); Imiquimod and control ODN treated skin (C); DMSO treated skin (D); DMSO and IRS661 treated skin (E); DMSO and control ODN treated skin (F); Untreated skin (G). (H) Quantification of the average nucleated cell number per 10 μm thickness of the IFE. ns: no significance.
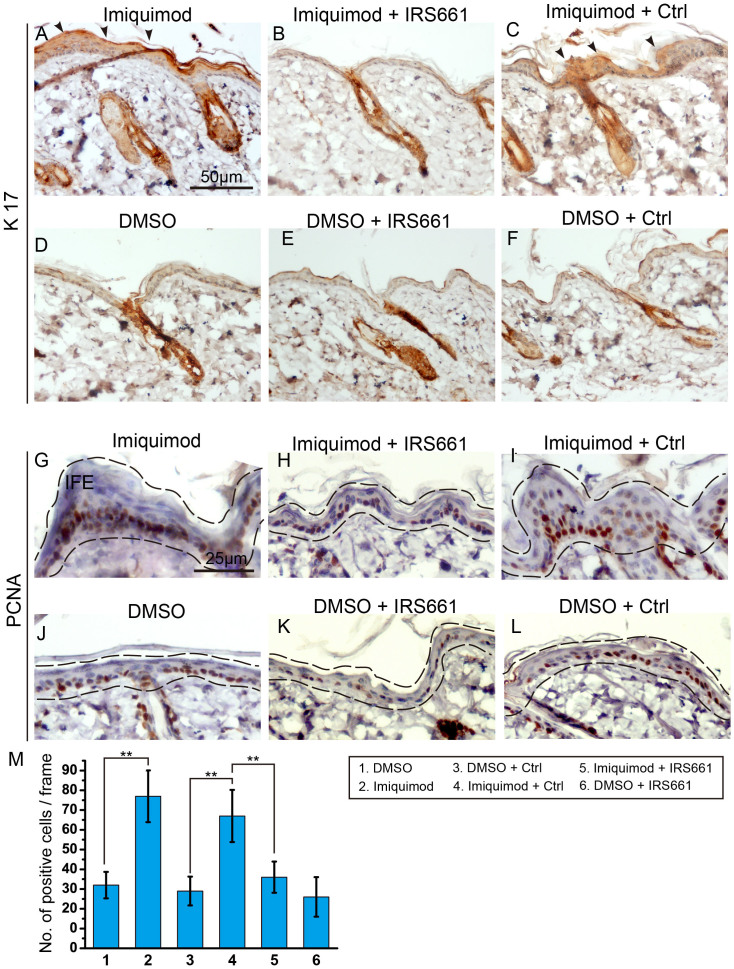
Careful examination of the murine skin sections showed that after imiquimod treatment, more proliferating cells were identified by the PCNA staining in the hyperplasitc skin regions (Fig. 2G, J). The treatment of IRS661 was strongly able to inhibit the abnormal proliferation of keratinocytes induced by imiquimod (Fig. 2H, I). A similar result was found in skin by the H3pS10 labelling (Fig. S2). To verify the results obtained by PCNA and H3pS10 staining, we injected EdU 1 hour before collecting skin samples. There was a marked increase in EdU-labeled cells of the imiquimod treatment group, confirming those cells' proliferative status. Most EdU-labeled cells were detected in IFE, especially in the basal layer highlighted by α6 integrin. The result showed that imiquimod induced the proliferation of basal cells. The effect of imiquimod on basal cells was inhibited by IRS661 (Fig. S3). K17, a marker which exclusively labels hair follicles of normal skin18, was ectopically expressed in the proliferating murine skin treated with imiquimod (Fig. 2A–F and S4A–C). Together, the results indicate that activation of TLR7 causes proliferation of keratinocytes in skins.
Figure 2. TLR7 activated by imiquimod induced ectopic expression of K17 and stimulated interfollicular cell proliferation.
(A–F) Immunohistochemistry staining for K17 highlighted its expression pattern in epidermis. Arrowheads indicated the ectopic expression of K17 in A and C. (G–L) Immunohistochemistry with the proliferation associated marker PCNA showed the proliferative epidermal cells. Black dashed lines outlined the epidermal region. Imiquimod treated skin (G); Imiquimod and IRS661 treated skin (H); Imiquimod and control ODN treated skin (I); DMSO treated skin (J); DMSO and IRS661 treated skin (K); DMSO and control ODN treated skin (L). IFE, interfollicular epidermis. (M) Quantification of the proliferation of interfollicular cells in G–L. **: P < 0.01, t-test.
