Abstract
Photodynamic therapy has shown promise in the treatment of early head and neck squamous cell carcinoma (SCC). In photodynamic therapy (PDT), a light sensitive drug (photosensitizer) and visible light cause cancer cell death by the creation of singlet oxygen and free radicals, inciting an immune response, and vascular collapse. In this paper, we review several studies that demonstrate the effectiveness of PDT in the treatment of early stage SCC of the head and neck, with some showing a similar response rate to surgery. Two cases are presented to illustrate the effectiveness of PDT. Then, new advances are discussed including the discovery of STAT3 crosslinking as a potential biomarker for PDT response and interstitial PDT for locally advanced cancers.
Keywords: Photodynamic therapy, PDT, Squamous cell carcinoma, Head and neck cancer
Introduction
In photodynamic therapy (PDT), a light sensitive drug (photosensitizer, PS), oxygen and visible light are required to achieve a therapeutic response. To activate the PS, a laser light is used to illuminate the treatment area. The specific laser wavelength depends on the PS. Most clinically approved PSs are activated with red light. PDT has 3 primary mechanisms of action: creation of singlet oxygen and free radicals, inciting an immune response, and vascular collapse.
Modern PDT came to the forefront with the work of Dougherty et al in the 1970s at the Roswell Park Cancer Institute.1 Their work led to the development of porforin sodium (Photofrin), a hematoporphyrin derivative, and its subsequent FDA approval. Porforin sodium is injected intravenously, and requires 24–48 h to preferentially retain in cancerous tissues. The other FDA approved photosensitizer, 5-aminolevulinic acid (5-ALA, Levulan) is used topically and mainly for dermatologic conditions. The ALA is absorbed through the skin or mucosa and is then converted to the active drug within tissues. Temoporfin (mTHPC, Foscan) is approved for use in Europe.
Multiple studies have demonstrated the effectiveness of PDT in the treatment of early stage squamous cell carcinoma (SCC) of the head and neck. In particular, it has been shown to result in acceptable response when used to treat T1/T2 SCCs of the oral cavity and glottic larynx.2, 3, 4, 5, 6 The main advantages of PDT are that it can be safely repeated, the PS itself has few minor side effects, does not preclude further radiation or surgery, and heals with minimal scarring. The main disadvantages are photosensitivity (up to 6 weeks), pain, and cost of the FDA approved drug.
PDT for early stage SCC
PDT is ideally suited to superficial SCCs which can be easily accessed by the treating surgeon. This has been investigated most extensively in the oral cavity and larynx, where PDT has been used to treat lesions ranging from dysplasia to locally advanced squamous cell carcinoma. Rigual et al demonstrated the efficacy of porfimer sodium for the treatment of oral cavity squamous cell carcinoma in 2006, and of HPPH in 2013.6, 7 Complete response rates in the 23 patient cohort of T1 SCCA treated with HPPH reached 82%. Durbec et al in 2013 examined the efficacy and safety of PDT in recurrent carcinoma of the oral cavity and oropharynx using Foscan, and observed a 93% complete response rate.8 Jerjes observed a 5 year survival of 84.2% and a recurrence rate of 15.8% in T1–2 SCCA of the oral cavity treated with up to three rounds of mTHPC.9 Biel, in a series of 113 oral cavity PDT patients, with 93.8% of patients with Cis-T2 tumors and 89.6% of patients with superficial T2–3 tumors remaining free of disease after a mean follow up of 90 months.3 A recent meta-analysis by Cerrati et al found 24 studies comparing T1–2N0M0 squamous cell carcinoma of the oral cavity to surgery.10 They found no statistically significant difference between rates of complete response to PDT, locoregional control with surgery, or recurrence rate between the two modalities. The similar response rates between surgery and PDT were supported by the work of de Visscher et al, who compared response rates, disease free survival and need for re-treatment in superficial (<5 mm) T1–2 squamous cell carcinoma of the oral cavity.11 In tumors treated by PDT, an 86% complete response rate was observed in T1 lesions, and a 63% complete response rate was observed for T2 lesions. This was not significantly different than the control rates observed in surgically treated T1 and T2 tumors, which were 76% and 79%, respectively. Overall survival was similar between the two treatment modalities when controlled for stage.
