Abstract
Methotrexate (MTX) is an important component in the therapy used to treat childhood acute lymphoblastic leukemia (ALL). Methylenetetrahydrofolate reductase (MTHFR) is a key enzyme for MTX pharmacokinetics. Two single-nucleotide polymorphisms in MTHFR gene, C677T and A1298C, affecting MTHFR activity, have been widely studied as potential markers of MTX toxicity and/or outcome in pediatric ALL. In this review, we show that the majority of published reports do not find association or present opposite effect. Therefore, MTHFR C677T and A1298C polymorphisms do not seem to be good markers of MTX-related toxicity and/or outcome in pediatric ALL. The efforts should be focused on other genes, such as transporter genes or microRNA-related genes.
Keywords: MTHFR, methotrexate, toxicity, outcome, C677T, A1298C
Introduction
Acute lymphoblastic leukemia (ALL) is the most common type of cancer in children, representing ~30% of all childhood malignancies.1,2 Survival rates have increased dramatically over the last years because of chemotherapy progress, with expected cure rates higher than 80%.3 Methotrexate (MTX) is an important drug used in the treatment protocols for ALL. However, MTX can cause toxicity, leading to a dose reduction or treatment interruption, which could compromise the survival.
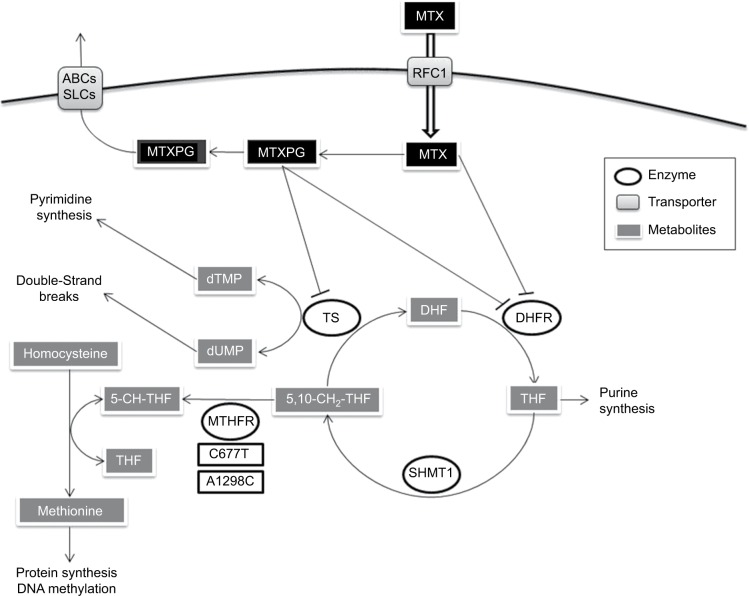
MTX is a folate analog that enters the cell via active transport mediated by the reduced folate carrier (RFC1).4 Then, MTX inhibits dihydrofolate reductase, arresting the folic acid cycle and affecting other important enzymes such as methylenetetrahydrofolate reductase (MTHFR), an enzyme that interferes with nucleic acid synthesis and favors cell death.5 Thus, MTHFR is a key enzyme for intracellular folate homeostasis and metabolism, because it catalyzes the irreversible conversion of 5,10-methylenetetrahydrofolate, required for purine and thymidine synthesis, to 5-methyltetrahydrofolate, required for protein synthesis and nucleic acid methylation. Subsequently, changes in the activity of MTHFR provoking an impaired conversion of 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate could modify folate pools and in turn alter the response of malignant and nonmalignant cells to MTX and influence its toxicity6 (Figure 1).
Figure 1.
MTX pathway.
Note: MTX and its metabolites are indicated.
