Abstract
This review summarizes the key role of Toll-Like Receptor (TLRs) molecules for igniting the immune system. Activated by a broad spectrum of pathogens, cytokines or other specific molecules, TLRs trigger innate immune responses. Published data demonstrate that the targeting and suppression of TLRs and TLR-related proteins with particular inhibitors may provide pivotal treatments for patients with cancer, asthma, sepsis, Crohn's disease and thrombosis. Many drugs that target cytokines act in the late phases of the activated pathways, after the final peptides, proteins or glycoproteins are formed in the cell environment. TLR activity occurs in the early activation of cellular pathways; consequently inhibiting them might be most beneficial in the treatment of human diseases.
Keywords: TLR, signaling pathways, therapy
HUMAN TOLL-LIKE RECEPTORS (TLRS)
The first report about Toll-Like Receptors (TLRs) was published in 1997, when the ortholog of Drosophila Toll molecule TLR4 was identified [1–3]. Prior to this time11 human TLRs and 13 TLR genes in mice had been identified [4]. All TLR - type transmembrane glycoproteins are critical for the innate immune response in mammals. They have very similar basal structure that contain extracellular fragments with 16–28 horseshoe-like shape leucine-rich repeat (LRR) modules and are responsible for binding “pathogen associated molecular patterns” (PAMPs) [5]. The features of these modules are determined by a conserved sequence pattern in the LRR modules ligand-induced dimerization of TLRs and this stabilizes the protein by protecting its hydrophobic core from solvents. Adaptor proteins trigger the intracellular TIR (Toll interleukin-1 receptor) domains and initiate signaling. They are followed by the transmembrane domain and intracellular signaling domain composed of about 150 amino acid residues (Fig. 1).
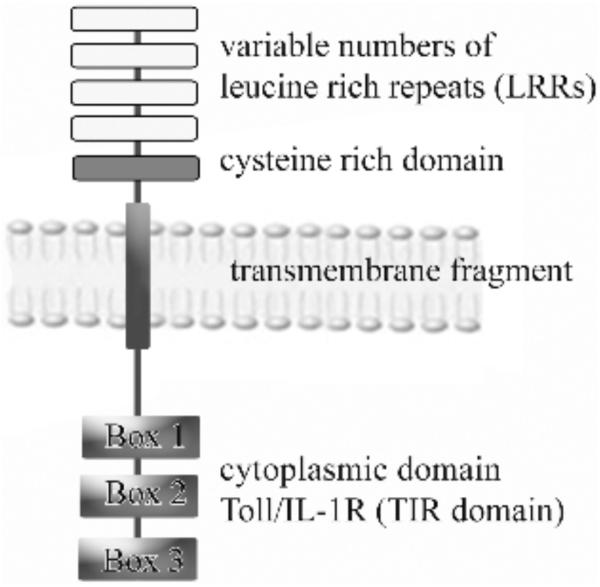
Fig. (1).
Structure of TLRs. One of major TLRs components is conserved cytoplasmic domain - Toll/IL-1R (TIR) domain, which contains three highly homologous regions (Box 1, Box 2 and Box3). This domain is linked by transmembrane fragment to variable number of N-terminal leucine rich repeats (LRRs) followed by a cysteine rich domain.
Classification of TLRs divides them into five subfamilies: TLR2, TLR3, TLR4, TLR5, and TLR9 [6] (Table 1). Although TLR subfamilies have a similar structure, it does not mean that they are similar in their function. The common feature of all TLRs is that they are structurally modified by posttranslational modification of N-linked glycosylation consensus sites. This modification affects receptor surface representation, trafficking, and pattern recognition (Table 2). It has been shown that gp96 (endoplasmin) is required for cell-surface expression of TLR1, TLR2, and TLR4. Placed on endoplasmatic reticulum, gp96 is necessary for proper protein folding. This chaperonin protein is required for post-translational folding of TLRs to produce functional receptors and for expression of both intracellular and cell surface TLRs. Deletion of gp96 resulted in functional deficiency in all studied TLRs [7,8]. TLR1, TLR2, TLR4, TLR5, and TLR6 are found on the plasma membrane. In contrast, TLR3, TLR7, TLR 8, and TLR9, which detect double-stranded RNA (dsRNA), single stranded RNA (ssRNA), and un-methylated DNA, mainly reside in the endoplasmic reticulum, endosomes and lysosomes [7].
Table 1.
Members of TLR Family with Assigned Gene Location
| Subfamily | Members | Gene Location |
|---|---|---|
| TLR2 | TLR1 | 4p14 |
| TLR2 | 4q32 | |
| TLR6 | 4p14 | |
| TLR10 | ------- | |
| TLR3 | TLR3 | 4q35 |
| TLR4 | TLR4 | 9q33–35 |
| TLR5 | TLR5 | 1q33.3 |
| TLR9 | TLR7 | Xp22 |
| TLR8 | Xp22 | |
| TLR9 | 3p21.3 |
Table 2.
Characteristic of TLRs Showing Their Tissue and Cell Localization, Specific Ligand Interactions and Disease Involvement
| Toll Like Receptor Type | Cell Type | Ligands | Involvement in the Disease Development |
|---|---|---|---|
|
| |||
| TLR1 | • Neutrophils, eosinophils, | • Soluble factors of bacterial and mycobacterial cell wall | |
| • Mast cells | • G+, G− bacterial triacylated lipopeptides | ||
| • monocytes/macrophages | |||
| • human blood dendritic cells | |||
| • B cells | |||
| • Keranocytes | |||
| • Mucosal epithelial cells | |||
|
| |||
| TLR2 | • neutrophils, eosinophils, basophils | • G+ bacterial peptidoglycan, lipoteichoic acids | • Inflammatory response in the pathogenesis of atherosclerotic plaque destabilization |
| • Mast cells | • Phenol-soluble modulin | • Intimal hyperplasia after arterial injury | |
| • small airway epithelial cells | • Di- and triacylated lipopeptides, lipoproteins | • Innate immunity | |
| • airway smooth muscle cells, | • Outer-membrane porins | • Autoimmune diabetes mellitus (DM1A) | |
| • type II alveolar epithelial cells | • Outer-surface protein – OspA | • cardiomyopathy | |
| • tracheal muscle layer, | • factors of mycobacterial cell wall | ||
| • monocytes/macrophages | • Zymosan | ||
| • glial cells | • Protozoan cell membrane glycolipids | ||
| • murine bone-marrow derived mast cells | • Wild-type H protein | ||
| • B cells | • HSV-1, CMV viruses envelope proteins | ||
| • Human blood dendritic cells | • Host HSP70 | ||
| • NK cells | • Parasites proteins | ||
| • Keranocytes | |||
| • Mucosal epithelial cells | |||
| • Human endothelial cells | |||
|
| |||
| TLR3 | • Mature human blood dendritic cells | • Viral and host double-stranded RNA | • Antiviral and immunostimulatory defense mechanism |
| • NK cells | • Polyinosinic-polycytidylic acid | • Autoimmune diabetes mellitus (DM1A) | |
| • Keranocytes | • cardiomyopathy | ||
| • Mucosal epithelial cells | |||
|
| |||
| TLR4 | • Neutrophils, eosinophils, basophils | • G− bacterial lipopolysaccharide (LPS) | • Inflammatory response in the pathogenesis of atherosclerotic plaque destabilization |
| • monocytes/macrophages | • Fusion protein of respiratory syncytial virus | • Intimal hyperplasia after arterial injury | |
| • human blood dendritic cells | • Murine mammary tumour virus | • unstable angina | |
| • B cells | • Moloney murine leukaemia virus | • asthma | |
| • Keranocytes | • Paclitaxel | • Innate immunity | |
| • Pulmonary epithelial cells | • Host extravascular fibrinogen/fibrin | • Systemic Lupus Erythematosus (SLE) | |
| • intestinal epithelial cells | • Host oligosaccharide fragments of hyaluronan | • Autoimmune diabetes mellitus (DM1A) | |
| • corneal epithelial cells | • Host extra domain A of fibronectin | • cardiomyopathy | |
| • Human endothelial cells | • Host polysaccharide fragments of heparan sulphate | ||
| • Heat-shock protein 60 | |||
| • Heat-shock protein 70 | |||
|
| |||
| TLR5 | • neutrophils | • Flagellin | |
| • monocytes/macrophages | • Single stranded DNA | ||
| • human blood dendritic cells | |||
| • NK cells | |||
| • Keranocytes | |||
| • Basolateral intestinal epithelial cells | |||
| • Mucosal epithelial cells | |||
|
| |||
| TLR6 | • Neutrophils, eosinophils, | • Diacylated lipopeptides | |
| • Mast cells | • Zymosan | ||
| • monocytes/macrophages | |||
| • B cells | |||
| • Mucosal epithelial cells | |||
|
| |||
| TLR7 | • Neutrophils, eosinophils, | • Imidazoquinolines | |
| • monocytes/macrophages | • Flagellin | ||
| • myeloid human blood dendritic cells | • Single stranded DNA | ||
| • B cells | |||
|
| |||
| TLR8 | • Neutrophils | • Viral single-stranded RNA | |
| • monocytes/macrophages | • Guanosine and uridine-rich ssRNA HIV-1 oligonucleotides | ||
|
| |||
| TLR9 | • Neutrophils, eosinophils, | • Unmethylated CpG oligodeoxynucleotides | • Autoimmune diabetes mellitus (DM1A) |
| • monocytes/macrophages | • Viral genomic DNA | • cardiomyopathy | |
| • myeloid human blood dendritic cells | |||
| • B cells | |||
|
| |||
| TLR10 | • Neutrophils, eosinophils, | • not yet identified | |
| • monocytes/macrophages | |||
| • B cells | |||
|
| |||
| TLR11 | • Neutrophils | • Uropathogenic bacteria | • preventing infection of internal organs of the urogenital system |
| • monocytes/macrophages | • soluble extract of the tachyzoite | ||
| • liver, kidneys, and bladder epithelial cells | |||
|
| |||
| TLR12 | • Neutrophils | • not yet identified | |
| • monocytes/macrophages | |||
|
| |||
| TLR13 | • Neutrophils | • not yet identified | |
| • monocytes/macrophages | |||
TOLL LIKE RECEPTORS SIGNALING PATHWAYS
TLRs intracellular fragments are structurally similar to parts of IL-1 receptors, thus they are likely to share the same signaling pathways. To be activated some TLRs appear to require dimerization. The TLR2 receptor is an excellent example of such a heterodimer connection establishing heterodimers with TLR1 or TLR6 (Fig. 2). The signaling cascades via the TIR domains are mediated by specific adaptor molecules including MyD88, MAL (also known as TIRAP), TRI, and TRAM [9]. The adaptor proteins containing TIR domains and TIR-TIR interactions between receptor-receptor, receptor-adaptor, and adaptor-adaptor are critical for activation and signaling [9]. Upon ligand binding to TLRs, the adaptor molecule MyD88 is recruited to the TLR complex as a dimer. The binding of MyD88 promotes association with IL-1 receptor–associated kinase-4 (IRAK-4) and IRAK-1. TNF-α associated factor 6 (TRAF6) is recruited to IRAK-1. The IRAK-4/IRAK-1/TRAF6 complex dissociates from the receptor and then interacts with another complex consisting of TGF-β-activated kinase (TAK1), TAK1-binding protein 1 (TAB1), and TAB2. TAK1 is subsequently activated in the cytoplasm, leading to the activation of IκB kinase kinases (IKKs). IKK activation leads to the phosphorylation and degradation of IκB and the consequent release of Nuclear Factor-κB (NF-κB). Translocation of NF- κB into the nucleus activates inflammatory chemokines and cytokines. TRIF (TIR-domain-containing adaptor protein inducing interferon-β), and TRAM (TRIF-related adaptor molecule) both mediate MyD88-independent induction of interferon by activating the expression of different types of interferon-inducible genes.