PDT treatment of laryngeal squamous cell carcinoma is attractive because of the potential to preserve both voice and swallowing integrity. Biel reported on 115 patients with recurrent or primary laryngeal lesions, ranging from Cis to T2N0 SCC, and observed a 5 year cure rate of 91%.2 All recurrences were successfully salvaged by either PDT or conventional means. A Phase Ib study carried out at Roswell Park Cancer Institute examined response of laryngeal T1 squamous cell carcinoma of the larynx to HPPH treatment as a secondary outcome, and observed an 82% complete response rate.12
Case examples
Case 1 – oral SCC
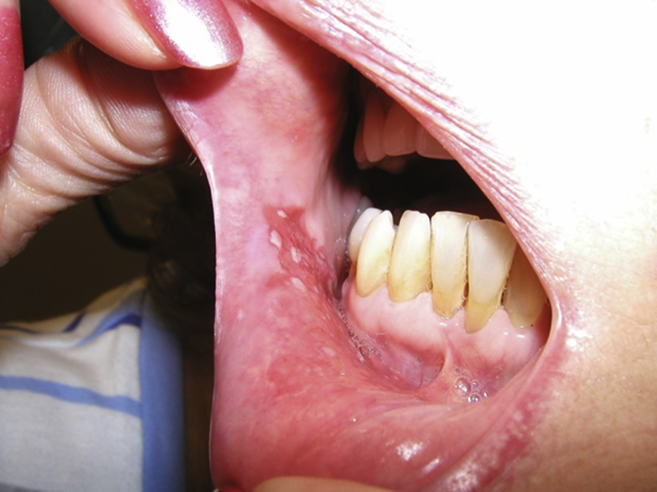
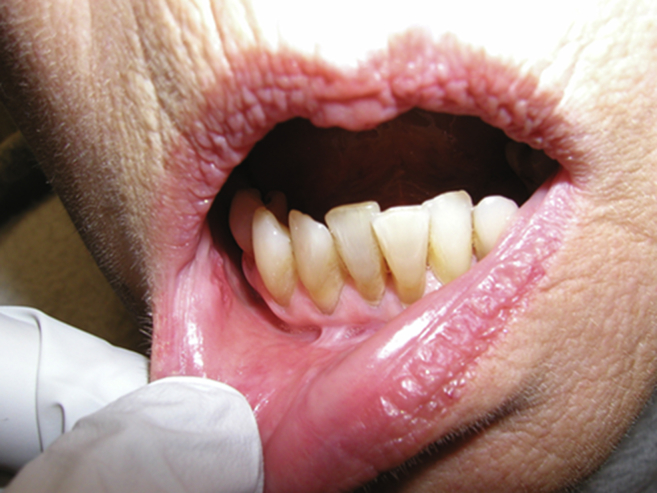
A 65 year old woman presented with a T2 SCC of the mucosa of the lower lip and vestibule. She had no significant past medical history except for a 40 pack/yr smoking history. The lesion was superficial, but extended onto the vestibule and lower gingiva (Fig. 1). She was given the option of surgery, radiation, or PDT and chose PDT. Two days before the procedure she was injected with 2 mg/kg of porfimer sodium. The procedure was done in the operating room under general anesthesia. A laser was used to illuminate the treatment area with a wavelength of 639 nm and a light dose of 50 J/cm2 and 150 mW/cm.2 6 months later, she remained disease free and had minimal scarring (Fig. 2).
Fig. 1.
Pre-PDT picture of a woman with a superficial T2N0 oral SCC.
Fig. 2.
Post-treatment (Fig. 1 patient), 6 months after PDT.