Abbreviations: ABCs, ABC family transporters; DHF, dihydrofolate; DHFR, dihydrofolate reductase; MTHFR, methylenetetrahydrofolate reductase; MTX, methotrexate; MTXPG, methotrexate polyglutamated forms; RFC1, reduced folate carrier; SHMT1, serine hydromethyl transferase; SLCs, SLC family transporters; THF, tetrahydrofolate; TS, thymidilate synthase; 5-CH-THF, 5-methyltetrahydrofolate; 5,10-CH2-THF, 5,10-methylenetetrahydrofolate.
In this context, two of the MTHFR polymorphisms most widely studied in relation to the toxicity of MTX are C677T (causing Ala222Val) and A1298C (causing Glu429Ala). Both polymorphisms have been associated with reduced enzyme activity. In the case of C677T, 677CT and 677TT individuals exhibit 60% and 30% of the normal MTHFR activity, respectively.7,8 In the case of A1298C, 1298CC individuals show 60% of the normal activity.9 Therefore, the patients carrying the variant alleles might have a higher intolerance to MTX or an increased risk of progression.8
MTX intolerance can manifest through adverse reactions of several organ systems such as hematological (anemia, thrombocytopenia, leukopenia, neutropenia), gastrointestinal (mucositis), hepatobiliary, urogenital, or central nervous system.10 Also, MTX plasma levels are used as an objective MTX-related toxicity marker.11 In some patients, the toxic effects are so serious that the dose must be cut down or the treatment paused, which besides the problems related to toxicity, can also have a negative impact on survival.12
As we have mentioned herein, variants that alter MTHFR activity may increase the availability of 5,10-methylenetetrahydrofolate and decrease 5-methyltetrahydrofolate. The reduction of this last would lead to DNA hypomethylation, which could invoke carcinogenesis through three different mechanisms: chromosomal instability, reactivation of elements, and loss of imprinting.13 The loss of methylation might favor mitotic recombination, leading to loss of heterozygosity as well as promoting karyotypically detectable rearrangements. Intragenomic parasitic DNA and other previously silent transposons may then be transcribed and even “jump” to other parts of the genome, leading to disruption of normal genes. Hypomethylation can likewise affect imprinted genes, which have been shown to contribute to carcinogenesis. Finally, genomic DNA hypomethylation can also increase through all the tumorigenic steps,14 from the benign proliferations to the invasive cancers.13 Therefore, decreased MTHFR activity due to 677T or 1298C alleles could induce hypomethylation, and all these modifications can lead to an increase in carcinogenesis.
To date, several groups have investigated the potential role of MTHFR polymorphisms in relation to the toxicity of MTX, as well as outcome in pediatric ALL, but the conclusions remain controversial. Some studies do not find association, whereas others present opposite effect. In consequence, we consider that it would be interesting to clarify these discrepancies.
Toxicity and MTHFR C677T and A1298C polymorphisms
In 2013, our group performed an exhaustive search to identify studies that examined the association between the C677T and A1298C polymorphisms of MTHFR and MTX toxicity in pediatric ALL patients. We used the keywords and subject terms “MTHFR and acute leukemia”, and “MTHFR and polymorphism(s) and toxicity” to search PubMed (www.ncbi.nlm.nih.gov/pubmed) for articles published through November 2011. We also carried out a meta-analysis with those articles supplying enough information on toxicity by genotype. The study was performed in a population composed only of pediatric ALL patients for short-term toxic effects including (MTX plasma levels, mucositis, hepatic toxicity, neutropenia, thrombocytopenia, anemia, and leucopenia).
The meta-analysis included 24 studies for C677T and 16 studies for A1298C. For C677T, none of the analysis revealed a statistically significant association with toxicity. In the case of A1298C, only a slight protective effect of 1298CC genotype for leukopenia was observed.15 Therefore, we concluded that there was no evidence to support the use of either the MTHFR C677T or the A1298C single-nucleotide polymorphisms (SNPs) as MTX toxicity markers in pediatric ALL patients, and consequently the MTX dose should not be adjusted based on these variants.