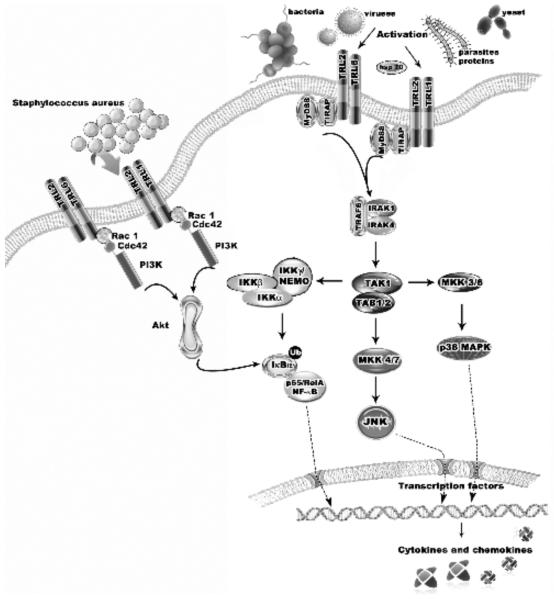
Fig. (2).
TLRs signaling pathway showed on TLR2 example. To be activated, TRL2 requires dimerization with TLR1 or TLR6. The signaling cascades via TIR domains are mediated by specific adaptor molecules including MyD88, TIRAP, TRIF, and TRAM. Upon ligand binding to TLRs, the adaptor molecule MyD88 is recruited to the TLR complex as a dimer. Binding of MyD88 promotes association with IL- 1, IRAK-4, and IRAK-1. TRAF6 is recruited to IRAK-1 and the IRAK-4/IRAK-1/TRAF6 complex dissociates from the receptor. It interacts with another complex consisting TAK1, TAB1, and TAB2. TAK1 is subsequently activated in the cytoplasm, leading to the activation of IKKs. IKK activation leads to the phosphorylation and degradation of IκB and consequent release of NF-κB. Translocation of NF-κB into the nucleus activates inflammatory chemokines and cytokines. The MyD88-independent pathway induces NF- B and MAPKs with delayed kinetics. It is possible that product of this pathway will have different targets from those activated by MyD88 dependent pathway. For example it can induce dendritic cell maturation instead cytokine production [136]. This pathway induces a fast and transient activation of the Rho GTPases - Rac1 and Cdc42. Recruitment of active Rac1 and phosphatidylinositol-3 kinase (PI3K) to the TLR2 cytosolic domain allows activation of Akt kinase, activation and translocation of the p65 subunit of NF-κB into the cell nucleus and specific cytokines release.
Toll like receptors have ability to bind a wide spectrum of agonists. This bond initiates variety of cell responses. A wide of spectrum of TLRs may serve as biomarkers of various diseases. TLRs are expressed by many cell types including endothelium, fibroblasts, vascular smooth muscle cells, memory T-cells, regulatory T-cells, mast cells and dendritic cells. Some TLRs such as TLR2, TLR3, TLR4 and TLR6, are also detectable in cardiac myocytes. Activated TLRs are also able to mediate pro-inflammatory signaling in pulmonary inflammatory disease. Respiratory epithelial cells are the initial site of bacterial colonization in the respiratory tract. Airway and alveolar type II epithelial cells are able to up-regulate TLR expression on the apical surface enhancing antimicrobial responses [10]. Subsequently TLR-driven responses can activate airway smooth muscle cells and neutrophils. Pharmacologic regulation of such a mechanism may be effective in the treatment of lung diseases.
It has been shown in the development of many diseases that polymorphism of TLRs may intensify the process. The association between TLR polymorphisms and infectious diseases or cancers has been reported in relation to TLR2, TLR4, TLR5 and TLR9. Other TLRs, like TLR1, TLR3, TLR6 or TLR10 seem to be very rare and are poorly understood. TLR2, TLR4, TLR6, and TLR9 polymorphisms are associated with the regulation of TLR expression and development of active tuberculosis [11]. TLR 9 gene polymorphism may be involved in the pathogenesis of Hodgkin's lymphoma through altered inflammatory responses [12]. It was also shown that TLR polymorphisms influence COPD development and severity in smokers. Single nucleotide polymorphism impaired TLR4 signaling and allowed receptor hyporesponsiveness in alveolar macrophages, epithelial cells, and peripheral blood mononuclear cells. In this manner TLR4 polymorphism dysfunction may contribute to COPD pathophysiology [13].
Data from the literature indicate that TLR-mediated recognition is mainly involved in the TH1 immune response [14–17]. The TH1 response is involved in inflammation and in fighting viruses and bacteria, and also it is also important in specific autoimmune diseases e.g. insulin-dependent diabetes mellitus. The antinflamatory action of the TH2 response counteracts the effect of the TH1 cytokines and engaged in allergy development. Allergens lack PAMPs, but some of them may be recognized by TLRs. They probably initiate adaptive immune responses by TLR-independent mechanisms. It is also possible that TH2 responses are TLR dependent, by the MyD88 independent pathway [5] (Fig. 2). Studies demonstrate that smoking during pregnancy significantly attenuates TLR-mediated immune responses. It increases the risk of the offspring's development of allergies and asthma [18,19]. Overexpression of TLR4 has been shown during atherosclerotic disease progression. This is demonstrated by clinical manifestations of atherosclerotic disease that correlate with cytokine release after TLR4 stimulation. Also TLR4 expression is significantly elevated in patients suffering from recurrent unstable angina, compared with healthy controls [20]. It has also been found that strong changes in cellular TLR responsiveness are induced by local vascular injury. TLRs, by signaling the presence of infection, can direct the adaptive immune responses against antigens of microbial origin. Immature Blood Dendritic Cells (DCs) express a full set of TLRs that actually mediate their own maturation. Mature DCs express high levels of MHC, CD80, CD86 and migrate to draining lymph nodes to present pathogen-derived antigens to naive T cells. Dendritic Cell TLRs are then able to induce expression of a number of cytokines e.g. Interleukin 12 (IL-12) [21–25]. In the presence of activating factors several mammalian TLRs increase synthesis and release of vascular endothelial growth factors (VEGF) [26].
TLR2 AS EXAMPLE OF TLR INVOLVEMENT IN A WIDE SPECTRUM OF DISEASES
Recently, TLR2, has been thought to have an impact on the development of some inflammatory diseases like acute lung injury (ALI). ALI and its more severe form, the acute respiratory distress syndrome (ARDS), derive from acute pulmonary edema and inflammation caused by trauma, sepsis, acute pancreatitis and drug overdose. The TLR2 pathway may be activated by the Toll interacting protein (Tollip) and subsequently suppress the activity of IRAK [27]. Tollip is known as a TLR regulator that is presented in a complex with IRAK-1. Upon stimulation with IL-1, the Tollip-IRAK-1 complex is recruited to the IL-1 receptor complex, allowing for IRAK-1 phosphorylation, dissociation from Tollip and TRAF6 activation. The mechanism of Tollip overexpression that leads to the inhibition of NF-βB activation is still poorly understood. [28] (Fig. 3). Negative regulation of TLR signaling by Tollip may limit the production of proinflammatory mediators during the inflammation process following an infection [29,30]. Although TLR2 shares the MyD88 signaling pathway with other TLRs, it may possess a unique signaling track. The most well known pathway is the TLR2-mediated signaling of human monocytes by Staphylococcus aureus. TLR2 is the receptor that also mediates signaling on numerous other ligands including: peptidoglycan from Gram-positive bacteria [31], bacterial lipoproteins [32–35], the mycobacterial cell wall [36–40], lipoarabinomannan [39], glycosylphosphatidylinositol (a lipid from Trypanosoma Cruzi) [41,42], yeast cell walls [43–49], atypical LPS from Leptospira interrogans [50] and Porphyromonas gingivitis [51,52]. It induces a fast and transient activation of the Rho GTPases - Rac1 and Cdc42 [53,54]. Recruitment of active Rac1 and phosphatidylinositol-3 kinase (PI3K) to the TLR2 cytosolic domain leads to activation of Akt kinase [53,54]. This allows activation and translocation of the p65 subunit of NF-κB into the cell nucleus in a process that is independent of IκB degradation (Fig. 2).
