Abstract
Phenotypic plasticity is posed to be a vital trait of cancer cells such as circulating tumor cells, allowing them to undergo reversible or irreversible switching between phenotypic states important for tumorigenesis and metastasis. While irreversible phenotypic switching can be detected by studying the genome, reversible phenotypic switching is often difficult to examine due to its dynamic nature and the lack of knowledge about its contributing factors. In this study, we demonstrate that culturing cells in different physical environments, stiff, soft, or suspension, induced a phenotypic switch in prostate cancer cells via mechanotransduction. The mechanosensitive phenotypic switching in prostate cancer cells was sustainable yet reversible even after long-term culture, demonstrating the impact of mechanical signals on prostate cancer cell phenotypes. Importantly, such a mechanotransduction-mediated phenotypic switch in prostate cancer cells was accompanied by decreased sensitivity of the cells to paclitaxel, suggesting a role of mechanotransduction in the evolution of drug resistance. Multiple signaling pathways such as p38MAPK, ERK, and Wnt were found to be involved in the mechanotransduction-induced phenotypic switching of prostate cancer cells. Given that cancer cells experience different physical environments during disease progression, this study provides useful information about the important role of mechanotransduction in cancer, and how circulating tumor cells may be capable of continuously changing their phenotypes throughout the disease process.
Introduction
The ability of cancer cells to undergo phenotypic switching as result of inherent plasticity at the gene expression, metabolic, and mechanical level/s has been associated with their resistance to cancer drugs and eventual therapy failure. Phenotypic switching of cancer cells occurs at various stages of the disease, can be irreversible or reversible, and may result from multiple factors ranging from genetic to epigenetic and environmental influences (1). While irreversible changes to cancer cell phenotypes are passed down to progeny cells and can be detected by molecular techniques such as sequencing, reversible phenotypic changes of cancer cells are difficult to study due to their dynamic nature. Reversible phenotypic switching in cancer has been associated with cancer cells with stem cell-like properties and in support of this view, a 2010 study has demonstrated that melanoma cells capable of reversibly expressing JARID1B are more capable of sustaining tumor growth compared with those do not (2). Phenotypic plasticity of cancer cells is also found within a large percentage of tumor population instead of being limited to a small subpopulation of rare, stem cell-like cells. Using xenograft transplantation experiments, a significant percentage of single melanoma cells isolated from patient tumors is found capable of forming tumors with unlimited tumorigenic capability (3). Regardless of the initial phenotype of the subpopulation used to form a xenograft tumor, tumor heterogeneity was reestablished at high frequencies, suggesting that phenotypic plasticity can be a general property of tumor cell population (3).
In addition to tumorigenesis, reversible phenotypic plasticity plays an important role in metastasis. During the epithelial-mesenchymal transition (EMT), cancer cells transition from an epithelial to a mesenchymal phenotype, which is associated with invasion, motility, and stem cell-like properties (4). However, the ability to undergo the reverse process of mesenchymal-epithelial transition (MET) by cancer cells is also key in determining whether metastasis to a secondary site can succeed. Cancer cells unable to revert to the epithelial phenotype (loss of plasticity) are less likely to form metastatic lesions (5, 6). Analysis of circulating tumor cells (CTCs) from metastatic breast cancer patients and castration-resistant prostate cancer patients also show that CTCs express both epithelial and mesenchymal markers, supporting an association of phenotypic plasticity of CTCs with their metastatic potential (7). Further supporting this view is evidence from clinical specimens demonstrating that metastatic lesions in prostate cancer patients reexpress E-cadherin (E-cad), despite loss of E-cad expression in primary tumor, suggesting that CTCs undergo MET and revert their phenotype upon successfully colonizing a distal metastatic site (8). While progress has been made in understanding phenotypic reversibility and its clinical relevance in cancer, the factors contributing to reversible phenotypic switching in CTCs remain unclear. Furthermore, characterization of reversible phenotypic switching, such as identifying target genes that possess a reversible expression profile, needs to be examined in detail. Moreover, little is known about how reversible phenotypic switching occurs within the general tumor cell population.
There is increasing evidence that mechanical signals from the local cell microenvironment can influence cellular phenotypes via mechanotransduction. CTCs, in particular, experience a drastic change in their physical environment as they invade from a tumor microenvironment before extravasating into the circulatory system. In this study, we sought to examine the phenotypic switch prostate cancer cells undergo (by performing gene and functional analysis) when grown in different physical environments by utilizing multiple culture platforms that provide a rigid or soft attached environment and suspension growth (as experienced by CTCs). We focused our study on the effect of mechanotransduction on prostate cancer, as prostate cancer cell biology is not as well understood as breast cancer. We demonstrated the reversibility of mechanotransduction-induced reversible phenotypic switching in prostate cancer cells and showed how this phenotypic switch involved activation of multiple signaling pathways important in gene regulation. Importantly, mechanoregulation of phenotypic switching in prostate cancer cells was associated with decreased sensitivity to paclitaxel. This decreased sensitivity of prostate cancer cells to paclitaxel was also reversible, supporting a functional role of mechanotransduction in regulating development of drug resistance in CTCs.
Materials and Methods
Cell culture
PC3 and DU145 cell lines were purchased from American Type Culture Collection (ATCC, Manassas, VA). Cells were grown in 1× RPMI 1640 medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum and 50 μg mL−1 penicillin/streptomycin (Invitrogen) and incubated at 37°C and 5% CO2. Growth media was changed every 2 days and cells were subcultured at a ratio of 1:6 every 4 days. Media conditions for culture under different substrate rigidities were the same.
Fabrication of poly(dimethylsiloxane) micropost array
Fabrication of the poly(dimethylsiloxane) (PDMS) micropost array (PMA) system was performed as reported in Fu et al. (9). By keeping the ratio of cross linker to PDMS monomer constant at 1:10, the bulk Young’s modulus of PDMS could be kept constant, thus allowing different PMA substrates of varying effective Young’s moduli to be generated by only altering the micropost height. Stiff culture conditions included tissue culture plate (TCP) dishes (with a Young’s modulus E of 2 × 106 kPa), a flat PDMS surface (with a Young’s modulus E of 1.3 × 103 kPa), and a 0.7 μm high PMA (with effective Young’s modulus of 1.2 × 103 kPa) (9). Soft cell culture conditions included a 14.5 μm high PMA (with effective Young’s modulus of ∼1 kPa) and suspension growth in liquid medium. To promote cell adhesion to PDMS, flat PDMS surfaces and PMAs were first treated with UV ozone before coated with collagen I (BD Biosciences, San Jose, CA) at a concentration of 50 μg cm−2 using microcontact printing (9). To ensure that phenotypic switch was not due to the presence of collagen, all TCP dishes were coated with collagen I (50 μg cm−2; BD Biosciences) as well before cell seeding.