At the same time, Yang et al16 performed another meta-analysis studying the effect of C677T and A1298C MTHFR polymorphisms on MTX-induced myelosuppression, oral mucositis, liver, hematological, gastrointestinal, and neurological toxicities. They only found a slight association between C677T and gastrointestinal toxicity in pediatric ALL. None of the polymorphisms was associated to any of the other studied toxicities, so again in this study, neither C667T nor A1298C were proved to be good toxicity markers for MTX dose reduction.
In spite of all this evidence, new studies are being published every year analyzing the involvement of MTHFR C677T and A1298C polymorphisms in MTX toxicity in pediatric ALL treatment. Herein, we have performed an exhaustive search to identify new studies that explore the association between the C677T and A1298C polymorphisms of MTHFR and MTX-induced toxicity from November 2011 to November 2016, following the same strategy as before.15 Keywords “MTHFR” and “acute lymphoblastic leukemia” for PubMed database were used.
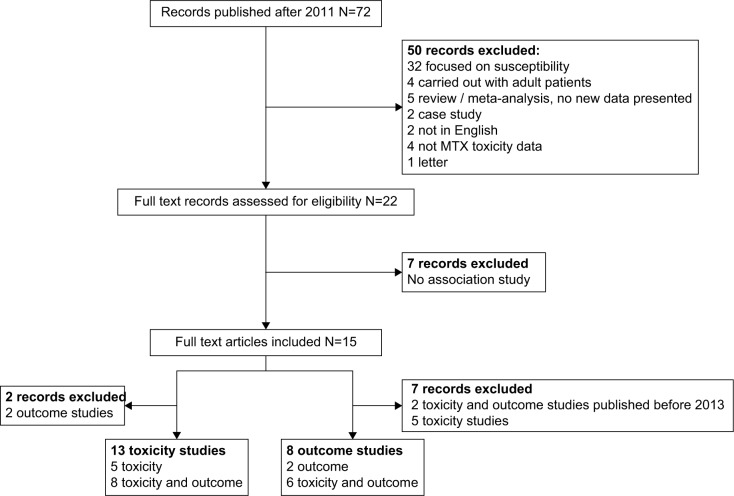
The search provided 186 records, 114 of them were directly discarded because they were published before 2011. From the remaining 72 papers, after abstract screening, 50 were excluded for not analyzing C677T and A1298C polymorphisms in pediatric ALL. For the other 22, full lecture was performed. Seven out of 22 articles were discarded, 4 because they were not focused on the analysis of C677T and A1298C polymorphism and MTX-induced toxicities,17–20 and other 3 because they analyzed the effect of the C677T/A1298C haplotype in MTX-related toxicity and each genotype could not be evaluated separately.21–23 We finally reviewed 15 articles that analyzed the MTHFR polymorphisms in relation to MTX-induced toxicities in pediatric ALL patients. Of the 15 reviewed papers, 2 were discarded for analyzing only outcome, and 13 studies that analyzed the association with MTX induced toxicities remained. The search and study selection process are shown in Figure 2.
Figure 2.
Flow diagram of study selection.
Abbreviation: MTX, methotrexate.
C677T polymorphism analysis
The 13 selected articles analyzed the C677T polymorphism in relation to MTX-induced toxicities. From them, only 5 found significant associations with toxicity, but with contradictory results: 2 studies related the T variant allele to a decrease in toxicity risk,24,25 whereas the other 3 associated it to increased risk (Table 1).26–28
Table 1.