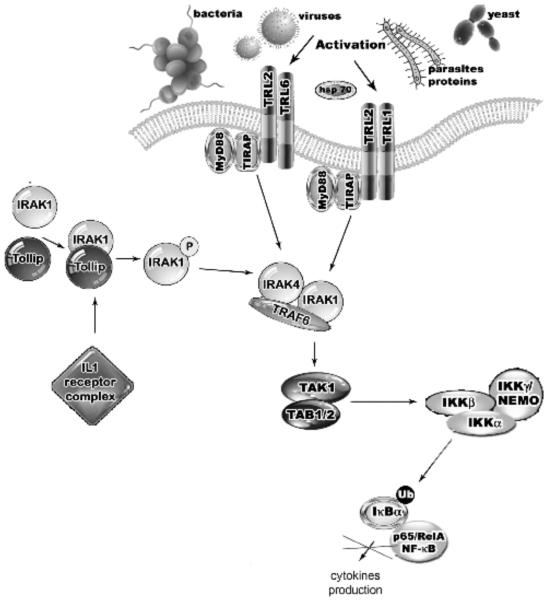
Fig. (3).
The TRL2 pathway is activated by Tollip and results in IRAK suppression. Upon stimulation with IL-1, the Tollip-IRAK-1 complex is recruited to the IL-1 receptor complex causing IRAK-1 phosphorylation, dissociation from Tollip and TRAF6 activation. Tollip overexpression leads to the suppression of NF-kβ inhibition. Negative regulation of TLR signaling by Tollip may therefore serve to limit the production of pro-inflammatory mediators during the inflammation process following an infection.
A wide spectrum of agonist activity is possible because TLR2 is supported either by TLR1 or TLR6 ability to create heterodimers [5]. Some other TLRs also create heterodimers e.g. TLR7 with TLR8. In addition TLR4 also establishes homodimers. In contrast, TLRs like TLR3, TLR5 and TL9 do not necessary require other TLRs to be activated. These differences demonstrate possible ways to regulate the activity of TLRs. Both TLR1 and TLR6 are expressed constitutively on many cell types, whereas the expression of TLR2 is regulated and seems to be restricted to antigen-presenting cells, smooth muscle cells, fibroblasts and endothelial cells [5], [20]. TLR2 is mainly known as a mediator of macrophage recognition of mycobacteria and gram-positive organisms [55]. Both TLR2 and TLR4 are associated with the inflammatory responses in the pathogenesis of atherosclerotic plaque destabilization and intimal hyperplasia after arterial injury [20,56–58]. TLR2 detects circulating, immunologically active cells, thus it could play role in innate immunity. TLR2 also has the ability to bind with the lipid scavenger receptor molecule, CD36 in response to diacylated lipoproteins [32,59–62]. Heterodimers TLR2/TLR1 and TLR2/TLR6 are pre-existing and internalize the corresponding ligand since their formation does not seem to be induced by the TLR2/1/6-binding molecules or PAMPs [63]. In contrast, interaction of TLR2/6 with CD36 is not activated unless it is induced by the ligand [32,63]. The ligand-binding pockets of TLR1 and TLR2 are located at the boundary of the central and C-terminal domain in the convex region. The flexible loops at the domain boundaries are separated, forming crevices that are connected to large internal pockets. The pockets of TLR1 and TLR2 are bridged by the bounded ligand and therefore form a long continuous hydrophobic pocket [64]. Due to the molecules diversity, it seems unlikely that TLR2 has the capability to react with all agonists to the same degree [6]. Nevertheless, TLR2 involvement in a large number of inflammatory diseases has been reported: rheumatoid arthritis, type I diabetes, inflammatory bowel disease, ischemia/reperfusion injury, vascular injury and atherosclerosis. There are also some suggestions that this genetic variation in TLR2 is a major determinant of susceptibility to asthma and allergies [3]. Genetic variations in TLR2 are responsible for an observed protection of farmers' children from allergy and asthma [18]. Prenatal exposure to farm stables upregulates TLR2 expression in neonatal cells [19]. TLR2 and TLR4 activation has been shown to reduce apoptosis of cardiac myocytes, which suggests that TLR signaling may be important in the development of myocardial diseases [3]. Some data link alcohol with upregulation of TLR2 followed by the production of an inflammatory response in the airway epithelium after a bacterial challenge. Cells were shown to double their IL-8 release when exposed to low stimulation levels of the TLR2 ligand. It has been shown that IL-8 secretion is increased by oxidant stress and conversely, IL-8, by causing recruitment of inflammatory cells, which induce a further increase in oxidant stress mediators, making it a key parameter in localized inflammation [65]. The TLR2 pathway was also noted to increase in bronchitis after alcohol consumption. Alcohol seems to prime airway cells and enhance airway inflammation inevitably leading to severe airway disease. The intensity of response with alcohol is dependent on the alcohol dose and upon the cell type [66]. Takagi et al. showed that the alleles and genotypes that included short GT repeats in intron 2 of the TLR2 gene were associated with acute pancreatitis in the Japanese population [67]. During carcinogenesis the nuclear factor–κB signaling pathway is activated [68]. Yim et al. reported that alleles with high and low numbers of GT repeats had greater promoter activities than those with medium numbers of repeats when stimulated with interferon [69]. However, the same investigators reported that shorter GT repeats were associated with weaker promoter activities and lower TLR2 expression on CD14 positive peripheral blood mononuclear cells. It was shown that uric acid crystals, product of purines catabolism, acting through TLR2 or TLR4 initiate inflammation in tumors [70]. Elevated levels of uric acid are produced by injured tissue and at high concentration uric acid precipitates and forms crystals causing inflammation [71,72]. Recent studies show that uric acid, acting on TLR2 and TLR4, is responsible for inflammation in lungs [71]. TLR2 and TLR4 acting through CD14 regulate uptake of uric acid crystals by alveolar macrophages [72]. A published study demonstrated that combined action of TLR2 and TLR4 is required for optimal inflammation in response to uric acid crystals during lung injury [71].
CLINICAL SIGNIFICANCE OF TLRS - HOW WE MIGHT TARGET TLRS THERAPEUTICALLY
Structurally, the domains of the TLRs may potentially be used as drug targets (Fig. 4). The ectodomain, which carries the site of ligand recognition, is unique to each TLR. Hsp10 treated cells reduced lipopolysaccharide (LPS)-induced NF-κB activation, regulated by TLRs and the secretion of several inflammatory mediators. Hsp10 treatment also delayed mortality [73]. Hsp10 acting through TLR4 has been shown to reduce symptoms of rheumatoid arthritis [74]. Literature also supports the possibility of limiting TLR2 signaling by blocking TLR2 with a neutralizing antibody or antisense oligonucleotides, or to regulate them by small-molecule inhibitors [75–78]. Rapid action by these inhibitors may be a possible tool in fighting sepsis or inflammatory disease [79,80]. For example TLR2 knockout mice are hyporesponsive to Gram-positive bacteria cell wall components [31]. Thus targeting TLRs could thus be important in the development of disease treatment. Many of the drugs, that target cytokines, act in the late phase - at the end of activated pathways. This may make treatment less effective, because final pathogenic peptides, proteins or glycoproteins are already in the cell and deregulating the cell environment. TLRs activity occurs in the early phase, thus inhibition of TLRs in the early phase might be more effective in activating or inhibiting the cascade that leads to inflammation and disease.
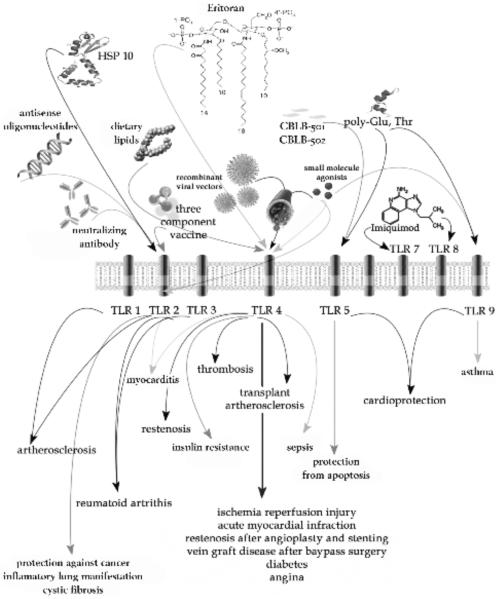
Fig. (4).
Scheme depicting drugs acting on designated TLRs with possible therapeutic effect and clinical significance in given disease.