Suspension growth
Suspension growth was achieved as described in Aw Yong et al. (10). In brief, 100 mm TCP dishes were first coated with 0.8 mg cm−2 polyhydroxyethylmethylacrylate (PHEMA; Sigma-Aldrich, St. Louis, MO) dissolved in ethanol. PHEMA-coated TCP dishes were dried in a 60°C oven before being stored at room temperature. Before seeding cells, TCP dishes were rinsed twice with 1− phosphate-buffered saline (PBS). Cells were seeded onto TCP dishes at a concentration of 0.4 × 106 cells mL−1 and incubated at 37°C and 5% CO2. For long-term culture of PC3 cells in suspension, cells were subcultured every 4 days at a 1:6 ratio by first pelleting the suspension culture by centrifugation at 300 × g. The supernatant was removed by aspiration and the cell pellet was resuspended in fresh medium before being subcultured onto a fresh PHEMA-coated TCP dish.
Quantitative real-time PCR
Total cell RNA was isolated using RNeasy (Qiagen, Hilden, Germany), and complementary DNA (cDNA) was synthesized using the iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA) using 1 μg of total RNA. Primer sequences used for quantitative real-time PCR (qRT-PCR) are as described. AGR2 forward: 5′-TTGTGGCCCTCTCCTACACT-3′; AGR2 reverse: 5′-AGTTGGTCACCCCAACCTCT-3′; ALDH3A1 forward: 5′-AACTACCCCTTCAACCTCACCAT-3′; ALDH3A1 reverse: 5′-TTACTGGGTACAGATCCTTGTCC-3′; E-cad forward: 5′-TCTTCAATCCCACCACGTACA-3′; E-cad reverse: 5′-TGCCATCGTTGTTCACTGGA-3′; N-cadherin (N-cad) forward: 5′-ATCAACCCCATACACCAGCC-3′; N-cad reverse: 5′-GTCGATTGGTTTGACCACGG-3′; GDF15 forward: 5′-TCAAGGTCGTGGGACGTGACA-3′; GDF15 reverse: 5′-GCCGTGCGGACGAAGATTCT-3′; Reference gene TBP forward: 5′-GAATATAATCCCAAGCGGTTTG-3′; TBP reverse: 5′-ACTTCACATCACAGCTCCCC-3′; fibronectin (FN1) forward: 5′-AACAAGCATGTCTCTCTGCCAA-3′; FN1 reverse: 5′-AGAAAACCCTCAGGAAACTCCC-3′; S100A4 forward: 5′-CACACGCTGTTGCTATAGTACG-3′; S100A4 reverse: 5′-TCTGTCCTTTTCCCCAAGAAGC-3′; mucin (MUC-1) forward: 5′-CATTTCACCACCACCATG-3′; MUC-1 reverse: 5′-GAGACCCCAGTAGACAAA-3′; vimentin 1 (VIM) forward: 5′-GGACCAGCTAACCAACGA-3′; VIM reverse: 5′-ACGCATTGTCAACATCCT -3′; desmoplakin (DSP) forward: 5′- GCAGAAGGAAGAGGATAC -3′; DSP reverse: 5′- TGAGATTCTCTGTGGTCT -3′; cytokeratin 18 (KRT18) forward: 5′- TCTCAGCAGATTGAGGAG -3′; and KRT18 reverse: 5′- AAGCTGGCCTTCAGATTT-3′. Working concentration of primers was 500 nM and 10 ng of cDNA template was used for qRT-PCR. Fold change in gene expression was calculated using the 2−ΔΔCT method using TBP as the reference gene.
Immunoblot
Cells grown on adherent substrates (TCP dish, or Dish; flat PDMS surface, or PMA-flat; 0.7 μm high PMA, or PMA-0.7; and 14.5 μm high PMA, or PMA-14.5) were washed twice with ice-cold 1× PBS to remove floating cells before harvesting cell lysate. Cell lysates were prepared by scraping the cells off the substrates using RIPA lysis buffer (Thermo Fisher Scientific, Waltham, MA) supplemented with 1× HALT protease and phosphatase inhibitors (Thermo Fisher Scientific) and further sonicated to improve protein yield. Cell lysates were mixed with 2× Lamelli buffer before being subjected to electrophoresis using a 4–20% TGX polyacrylamide gel (Bio-Rad) at 100 V. Proteins were transferred to a nitrocellulose membrane using 1× Tris/Glycine and 20% methanol at 100 V for 1 h. Membranes were blocked with a blocking solution (LI-COR Biosciences, Lincoln, NE) or 1 h at room temperature before adding primary antibody for overnight at 4°C. Working concentrations of primary antibodies are 1:1000 for AGR2 (Sigma-Aldrich), 1:1000 for ALDH3A1 (Millipore, Billerica, MA), 1:500 for GDF15 (Sigma-Aldrich), 1:100 for E-cad (BioLegend, San Diego, CA), and 1:100 for N-cad (BioLegend). Lamin A/C (Sigma-Aldrich) was used as a loading control at a 1:2000 working concentration. Membranes were washed with wash buffer (1× PBS/0.1% Tween-20) for 5 min for five times before adding fluorescent secondary antibody (LI-COR Biosciences) at a 1:10,000 dilution and incubated for 1 h at room temperature. Membranes were washed with wash buffer for 5 min, five times, before a final rinse with 1− PBS. Membranes were subsequently scanned using the Odyssey system (LI-COR Biosciences).
Image analysis
Cells grown on adherent substrates (Dish, PMA-flat, PMA-0.7, and PMA-14.5) were washed twice with ice-cold 1× PBS to remove floating cells before imaging using an AxioObserver (Carl Zeiss, Oberkochen, Germany). Cell shape analysis was performed using the software ImageJ (National Institutes of Health, Bethesda, MD) to measure cell circularity and spread area.