List of 13 studies that analyzed association between the MTHFR C677T polymorphism and MTX toxicity in pediatric ALL, grouped according to the level of association between the SNP and MTX toxicity
|
MTHFR C677T
| ||||
|---|---|---|---|---|
| Patient population | MTX dose | Population | Association with toxicity | Reference, year |
| 21 ALLC | High | Indian | NA | Moulik et al,31 2016 |
| 106 ALLC | High | Turkish | NA | Yazıcıoğlu et al, 32 2016 |
| 56 ALL or LymphomaA | High | Japanese | NA | Tsujimoto et al,47 2016 |
| 53 ALLA | Low | Japanese | NA | Suzuki et al,30 2015 |
| 91 ALLC | High | Chinese | NA | Wang et al,48 2014 |
| 499 ALLA | High | German | NA | Radtke et al,39 2013 |
| 18 ALLC | High | Brazilian | NA | de Deus et al,49 2012 |
| 100 ALLA | ND | Korean | NA | Kim et al,50 2012 |
| 109 ALLC | High | Mexican | −T | Ramírez-Pacheco et al,24 2016 |
| 35 ALLB | High | Caucasian | −T | Haase et al,25 2012 |
| 161 ALLC | High | Argentinian | +T | Aráoz et al,26 2015 |
| 127 ALLC | High | Lebanese | +T | Zgheib et al,27 2014 |
| 103 ALL and NHLA | High | Japanese | +T | Fukushima et al,28 2013 |
Notes: Type of sample: A, normal; B, tumor; C, unknown. High MTX dose =1.5–5 g m−2; Low MTX dose =25 mg m−2.
Abbreviations: MTHFR, methylenetetrahydrofolate reductase; SNP, single-nucleotide polymorphism; MTX, methotrexate; ALL, acute lymphoblastic leukemia; NHL, non-Hodgkin Lymphoma; ND, no data; NA, no association between SNP and toxicity; +T, SNP is associated with increased toxicity; −T, SNP is associated with decreased toxicity.
When we analyzed those studies in depth, we again found discordant results (Table 2). The association study between MTX pharmacokinetics and C677T polymorphism showed that 7 out of 8 studies did not find significant association. Haase et al25 was the only study in which MTX levels were significantly increased in patients with the MTHFR C677T wild type compared to CT/TT genotype variant carriers. However, the number of patients included in this study was very low (n=35). In our previous meta-analysis, only one study found association, but the result was just the opposite.29 Until now, taking into account our previous meta-analysis and the studies included in this review, a total of 13 studies have been performed about MTX pharmacokinetics, 11 with no association, and 2 with association but with opposite results. Therefore, C677T could not be considered as a good marker for MTX pharmacokinetics.
Table 2.
Types of toxicities analyzed and the findings in each study of the association between the MTHFR C677T polymorphism and MTX toxicity
|
MTHFR C677T
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Reference, year | MTX kinetics | Hematologic toxicity
|
Hepatic toxicity | Mucositis | Renal toxicity | Neurotoxicity | Other | |||
| Anemia | Thrombocytopenia | Leukopenia | Neutropenia | |||||||
| Moulik et al,31 2016 | – | NA | NA | – | NA | NA | NA | NA | – | NAa,b |
| Yazıcıoğlu et al,32 2016 | NAc | NA | NA | NA | NA | NA | NA | NA | NA | – |
| Tsujimoto et al,47 2016 | – | – | – | – | – | – | – | – | NA | – |
| Suzuki et al,30 2015 | – | – | – | NA | – | NA | – | – | – | NAb |
| Wang et al,48 2014 | NAd,e | – | – | – | – | – | – | – | – | – |
| Radtke et al,39 2013 | NAf,g,h | – | – | – | – | – | NA | – | – | – |
| de Deus et al,49 2012 | NAc | – | – | – | – | NA | – | NA | – | – |
| Kim et al,50 2012 | – | – | – | – | – | NA | – | – | – | NA b,i |
| Ramírez-Pachecoet al,24 2016 | NAc | – | – | NA | – | – | −T | – | – | – |
| Haase et al,25 2012 | −Tc | −T | – | −T | – | – | – | – | – | −Tj |
| Aráoz et al,26 2015 | – | NA | NA | +T | +T | NA | NA | – | – | – |
| Zgheib et al,27 2014 | NAf,g,k | +T | – | – | NA | – | – | – | – | NAb |
| Fukushima et al,28 2013 | NAc | – | – | – | – | +T | – | – | – | +Tl |
Notes: Identifiers:
febrile neutropenia;
MTX dose reduction;
MTX levels;
dose corrected MTX level;
percent of MTX >40 mmol/L;
MTX clearance;
MTX AUC;
peak MTX plasma level;
sepsis;
rate of cycles with infection;
time to reach MTX level <0,1 mmol/L;
MTX administration delay; –, not evaluated.