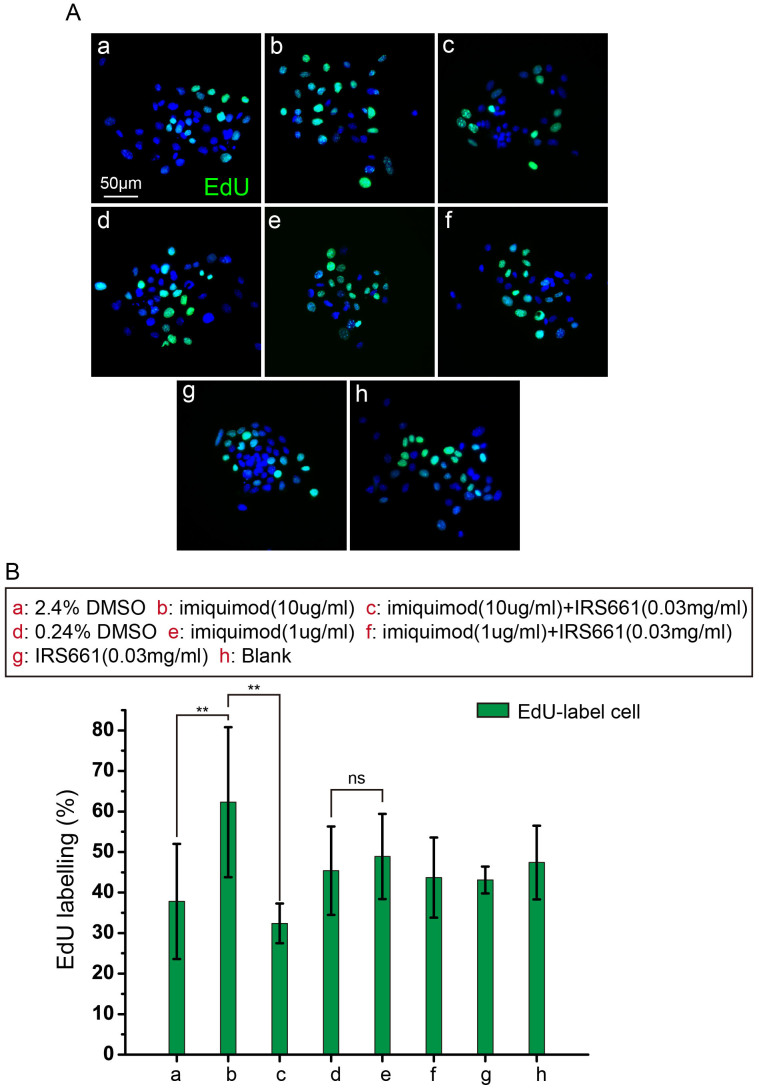
To examine whether the proliferation was due to a direct effect of imiquimod on TLR7-positive cells, the keratinocytes from the newborn C57 mouse epidermis expressing TLR7 were isolated by immunolabeling with TLR7 antibodies. Approximately 4%–6% of the keratinocytes were identified as TLR7-positive cells (Fig. S5A, B). The sorted TLR7-positive cells showed membrane-bound staining for TLR7 (Fig. S5C). TLR7-positive cells isolated were seeded at a density of 2 × 104 cells per well in 6-well plates and maintained in proliferation medium containing low concentrations Ca2+ (CnT-07 medium, CELLnTEC). Three days after primary culture, TLR7-positive cells were treated with 1 μg/ml, 10 μg/ml imiquimod. Cells treated with equivalent volume of DMSO and cells untreated served as control. To inhibit the proliferation induced by imiquimod, endotoxin-free oligodeoxyribonucleotides (ODN) IRS661 was used at a final concentration of 0.03 mg/ml. Cells treated with IRS661 alone served as control. All treatments were performed on an alternate day till 96 hours. To discern cells of proliferative status, TLR7-positive cells were incubated in EdU (10 μM) for 4 hours prior to fixation. Neither DMSO (2.4% and 0.24%) nor IRS661 (0.03 mg/ml) alone diluted in medium had effect on cell growth. Examination of the number of EdU-labeled cells in cultures exposed to 10 μg/ml imiquimod demonstrated that imiquimod-subjected cultures contained significantly more EdU-labeled cells than in control cultures (Fig. 3A, B). 1 μg/ml imiquimod treatment group showed no significant differences from control cultures, suggesting that 1 μg/ml imiquimod wasn't enough to activate TLR7-positive cells excessively proliferating (Fig. 3B). Cultures treated with 10 μg/ml imiquimod and IRS661 were decreased in EdU-labeled cell number from control levels (Fig. 3B). Cultures treated with 1 μg/ml imiquimod and IRS661 had a comparable EdU-labeled cell number as controls (Fig. 3B). It indicated that IRS661 blocked the excessive proliferation in cultures with 10 μg/ml imiquimod. These data suggest that imiquimod directly stimulate TLR7-positive cells to proliferate independently from immune system in vitro.
Figure 3. Imiquimod induced the proliferation of TLR7 positive keratinocytes in vitro.
(A) Cells were treated with imiquimod at the indicated concentrations. EdU highlighted the proliferation of TLR7 positive cells. 2.4% DMSO in a, 10 ug/ml imiquimod (dissolved in 100% DMSO) in b, 10 ug/ml imiquimod (dissolved in 100% DMSO) and 0.03 mg/ml IRS661 in c, 0.24% DMSO in d, 1 ug/ml imiquimod in e, 1 ug/ml imiquimod and 0.03 mg/ml IRS661 in f, 0.03 mg/ml IRS661 in g, untreated group as blank group in h. (B) Quantification of the proliferation of interfollicular cells in A. These data are representative of at least three independent experiments with similar findings. **: P < 0.01, t-test. ns: no significance.
TLR7 is expressed in murine hair follicles and interfollicular epidermis
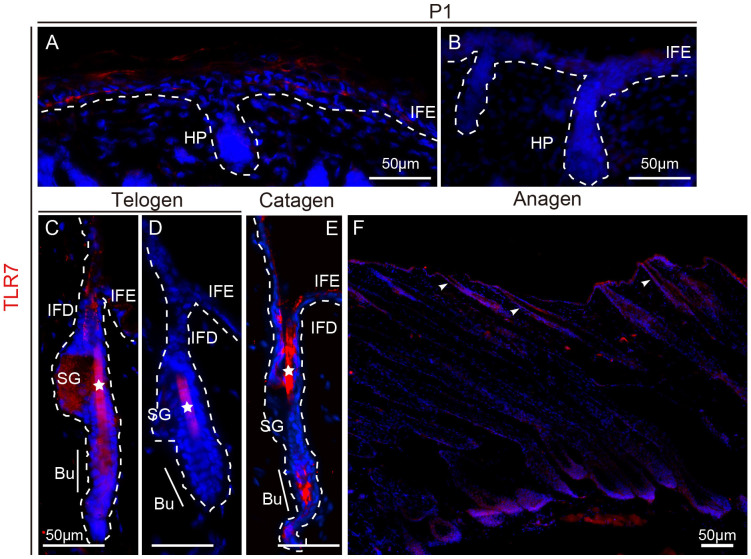
The relationship between TLR7 expression and murine skin was examined by immunofluorescent labeling of dorsal skin sections from wild-type, C57 mice at various hair follicle growing stages. From postnatal day 1 (P1), the hair follicles begin to grow as hair pegs. At P1, TLR7 was visualized at high levels in the IFE (Fig. 4A). As the follicle entered the first telogen phase (quiescent stage) at P21, TLR7 was detectable in the infundibulum (the upper part of HF), extending upwards to IFE, as well as the secondary germ (Fig. 4C). In the subsequent anagen phase at P37, most of the TLR7-labeled cells were in the IFE, infundibulum and hair bulb, where the proliferating matrix cells reside (Fig. 4F). During the catagen phase, TLR7-positive cells persisted in the IFE and infundibulum (Fig. 4E). The asterisks in figure 4C–E mark the red staining of the hair shaft caused by non-specific staining which was verified by isotype control (Fig. 4B and D).