Some medications targeting TLRs have already been approved by the Food and Drug Administration (FDA) and even more are being tested in numerous clinical trials [81–87]. Imiquimod, approved in 1997 acts as an immune response modifier through TLR7 [88–92]. It leads cells to secret cytokines, IFN-α, IL-6 and TNF-α. This medication treats certain diseases of the skin, superficial malignant melanomas, and genital warts [93]. In 2005 the successful phase ll clinical trial results of E5564 (generic name eritoran), a drug candidate for treating severe sepsis, was reported [94–97]. The results showed a certain reduction in the mortality rate of the group with high-dose eritoran. Specifically, the rate was reduced by 6.4% compared to the placebo group. E5564, is a Lipid A derivative, which by antagonizing TLR4, blocks the receptor signal transduction. Subsequently it inhibits the release of the inflammatory cytokines IL-1 and TNF-α, and suppresses the development of severe sepsis [96–98]. TLRs are also known to be directly related to cancer development and progression. TLRs heterogeneity allows different regulation and expression of the genes related to inflammation and immunity. Understanding and proper targeting of TLRs could result in novel treatments for cancers [99]. Recently, it has been shown that carbohydrate-based cancer vaccines could act by an artificial linker to a carrier protein [100,101]. One of the components of the three component vaccine is a TLR2 activator faciliating incorporation of TLR activators. It induces cytokines necessary for maturation of immune cells, leading to a strong antigenic response against tumor-associated glycopeptides antigens. Improved cancer cell recognition was observed when the TLR2 epitope was covalently attached to the glycopeptide on the lymphocyte surface [100,101]. The search for an efficient cancer vaccine focuses on the development of a link between two components: an antigen and an adjuvant. A TLR activator could be a part of a designed vaccine, by determination of maturation of dendritic cells, natural killer cell activation for cellular immune response and an antibody necessary for humoral immune response. It could have both therapeutic and preventive effects in cancer treatment. It was shown that cancer treated with peptide chimeras generated from a TLR2 agonist (small lipopeptides) and anti-tumour antibodies show a decrease in development [102]. Pretreatment of tumor cells with TLR ligands resulted in an increased production of granzymes and an enhanced killing capacity of T-cell lines [103]. It was also shown that TLR5 ligation with flagellin can convert dendritic cells from tolerogenic into activating antigen-presenting cells providing material for cancer immunotherapy [104]. The targeting of TLR9 reveals a possible treatment an complex pathology in diseases like asthma [105]. G oligonucleotides (CpG–ODN, resembling bacterial DNA) have been shown to activate TLR-9 on leukocytes B and dendritic cells, resulting in the induction of Th1-immune response and an interruption of mast cell signaling and the blocking of IgE mediated pathways [106]. IMO-2055, a TLR-9 agonist, is under clinical evaluation in oncology patients. Another TLR-9 agonist IMO-2125 induces high and sustained levels of IFN and is being evaluated in Hepatitis C infected human subjects [107].
Another therapeutic potential of TLRs may be to target the inflammatory lung manifestations of cystic fibrosis [108]. For example, TLR3 affects osteoclastogenic activity and thus may well have an impact on rheumatoid arthritis (RA) development or progression [109]. Expression of TLR3 is much higher in the RA synovium than in osteoarthritis synovium [110,111]. TLR3 may also affect human monocyte osteoclast differentiation Targeting the TLR3 pathway with for example Polyinosine-polycytidylic acid (poly(I:C) – a synthetic analog of double-stranded RNA, could block inflammatory bone destruction in RA or development of cancer [112] [109].
TLRs e.g. TLR2 and TLR4 are also involved in primarily non-immune-related diseases like hepatic ischemia reperfusion injury (IRI) [113–115]. TLRs activation of Kupffer cells trigger pro-inflammatory signaling responses that lead to liver IRI. This pathway offers another potential for cure and signal modulation could have a beneficial effect in patients with liver IRI [116]. Very recently it was shown that ischemia reperfusion injury following kidney transplantation in humans involves signaling through TLR4 expressed in donor kidney cells [117]. As Kruger et al. pointed in their paper, targeting TLR signaling by specific inhibitors may have several potential implications beyond protection against ischemia and reperfusion injury and increase the success of tolerogeneic protocols in the long-term maintenance of graft function and survival [117].
There are several reports that TLRs are highly expressed in endothelium and heart muscle cells, suggesting a functional importance of TLRs in the cardiovascular system [86,118,119]. Acting with specific inhibitors through TLRs could have a therapeutic effect in patients diagnosed with cardiac disorders including atherosclerosis, heart failure, myocarditis, septic myocardial dysfunction and diabetic antipathies [120]. Human studies have established that unstable angina and acute myocardial infarction are associated with enhanced expression and signaling events downstream from human TLR4 in circulating monocytes [3,121]. In monocytes TLR4 has been shown to be activated in acute heart failure after myocardial infarction [122]. Interestingly, therapeutic use of TLR4 inhibition is suggested in thrombosis, transplant atherosclerosis, restenosis after angioplasty with stenting, and in vein-graft disease after bypass surgery [123]. The vascular delivery of TLR4 or MyD88 inhibitors can be accomplished by coating them with compound inhibitors, and by administering recombinant viral vectors that deliver genes expressing antisense TLR4 RNA, or by small-molecule antagonists [123]. Other signaling inhibitors including antiapoptotic protein A20, agonistic lipid A and its analogues, inhibitors of IkB2, salicylate and parthenolide, NF-B kinases inhibitor PS-1145, inhibitors of p38, JNK, anti-TLR, anti-MyD88 antibodies, are able to target the TIR domain of the TLR4 or the MyD88 [79,124,125].
TLR2 and TLR1 expression is elevated in human atherosclerotic lesions and injection of exogenous TLR2/1 agonist exacerbated atherosclerosis [126]. Accumulation and upregulation of TLR2, TLR4 and its signaling pathway products, have been reported in myocarditis. Recent studies demonstrate that it is possible to develop effective therapies with a cardioprotective effect, which acts through T regulatory cells via the TLR5 and TLR9 [120,127,128]. Others compounds such as CBLB-501 and CBLB-502, recombinant flagellin proteins have been isolated from activated NFkB and act via TLR5 for protection against tissue injury in conditions involving high levels of apoptosis caused by radiotherapy, other radiation exposure and hypoxia. TLRs can also activate vascular damage in diabetic patients, as well as affect other organs [18,129–133]. Ligation of TLRs allows for ongoing inflammatory processes that run independently from the glucose level, even when homeostasis is restored. Some anti-inflammatory agents, TLR4, TLR2, TLR3, and MyD88 inhibitors as well as statins and thiazolidines have been suggested to treat diabetes and obesity-associated cardiovascular disorders [134]. Interference with TLR signaling by these agents may turn off the inflammatory process that triggers diabetic complications or reduce the extent to which complications occur. TLR4 has been shown to participate in the regulation of energy balance and insulin resistance in response to changes in the nutritional environment. Certain dietary lipids activate TLR4 and can promote insulin resistance influencing the development of type 2 diabetes. An interaction with TLR4 or MyD88 signaling pathways was demonstrated during developmental research for effective medications like fluvastatin, simvastatin, and atorvastatin. TLR1, TLR2, TLR3, and TLR7 seem to have a double nature: they play a beneficial role in host defense but may also trigger a strong autoimmune response that can lead to diabetes or other autoimmune diseases. These findings demonstrate that TLR pathways are involved in mediation of islet inflammation. This link between TLR upregulation and autoimmunity is another area of potential new therapeutic modalities in the pathways involving TLR agonists, especially as vaccine adjuvants [135].
Developmental efforts are focused primarily on compounds targeting specific TLRs such as TLR4, TLR2, TLR3, TLR5, TLR7 and TLR9. Because of multiple molecular links between chronic infection, inflammation, cardiovascular disease and TLRs, modulating just one receptor might not produce a complete immune response. Effective treatment and prevention may require an integrated approach that utilizes a combination of strategies to target the underlying inflammatory processes. The TLR family has been discovered only recently and further study is needed to focus on other possible pathways regulated by specific TLRs, such as the connection of TLR2 to the development of ALI and ARDS.
In summary, the association of toll-like transmembrane receptors with a large number of diseases creates a possibility for treatment with specific substances, compounds and biological particles. Although such a treatment could be beneficial for the patients, it seems to have a double nature, it could either trigger a strong autoimmune response resulting in diabetes, asthma and allergies or inhibit positive immune response against cancer cells or sepsis. Finding balance in regulation of TLRs pathway is crucial for efficient treatment.
ACKNOWLEDGEMENTS
We thank Joyce Gonzales, MD (Medical College of Georgia, Augusta, USA), for comments and valuable assistance on earlier versions of the manuscript. Manuscript was supported by grants HL083327, HL067307, HL08675 from NIH (ADV) and start-up funds from Medical College of Georgia (ADV).