Abbreviations: MTHFR, methylenetetrahydrofolate reductase; SNP, single-nucleotide polymorphism; MTX, methotrexate; NA, no association between SNP and toxicity; +T, SNP is associated with increased toxicity; −T, SNP is associated with decreased toxicity.
With regard to hematological toxicity, when anemia was analyzed, only 2 out of 5 studies found association. Haase et al25 showed that the variant of C677T was associated with a decreased toxicity, whereas Zgheib et al27 found the opposite effect. The other 3 studies found no association with anemia (Table 2). Contradictory results were also seen for leukopenia, for which Haase et al25 showed that the C677T T variant allele was associated with a decreased toxicity, whereas Aráoz et al26 found that this allele increased the risk of severe leukopenia, but only in carriers who received 2 g/m2 of MTX. However, Aráoz et al26 also observed that the 677T allele did not seem to modulate the presence of severe adverse events in patients who received 5 g/m2 of MTX. The analysis of leukopenia in the other 3 studies showed no association with toxicity (Table 2). Neutropenia was analyzed in 4 studies. Only the study of Aráoz et al26 found risk 677T allele associated to neutropenia in patients who received low doses of MTX (2 g/m2). None of the other 3 studies found association. Again, these results are in line with the results of our previous meta-analysis in which we proposed that C677T polymorphism does not seem to be a good marker for hematological toxicity.
Hepatotoxicity was analyzed in 7 studies, and only the study of Fukushima et al28 found significant association for 677CT/TT carriers. This study was conducted in a cohort of 103 Japanese patients, that mixed ALL (n=82) and non-Hodgkin lymphoma (NHL) (N=21) patients. In contrast, another study carried out in another Japanese population found no significant association.30 The other 5 studies performed in other populations found no significant association either. Once more, these results are in line with the results found by our group in the previous review, in which 13 studies out of 16 were not significant.
Mucositis was analyzed in 5 studies, and only Ramírez-Pacheco et al24 concluded that CC genotype was associated with a higher risk of developing mucositis in a cohort of 109 children with ALL. The rest of the 4 studies found no association, in line with our meta-analysis results in which 8 out of 10 studies were not significant.
Finally, for thrombocytopenia and renal toxicity with 3 studies and neurotoxicity with 2 studies, none of them found significant association. Once more, these results were in agreement with the previous ones.
In conclusion, after analyzing thoroughly a total of 37 articles and 4,583 patients (24 studies with 3,104 patients from the previous meta-analysis and 13 studies with 1,479 patients from our actual review) and considering that the majority of studies were not significant, the significant results were often contradictory or with low statistical power, we can conclude that C677T is not a good MTX toxicity marker in pediatric ALL.
A1298C polymorphism analysis
Of the 13 articles reviewed, 10 analyzed the association of A1298C polymorphism with MTX-induced toxicity (Table 3). From them, only 3 studies found significant association. Moulik et al31 and Haase et al25 showed the C variant allele favoring an increase in toxicity, whereas Fukushima et al28 showed a protective role for the C variant allele. When the different toxicities were considered, almost all the results found no significant associations (Table 4).
Table 3.