Figure 4. TLR7 expression in mouse epidermis.
(A–F) Immunofluorescence staining of TLR7 (red) in skin obtained from wild-type mice at P1 (A and B) and in telogen (C and D), catagen (E) and anagen (F). Isotype control at P1 (B) and in telogen (D). Arrowheads indicate positively stained calls in infundibulum (D). The asterisksin (C–E) mark the red staining of the hair shaft caused by non-specific stainingwhich was verified by isotype control (D). White dashed lines represent the boundary between epidermis and dermis or edge of the hair follicles. IFE, interfollicular epidermis; HP, hair peg; IFD, infundibulum; SG, sebaceous gland; Bu, bulge.
Because TLR7 expression in keratinocytes has not been previously reported, we confirmed the specificity of the TLR7 antibodies used in these experiments by western blot analysis. The specificity of the method was determined by detecting the expression of TLR7 in brain and spleen tissues. According to the molecular weight of TLR7, 120.9 kDa, we found the specific band appeared in spleen tissue, and the expression of TLR7 was too low to be detected in brain (Fig. S6A). Similarly, immune cells in spleen expressed high level of TLR7 (Fig. S6B). In contrast, the brain cortex displayed much lower TLR7 expression by immunofluorescent staining (Fig. S6C). Thus, we conclude that the antibodies used in immunofluorescence, immunohistochemistry and FACS are specifically recognizing the TLR7 epitope on cells.
Properties of TLR7-positive keratinocytes
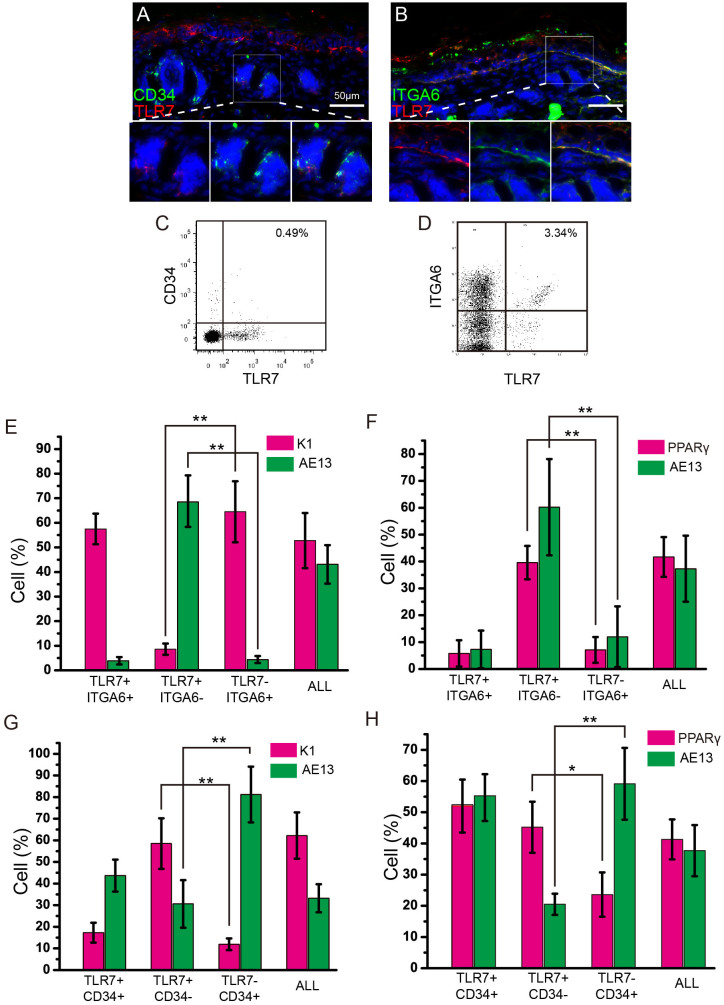
To characterize the TLR7-positive keratinocytes, we used two described markers, α6 integrin and CD34, to examine the cell types of TLR7-positive keratinocytes. α6 integrin is a valuable marker of the basal cells3,4,19. CD34 is an excellent bulge stem cell marker. CD34 positive keratinocytes can express either low or high level of α6 integrin4. In the frozen sections of dorsal skin from C57 mice co-labelled with α6 integrin or CD34 and TLR7, we observed that TLR7 positive population was predominantly located in the IFE and substantial overlap with the α6 integrin positive population that was present in the basal epidermis and throughout the hair follicle (Fig. 5B). A few TLR7-positive cells were also detected in the developing hair follicles and a small fraction of them showed overlap with the CD34 positive population (Fig. 5A).
Figure 5. Characterization of TLR7-positive population.
(A, B) Immunofluorescence was performed on dorsal skin sections of wild-type mice at P1. Co-staining of TLR7 and CD34 (A), TLR7 and ITGA6 (B). (C) FACS analysis of P1 epidermis from wild-type micelabelled with antibodies to ITGA6 and TLR7. (D) FACS analysis of P1 epidermis from wild-type micelabelled with antibodies to CD34 and TLR7. (E–H) Analysis of the differentiation potential of different populations from P1 skin. Four distinct populations: TLR7+/CD34+, TLR7+/CD34−, TLR7−/CD34+ and All (the unfractionated population) in E and F; TLR7+/ITGA6+, TLR7+/ITGA6−, TLR7−/ITGA6+ and All (the unfractionated population) in G and H. AE13: follicular marker; PPARγ: sebaceous gland marker; K1: epidermal marker. **: P < 0.01, t-test.