Flow cytometry
Cells grown both on TCP dish and in suspension were first trypsinized and resuspended in growth medium before washing twice with ice-cold BD Pharmingen Stain Buffer (BD Biosciences). Cells were pelleted by centrifugation at 300 × g for 5 min and resuspended in BD Pharmingen Stain Buffer to a final concentration of 2 × 106 cells μL−1. E-cad-FITC and N-cad-FITC antibodies were diluted 1:100 and used for cell staining at 4°C for 20 min. After staining, cells were washed twice with ice-cold BD Pharmingen Stain Buffer and analyzed by flow cytometry (Attune; Thermo Fisher Scientific). To quantify differences in fluorescence intensities between cells grown on TCP dish and in suspension, the ratio of the median fluorescence intensity (MFI) between stained cells and negative controls was first calculated. The ratio between various growth conditions (TCP dish versus suspension) was then determined.
WST-1 assay
Cells were seeded into a 96-well plate coated with or without PHEMA (Sigma-Aldrich) at a density of 5 × 103 cells per well and incubated at 37°C and 5% CO2 for 24 h. Paclitaxel (Sigma-Aldrich) was added to wells starting from a concentration of 1 nM and increased in a twofold manner. Cells were exposed to paclitaxel for 48 h before adding WST-1 reagent (Roche Holding, Basel, Switzerland) and further incubated at 37°C and 5% CO2 for 3 h. Absorbance readings were obtained using a plate reader (BioTek Instruments, Winooski, VT) at 480 nm with a wavelength correction at 650 nm. All absorbance readings were further corrected using blank readings, before IC-50 curves were calculated using nonlinear regression (GraphPad; GraphPad Software, https://www.graphpad.com/).
Trypan blue exclusion assay
Cells were first allowed to grow on TCP dishes or in suspension for 24 h before being exposed to 25 nM paclitaxel or vehicle for an additional 48 h. Cells were harvested and resuspended in 1 mL ice cold 1× PBS. A quantity of 50 μL of resuspended cells was mixed with an equal volume of trypan blue, and viable cells were counted using an automated cell counter (Logos Biosystems, Seoul, South Korea). Viability of paclitaxel-treated cells was calculated as a percentage of viable cells compared with vehicle-treated cells.
Signaling pathway inhibitors
Inhibitors were prepared as stock solution dissolved in DMSO (Sigma-Aldrich) as described by the manufacturers. Working concentration of p38MAPK inhibitors was 1 μM for both SB203580 and SB220025 (Enzo Life Sciences, Farmingdale, NY). A quantity of 10 μM PD98059 (Cell Signaling Technology, Danvers, MA) was used for inhibiting ERK signaling, while 10 μM CHIR99021 (Sigma-Aldrich) was used for inhibiting GSKβ as a mechanism of activating β-catenin. A quantity of 15 μM FH535 (EMD-Millipore/Merck, Darmstadt, Germany) was used for β-catenin inhibition. Before treatment with inhibitors, cells were allowed to grow in suspension for 24 h before exposed to inhibitors for an additional 48 h.
Results
Mechanotransduction-induced phenotypic switching characterized by changes in gene expression and cell morphology
To study the role of mechanotransduction in phenotypic switching of prostate cancer cells, we utilized various culture methods to provide three physical growth conditions. Rigid or soft conditions were provided using tissue culture plastic and the micromolded PMA system, while suspension culture was achieved by growing cells on PHEMA-coated TCP dishes. In particular, the PMA has a uniform surface geometry and different post heights, thus allowing modulation of substrate rigidity independently of effects on adhesion and other material surface properties (9). An alternative strategy for modulating substrate stiffness can involve altering stromal stiffness by changing stromal protein concentration (11). However, altering stromal protein concentration would impact not only bulk substrate mechanics but also molecular-scale material properties including porosity and binding properties of adhesive ligands (12). We further used a TCP dish and flat PDMS surface as additional rigid surfaces for cell culture. Effective Young’s moduli E of adherent culture conditions used in this work thus span a broad range (TCP (Dish), E = 2 × 106 kPa; flat PDMS (PMA-flat), E = 1.3 × 103 kPa; PMA with a post height of 0.7 μm (PMA-0.7), E = 1.2 × 103 kPa; PMA with a post height of 14.5 μm (PMA-14.5), E = 1.0 kPa). While suspension culture was different from growth on tissue culture plastic and PMA, some minimal physical contact between cells and PHEMA-coated surfaces may still occur. We assumed the effective Young’s moduli E for suspension culture (Susp) as <1 kPa, because the cells would remain rounded and barely spread out.
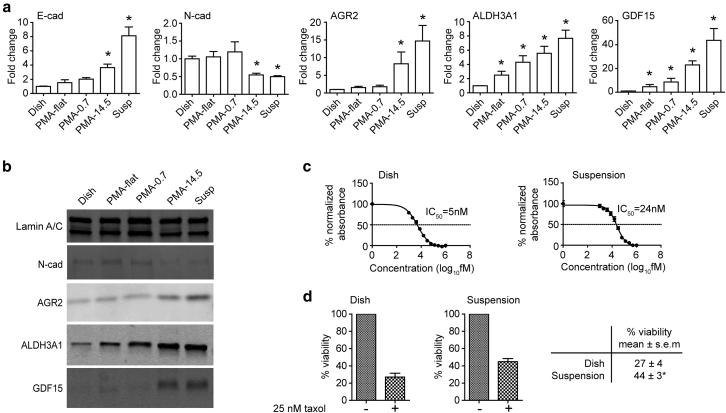
As plasticity of E-cad expression has been associated with a more invasive prostate cancer phenotype (13), we first examined expression of E-cad and N-cad in prostate carcinoma cell lines PC3 and DU145 grown in different culture conditions for 72 h. For PC3 cells, E-cad mRNA expression level increased while N-cad expression decreased under soft and suspension culture conditions (PMA-14.5 and Susp), suggesting phenotypic switch such as MET for prostate cancer cells (Fig. 1 a). Further gene expression analysis of epithelial markers KRT18, MUC-1, and DSP in PC3 cells demonstrated increased expression under PMA-14.5 and Susp conditions (Fig. S1 in the Supporting Material), further supporting the likelihood of ongoing MET under soft or suspension culture environment. However, gene expression analysis of mesenchymal markers such as VIM-1, FN1, and S100A4 (also known as fibroblast-specific protein 1) demonstrated increased expression under soft PMA-14.5 and Susp conditions as well (Fig. S1). These observations suggested that phenotypic switching observed in PC3 cells induced by soft growth conditions was not aligned to a specific canonical MET program, but might involve different signaling pathways simultaneously driving phenotypic changes.
Figure 1.