Case 2 – T2 glottic SCC
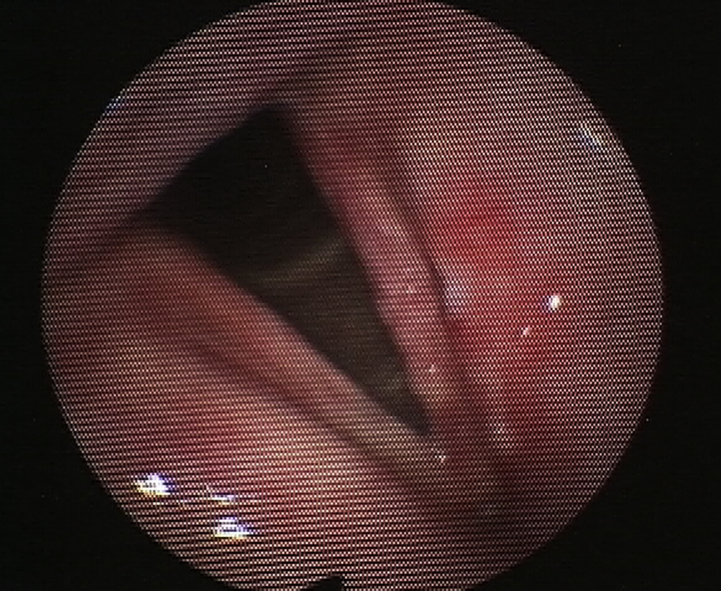
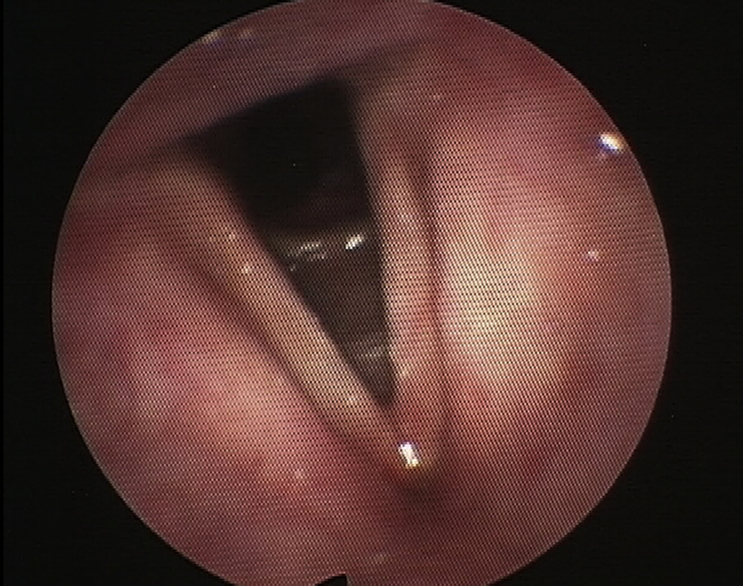
A 63 year old man presented with a several month history of hoarseness and dysphagia. Office examination revealed an ulcerative left tonsil mass. Flexible laryngoscopy revealed a lesion on the left false and true vocal cords (Fig. 3). Direct laryngoscopy and biopsy showed a T2 SCC involving the left false cord and extending to the true cord and anterior commissure. He also had a concurrent T4N0 SCC of the left tonsil. He chose to have a standard treatment for the tonsil SCC (surgery and adjuvant treatment) and PDT for the larynx. Two days before the procedure he was injected with 2 mg/kg of porfimer sodium. He then underwent a suspension laryngoscopy and was treated with surface illumination via a red laser at 630 nm wavelength, 100 J/cm2 and 150 mW/cm.2 Flexible laryngoscopy done 2 months post procedure showed a complete response (Fig. 4). He has shown no signs of recurrence after 2.5 years.
Fig. 3.
Pre-PDT picture of a man with T2N0 laryngeal SCC.
Fig. 4.
Post treatment (Fig. 3 patient), 2 months after PDT.
Recent advancements
A biomarker for quantifying PDT-induced photoreaction
Recent work at our center has shown that STAT3 crosslinking has the potential to predict the response to PDT. The assay is done on a tissue biopsy collected from the treatment area, immediately after PDT. In a recent study, we found that a threshold of ≥10% STAT3 crosslinking is associated with complete response in patients with T1N0 SCC of the oral cavity treated with HPPH-PDT.6 In patients with carcinoma-in-situ and leukoplakia, we observed <10% degree of STAT3 crosslinking, and this was associated with a lower rate response (46% versus 82%) when compared to T1 SCC treated at the same PDT settings.