To examine the properties of TLR7 positive cell population further, we performed FACS analysis. When disaggregated epidermal cells were double-labelled with antibodies of TLR7 and α6 integrin, 55%–80% of the TLR7 positive cells carried high levels of α6 integrin (Fig. 5D). As expected, very small fraction of TLR7 positive cells expressed CD34 (8%–12%) (Fig. 5C). Then, cells were isolated and 3000 cells were seeded per well in 6-well plate. After primary culture for 14 days, colonies derived from a single keratinocyte formed and were induced to terminally differentiate in vitro. Major of differentiated cells derived from the TLR7, α6 integrin double-positive cells expressed K1, an IFE-terminal differentiation marker, suggesting the TLR7, α6 integrin double-positive cells are prone to undergo epidermal differentiation and are similar to epidermal stem cells (Fig. 5E and S7A). In contrast,most of the TLR7 positive, α6 integrin negative population underwent HF and SG differentiation as measured by markers specific for hair keratins (AE13) and for SG (PPARγ) respectively (Fig. 5F and S7B). A large fraction of the TLR7, CD34 double-positive population preferred to differentiate to HF and SG rather than epidermis (Fig. 5G, H and S7C, D). These cells seemed to possess differentiation potential as HF stem cells in vitro. Together, these observations suggest that the TLR7 positive population probably represent a subset of the total epidermal stem cell population in the epidermis and a small subset of the HF stem cell population in the HFs.
TLR7-expressing cells possess the skin generative ability in vitro and in vivo
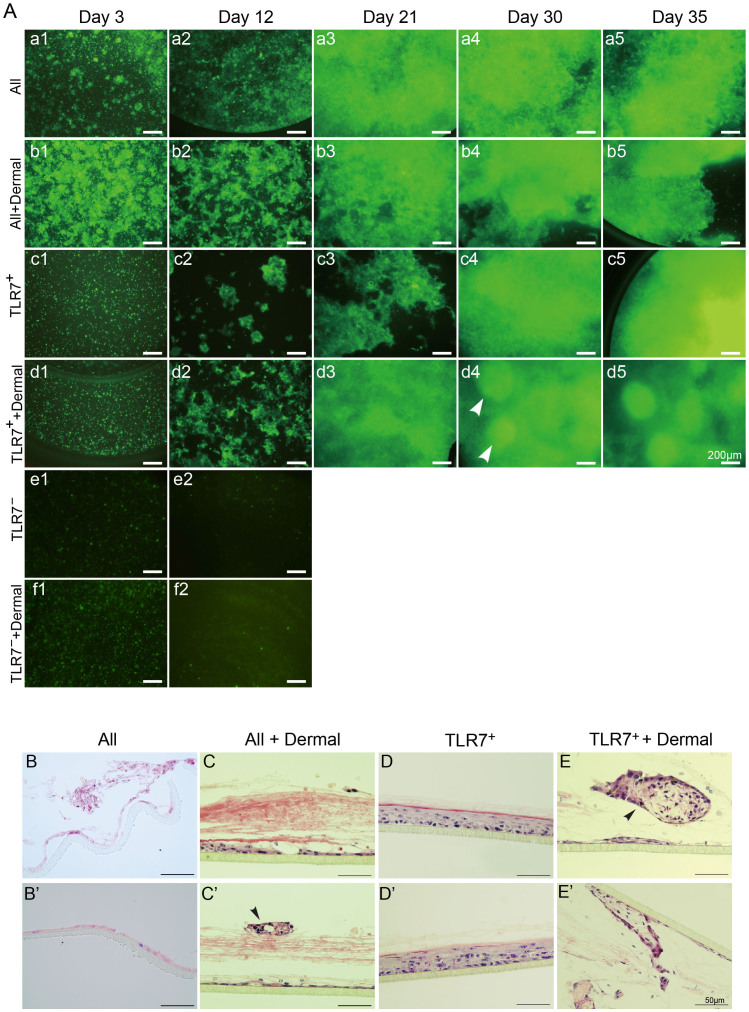
To examine whether TLR7-positive cells are able to generate the skin structures, we performed three-dimensional organotypic cultures in vitro. The three-dimensional architecture of the reconstructed epidermis can mimic the morphologic, differentiation, and growth processes of the epidermis in vivo20. Because FACS usually reduced the viability of TLR7-positive cells in our previous experiments, we chose magnetic cell sorting to perform enrichment of TLR7-positive cells. To examine the efficiency of magnetic cell sorting, we performed FACS after the magnetic sorting for quantification of TLR7-positive cell enrichment. The positive fraction was labeled with another TLR7 antibody with different binding site to TLR7 and was analyzed by FACS. The proportion of TLR7-positive cell was 75%–85% (Fig. S8). Briefly, freshly isolated primary keratinocytes from newborn EGFP mice by magnetic cell sorting expressing enhanced green fluorescent protein (EGFP) were cultivated on PCF membranes of inserts in proliferation medium. When the keratinocytes reached confluence, the culture medium was exchanged with differentiation medium. After 15–16 hours, the cells subsequently began stratification at the air-liquid interface by aspirating out the medium inside the inserts. Figure 6A illustrates the outgrowth of cells, viewed with an inverted fluorescent microscope. Within the first 3 days, some TLR7-positive cells adhered, although many cell died (Fig. 6Ac1), presumably as a result of the pressure inherent in the sorting process. By 30 days, TLR7-positive cells were nearly confluent (Fig. 6Ac4), following stratification, organization and differentiation.
Figure 6. Three-dimensional organotypic cultures in vitro.