Mechanotransduction-induced phenotypic transition in PC3 cells. (a) qRT-PCR analysis of E-cad, N-cad, AGR2, ALDH3A1, and GDF15 expression for PC3 cells cultured under different conditions as indicated for 72 h. Rigid adherent substrates included TCP (Dish), flat PDMS (PMA-flat), and PDMS micropost array (PMA) with a post height of 0.7 μm (PMA-0.7). Soft culture conditions included PMA with a post height of 14.5 μm (PMA-14.5) and suspension (Susp) culture. (b) Immunoblot assays of N-cad, AGR2, ALDH3A1, GDF15, and lamin A/C in PC3 cells grown under different conditions as indicated for 72 h. E-cad was undetectable by immunoblot. (c). Paclitaxel IC-50 value determined by nonlinear regression analysis of cell viability curve (measured by WST-1) against concentrations of paclitaxel. PC3 cells cultured on TCP dish and in suspension had IC-50 values of 5 and 24 nM, respectively. (d). Trypan blue exclusion assays to determine viability of PC3 cells grown on TCP dish (27 ± 4%) or in suspension (44 ± 3%) treated with 25 nM paclitaxel for 48 h. Vehicle controls (−) were included for comparison. Data represents the mean ± SE with n = 3. P-values were calculated using Student’s t-test. ∗p < 0.05.
To further examine molecular phenotypic changes induced by soft culture conditions, we assayed several additional markers clinically associated with metastatic prostate cancer differentiation and/or stem cell-like properties such as anterior gradient-2 (AGR2), aldehyde dehydrogenase 3A1 (ALDH3A1), and growth differentiation factor 15 (GDF15) (14, 15, 16). Both mRNA and protein expression levels of AGR2, ALDH3A1, and GDF15 increased under PMA-14.5 and Susp conditions (Fig. 1, a and b), with highest expression in suspension culture, further supporting that mechanotransduction likely activated different phenotypic switching programs in addition to EMT and MET. A similar mechanosensitive response in gene expression to rigid, soft, and suspension culture conditions was observed in DU145 cells (Fig. S2). Given that cells grown on PMA-14.5 shared a similar gene expression profile with those in suspension culture despite the difference between the two growth conditions, we decided to further study the mechanotransductive phenotypic switch in prostate cancer cells under suspension culture as that may be relevant to CTC biology.
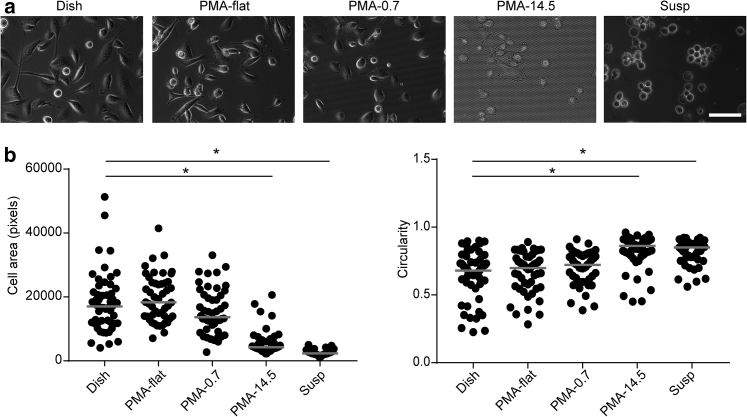
Changes in gene expression profiles in PC3 and DU145 were further accompanied by observable changes in cell morphology for both cell types. Specifically, PC3 cells possessed a larger cell spread area on rigid adherent surfaces (Dish, PMA-flat, and PMA-0.7) but adopted a smaller and rounded morphology on a soft PMA-14.5 surface or in a suspension culture (Fig. 2). A similar change in cell morphology was observed for DU145 cells, although DU145 cells tended to form spheroids on a compliant PMA-14.5 surface as well as in a suspension culture (Fig. S2).
Figure 2.
Effect of mechanotransduction on PC3 cell morphology. (a) Phase images of PC3 cells grown under different conditions as indicated. (b) Cell morphology parameters such as cell area and circularity as a function of culture conditions as indicated. Scale bar, 100 μm. Data represents the mean × SE with n = 50. P-values were calculated using Student’s t-test. ∗p < 0.05.
Mechanotransduction-induced phenotypic switch is associated with sensitivity to paclitaxel
Recent studies have shown phenotypic switching programs such as EMT to be one of the cellular mechanisms crucial for conferring resistance to cancer therapies (17, 18). Because prostate cancer cells grown in suspension demonstrated the highest degree of phenotypic change (as indicated by gene expression) compared with TCP dish, we next examined whether the mechanosensitive phenotypic switching induced by suspension culture might be associated with differential response of prostate cancer cells to paclitaxel treatment. PC3 cells grown in suspension for 24 h before exposed to paclitaxel for an additional 48 h showed a decreased sensitivity to paclitaxel (IC-50 value of 24 nM) compared with the cells grown on a TCP dish (IC-50 value of 5 nM) (Fig. 1 c). Trypan blue exclusion assays were further conducted to measure cell viability after exposure of PC3 cells to 25 nM paclitaxel. PC3 cells grown in suspension for 24 h before being exposed to 25 nM paclitaxel for an additional 48 h had a survival rate (viability) of 44.90 ± 3.52%, significantly greater than the 27.04 ± 4.45% survival rate of PC3 cells grown on TCP dishes (Fig. 1 d).
Mechanotransduction-induced phenotypic switch occurs throughout the cell population
To investigate whether the mechanosensitive phenotypic switch observed in prostate cancer cells occurred throughout the entire cell population or was the result of selecting for subpopulations of cells with distinct E-cad or N-cad expression, PC3 cells cultured in suspension or on TCP dishes were stained with fluorescently labeled anti-E-cad or anti-N-cad antibodies before being analyzed by flow cytometry (Fig. S3). PC3 cells cultured in suspension had ∼1.5-fold higher E-cad expression compared with TCP dish condition, as evidenced by MFI ratio calculations. Consistently, PC3 cells grown in suspension had approximately twofold lower N-cad MFI ratio compared with the TCP dish (Fig. S3). Importantly, changes in E-cad and N-cad expression occurred throughout the entire PC3 cell population as seen by a shift of the entire flow cytometry fluorescence intensity histograms, rather than appearance of distinct cell subpopulations with increased E-cad and decreased N-cad expression (Fig. S3).