ABBREVIATIONS
- ALI
Acute Lung Injury
- ARDS
Acute Respiratory Distress Syndrome
- COPD
Chronic Obstructive Pulmonary Disease
- DC
Blood Dendritic Cells
- dsRNA
double stranded RNA
- FDA
Food and Drug Administration
- HSP
Heat Shock Protein
- IKKs
Iκβkinase kinase
- IL1
Interleukin 1
- IL6
Interleukin 6
- IL8
Interleukin 8
- IL12
Interleukin 12
- INF-α
Interferon-α
- IRI
Ischemia Reperfusion Injury
- IRAK-4
IL-1 receptor–associated kinase-4
- LPS
Lipopolysaccharide
- LRR
Leucine-rich repeat modules
- MAL
T-lymphocyte maturation-associated protein
- MyD88
Myeloid Differentiation primary response gene 88
- NF-κB
Nuclear factor κB
- PAMP
Pathogen Associated Molecular Pattern
- PI3K
Phosphatidylinositol-3 Kinase
- RA
Rheumatoid Arthritis
- ssRNA
single stranded RNA
- TAK1
TGF-β-activated kinase 1
- TAB1
TAK1-binding protein 1
- TIR
Toll Interleukin-1 Receptor
- TLR
Toll-Like Receptor
- TIRAP
TIR-domain-containing Adaptor Protein
- TNF-α
Tumor Necrosis Factor α
- Tollip
Toll Interacting Protein
- TRAF6
TNF - associated factor 6
- TRIF
TIR-domain-containing Adaptor Protein Inducing Interferon-β
- TRAM
TRIF-related Adaptor Molecule
- VEGF
Vascular Endothelial Growth Factor
REFERENCE
- [1].Gay NJ, Packman LC, Weldon MA, Barna JC. A leucine-rich repeat peptide derived from the Drosophila Toll receptor forms extended filaments with a beta-sheet structure. FEBS Lett. 1991;291:87–91. doi: 10.1016/0014-5793(91)81110-t. [DOI] [PubMed] [Google Scholar]
- [2].Gay NJ, Keith FJ. Drosophila Toll and IL-1 receptor. Nature. 1991;351:355–6. doi: 10.1038/351355b0. [DOI] [PubMed] [Google Scholar]
- [3].Frantz S, Ertl G, Bauersachs J. Mechanisms of disease: Toll-like receptors in cardiovascular disease. Nat Clin Pract Cardiovasc Med. 2007;4:444–54. doi: 10.1038/ncpcardio0938. [DOI] [PubMed] [Google Scholar]
- [4].Jiang D, Liang J, Noble PW. Hyaluronan in tissue injury and repair. Annu Rev Cell Dev Biol. 2007;23:435–61. doi: 10.1146/annurev.cellbio.23.090506.123337. [DOI] [PubMed] [Google Scholar]
- [5].Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–45. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- [6].Sandor F, Buc M. Toll-like receptors. I. Structure, function and their ligands. Folia Biol (Praha) 2005;51:148–57. [PubMed] [Google Scholar]
- [7].Saitoh S, Miyake K. Regulatory molecules required for nucleotide-sensing Toll-like receptors. Immunol Rev. 2009;227:32–43. doi: 10.1111/j.1600-065X.2008.00729.x. [DOI] [PubMed] [Google Scholar]
- [8].Harding CV. gp96 leads the way for toll-like receptors. Immunity. 2007;26:141–3. doi: 10.1016/j.immuni.2007.02.003. [DOI] [PubMed] [Google Scholar]
- [9].O'Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353–64. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- [10].Chaudhuri N, Dower SK, Whyte MK, Sabroe I. Toll-like receptors and chronic lung disease. Clin Sci (Lond) 2005;109:125–33. doi: 10.1042/CS20050044. [DOI] [PubMed] [Google Scholar]
- [11].Velez DR, Wejse C, Stryjewski ME, et al. Variants in toll-like receptors 2 and 9 influence susceptibility to pulmonary tuberculosis in Caucasians, African-Americans, and West Africans. Hum Genet. 127:65–73. doi: 10.1007/s00439-009-0741-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mollaki V, Georgiadis T, Tassidou A, et al. Polymorphisms and haplotypes in TLR9 and MYD88 are associated with the development of Hodgkin's lymphoma: a candidate-gene association study. J Hum Genet. 2009;54:655–9. doi: 10.1038/jhg.2009.90. [DOI] [PubMed] [Google Scholar]
- [13].Speletas M, Merentiti V, Kostikas K, et al. Association of TLR4-T399I polymorphism with chronic obstructive pulmonary disease in smokers. Clin Dev Immunol. 2009;2009:260286. doi: 10.1155/2009/260286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Rad R, Brenner L, Krug A, et al. Toll-like receptor-dependent activation of antigen-presenting cells affects adaptive immunity to Helicobacter pylori. Gastroenterology. 2007;133:150–163. e3. doi: 10.1053/j.gastro.2007.04.071. [DOI] [PubMed] [Google Scholar]
- [15].Bauer S, Hangel D, Yu P. Immunobiology of toll-like receptors in allergic disease. Immunobiology. 2007;212:521–33. doi: 10.1016/j.imbio.2007.03.011. [DOI] [PubMed] [Google Scholar]
- [16].Eisenbarth SC, Cassel S, Bottomly K. Understanding asthma pathogenesis: linking innate and adaptive immunity. Curr Opin Pediatr. 2004;16:659–66. doi: 10.1097/01.mop.0000145920.00101.e4. [DOI] [PubMed] [Google Scholar]
- [17].Imanishi T, Hara H, Suzuki S, et al. Cutting edge: TLR2 directly triggers Th1 effector functions. J Immunol. 2007;178:6715–9. doi: 10.4049/jimmunol.178.11.6715. [DOI] [PubMed] [Google Scholar]
- [18].Bjornvold M, Munthe-Kaas MC, Egeland T, et al. A TLR2 polymorphism is associated with type 1 diabetes and allergic asthma. Genes Immun. 2009 doi: 10.1038/gene.2008.100. [DOI] [PubMed] [Google Scholar]
- [19].Smit LA, Bongers SI, Ruven HJ, et al. Atopy and new-onset asthma in young Danish farmers and CD14, TLR2, and TLR4 genetic polymorphisms: a nested case-control study. Clin Exp Allergy. 2007;37:1602–8. doi: 10.1111/j.1365-2222.2007.02831.x. [DOI] [PubMed] [Google Scholar]
- [20].Pasterkamp G, Versteeg D, de Kleijn DP. Immune regulatory cells: circulating biomarker factories in cardiovascular disease. Clin Sci (Lond) 2008;115:129–31. doi: 10.1042/CS20080089. [DOI] [PubMed] [Google Scholar]
- [21].Kamath AT, Sheasby CE, Tough DF. Dendritic cells and NK cells stimulate bystander T cell activation in response to TLR agonists through secretion of IFN-alpha beta and IFN-gamma. J Immunol. 2005;174:767–76. doi: 10.4049/jimmunol.174.2.767. [DOI] [PubMed] [Google Scholar]
- [22].Siegemund S, Schutze N, Freudenberg MA, et al. Production of IL-12, IL-23 and IL-27p28 by bone marrow-derived conventional dendritic cells rather than macrophages after LPS/TLR4-dependent induction by Salmonella Enteritidis. Immunobiology. 2007;212:739–50. doi: 10.1016/j.imbio.2007.09.004. [DOI] [PubMed] [Google Scholar]
- [23].Yanagawa Y, Onoe K. Enhanced IL-10 production by TLR4- and TLR2-primed dendritic cells upon TLR restimulation. J Immunol. 2007;178:6173–80. doi: 10.4049/jimmunol.178.10.6173. [DOI] [PubMed] [Google Scholar]
- [24].Nakamura K, Miyazato A, Koguchi Y, et al. Toll-like receptor 2 (TLR2) and dectin-1 contribute to the production of IL-12p40 by bone marrow-derived dendritic cells infected with Penicillium marneffei. Microbes Infect. 2008;10:1223–7. doi: 10.1016/j.micinf.2008.06.011. [DOI] [PubMed] [Google Scholar]
- [25].Pabst R, Durak D, Roos A, Luhrmann A, Tschernig T. TLR2/6 stimulation of the rat lung: effects on lymphocyte subsets, natural killer cells and dendritic cells in different parts of the air-conducting compartments and at different ages. Immunology. 2009;126:132–9. doi: 10.1111/j.1365-2567.2008.02886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kawamura T, Murakami K, Bujo H, Unoki H, Jiang M, Nakayama T, Saito Y. Matrix metalloproteinase-3 enhances the free fatty acids-induced VEGF expression in adipocytes through toll-like receptor 2. Exp Biol Med (Maywood) 2008;233:1213–21. doi: 10.3181/0801-RM-20. [DOI] [PubMed] [Google Scholar]
- [27].Rebl A, Hoyheim B, Fischer U, Kollner B, Siegl E, Seyfert HM. Tollip, a negative regulator of TLR-signalling, is encoded by twin genes in salmonid fish. Fish Shellfish Immunol. 2008;25:153–62. doi: 10.1016/j.fsi.2008.04.002. [DOI] [PubMed] [Google Scholar]
- [28].Takeda K, Akira S. TLR signaling pathways. Semin Immunol. 2004;16:3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- [29].Zhang G, Ghosh S. Negative regulation of toll-like receptor-mediated signaling by Tollip. J Biol Chem. 2002;277:7059–65. doi: 10.1074/jbc.M109537200. [DOI] [PubMed] [Google Scholar]
- [30].Sandor F, Buc M. Toll-like receptors. II. Distribution and pathways involved in TLR signalling. Folia Biol (Praha) 2005;51:188–97. [PubMed] [Google Scholar]
- [31].Takeuchi O, Hoshino K, Kawai T, et al. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–51. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- [32].Drage MG, Pecora ND, Hise AG, et al. TLR2 and its co-receptors determine responses of macrophages and dendritic cells to lipoproteins of Mycobacterium tuberculosis. Cell Immunol. 2009;258:29–37. doi: 10.1016/j.cellimm.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hasebe A, Pennock ND, Mu HH, et al. A microbial TLR2 agonist imparts macrophage-activating ability to apolipoprotein A-1. J Immunol. 2006;177:4826–32. doi: 10.4049/jimmunol.177.7.4826. [DOI] [PubMed] [Google Scholar]
- [34].Cabral ES, Gelderblom H, Hornung RL, et al. Borrelia burgdorferi lipoprotein-mediated TLR2 stimulation causes the down-regulation of TLR5 in human monocytes. J Infect Dis. 2006;193:849–59. doi: 10.1086/500467. [DOI] [PubMed] [Google Scholar]