(A) Various developmental stages of organotypic cultures viewed with an inverted fluorescent microscope (D3, D12, D21, D30 and D35). (a1–a5) The unfractionated population of epidermis without dermal cells (All), (b1–b5) the unfractionated population of epidermis mixed with dermal cells (All + dermal), (c1–c5) TLR7-positive cells in the absence of dermal cells (TLR7+), (d1–d5) TLR7-positive cells with dermal cells (TLR7+ + dermal), (e1–e2) TLR7-negative cells without dermal cells (TLR7−), (f1–f2) TLR7-negative cells with dermal cells (TLR7− + dermal). The white arrowhead indicates two bright elevated spheroids in epidermal equivalent. (B–E′) Paraffin section of epidermal equivalent on PCF membranes labeled with hematoxylin stain. Structures like cutaneous appendages are indicated by the black arrowhead.Data are means of triplicate determinations from a single representative experiment.
As previously described, dermal cells can secrete keratinocyte growth factors that are necessary for keratinocyte proliferation and differentiation21. When TLR7-positive cells were combined with EGFP-negative dermal cells from P1 newborn wild-type C57 mice, they showed better growth (Fig. 6Ad1–5). TLR7-positive cells combined with dermal cells more efficiently generated colonies than cells without dermal cells (Fig. 6Ad2). By 21 days of culture, TLR7-positive cells with dermal cells had reached confluence (Fig. 6Ad3) and initiated stratification, whereas TLR7-positive cells without dermal cells only formed a thin layer with some large hollows. On day 30, the bright elevated spheroids were visible in the epidermis constructed by TLR7-positive cells with dermal cells (Fig. 6Ad4). Although the unfractionated population formed more complete layer before, they had atrophied at this time (Fig. 6Aa4, b4). After 35 days, keratinocytes stratified and completely differentiated. Contrast to TLR7-positive population and unfractionated population, TLR7-negative population died and failed to generate outgrowth whether they were mixed with dermal cells or not (Fig. 6Ae, f). Cultured membranes were harvested for histology and immunohistochemistry analysis at various times until a complete epidermis had formed. Hematoxylin staining of the organotypic cultured tissues showed that the unfractionated population of the epidermis without dermal cells failed to form the normal basal cells and stratified structure. The majority of the cells had differentiated and assembled rough-and-tumble masses, but failed to organize the structure as the IFE (Fig. 6B, B′). In comparison, organotypic tissues generated from TLR7-positive cells showed nice stratification and differentiation, attaching to PCF membranes. These tissues exhibited an intact developed basal layer, suprabasal layers, and cornified layers, indicating that TLR7-positive cells carry a better ability to form IFE structures in vitro than unsorted cells (Fig. 6D, D′).
The epidermis derived from TLR7-positive cells with growth support and spatial features provided by dermal cells displayed abnormal morphologic stratification and only assemble a fragmentary basal layer (Fig. 6E, E′). Similar result was observed in the production of the unfractionated population of the epidermis mixed with dermal cells, implying that TLR7-positive cells and unfractionated cells with dermal cells didn't differentiate to IFE overall. Together, these results suggested that TLR7-expressing cells have potential to proliferate and execute IFE differentiation, especially without the presence of dermal cells in vitro.
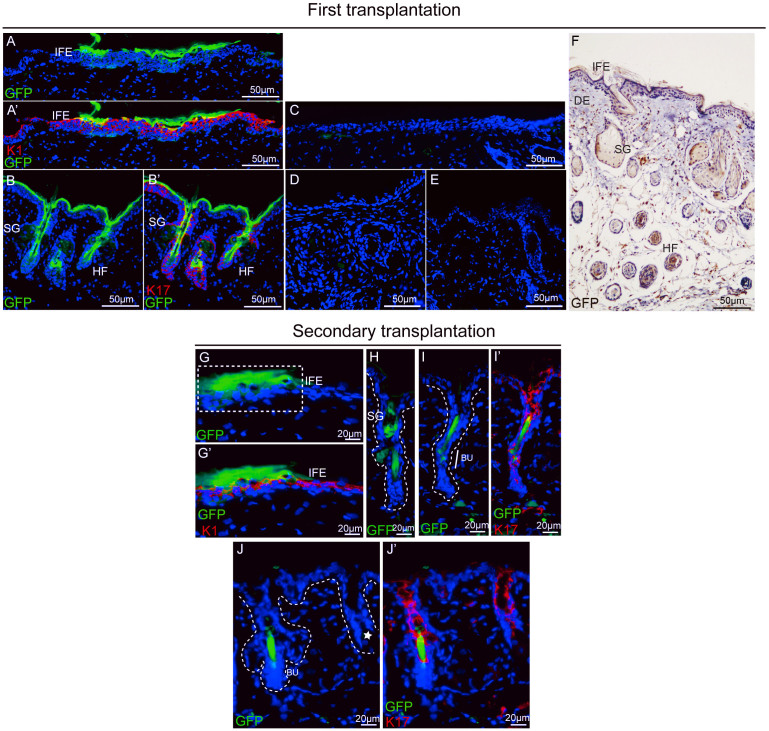
To test the capacity of TLR7-expressing cells to reconstitute all of the epidermal lineage and HFs in vivo, we isolated cells by magnetic cell sorting from newborn EGFP mice based on TLR7 expression. The isolated TLR7-positive cells (2 × 105) and EGFP-negative dermal cells from P1 newborn wild-type, C57 mice (2 × 105) were mixed and surgically transplanted onto the upper backs of recipient nude mice at a site where the full-thickness skin had been removed. Black hair formation from TLR7-positive cells was typically visible at 3–4 weeks post-transplantation, showing the same color as the donor (Fig. S9A, B), whereas only scar in graft derived from control mice with no transplantation and mice transplanted with TLR7-negative cells (Fig. S9C). At 4–5 weeks post-implantation, skin grafts were harvested and analyzed. When viewed microscopically from the epidermal side, black hairs from the highlight follicle were observed to penetrate the green fluorescent epidermis (Fig. S9C, D). Sections of grafts were processed for immunofluorescence staining with GFP antibody. Microscopy of skin sections from mice transplanted with TLR7-positive cells revealed the contribution of EGFP-positive cells to IFE, HFs, and SGs (Fig. 7A, A′ and B, B′). Isotype control in figure 7C verified that green staining was not due to non-specific staining. By contrast, no GFP-positive cell was detected in sections of grafts from mice transplanted with TLR7-negative cells or control mice with no transplantation (Fig. 7D and E). To further validate that these regions originated from EGFP-expressing keratinocytes, we stained paraffin embedded sections with EGFP antibodies. EGFP-positive cells were present in regenerated IFE, HFs, and SGs (Fig. 7F). These data suggest that the TLR7-expressing population displays enrichment for the cells that contribute to all epidermal lineages in the regenerated epidermis in vivo.