Mechanotransduction-induced phenotypic switch is reversible
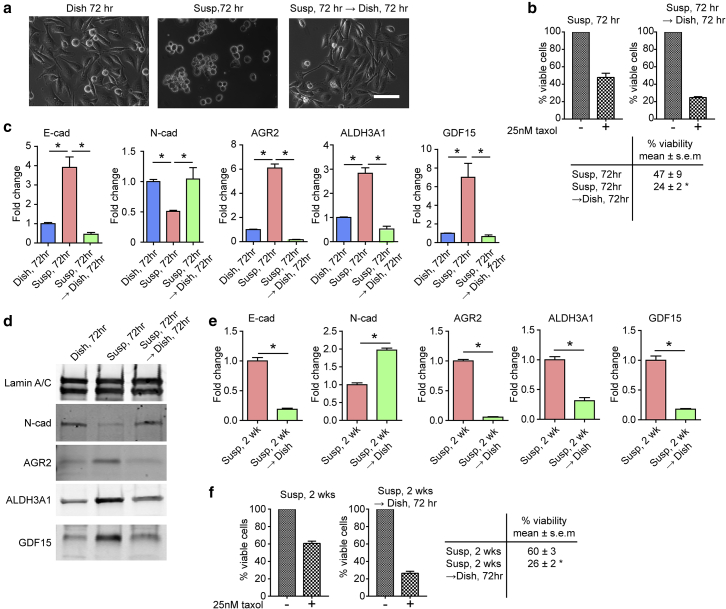
Next, we examined whether a mechanotransduction-induced phenotypic switch in prostate cancer cells was reversible. To this end, PC3 cells grown in suspension for 72 h were seeded back onto TCP dishes and allowed to reattach for an additional 72 h. After 72 h suspension culture, PC3 cells still retained the ability to reattach to TCP dishes with cell morphology comparable with cells directly grown on TCP dishes (Fig. 3 a). In addition, mRNA expression levels of E-cad, N-cad, AGR2, ALDH3A1, and GDF15 in PC3 cells cultured for 72 h in suspension reversed once the cells were allowed to reattach to TCP dishes (Fig. 3 c). Similar observations were made at the protein levels for N-cad, AGR2, ALDH3A1, and GDF15 in PC3 cells using immunoblots (Fig. 3 d), supporting that mechanotransduction-induced phenotypic switch of prostate cancer cells was reversible.
Figure 3.
Mechanosensitive phenotypic transition of PC3 cells is reversible. (a) Phase images of PC3 cells grown under different conditions as indicated for 72 h. After 72 h, PC3 cells in suspension were reseeded back onto the TCP dish and allowed to reattach for another 72 h (Susp → Dish; right). Scale bar, 100 μm. (b) Decreased sensitivity to paclitaxel in suspension culture was reversible when PC3 cells in suspension were allowed to reattach to TCP dish. Control cells were PC3 cells continuously cultured in suspension for 72 h (left). Viability was measured after an incubation with paclitaxel (25 nM) for 48 h using trypan-blue exclusion viability assay. The Susp condition for 72 h showed a viability of 49 ± 9%, while Susp 72 h → Dish 72 h had a viability of 24 ± 2%. (c) qRT-PCR analysis of E-cad, N-cad, AGR2, ALDH3A1, and GDF15 expression for PC3 cells cultured under different conditions as indicated. (d) Immunoblot assays of N-cad, AGR2, ALDH3A1, and GDF15 for PC3 cells cultured under different conditions as indicated. E-cad was not detected by immunoblot. (e) qRT-PCR analysis of E-cad, N-cad, AGR2, ALDH3A1, and GDF15. PC3 cells were cultured in suspension for 2 weeks (Susp, 2 weeks) before being allowed to reattach to TCP dish for 72 h (Susp, 2 weeks → Dish). (f) Decreased sensitivity to paclitaxel observed in Susp was sustainable in long-term suspension culture and remained reversible. PC3 cells grown in suspension for 2 weeks were allowed to reattach to TCP dish for 72 h. Control cells were PC3 cells continuously cultured in suspension for 2 weeks. Viability was measured after an incubation with paclitaxel (25 nM) for 48 h using a trypan-blue exclusion viability assay. Susp 2 weeks had a viability of 60 ± 3%, while Susp 2 weeks → Dish 72 h had a viability of 26 ± 2%. Data represents the mean ± SE with n = 3. P-values were calculated using Student’s t-test. ∗p < 0.05. To see this figure in color, go online.
Importantly, PC3 cells cultured for 72 h in suspension regained sensitivity to paclitaxel once the cells were allowed to reattach to TCP dishes, demonstrating that the molecular and functional phenotypes associated with the mechanotransduction-induced phenotypic switch of prostate cancer cells were both reversible (Fig. 3 b). To further examine whether this phenotypic reversibility (thus cancer cell plasticity) could be retained after long-term suspension culture, PC3 cells were grown in suspension for 2 weeks before being reseeded onto TCP dishes for 72 h. mRNA expression levels of E-cad, N-cad, AGR2, ALDH3A1, and GDF15 in PC3 cells cultured for 2 weeks in suspension reversed once the cells were allowed to reattach to TCP dishes (Fig. 3 e). Consistently, the reversibility of decreased sensitivity to paclitaxel for PC3 cells cultured in suspension was retained even after 2 weeks of culture in suspension (Fig. 3 f), underscoring the plastic nature of prostate cancer cells and its regulation by mechanotransduction.
Mechanotransduction-induced phenotypic switch involves multiple signaling pathways
In breast cancer, mechanotransduction has been demonstrated to play a role in regulating tumorigenesis, as well as in enhancing initiation of the EMT program via mechanosensitive nuclear translocation of TWIST (19). Other important mechanosensitive pathways including YAP and p38MAPK have also been identified in cancer progression. Increasing YAP expression or generating constitutively active YAP mutants have been shown to promote tumor growth and increase invasion, while p38MAPK signaling in cancer cells has been shown to be sensitive to shear stress (20, 21, 22). These examples suggest that mechanotransduction may activate key signaling pathways to drive phenotypic switching of cancer cells.
We specifically investigated different mechanotransductive pathways responsible for inducing the mechanosensitive phenotypic switching observed in prostate cancer cells. YAP signaling in PC3 cells grown under different culture conditions was first analyzed by YAP phosphorylation and subcellular localization. Phosphorylation of YAP, as well as nuclear and cytoplasmic YAP levels, was unchanged in PC3 cells between rigid (Dish, PMA-flat, and PMA-0.7), soft (PMA-14.5), or Susp culture conditions (Fig. S4). Furthermore, inhibition of Smad signaling using SB431542 did not prevent the mechanosensitive phenotypic switch of PC3 cells (Fig. S4).