Pretreatment planning and real-time dosimetry
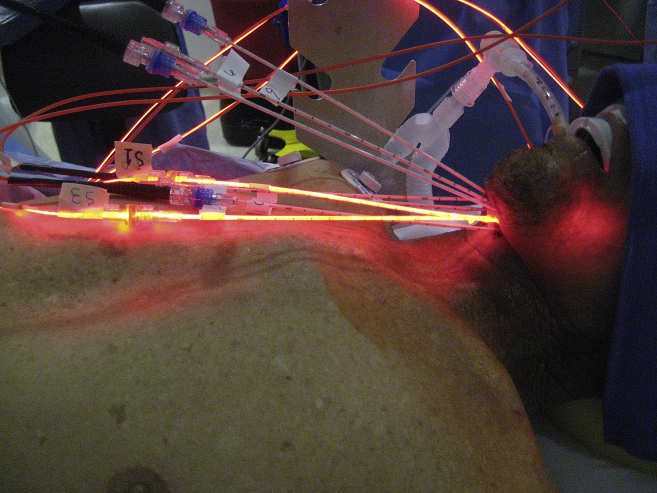
One of the drawbacks of conventional PDT is that is it difficult to treat deep or thick lesions >1 cm in depth. Interstitial PDT can circumvent this depth problem. For patients who are unable to undergo further surgery or radiation, this may be a viable option. Light fibers are placed directly into the tumor either by direct visualization or by image guidance (computerized tomography or ultrasound). Several groups in Europe have demonstrated the benefits of interstitial PDT with Foscan for the treatment of patients with locally advanced head and neck SCC.13, 14 Our group has used interstitial PDT with Photofrin to treat these patients. Fig. 5 demonstrates one of our patients undergoing interstitial PDT for a recurrent base of tongue SCC.
Fig. 5.
Patient with recurrent and locally advanced oropharyngeal SCC, undergoing interstitial PDT.
We have recently presented a new near real-time pretreatment planning algorithm to simulate the light propagation in the tumor and adjacent structures during treatment, which could help physicians to visualize the light dose distribution within tumors and adjacent tissues.15 In this approach, for patients that are candidates for interstitial PDT, a preoperative virtual 3D model of the tumor and margins is created from a thin cut CT scan. This model is used for pretreatment planning, to suggest fiber placement and locations that will maximized light dose in the target tumor while sparing adjacent healthy and sensitive tissues, as we previously described in Oakley et al.15 In our institute, treatment is done in the operating room with ultrasound helping to guide fiber placement. During treatment, we use our real-time light dosimetry system to monitor how much light is being delivered to the tumor.
Summary
PDT can be an effective treatment for early stage SCCs of the head and neck. The treatment is relatively easy to perform, can be repeated, and has minimal scarring. With new photosensitizers, the period of photosensitivity will be reduced from weeks to days. Pre-treatment planning, real-time dosimetry, and interstitial PDT have the potential to help treat locally advanced head and neck SCCs.
Conflicts of interest
Dr. Gal Shafirstein, is a co-inventor of a patent application owned by Roswell Park Cancer Institute that describes some of the methods mentioned in this paper. Dr. Gal Shafirstein and Dr. Hassan Arshad receive support from Pinnacle Biologics Inc. for a clinical trial that uses the techniques reported in this manuscript to treat patients with locally advanced head and neck cancer. There are no other financial disclosures or conflicts of interest.
Contract grant sponsor: National Cancer Institute; Contract grant number: P01CA55791, S. Gollnick; National Cancer Institute; Contract grant number: R01 CA193610G. Shafirstein; and National Cancer Institute; Contract grant number: P30CA16056, C. Johnson.