- [35].Shimizu T, Kida Y, Kuwano K. A dipalmitoylated lipoprotein from Mycoplasma pneumoniae activates NF-kappa B through TLR1, TLR2, and TLR6. J Immunol. 2005;175:4641–6. doi: 10.4049/jimmunol.175.7.4641. [DOI] [PubMed] [Google Scholar]
- [36].Bowdish DM, Sakamoto K, Kim MJ, et al. MARCO, TLR2, and CD14 are required for macrophage cytokine responses to mycobacterial trehalose dimycolate and Mycobacterium tuberculosis. PLoS Pathog. 2009;5:e1000474. doi: 10.1371/journal.ppat.1000474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Doz E, Rose S, Nigou J, et al. Acylation determines the toll-like receptor (TLR)-dependent positive versus TLR2-, mannose receptor-, and SIGNR1-independent negative regulation of proinflammatory cytokines by mycobacterial lipomannan. J Biol Chem. 2007;282:26014–25. doi: 10.1074/jbc.M702690200. [DOI] [PubMed] [Google Scholar]
- [38].Quesniaux VJ, Nicolle DM, Torres D, et al. Toll-like receptor 2 (TLR2)-dependent-positive and TLR2-independent-negative regulation of proinflammatory cytokines by mycobacterial lipomannans. J Immunol. 2004;172:4425–34. doi: 10.4049/jimmunol.172.7.4425. [DOI] [PubMed] [Google Scholar]
- [39].Tapping RI, Tobias PS. Mycobacterial lipoarabinomannan mediates physical interactions between TLR1 and TLR2 to induce signaling. J Endotoxin Res. 2003;9:264–8. doi: 10.1179/096805103225001477. [DOI] [PubMed] [Google Scholar]
- [40].Sugawara I, Yamada H, Li C, et al. Mycobacterial infection in TLR2 and TLR6 knockout mice. Microbiol Immunol. 2003;47:327–36. doi: 10.1111/j.1348-0421.2003.tb03404.x. [DOI] [PubMed] [Google Scholar]
- [41].Carrera-Silva EA, Carolina CR, Natalia G, et al. TLR2, TLR4 and TLR9 are differentially modulated in liver lethally injured from BALB/c and C57BL/6 mice during Trypanosoma cruzi acute infection. Mol Immunol. 2008;45:3580–8. doi: 10.1016/j.molimm.2008.05.004. [DOI] [PubMed] [Google Scholar]
- [42].Bafica A, Santiago HC, Goldszmid R, et al. Cutting edge: TLR9 and TLR2 signaling together account for MyD88-dependent control of parasitemia in Trypanosoma cruzi infection. J Immunol. 2006;177:3515–9. doi: 10.4049/jimmunol.177.6.3515. [DOI] [PubMed] [Google Scholar]
- [43].Lamkanfi M, Malireddi RK, Kanneganti TD. Fungal zymosan and mannan activate the cryopyrin inflammasome. J Biol Chem. 2009;284:20574–81. doi: 10.1074/jbc.M109.023689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Ikeda Y, Adachi Y, Ishii T, et al. Dissociation of Toll-like receptor 2-mediated innate immune response to Zymosan by organic solvent-treatment without loss of Dectin-1 reactivity. Biol Pharm Bull. 2008;31:13–8. doi: 10.1248/bpb.31.13. [DOI] [PubMed] [Google Scholar]
- [45].Kelly MM, McNagny K, Williams DL, et al. The lung responds to zymosan in a unique manner independent of toll-like receptors, complement, and dectin-1. Am J Respir Cell Mol Biol. 2008;38:227–38. doi: 10.1165/rcmb.2007-0045OC. [DOI] [PubMed] [Google Scholar]
- [46].Mariani CL, Rajon D, Bova FJ, Streit WJ. Nonspecific immunotherapy with intratumoral lipopolysaccharide and zymosan A but not GM-CSF leads to an effective anti-tumor response in subcutaneous RG-2 gliomas. J Neurooncol. 2007;85:231–40. doi: 10.1007/s11060-007-9415-2. [DOI] [PubMed] [Google Scholar]
- [47].Ikeda Y, Adachi Y, Ishibashi K, Miura N, Ohno N. Activation of toll-like receptor-mediated NF-kappa beta by zymosan-derived water-soluble fraction: possible contribution of endotoxin-like substances. Immunopharmacol Immunotoxicol. 2005;27:285–98. doi: 10.1081/iph-200067943. [DOI] [PubMed] [Google Scholar]
- [48].Sato M, Sano H, Iwaki D, et al. Direct binding of Toll-like receptor 2 to zymosan, and zymosan-induced NF-kappa B activation and TNF-alpha secretion are down-regulated by lung collectin surfactant protein A. J Immunol. 2003;171:417–25. doi: 10.4049/jimmunol.171.1.417. [DOI] [PubMed] [Google Scholar]
- [49].Thivierge M, Stankova J, Rola-Pleszczynski M. Cysteinyl-leukotriene receptor type 1 expression and function is down-regulated during monocyte-derived dendritic cell maturation with zymosan: involvement of IL-10 and prostaglandins. J Immunol. 2009;183:6778–87. doi: 10.4049/jimmunol.0901800. [DOI] [PubMed] [Google Scholar]
- [50].Boon Hinckley M, Reynolds CM, Ribeiro AA, et al. A Leptospira interrogans enzyme with similarity to yeast Ste14p that methylates the 1-phosphate group of lipid A. J Biol Chem. 2005;280:30214–24. doi: 10.1074/jbc.M506103200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Burns E, Eliyahu T, Uematsu S, Akira S, Nussbaum G. TLR2-Dependent Inflammatory Response to Porphyromonas gingivalis Is MyD88 Independent, whereas MyD88 Is Required To Clear Infection. J Immunol. 184:1455–62. doi: 10.4049/jimmunol.0900378. [DOI] [PubMed] [Google Scholar]
- [52].Nemoto E, Darveau RP, Foster BL, Nogueira-Filho GR, Somerman MJ. Regulation of cementoblast function by P. gingivalis lipopolysaccharide via TLR2. J Dent Res. 2006;85:733–8. doi: 10.1177/154405910608500809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Schmeck B, Huber S, Moog K, et al. Pneumococci induced TLR-and Rac1-dependent NF-kappaB-recruitment to the IL-8 promoter in lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2006;290:L730–L737. doi: 10.1152/ajplung.00271.2005. [DOI] [PubMed] [Google Scholar]
- [54].Arbibe L, Mira JP, Teusch N, et al. Toll-like receptor 2-mediated NF-kappa B activation requires a Rac1-dependent pathway. Nat Immunol. 2000;1:533–40. doi: 10.1038/82797. [DOI] [PubMed] [Google Scholar]
- [55].Underhill DM, Ozinsky A, Smith KD, Aderem A. Toll-like receptor-2 mediates mycobacteria-induced proinflammatory signaling in macrophages. Proc Natl Acad Sci U S A. 1999;96:14459–63. doi: 10.1073/pnas.96.25.14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].den Dekker WK, Cheng C, Pasterkamp G, Duckers HJ. Toll like receptor 4 in atherosclerosis and plaque destabilization. Atherosclerosis. 2009 doi: 10.1016/j.atherosclerosis.2009.09.075. [DOI] [PubMed] [Google Scholar]
- [57].Hajishengallis G, Sharma A, Russell MW, Genco RJ. Interactions of oral pathogens with toll-like receptors: possible role in atherosclerosis. Ann Periodontol. 2002;7:72–8. doi: 10.1902/annals.2002.7.1.72. [DOI] [PubMed] [Google Scholar]
- [58].Schoneveld AH, Oude Nijhuis MM, van Middelaar B, Laman JD, de Kleijn DP, Pasterkamp G. Toll-like receptor 2 stimulation induces intimal hyperplasia and atherosclerotic lesion development. Cardiovasc Res. 2005;66:162–9. doi: 10.1016/j.cardiores.2004.12.016. [DOI] [PubMed] [Google Scholar]
- [59].Erdman LK, Cosio G, Helmers AJ, et al. CD36 and TLR interactions in inflammation and phagocytosis: implications for malaria. J Immunol. 2009;183:6452–9. doi: 10.4049/jimmunol.0901374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Niebuhr M, Langnickel J, Sigel S, Werfel T. Dysregulation of CD36 upon TLR-2 stimulation in monocytes from patients with atopic dermatitis and the TLR2 R753Q polymorphism. Exp Dermatol. 2009 doi: 10.1111/j.1600-0625.2009.00989.x. [DOI] [PubMed] [Google Scholar]
- [61].Taront S, Dieudonne A, Blanchard S, et al. Implication of scavenger receptors in the interactions between diesel exhaust particles and immature or mature dendritic cells. Part Fibre Toxicol. 2009;6:9. doi: 10.1186/1743-8977-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Melkamu T, Squillace D, Kita H, O'Grady SM. Regulation of TLR2 expression and function in human airway epithelial cells. J Membr Biol. 2009;229:101–13. doi: 10.1007/s00232-009-9175-3. [DOI] [PubMed] [Google Scholar]
- [63].Triantafilou M, Gamper FG, Haston RM, et al. Membrane sorting of toll-like receptor (TLR)-2/6 and TLR2/1 heterodimers at the cell surface determines heterotypic associations with CD36 and intracellular targeting. J Biol Chem. 2006;281:31002–11. doi: 10.1074/jbc.M602794200. [DOI] [PubMed] [Google Scholar]
- [64].Jin MS, Lee JO. Structures of the toll-like receptor family and its ligand complexes. Immunity. 2008;29:182–91. doi: 10.1016/j.immuni.2008.07.007. [DOI] [PubMed] [Google Scholar]
- [65].Vlahopoulos S, Boldogh I, Casola A, Brasier AR. Nuclear factor-kappaB-dependent induction of interleukin-8 gene expression by tumor necrosis factor alpha: evidence for an antioxidant sensitive activating pathway distinct from nuclear translocation. Blood. 1999;94:1878–89. [PubMed] [Google Scholar]
- [66].Bailey KL, Wyatt TA, Romberger DJ, Sisson JH. Alcohol Functionally Upregulates Toll-Like Receptor 2 in Airway Epithelial Cells. Alcohol Clin Exp Res. 2008 doi: 10.1111/j.1530-0277.2008.00862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Takagi Y, Masamune A, Kume K, et al. Microsatellite polymorphism in intron 2 of human Toll-like receptor 2 gene is associated with susceptibility to acute pancreatitis in Japan. Hum Immunol. 2009 doi: 10.1016/j.humimm.2009.01.006. [DOI] [PubMed] [Google Scholar]