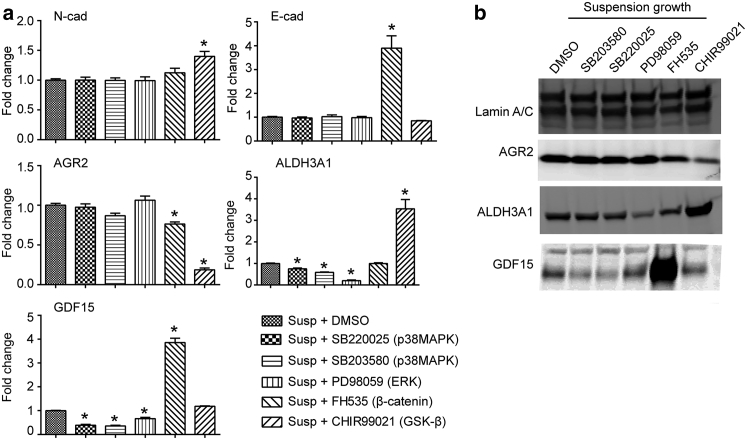
Possible involvement of other mechanotransductive signaling pathways was also examined by treating PC3 cells in suspension with general p38MAPK signaling pathway inhibitors SB203580 and SB220025, ERK inhibitor PD98059, GSK-β inhibitor CHIR99021, and β-catenin inhibitor FH535 (23, 24, 25). qRT-PCR analysis and immunoblot assays showed that inhibition of p38MAPK by SB203580 and SB220025 was only able to rescue GDF15 overexpression, and inhibiting ERK by PD98059 was only able to rescue ALDH3A1 overexpression (Fig. 4). Activating β-catenin with CHIR99021 rescued AGR2 overexpression, while inhibiting β-catenin using FH535 had no significant effect on AGR2 upregulation, although it further increased GDF15 expression (Fig. 4). In addition, activation of β-catenin signaling with CHIR99021 induced further upregulation of ALDH3A1 expression (Fig. 4), in agreement with a previous study suggesting that activation of β-catenin signaling could induce expression of ALDH3A1 (26). However, inhibiting β-catenin in PC3 cells grown in suspension using FH535 was unable to rescue overexpression of ALDH3A1, suggesting that ALDH3A1 upregulation by mechanotransduction was not due to activation of β-catenin signaling, but, at least in part, via the ERK pathway (Fig. 4). Neither upregulation of E-cad nor downregulation of N-cad could be reversed with the inhibitors used (Fig. 4 a). The unique response of each gene to different inhibitors assayed highlights the molecular complexity of mechanosensitive phenotypic switch in prostate cancer cells, which likely involves simultaneous activation of multiple signaling pathways.
Figure 4.
Signaling activation in mechanotransduction-induced phenotypic switch of PC3 cells. (a) qRT-PCR analysis of N-cad, E-cad, AGR2, ALDH3A1, and GDF15 expression in PC3 cells grown in suspension and treated with inhibitors of p38MAPK (SB203580 or SB220025), ERK (PD98059), β-catenin (FH535), and GSK-β (CHIR99021). Vehicle (DMSO) was used as control. (b) Immunoblot assays to detect AGR2, ALDH3A1, and GDF15 protein levels in PC3 cells grown in suspension and treated with SB203580, SB220025, PD98059, FH535, and CHIR99021 as indicated. Data represents the mean ± SE with n = 3. P-values were calculated using Student’s t-test. ∗p < 0.05.
Given the association of ALDH3A1 with stem cell-like properties and thus resistance to paclitaxel, we further examined whether inhibiting ERK using PD98059 (to suppress ALDH3A1 expression) would restore sensitivity of PC3 cells to paclitaxel. However, PC3 cells in suspension treated with ERK inhibitor PD98059 did not restore sensitivity to paclitaxel treatment (Fig. S5).
Discussion
In this study, we report that mechanotransduction can induce a common, reversible phenotypic switching program in phenotypically plastic prostate carcinoma cells PC3 and DU145. This was achieved using three different culture conditions to examine mechanosensitive phenotypic switching of prostate cancer cells: rigid culture surfaces (tissue culture plastic, flat PMA, or PMA-0.7), soft culture surfaces (PMA-14.5), and suspension growth. This mechanosensitive phenotypic switching of prostate cancer cells at the cell population level could be partially characterized by upregulation of E-cad, AGR2, ALDH3A1, and GDF15 and downregulation of N-cad under soft and suspension culture conditions (Figs. 1, a and b and 2). While soft culture surfaces and suspension culture are different culture conditions, they elicited a common phenotypic switch for prostate cancer cells (defined by the affected genes) in this study. However, it is likely that undiscovered phenotypic differences may exist between these two different culture conditions, which is a goal of future work.
The mechanotransduction-induced phenotypic switch of prostate cancer cells consisted of a mix of EMT, MET, and other programs and did not appear to belong to canonical MET or EMT program alone (Fig. S1). This observation suggests mechanotransduction as a probable regulatory mechanism for CTC phenotype. Furthermore, the reversible mechanosensitive phenotypic switch took place within 72 h and could be maintained long term (for at least 2 weeks) without loss of reversibility, an indication of phenotypic plasticity of cancer cells (Fig. 3).
Our study provides new (to our knowledge) insights for reversible phenotypic switching observed in prostate cancer. It is known that metastatic lesions found in prostate cancer patients are capable of reverting back to their original epithelial phenotype, suggesting that phenotypic plasticity of prostate cancer cells is not likely due to irreversible genetic mutations (8). Based on our data, it seems probable that mechanotransduction could play a role in reverting CTCs back to their epithelial phenotype at the metastatic lesions (Fig. 5). Importantly, we observed an association between the mechanosensitive phenotypic switch of prostate cancer cells with decreased sensitivity to paclitaxel (Fig. 1, c and d), suggesting that mechanotransduction may be involved in regulation of drug resistance to taxanes. However, it should be noted that decreased paclitaxel sensitivity for prostate cancer cells cultured in suspension was not solely associated with EMT, as has been suggested previously for CTCs (17, 18). Study of the relationship between mechanotransduction and drug resistance in cancer cells including CTCs will surely lead to new knowledge of the importance of mechanotransduction within clinical cancer settings and will be the focus of future work.