Figure 7. The reconstituted skin inepidermal reconstitution assay.
(A–E) Sections of the fist grafts were processed for immunofluorescence staining. Epifluorescent EGFP was employed in conjunction with antibodies against the epidermal marker K1 (A, A′) or the hair follicular marker K17 (B, B′) to examine the contribution to skin lineages.Isotype control (C), section of graft from mice transplanted with TLR7-negative cells (D), section from un-transplantation mice (E). (F) Section of graft stained with GFP antibody (brown) and hematoxylin counterstain (blue) showing the contribution of TLR7-positive cells to the graft. IFE, interfollicular epidermis; SG, sebaceous gland; HF, hair follicle; DE, dermis. (G–J′) Sections of the second graft were stained with antibodies against the epidermal marker K1 (G, G′) or the hair follicular marker K17 (I, I′, J, J′) to show the contribution of EGFP-positive cells. EGFP-positive cells contributed to the sebaceous gland (H). The region of reconstituted IFE is demarcated by white dashed lines in G. The asterisk marks thehair follicles that did notreconstitute by EGFP-positive cells (J). White dashed lines represent the boundary between epidermis and dermis or edge of the hair follicles in (H–J). IFE, interfollicular epidermis; SG, sebaceous gland; Bu, bulge.
TLR7-expressing cells carry self-renewal ability in vivo
It is known that one of the fundamental properties of epidermal stem cells carry self-renew ability. The original stem cell can divide asymmetrically into transit-amplifying (TA) cell. TA cells are short-term repopulating cells that undergo limited divisions. Serial transplantation of the cells originating from the primary graft ensures long-term regeneration and experimentally excludes the TA cells22,23. TLR7-positive cells from EGFP mice combined with EGFP-negative dermal cells from P1 newborn wild-type, C57 mice were first grafted to a nude mouse. 4 weeks after primary transplantation, regenerated skin with tufts of hair was observed (Fig. S10C). Until the regenerated area entered the resting phase of the hair cycle, epidermal cells were collected after enzymatic digestion of the regenerated skin. Fluorescence imaging showed the majority of dissociated epidermal cells exhibited green fluorescence, suggesting they were progeny of TLR7-positive cells (Fig. S10E). The isolated epidermal cells were mixed with dermal cells and delivered immediately to the full-thickness wound on the back skin of another recipient nude mouse. At 3 weeks post-grafting, while daughter from unsorted epidermal cells couldn't form new epidermis (Fig. S10B), the secondary graft from TLR7-positive daughter cells displayed a normal epidermis, as viewed from above (Fig. S10D). The area of reconstituted skin was smaller than the primary grafts because of small number and low viability of disaggregated epidermal cells. The graft was analyzed four weeks after surgery. When viewed from the epidermal side, most EGFP-positive cells observed contributed to the epidermis, showing typical appearance of epidermal cells (Fig. S9E, arrowhead and F). In addition to numerous EGFP-positive epidermal cells, there were a few short black hairs sprouted from the graft, implicating the contribution of TLR7-positive cells to hair follicles (Fig. S9Ea). Immunofluorescence microscopy of the graft section revealed a noticeable EGFP fluorescence in the IFE (Fig. 7G, G′) and showed that a few EGFP-positive cells were scattered in the reconstituted HFs (Fig. 7H) and SGs (Fig. 7I–J′). The weak ability of the EGFP-positive cells committed to HFs and SGs presumably represents progeny of transit-amplifying cells that originally derived from TLR7-expressing cells. After these cells complete a limited number of divisions over the short term, they terminally differentiate and are sloughed into the environment, suggesting that TLR7-expressing cells contain the HF progenitors rather than stem cells. These results show that a fraction of cells in the TLR7-expressing cellular population exhibits self-renewal and long-term proliferation characteristics and is able to differentiate to IFE. Combined the in vitro and in vivo experiments, we conclude that the TLR7-expressing cellular population contains the interfollicular epidermal stem cells.
Discussion
The skin is the largest organ in the mammalian body and consists of the interfollicular epidermis (IFE) and appendages, including hair follicles (HF), sebaceous glands (SG) and sweat glands1,2. Mouse HF stem cells are identified in the hair follicle bulge, carrying the CD34, cytokeratin 15 (K15) and Lgr5, which include a subpopulation of infrequently dividing, DNA label-retaining cells2,11,13. Another HF stem cells marker Lgr6 is expressed in the earliest murine embryonic hair placodes and the region directly above the follicle bulge in adult murine skin. Prenatal Lgr6-positive cells produce the cellular lineages of the HF, SG, and IFE24. Normal IFE homeostasis is maintained throughout the entire life by its own stem cells. Most recently, Lrig1-positive cells have been found in the HF junctional zone adjacent to the SGs, and infundibulum can give rise to all of the adult epidermal lineages in skin reconstitution assays, suggesting that a reservoir of adult mouse IFE stem cells25. Although α6 integrin is a described basal cell marker, subsets in α6 integrin expressing population were not entirely clear yet. The β1 integrin-positive cells reside in clusters of the basal layer and have high proliferative potential in vitro, but these cells are far from pure1. Our results reveal TLR7 as a novel marker that defines keratinocytes possessing the fundamental characteristics of interfollicular epidermal stem cells.