- [68].Takagi Y, Masamune A, Kume K, et al. Microsatellite polymorphism in intron 2 of human Toll-like receptor 2 gene is associated with susceptibility to acute pancreatitis in Japan. Human Immunology. 2009;70:200–4. doi: 10.1016/j.humimm.2009.01.006. [DOI] [PubMed] [Google Scholar]
- [69].Yim JJ, Ding L, Schaffer AA, et al. A microsatellite polymorphism in intron 2 of human Toll-like receptor 2 gene: functional implications and racial differences. FEMS Immunol Med Microbiol. 2004;40:163–9. doi: 10.1016/S0928-8244(03)00342-0. [DOI] [PubMed] [Google Scholar]
- [70].Liu-Bryan R, Scott P, Sydlaske A, Rose DM, Terkeltaub R. Innate immunity conferred by Toll-like receptors 2 and 4 and myeloid differentiation factor 88 expression is pivotal to monosodium urate monohydrate crystal-induced inflammation. Arthritis Rheum. 2005;52:2936–46. doi: 10.1002/art.21238. [DOI] [PubMed] [Google Scholar]
- [71].Gasse P, Riteau N, Charron S, et al. Uric acid is a danger signal activating NALP3 inflammasome in lung injury inflammation and fibrosis. Am J Respir Crit Care Med. 2009;179:903–13. doi: 10.1164/rccm.200808-1274OC. [DOI] [PubMed] [Google Scholar]
- [72].Scott P, Ma H, Viriyakosol S, Terkeltaub R, Liu-Bryan R. Engagement of CD14 mediates the inflammatory potential of monosodium urate crystals. J Immunol. 2006;177:6370–8. doi: 10.4049/jimmunol.177.9.6370. [DOI] [PubMed] [Google Scholar]
- [73].Johnson BJ, Le TT, Dobbin CA, et al. Heat shock protein 10 inhibits lipopolysaccharide-induced inflammatory mediator production. J Biol Chem. 2005;280:4037–47. doi: 10.1074/jbc.M411569200. [DOI] [PubMed] [Google Scholar]
- [74].Vanags D, Williams B, Johnson B, et al. Therapeutic efficacy and safety of chaperonin 10 in patients with rheumatoid arthritis: a double-blind randomised trial. Lancet. 2006;368:855–63. doi: 10.1016/S0140-6736(06)69210-6. [DOI] [PubMed] [Google Scholar]
- [75].Chang YC, Kao WC, Wang WY, et al. Identification and characterization of oligonucleotides that inhibit Toll-like receptor 2-associated immune responses. Faseb J. 2009;23:3078–88. doi: 10.1096/fj.09-129312. [DOI] [PubMed] [Google Scholar]
- [76].Arslan F, Smeets MB, O'Neill LA, et al. Myocardial ischemia/reperfusion injury is mediated by leukocytic toll-like receptor-2 and reduced by systemic administration of a novel anti-toll-like receptor-2 antibody. Circulation. 121:80–90. doi: 10.1161/CIRCULATIONAHA.109.880187. [DOI] [PubMed] [Google Scholar]
- [77].Cervantes-Barragan L, Gil-Cruz C, Pastelin-Palacios R, et al. TLR2 and TLR4 signaling shapes specific antibody responses to Salmonella typhi antigens. Eur J Immunol. 2009;39:126–35. doi: 10.1002/eji.200838185. [DOI] [PubMed] [Google Scholar]
- [78].Moreno M, Mol BM, von Mensdorff-Pouilly S, et al. Toll-like receptor agonists and invariant natural killer T-cells enhance antibody-dependent cell-mediated cytotoxicity (ADCC) Cancer Lett. 2008;272:70–6. doi: 10.1016/j.canlet.2008.06.028. [DOI] [PubMed] [Google Scholar]
- [79].O'Neill LA. Therapeutic targeting of Toll-like receptors for inflammatory and infectious diseases. Curr Opin Pharmacol. 2003;3:396–403. doi: 10.1016/s1471-4892(03)00080-8. [DOI] [PubMed] [Google Scholar]
- [80].O'Neill LA, Bryant CE, Doyle SL. Therapeutic Targeting of Toll-Like Receptors for Infectious and Inflammatory Diseases and Cancer. Pharmacol Rev. 2009 doi: 10.1124/pr.109.001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Decker WK, Safdar A. Bioimmunoadjuvants for the treatment of neoplastic and infectious disease: Coley's legacy revisited. Cytokine Growth Factor Rev. 2009;20:271–81. doi: 10.1016/j.cytogfr.2009.07.004. [DOI] [PubMed] [Google Scholar]
- [82].Shammas NW, Shammas GA, Hahn A, et al. In-hospital complications and long-term outcomes of the paclitaxel drug-eluting stent in acute ST-elevation myocardial infarction: a real-world experience from a high-volume medical center. Cardiovasc Revasc Med. 2009;10:151–5. doi: 10.1016/j.carrev.2009.01.007. [DOI] [PubMed] [Google Scholar]
- [83].Othoro C, Johnston D, Lee R, et al. Enhanced immunogenicity of Plasmodium falciparum peptide vaccines using a topical adjuvant containing a potent synthetic Toll-like receptor 7 agonist, imiquimod. Infect Immun. 2009;77:739–48. doi: 10.1128/IAI.00974-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Wu D, Zhang J, Sun R, Wei H, Tian Z. Preferential distribution of NK cells into uteri of C57B1/6J mice after adoptive transfer of lymphocytes. J Reprod Immunol. 2007;75:120–7. doi: 10.1016/j.jri.2007.05.003. [DOI] [PubMed] [Google Scholar]
- [85].Gaspari AA. Mechanism of action and other potential roles of an immune response modifier. Cutis. 2007;79:36–45. [PubMed] [Google Scholar]
- [86].Kast RE, Foley KF, Focosi D. Doxorubicin cardiomyopathy via TLR-2 stimulation: potential for prevention using current antiretroviral inhibitors such as ritonavir and nelfinavir. Hematol Oncol. 2007;25:96–7. doi: 10.1002/hon.811. [DOI] [PubMed] [Google Scholar]
- [87].Smith KJ, Hamza S, Skelton H. The imidazoquinolines and their place in the therapy of cutaneous disease. Expert Opin Pharmacother. 2003;4:1105–19. doi: 10.1517/14656566.4.7.1105. [DOI] [PubMed] [Google Scholar]
- [88].Herrera JL, Gonzalez-Rey E, Fernandez-Montesinos R, et al. Toll-like receptor stimulation differentially regulates vasoactive intestinal peptide type 2 receptor in macrophages. J Cell Mol Med. 2009 doi: 10.1111/j.1582-4934.2009.00662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Prescott SL, Noakes P, Chow BW, et al. Presymptomatic differences in Toll-like receptor function in infants who have allergy. J Allergy Clin Immunol. 2008;122:391–9. 399, e1–5. doi: 10.1016/j.jaci.2008.04.042. [DOI] [PubMed] [Google Scholar]
- [90].Cheung PF, Wong CK, Ip WK, Lam CW. FAK-mediated activation of ERK for eosinophil migration: a novel mechanism for infection-induced allergic inflammation. Int Immunol. 2008;20:353–63. doi: 10.1093/intimm/dxm146. [DOI] [PubMed] [Google Scholar]
- [91].Wong CK, Cheung PF, Ip WK, Lam CW. Intracellular signaling mechanisms regulating toll-like receptor-mediated activation of eosinophils. Am J Respir Cell Mol Biol. 2007;37:85–96. doi: 10.1165/rcmb.2006-0457OC. [DOI] [PubMed] [Google Scholar]
- [92].Demedts IK, Bracke KR, Maes T, Joos GF, Brusselle GG. Different roles for human lung dendritic cell subsets in pulmonary immune defense mechanisms. Am J Respir Cell Mol Biol. 2006;35:387–93. doi: 10.1165/rcmb.2005-0382OC. [DOI] [PubMed] [Google Scholar]
- [93].Miller RL, Gerster JF, Owens ML, Slade HB, Tomai MA. Imiquimod applied topically: a novel immune response modifier and new class of drug. Int J Immunopharmacol. 1999;21:1–14. doi: 10.1016/s0192-0561(98)00068-x. [DOI] [PubMed] [Google Scholar]
- [94].Sun Y, Pearlman E. Inhibition of Corneal Inflammation by the TLR4 antagonist Eritoran tetrasodium (E5564) Invest Ophthalmol Vis Sci. 2008 doi: 10.1167/iovs.08-2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Brodsky I, Medzhitov R. Two modes of ligand recognition by TLRs. Cell. 2007;130:979–81. doi: 10.1016/j.cell.2007.09.009. [DOI] [PubMed] [Google Scholar]
- [96].Rossignol DP, Wong N, Noveck R, Lynn M. Continuous pharmacodynamic activity of eritoran tetrasodium, a TLR4 antagonist, during intermittent intravenous infusion into normal volunteers. Innate Immun. 2008;14:383–94. doi: 10.1177/1753425908099173. [DOI] [PubMed] [Google Scholar]
- [97].Rossignol DP, Lynn M. TLR4 antagonists for endotoxemia and beyond. Curr Opin Investig Drugs. 2005;6:496–502. [PubMed] [Google Scholar]
- [98].Rossignol DP, Wasan KM, Choo E, et al. Safety, pharmacokinetics, pharmacodynamics, and plasma lipoprotein distribution of eritoran (E5564) during continuous intravenous infusion into healthy volunteers. Antimicrob Agents Chemother. 2004;48:3233–40. doi: 10.1128/AAC.48.9.3233-3240.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Kimbro K, Parker S. Recent Patents in Toll-like Receptor Pathways and Relevance to Cancer. Recent Pat Anticancer Drug Discov. 2009 doi: 10.2174/157489209789206904. [DOI] [PubMed] [Google Scholar]