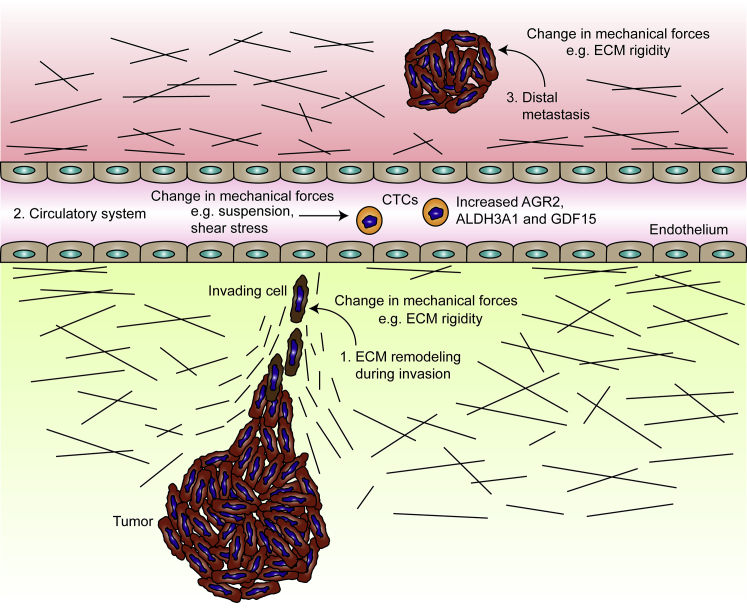
Figure 5.
Mechanotransduction-induced phenotypic changes of cancer cells at various stages of metastasis. (1) ECM remodeling during invasion: Mechanical signals arising from the environment can alter cancer cell phenotype without affecting the genotype under several possible scenarios. Examples include extracellular matrix remodeling during invasion, which can feed back in the form of mechanical signals to regulate cancer cell phenotype. (2) Circulatory system: In the circulatory system, CTCs exist in an environment where they are exposed to mechanical signals not experienced within the local tissue (e.g., shear stress due to blood flow, the absence of a Young’s modulus, as well as a lack of cell attachment). (3) Distal metastasis: Colonization in a distal metastatic site may subject cancer cells to a different mechanical environment than the primary tumor site. Depending on local tissue stiffness of the metastatic site, cancer cells may be induced to revert to their original phenotype via mechanotransduction. To see this figure in color, go online.
There was little phenotypic difference for prostate cancer cells among TCP dish, PMA-flat, and PMA-0.7 conditions, despite a 1000-fold difference in substrate rigidity. This is probably because the effective Young’s moduli E of TCP dish, PMA-flat, and PMA-0.7 are all >1.0 MPa, greater than most organs in the body (except bone). To mount a significant mechanosensitive biological response, a greater difference in mechanical stiffness of the cell culture environment will be needed, as was observed in compliant PMA-14.5.
Mechanistic investigation of phenotypic switching using pathway inhibitors suggested involvement of several different pathways such as activation of p38MAPK and ERK pathways and inhibition of β-catenin activity (Fig. 4). However, YAP signaling did not appear involved in mechanosensitive phenotypic switching of prostate cancer cells. TWIST, a known driver of EMT, has been reported to undergo nuclear translocation under the effect of mechanotransduction to upregulate N-cad and downregulate E-cad (19). However, our findings of a mixed phenotypic shift consisting of both ongoing EMT and MET did not point to a major involvement of TWIST (19). In addition, mechanosensitive TWIST nuclear translocation requires the addition of exogenous transforming growth factor-β (TGF-β), another molecular driver of EMT, which was not supplemented in our study. ALDH3A1 is a known marker for stem cell-like properties (15), rendering it a candidate gene potentially contributing to decreased sensitivity to paclitaxel. However, inhibition of ALDH3A1 in PC3 cells using ERK inhibitors failed to restore sensitivity of PC3 cells to paclitaxel in suspension culture (Fig. S5). This suggests that decreased sensitivity of PC cells to paclitaxel in suspension is not likely due to ALDH3A1 upregulation, and further implies involvement of multiple pathways. Furthermore, changes in E-cad and N-cad expression in PC3 cells cultured in suspension could not be restored using the inhibitors tested in this study (Fig. 4). Thus, a future multifocal approach to simultaneously interrogate different pathways is necessary to discern the exact molecular mechanisms and their complex cross talk and feedback regulation underlying the mechanosensitive phenotypic switch of prostate cancer cells (27).
The novel (to our knowledge) mechanosensitive and plastic nature of AGR2, ALDH3A1, and GDF15 expression in prostate cancer cells reported in this study implies that cancer cells are able to alter expression of these genes depending on mechanical signals from their local culture environment. This further implies that CTCs may undergo a mechanosensitive phenotypic switch as a result of entering the circulation, which confers additional survival advantages subsequently. Based on our data as well as the known functions of these genes, such advantages may include decreased sensitivity to the chemotherapeutic drug paclitaxel, increased aggressiveness of the cancer (AGR2), and the ability of cancer cells to modulate immune response (GDF15) and to adopt stem cell-like properties (ALDH3A1) (14, 15, 16, 26) (Fig. 5). Thus, the potential mechanosensitive properties of these genes would make them suitable as markers of CTCs in prostate cancer. Indeed, elevated levels of AGR2 and GDF15 have both been clinically associated with CTCs (14, 28), and this association can be explained by this study showing that CTCs may be capable of upregulating these genes via mechanotransduction.
It is clear that cancer cells are phenotypically plastic and that mechanotransduction plays a contributing role in determining the phenotype adopted by cancer cells in addition to established genetic, epigenetic, and biochemical perturbations. The mechanosensitive behavior of the genes identified in this study support their prognostic or therapeutic utility in prostate cancer and the characterization of such a mechanosensitive phenotypic switch of prostate cancer cells will be of use for future studies of the mechanobiology of prostate cancer and CTC biology.
Author Contributions
K.A.Y. designed and performed experiments, and wrote the article; Y.S. helped perform experiments and edit the article; S.D.M. helped write the article; and J.F. helped design experiments and write the article.
Acknowledgments
The Lurie Nanofabrication Facility at the University of Michigan, a member of the National Nanotechnology Infrastructure Network funded by the National Science Foundation, is acknowledged for support in microfabrication.
This work is supported by the National Science Foundation (grant nos. CMMI 1129611 and CBET 1149401), the American Heart Association (grant no. 12SDG12180025), and the Department of Mechanical Engineering at the University of Michigan, Ann Arbor.
Editor: Alexander Dunn.
Footnotes
Koh Meng Aw Yong’s present address is Department of Internal Medicine, University of Michigan Health System, Ann Arbor, Michigan.