The reconsitution fragmentary epidermis formed by TLR7-positive cells mixed with dermal cells is similar to the one by the unfractionated population with dermal cells. By contrast, TLR7-positive cells without dermal cells formed epidermis as that in vivo morphologically. These results suggest a subset thought as IFE stem cells in TLR7-expressing cells have potential to proliferate and differentiate, as well as to sufficiently generate intact epidermis in vitro, which are superior to unsorted cells. Dermal cells do not support TLR7-expressing cells differentiate to IFE, implying that proliferation and differentiation to IFE lineage of TLR7-expressing cells do not mediated by stroma cells.
It is known that epidermal stem cells can divide asymmetrically into two daughter cells with different fates. One of them reserves quiescent while another, called transit-amplifying (TA) cells commits along a differentiation pathway with a finite number of divisions2,23. We take the advantage of serial-transplantation assays to examine the self-renewal and to distinguish the true stem cells from TA cells, which were short-term repopulating cells. After TLR7-positive cells from EGFP mouse undergo serial-transplantation, GFP-positive cells substantially and nicely give rise to IFE with the full structures. It indicates that a subpopulation of TLR7-positive cells, which regenerates the intact IFE carried self-renewal capability, are IFE stem cells. In contrast to primary transplantation, GFP-positive cells are sporadic in the regenerative HF and SG, suggesting TLR7-positive cells are not able to contribute to complete structures of HF and SG during the secondary transplantation. Furthermore, the regenerative IFE is separated from the regenerative appendages and most GFP-positive cells, which slough into the canal of the regenerative HF and SG respectively. Together, it indicates that TLR7-positive cells, which regenerate HF and SG, are representing TA cells that are originally arising from initial TLR7-positive population in primary transplantation. They cannot maintain self-renewal and then undergo terminal differentiation.
TLR7 mainly resides in intracellular compartment. It is thought to be an essential sensor of microbial infection, which recognizes single-stranded RNA (ssRNA) derived from pathogen. TLR7 is mainly expressed in plasmacytoid dendritic cells (pDCs) and B lymphocytes9. In skin, different types of cells express distinct TLRs. Keratinocytes are reported to express TLR1-6 and 9. Langerhans cells express all TLRs. They are involved in activation of NF-κB, production of cytokines, and resistance to pathogens6,8. Our results reveal TLR7, as a novel marker, defines keratinocytes that possess the fundamental characteristics of interfollicular epidermal stem cells. It suggests a novel function of TLR7 that never uncover before. Although the mice null for the TLR7 gene can be born alive without any apparent abnormalities5, it indicates that under normal physiological condition, the development and maintenance of skin do not require TLR7 activation. Additional experiments are required to address the function roles of TLR7 in skin development and regeneration.
Imiquimod is used to treat numerous dermatologic conditions because of its pleiotropic effects. Clinical responsiveness to topical treatment with imiquimod is found for both primary skin tumors and cutaneous metastases26. There are reported cases relating to imiquimod treatment in which imiquimod induces the localized autoimmune skin disorders vitiligo and pemphigus27,28 and exacerbates the immune-mediated disease psoriasis29. Furthermore, imiquimod cream as medication for patients have some side effects including mild skin irritation, itching, dryness, flaking, scabbing, crusting and hardening of the skin where the medicine is applied. However, mechanisms of how imiquimod causes the skin side effects are unknown. In this study, TLR7-positive keratinocytes sorted were stimulated to proliferate by imiquimod in vitro. It suggests that imiquimod affects TLR7-positive keratinocytes independently from immune system in vitro. Although, whether the effect of imiquimod on TLR7-positive keratinocytes proliferation relates to immune system or not in vivo is unclear. In previous literature, TLR7 which activated by imiquimod can increase the production of type I IFNs, IL-6 and CCL2 in vivo30. Type I IFNs and IL-6 can influence IFN-γ by JAK-STAT signal pathway31,32,33,34,35. IFN-γ has been reported to be able to up-regulate K17 expression by activating STAT1-STAT3 complex, and this signal pathway in keratinocytes is likely involved in the hyperproliferative psoriatic epidermis36. Taken together, our data provide a novel function of TLR7 in skin, as well as a possible application of TLR7 for purification of skin stem cell for skin transplantation and regeneration. The relationship between the immune function and reform capability requires further studies.
Supplementary Material
Supplementary information
Acknowledgments
The authors thank the members of Mo's laboratory for their critical discussion and review of the manuscript. The English was edited by Christopher Brooks, Ph. D from Bioscience Editing Solutions. This work was supported by the Nature Science Foundation of China (to H. X. (31171384), the National Basic Research Program of China (2009CB941200) and the Key Project of Chinese Ministry of Education (to X.M. No. 109136), Natural Science Foundation Project of CQ CSTC (No.2007BB5296).
Footnotes
The authors declare no competing financial interests.
Author Contributions C.Y. and T.Z. designed, performed and analysed cellular and biochemical experiments, and wrote this manuscript; L.Q. helped biochemical experiments; J.D., S.L., H.Z., F.W. and Q.H. helped cellular experiments; W.M., H.Z. and H.B. helped experimental analysis; X.M. helped experimental analysis and writing; H.L. and H.X. conceived this study.