- [100].Ingale S, Wolfert MA, Buskas T, Boons GJ. Increasing the antigenicity of synthetic tumor-associated carbohydrate antigens by targeting Toll-like receptors. Chembiochem. 2009;10:455–63. doi: 10.1002/cbic.200800596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Ingale S, Wolfert MA, Gaekwad J, Buskas T, Boons GJ. Robust immune responses elicited by a fully synthetic three-component vaccine. Nat Chem Biol. 2007;3:663–7. doi: 10.1038/nchembio.2007.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Seya T, Akazawa T, Tsujita T, Matsumoto M. Role of Toll-like receptors in adjuvant-augmented immune therapies. Evid Based Complement Alternat Med. 2006;3:31–8. doi: 10.1093/ecam/nek010. discussion 133–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Shojaei H, Oberg HH, Juricke M, et al. Toll-like receptors 3 and 7 agonists enhance tumor cell lysis by human gammadelta T cells. Cancer Res. 2009;69:8710–7. doi: 10.1158/0008-5472.CAN-09-1602. [DOI] [PubMed] [Google Scholar]
- [104].Vicente-Suarez I, Brayer J, Villagra A, Cheng F, Sotomayor EM. TLR5 ligation by flagellin converts tolerogenic dendritic cells into activating antigen-presenting cells that preferentially induce T-helper 1 responses. Immunol Lett. 2009;125:114–8. doi: 10.1016/j.imlet.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Long AA. Immunomodulators in the treatment of asthma. Allergy Asthma Proc. 2009;30:109–19. doi: 10.2500/aap.2009.30.3203. [DOI] [PubMed] [Google Scholar]
- [106].Fonseca DE, Kline JN. Use of CpG oligonucleotides in treatment of asthma and allergic disease. Adv Drug Deliv Rev. 2009;61:256–62. doi: 10.1016/j.addr.2008.12.007. [DOI] [PubMed] [Google Scholar]
- [107].Agrawal S, Kandimalla ER. Synthetic agonists of Toll-like receptors 7, 8 and 9. Biochem Soc Trans. 2007;35:1461–7. doi: 10.1042/BST0351461. [DOI] [PubMed] [Google Scholar]
- [108].Greene CM, Branagan P, McElvaney NG. Toll-like receptors as therapeutic targets in cystic fibrosis. Expert Opin Ther Targets. 2008;12:1481–95. doi: 10.1517/14728220802515293. [DOI] [PubMed] [Google Scholar]
- [109].Kim KW, Cho ML, Oh HJ, et al. TLR-3 enhances osteoclastogenesis through upregulation of RANKL expression from fibroblast-like synoviocytes in patients with rheumatoid arthritis. Immunol Lett. 2009;124:9–17. doi: 10.1016/j.imlet.2009.02.006. [DOI] [PubMed] [Google Scholar]
- [110].Brentano F, Kyburz D, Gay S. Toll-like receptors and rheumatoid arthritis. Methods Mol Biol. 2009;517:329–43. doi: 10.1007/978-1-59745-541-1_20. [DOI] [PubMed] [Google Scholar]
- [111].Roelofs MF, Wenink MH, Brentano F, et al. Type I interferons might form the link between Toll-like receptor (TLR) 3/7 and TLR4-mediated synovial inflammation in rheumatoid arthritis (RA) Ann Rheum Dis. 2009;68:1486–93. doi: 10.1136/ard.2007.086421. [DOI] [PubMed] [Google Scholar]
- [112].Jiang Q, Wei H, Tian Z, Poly I. C enhances cycloheximide-induced apoptosis of tumor cells through TLR3 pathway. BMC Cancer. 2008;8:12. doi: 10.1186/1471-2407-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Katsargyris A, Klonaris C, Alexandrou A, et al. Toll-like receptors in liver ischemia reperfusion injury: a novel target for therapeutic modulation? Expert Opin Ther Targets. 2009;13:427–42. doi: 10.1517/14728220902794939. [DOI] [PubMed] [Google Scholar]
- [114].Zhang QJ, Shen H, Zhang W, Li YP, Li TS. Effects of combined naloxone and methylprednisolone on nuclear factor-kappaB expression in the lung in acute lung injury in rats. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2005;17:370–2. [PubMed] [Google Scholar]
- [115].Tsoulfas G, Takahashi Y, Ganster RW, et al. Activation of the lipopolysaccharide signaling pathway in hepatic transplantation preservation injury. Transplantation. 2002;74:7–13. doi: 10.1097/00007890-200207150-00003. [DOI] [PubMed] [Google Scholar]
- [116].Katsargyris A, Klonaris C, Alexandrou A, et al. Toll like receptors in liver ischemia reperfusion injury: a novel target for therapeutic modulation? Expert Opin Ther Targets. 2009;13:427–42. doi: 10.1517/14728220902794939. [DOI] [PubMed] [Google Scholar]
- [117].Kruger B, Krick S, Dhillon N, et al. Donor Toll-like receptor 4 contributes to ischemia and reperfusion injury following human kidney transplantation. Proc Natl Acad Sci U S A. 2009;106:3390–5. doi: 10.1073/pnas.0810169106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Liu YY, Cai WF, Yang HZ, et al. Bacillus Calmette-Guerin and TLR4 agonist prevent cardiovascular hypertrophy and fibrosis by regulating immune microenvironment. J Immunol. 2008;180:7349–57. doi: 10.4049/jimmunol.180.11.7349. [DOI] [PubMed] [Google Scholar]
- [119].Hadley JS, Wang JE, Michaels LC, et al. Alterations in inflammatory capacity and TLR expression on monocytes and neutrophils after cardiopulmonary bypass. Shock. 2007;27:466–73. doi: 10.1097/01.shk.0000245033.69977.c5. [DOI] [PubMed] [Google Scholar]
- [120].Foldes G, von Haehling S, Jankowska EA, Anker SD. Targeting the toll-system in cardiovascular sciences. Recent Pat Inflamm Allergy Drug Discov. 2007;1:57–67. doi: 10.2174/187221307779815084. [DOI] [PubMed] [Google Scholar]
- [121].Frantz S, Ertl G, Bauersachs J. Toll-like receptor signaling in the ischemic heart. Front Biosci. 2008;13:5772–9. doi: 10.2741/3114. [DOI] [PubMed] [Google Scholar]
- [122].Balistreri CR, Candore G, Mirabile M, et al. TLR2 and age-related diseases: potential effects of Arg753Gln and Arg677Trp polymorphisms in acute myocardial infarction. Rejuvenation Res. 2008;11:293–6. doi: 10.1089/rej.2008.0666. [DOI] [PubMed] [Google Scholar]
- [123].Krejsek J, Kunes P, Kolackova M, et al. Expression of Toll-like receptors 2 and 4 on innate immunity cells modulated by cardiac surgical operation. Scand J Clin Lab Invest. 2008;68:749–58. doi: 10.1080/00365510802233434. [DOI] [PubMed] [Google Scholar]
- [124].Gomariz RP, Gutierrez-Canas I, Arranz A, et al. Peptides targeting Toll-like receptor signalling pathways for novel immune therapeutics. Curr Pharm Des. 16:1063–80. doi: 10.2174/138161210790963841. [DOI] [PubMed] [Google Scholar]
- [125].O'Neill LA, Bryant CE, Doyle SL. Therapeutic targeting of Toll-like receptors for infectious and inflammatory diseases and cancer. Pharmacol Rev. 2009;61:177–97. doi: 10.1124/pr.109.001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Shinohara M, Hirata K, Yamashita T, et al. Local overexpression of toll-like receptors at the vessel wall induces atherosclerotic lesion formation: synergism of TLR2 and TLR4. Arterioscler Thromb Vasc Biol. 2007;27:2384–91. doi: 10.1161/ATVBAHA.106.139253. [DOI] [PubMed] [Google Scholar]
- [127].Zhu X, Bagchi A, Zhao H, et al. Toll-like receptor 2 activation by bacterial peptidoglycan-associated lipoprotein activates cardiomyocyte inflammation and contractile dysfunction. Crit Care Med. 2007;35:886–92. doi: 10.1097/01.CCM.0000256723.37586.A2. [DOI] [PubMed] [Google Scholar]
- [128].Boyd JH, Mathur S, Wang Y, Bateman RM, Walley KR. Toll-like receptor stimulation in cardiomyoctes decreases contractility and initiates an NF-kappaB dependent inflammatory response. Cardiovasc Res. 2006;72:384–93. doi: 10.1016/j.cardiores.2006.09.011. [DOI] [PubMed] [Google Scholar]
- [129].Du T, Zhou ZG, You S, et al. Regulation by 1, 25-dihydroxyvitamin D3 on altered TLRs expression and response to ligands of monocyte from autoimmune diabetes. Clin Chim Acta. 2009 doi: 10.1016/j.cca.2008.12.038. [DOI] [PubMed] [Google Scholar]
- [130].Karumuthil-Melethil S, Perez N, Li R, Vasu C. Induction of innate immune response through TLR2 and dectin 1 prevents type 1 diabetes. J Immunol. 2008;181:8323–34. doi: 10.4049/jimmunol.181.12.8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Devaraj S, Dasu MR, Rockwood J, Winter W, Griffen SC, Jialal I. Increased toll-like receptor (TLR) 2 and TLR4 expression in monocytes from patients with type 1 diabetes: further evidence of a proinflammatory state. J Clin Endocrinol Metab. 2008;93:578–83. doi: 10.1210/jc.2007-2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Vallois D, Grimm CH, Avner P, Boitard C, Rogner UC. The type 1 diabetes locus Idd6 controls TLR1 expression. J Immunol. 2007;179:3896–903. doi: 10.4049/jimmunol.179.6.3896. [DOI] [PubMed] [Google Scholar]
- [133].Santin I, Bilbao JR, de Nanclares GP, Calvo B, Castano L. No association of TLR2 and TLR4 polymorphisms with type I diabetes mellitus in the Basque population. Ann N Y Acad Sci. 2006;1079:268–72. doi: 10.1196/annals.1375.040. [DOI] [PubMed] [Google Scholar]
- [134].Holvoet P. Relations between metabolic syndrome, oxidative stress and inflammation and cardiovascular disease. Verh K Acad Geneeskd Belg. 2008;70:193–219. [PubMed] [Google Scholar]
- [135].Lien E, Zipris D. The role of toll-like receptor pathways in the mechanism of type 1 diabetes. Curr Mol Med. 2009;9:52–68. doi: 10.2174/156652409787314453. [DOI] [PubMed] [Google Scholar]
- [136].Horng T, Barton GM, Medzhitov R. TIRAP: an adapter molecule in the Toll signaling pathway. Nat Immunol. 2001;2:835–41. doi: 10.1038/ni0901-835. [DOI] [PubMed] [Google Scholar]