Yubing Sun’s present address is Department of Mechanical and Industrial Engineering, University of Massachusetts, Amherst, Massachusetts.
Five figures are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(17)30220-5.
Supporting Material
References
- 1.Meacham C.E., Morrison S.J. Tumour heterogeneity and cancer cell plasticity. Nature. 2013;501:328–337. doi: 10.1038/nature12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roesch A., Fukunaga-Kalabis M., Herlyn M. A temporarily distinct subpopulation of slow-cycling melanoma cells is required for continuous tumor growth. Cell. 2010;141:583–594. doi: 10.1016/j.cell.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quintana E., Shackleton M., Morrison S.J. Phenotypic heterogeneity among tumorigenic melanoma cells from patients that is reversible and not hierarchically organized. Cancer Cell. 2010;18:510–523. doi: 10.1016/j.ccr.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mani S.A., Guo W., Weinberg R.A. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsai J.H., Donaher J.L., Yang J. Spatiotemporal regulation of epithelial-mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer Cell. 2012;22:725–736. doi: 10.1016/j.ccr.2012.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tam W.L., Weinberg R.A. The epigenetics of epithelial-mesenchymal plasticity in cancer. Nat. Med. 2013;19:1438–1449. doi: 10.1038/nm.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armstrong A.J., Marengo M.S., Garcia-Blanco M.A. Circulating tumor cells from patients with advanced prostate and breast cancer display both epithelial and mesenchymal markers. Mol. Cancer Res. 2011;9:997–1007. doi: 10.1158/1541-7786.MCR-10-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Marzo A.M., Knudsen B., Epstein J.I. E-cadherin expression as a marker of tumor aggressiveness in routinely processed radical prostatectomy specimens. Urology. 1999;53:707–713. doi: 10.1016/s0090-4295(98)00577-9. [DOI] [PubMed] [Google Scholar]
- 9.Fu J., Wang Y.K., Chen C.S. Mechanical regulation of cell function with geometrically modulated elastomeric substrates. Nat. Methods. 2010;7:733–736. doi: 10.1038/nmeth.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aw Yong K.M., Zeng Y., Getzenberg R.H. Morphological effects on expression of growth differentiation factor 15 (GDF15), a marker of metastasis. J. Cell. Physiol. 2014;229:362–373. doi: 10.1002/jcp.24458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paszek M.J., Zahir N., Weaver V.M. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Baker B.M., Chen C.S. Deconstructing the third dimension: how 3D culture microenvironments alter cellular cues. J. Cell Sci. 2012;125:3015–3024. doi: 10.1242/jcs.079509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bae K.M., Parker N.N., Siemann D.W. E-cadherin plasticity in prostate cancer stem cell invasion. Am. J. Cancer Res. 2011;1:71–84. [PMC free article] [PubMed] [Google Scholar]
- 14.Kani K., Malihi P.D., Gross M.E. Anterior gradient 2 (AGR2): blood-based biomarker elevated in metastatic prostate cancer associated with the neuroendocrine phenotype. Prostate. 2013;73:306–315. doi: 10.1002/pros.22569. [DOI] [PubMed] [Google Scholar]
- 15.Yan J., De Melo J., Tang D. Aldehyde dehydrogenase 3A1 associates with prostate tumorigenesis. Br. J. Cancer. 2014;110:2593–2603. doi: 10.1038/bjc.2014.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kempf T., Zarbock A., Wollert K.C. GDF-15 is an inhibitor of leukocyte integrin activation required for survival after myocardial infarction in mice. Nat. Med. 2011;17:581–588. doi: 10.1038/nm.2354. [DOI] [PubMed] [Google Scholar]
- 17.Fischer K.R., Durrans A., Gao D. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature. 2015;527:472–476. doi: 10.1038/nature15748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng X., Carstens J.L., Kalluri R. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature. 2015;527:525–530. doi: 10.1038/nature16064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei S.C., Fattet L., Yang J. Matrix stiffness drives epithelial-mesenchymal transition and tumour metastasis through a TWIST1-G3BP2 mechanotransduction pathway. Nat. Cell Biol. 2015;17:678–688. doi: 10.1038/ncb3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lamar J.M., Stern P., Hynes R.O. The Hippo pathway target, YAP, promotes metastasis through its TEAD-interaction domain. Proc. Natl. Acad. Sci. USA. 2012;109:E2441–E2450. doi: 10.1073/pnas.1212021109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moroishi T., Hansen C.G., Guan K.L. The emerging roles of YAP and TAZ in cancer. Nat. Rev. Cancer. 2015;15:73–79. doi: 10.1038/nrc3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gee E., Milkiewicz M., Haas T.L. p38 MAPK activity is stimulated by vascular endothelial growth factor receptor 2 activation and is essential for shear stress-induced angiogenesis. J. Cell. Physiol. 2010;222:120–126. doi: 10.1002/jcp.21924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baker A.B., Ettenson D.S., Edelman E.R. Endothelial cells provide feedback control for vascular remodeling through a mechanosensitive autocrine TGF-beta signaling pathway. Circ. Res. 2008;103:289–297. doi: 10.1161/CIRCRESAHA.108.179465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Azuma N., Akasaka N., Sumpio B.E. Role of p38 MAP kinase in endothelial cell alignment induced by fluid shear stress. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H189–H197. doi: 10.1152/ajpheart.2001.280.1.H189. [DOI] [PubMed] [Google Scholar]
- 25.Rotherham M., El Haj A.J. Remote activation of the Wnt/β-catenin signalling pathway using functionalised magnetic particles. PLoS One. 2015;10:e0121761. doi: 10.1371/journal.pone.0121761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calderaro J., Nault J.C., Zucman-Rossi J. ALDH3A1 is overexpressed in a subset of hepatocellular carcinoma characterised by activation of the Wnt/β-catenin pathway. Virchows Arch. 2014;464:53–60. doi: 10.1007/s00428-013-1515-0. [DOI] [PubMed] [Google Scholar]
- 27.da Silva H.B., Amaral E.P., Correa R.G. Dissecting major signaling pathways throughout the development of prostate cancer. Prostate Cancer. 2013;2013:920612. doi: 10.1155/2013/920612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alonso-Alconada L., Muinelo-Romay L., Abal M., ENITEC Consortium Molecular profiling of circulating tumor cells links plasticity to the metastatic process in endometrial cancer. Mol. Cancer. 2014;13:223. doi: 10.1186/1476-4598-13-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.