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Dougherty T.J., Kaufman J.E., Goldfarb A., Weishaupt K.R., Boyle D., Mittleman A. Photoradiation therapy for the treatment of malignant tumors. Cancer Res. 1978;38:2628–2635. [PubMed] [Google Scholar]
- 2.Biel M.A. Photodynamic therapy treatment of early oral and laryngeal cancers. Photochem Photobiol. 2007;83:1063–1068. doi: 10.1111/j.1751-1097.2007.00153.x. [DOI] [PubMed] [Google Scholar]
- 3.Biel M.A. Photodynamic therapy of head and neck cancers. Methods Mol Biol. 2010;635:281–293. doi: 10.1007/978-1-60761-697-9_18. [DOI] [PubMed] [Google Scholar]
- 4.Karakullukcu B., van Oudenaarde K., Copper M.P. Photodynamic therapy of early stage oral cavity and oropharynx neoplasms: an outcome analysis of 170 patients. Eur Arch Otorhinolaryngol. 2011;268:281–288. doi: 10.1007/s00405-010-1361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rigual N.R., Thankappan K., Cooper M. Photodynamic therapy for head and neck dysplasia and cancer. Arch Otolaryngol Head Neck Surg. 2009;135:784–788. doi: 10.1001/archoto.2009.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rigual N., Shafirstein G., Cooper M.T. Photodynamic therapy with 3-(1′-hexyloxyethyl) pyropheophorbide a for cancer of the oral cavity. Clin Cancer Res. 2013;19:6605–6613. doi: 10.1158/1078-0432.CCR-13-1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mang T.S., Sullivan M., Cooper M., Loree T., Rigual N. The use of photodynamic therapy using 630nm laser light and porfimer sodium for the treatment of oral squamous cell carcinoma. Photodiagnosis Photodyn Ther. 2006;3:272–275. doi: 10.1016/j.pdpdt.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Durbec M., Cosmidis A., Fuchsmann C., Ramade A., Céruse P. Efficacy and safety of photodynamic therapy with temoporfin in curative treatment of recurrent carcinoma of the oral cavity and oropharynx. Eur Arch Otorhinolaryngol. 2013;270:1433–1439. doi: 10.1007/s00405-012-2083-7. [DOI] [PubMed] [Google Scholar]
- 9.Jerjes W., Upile T., Hamdoon Z., Alexander Mosse C., Morcos M., Hopper C. Photodynamic therapy outcome for T1/T2 N0 oral squamous cell carcinoma. Lasers Surg Med. 2011;43:463–469. doi: 10.1002/lsm.21071. [DOI] [PubMed] [Google Scholar]
- 10.Cerrati E.W., Nguyen S.A., Farrar J.D., Lentsch E.J. The efficacy of photodynamic therapy in the treatment of oral squamous cell carcinoma: a meta-analysis. Ear Nose Throat J. 2015;94:72–79. doi: 10.1177/014556131509400208. [DOI] [PubMed] [Google Scholar]
- 11.de Visscher S.A., Melchers L.J., Dijkstra P.U. mTHPC-mediated photodynamic therapy of early stage oral squamous cell carcinoma: a comparison to surgical treatment. Ann Surg Oncol. 2013;20:3076–3082. doi: 10.1245/s10434-013-3006-6. [DOI] [PubMed] [Google Scholar]
- 12.Shafirstein G., Rigual N.R., Arshad H. Photodynamic therapy with 3-(1′-hexyloxyethyl) pyropheophorbide-a for early-stage cancer of the larynx: Phase Ib study. Head Neck. 2016;38(suppl 1):E377–E383. doi: 10.1002/hed.24003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jerjes W., Upile T., Hamdoon Z. Photodynamic therapy: the minimally invasive surgical intervention for advanced and/or recurrent tongue base carcinoma. Lasers Surg Med. 2011;43:283–292. doi: 10.1002/lsm.21048. [DOI] [PubMed] [Google Scholar]
- 14.Karakullukcu B., Nyst H.J., van Veen R.L. mTHPC mediated interstitial photodynamic therapy of recurrent nonmetastatic base of tongue cancers: development of a new method. Head Neck. 2012;34:1597–1606. doi: 10.1002/hed.21969. [DOI] [PubMed] [Google Scholar]
- 15.Oakley E., Wrazen B., Bellnier D.A., Syed Y., Arshad H., Shafirstein G. A new finite element approach for near real-time simulation of light propagation in locally advanced head and neck tumors. Lasers Surg Med. 2015;47:60–67. doi: 10.1002/lsm.22313. [DOI] [PMC free article] [PubMed] [Google Scholar]