References
- Blanpain C. & Fuchs E. Epidermal stem cells of the skin. Annu Rev Cell Dev Biol 22, 339–373 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpain C. & Fuchs E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nat Rev Mol Cell Biol 10, 207–217 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani H., Morris R. J. & Kaur P. Enrichment for murine keratinocyte stem cells based on cell surface phenotype. Proc Natl Acad Sci U S A 97, 10960–10965 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trempus C. S. et al. Enrichment for living murine keratinocytes from the hair follicle bulge with the cell surface marker CD34. J Invest Dermatol 120, 501–511 (2003). [DOI] [PubMed] [Google Scholar]
- Hemmi H. et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol 3, 196–200 (2002). [DOI] [PubMed] [Google Scholar]
- Ermertcan A. T., Ozturk F. & Gunduz K. Toll-like receptors and skin. J Eur Acad Dermatol Venereol 25, 997–1006 (2011). [DOI] [PubMed] [Google Scholar]
- Garantziotis S., Hollingsworth J. W., Zaas A. K. & Schwartz D. A. The effect of toll-like receptors and toll-like receptor genetics in human disease. Annu Rev Med 59, 343–359 (2008). [DOI] [PubMed] [Google Scholar]
- Hari A., Flach T. L., Shi Y. & Mydlarski P. R. Toll-like receptors: role in dermatological disease. Mediators Inflamm 2010, 437246 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh S. & Miyake K. Regulatory molecules required for nucleotide-sensing Toll-like receptors. Immunol Rev 227, 32–43 (2009). [DOI] [PubMed] [Google Scholar]
- Lee J. et al. Activation of anti-hepatitis C virus responses via Toll-like receptor 7. Proc Natl Acad Sci U S A 103, 1828–1833 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaner D. E. & Masellis A. Toll-like receptor agonists in the treatment of chronic lymphocytic leukemia. Leukemia 21, 53–60 (2007). [DOI] [PubMed] [Google Scholar]
- Barrat F. J. et al. Nucleic acids of mammalian origin can act as endogenous ligands for Toll-like receptors and may promote systemic lupus erythematosus. J Exp Med 202, 1131–1139 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letada P. R., Sparling J. D. & Norwood C. Imiquimod in the treatment of alopecia universalis. Cutis 79, 138–140 (2007). [PubMed] [Google Scholar]
- Zhang Y. et al. Nuclear factor kappa B signaling initiates early differentiation of neural stem cells. Stem Cells 30, 510–524 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen K. B., Driskell R. R. & Watt F. M. Assaying proliferation and differentiation capacity of stem cells using disaggregated adult mouse epidermis. Nat Protoc 5, 898–911 (2010). [DOI] [PubMed] [Google Scholar]
- Pawar R. D. et al. Inhibition of Toll-like receptor-7 (TLR-7) or TLR-7 plus TLR-9 attenuates glomerulonephritis and lung injury in experimental lupus. J Am Soc Nephrol 18, 1721–1731 (2007). [DOI] [PubMed] [Google Scholar]
- Smits E. L., Ponsaerts P., Berneman Z. N. & Van Tendeloo V. F. The use of TLR7 and TLR8 ligands for the enhancement of cancer immunotherapy. Oncologist 13, 859–875 (2008). [DOI] [PubMed] [Google Scholar]
- Panteleyev A. A. et al. Keratin 17 gene expression during the murine hair cycle. J Invest Dermatol 108, 324–329 (1997). [DOI] [PubMed] [Google Scholar]
- Jones P. H. & Watt F. M. Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell 73, 713–724 (1993). [DOI] [PubMed] [Google Scholar]
- Carlson M. W., Alt-Holland A., Egles C. & Garlick J. A. Three-dimensional tissue models of normal and diseased skin. Curr Protoc Cell Biol Chapter 19, Unit 19 19 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangatirkar P., Paquet-Fifield S., Li A., Rossi R. & Kaur P. Establishment of 3D organotypic cultures using human neonatal epidermal cells. Nat Protoc 2, 178–186 (2007). [DOI] [PubMed] [Google Scholar]
- Kordon E. C. & Smith G. H. An entire functional mammary gland may comprise the progeny from a single cell. Development 125, 1921–1930 (1998). [DOI] [PubMed] [Google Scholar]
- Schneider T. E. et al. Measuring stem cell frequency in epidermis: a quantitative in vivo functional assay for long-term repopulating cells. Proc Natl Acad Sci U S A 100, 11412–11417 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snippert H. J. et al. Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science 327, 1385–1389 (2010). [DOI] [PubMed] [Google Scholar]
- Jensen K. B. et al. Lrig1 expression defines a distinct multipotent stem cell population in mammalian epidermis. Cell Stem Cell 4, 427–439 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schon M. P. & Schon M. Imiquimod: mode of action. Br J Dermatol 157 Suppl 2, 8–13 (2007). [DOI] [PubMed] [Google Scholar]
- Mashiah J. & Brenner S. Possible mechanisms in the induction of pemphigus foliaceus by topical imiquimod treatment. Arch Dermatol 141, 908–909; author reply 909 (2005). [DOI] [PubMed] [Google Scholar]
- Al-Dujaili Z. & Hsu S. Imiquimod-induced vitiligo. Dermatol Online J 13, 10 (2007). [PubMed] [Google Scholar]
- Gilliet M. et al. Psoriasis triggered by toll-like receptor 7 agonist imiquimod in the presence of dermal plasmacytoid dendritic cell precursors. Arch Dermatol 140, 1490–1495 (2004). [DOI] [PubMed] [Google Scholar]
- Pawar R. D. et al. Toll-like receptor-7 modulates immune complex glomerulonephritis. J Am Soc Nephrol 17, 141–149 (2006). [DOI] [PubMed] [Google Scholar]
- Aaronson D. S. & Horvath C. M. A road map for those who don't know JAK-STAT. Science 296, 1653–1655 (2002). [DOI] [PubMed] [Google Scholar]
- Darnell J. E. Jr STATs and gene regulation. Science 277, 1630–1635 (1997). [DOI] [PubMed] [Google Scholar]
- Darnell J. E. Jr, Kerr I. M. & Stark G. R. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 264, 1415–1421 (1994). [DOI] [PubMed] [Google Scholar]
- Platanias L. C. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol 5, 375–386 (2005). [DOI] [PubMed] [Google Scholar]
- Varinou L. et al. Phosphorylation of the Stat1 transactivation domain is required for full-fledged IFN-gamma-dependent innate immunity. Immunity 19, 793–802 (2003). [DOI] [PubMed] [Google Scholar]
- Jin L. & Wang G. Keratin 17: A Critical Player in the Pathogenesis of Psoriasis. Med Res Rev 34, 438–454 (2013). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information