List of 10 studies that analyzed association between the MTHFR A1298C polymorphism and MTX toxicity in pediatric ALL, grouped according to the level of association between the SNP and MTX toxicity
|
MTHFR A1298C
| ||||
|---|---|---|---|---|
| Patient population | MTX dose | Population | Association with toxicity | Reference, year |
| 106 ALLC | High | Turkish | NA | Yazıcıoğlu et al,32 2016 |
| 53 ALLA | Low | Japanese | NA | Suzuki et al,30 2015 |
| 161 ALLC | High | Argentinian | NA | Aráoz et al,26 2015 |
| 127 ALLC | High | Lebanese | NA | Zgheib et al,27 2014 |
| 499 ALLA | High | German | NA | Radtke et al,39 2013 |
| 18 ALLC | High | Brazilian | NA | de Deus et al,49 2012 |
| 100 ALLA | ND | Korean | NA | Kim et al,50 2012 |
| 103 ALL and NHLA | High | Japanese | −T | Fukushima et al,28 2013 |
| 21 ALLC | High | Indian | +T | Moulik et al,31 2016 |
| 35 ALLB | High | Caucasian | +T | Haase et al,25 2012 |
Notes: Type of sample: A, normal; B, tumor; C unknown. High MTX dose =1.5–5 g m−2; Low MTX dose =25 mg m−2.
Abbreviations: MTHFR, methylenetetrahydrofolate reductase; SNP, single-nucleotide polymorphism; MTX, methotrexate; ALL, acute lymphoblastic leukemia; NHL, non-Hodgkin Lymphoma; ND, no data; NA, no association between SNP and toxicity; +T, SNP is associated with increased toxicity; −T, SNP is associated with decreased toxicity.
Table 4.
Types of toxicities analyzed and the findings in each study of the associations between the MTHFR A1298C polymorphism and MTX toxicity
|
MTHFR A1298C
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Reference, year | MTX kinetics | Hematologic toxicity
|
Hepatic toxicity | Mucositis | Renal toxicity | Neurotoxicity | Other | |||
| Anemia | Thrombocytopenia | Leukopenia | Neutropenia | |||||||
| Yazıcıoğlu et al,32 2016 | NAa | NA | NA | NA | NA | NA | NA | NA | NA | – |
| Suzuki et al,30 2015 | – | – | – | NA | – | NA | – | – | – | NAb |
| Aráozetal,26 2015 | – | NA | NA | NA | NA | NA | NA | – | – | – |
| Zgheib et al 201427 | NAc,d,e | NA | – | – | NA | – | – | – | – | NAb |
| Radtkeetal,352013 | NAc,d,f | – | – | – | – | – | NA | – | – | – |
| de Deus et al,45 2012 | NAa | – | – | – | – | NA | – | NA | – | – |
| Kim et al,50 2012 | – | – | – | – | – | NA | – | – | – | NAb,g |
| Fukushima et al,28 2013 | NAa | – | – | – | – | -T | – | – | – | NAh |
| Mouliketal,31 2016 | – | +T | NA | – | NA | NA | NA | NA | – | NAi,b |
| Haaseetal,25 2012 | NAa | +T | – | NA | – | – | – | – | – | NAj |
Notes: Identifiers:
MTX levels;
MTX dose reduction;
MTX clearance;
MTX AUC;
time to reach MTX level <0,1 mmol/L;
peak MTX plasma level;
sepsis;
MTX administration delay;
febrile neutropenia;
rate of cycles with infection. –, not evaluated.
Abbreviations: MTHFR, methylenetetrahydrofolate reductase; SNP, single-nucleotide polymorphism; MTX, methotrexate; NA, no association between the SNP and toxicity; +T, SNP is associated with increased toxicity; −T, SNP is associated with decreased toxicity.
Regarding anemia, 3 of 5 studies found no significant results, whereas both Moulik et al31 and Haase et al25 found association with an increase in toxicity for patients carrying the CC genotype. These studies included a low number of patients (n=21 and n=35, respectively), whereas the studies that did not result in significance were performed in cohorts of 106,32 161,26 and 12727 patients. Since our previous review found no association in 5 of the 6 studies that analyzed anemia and the one that showed association was performed in a population of 37 patients,33 we can conclude that A1298C variant is not a good marker for anemia.
Hepatotoxicity was analyzed in 7 studies, only Fukushima et al28 showed significant results in their cohort of children, which as previously mentioned, mixed 82 ALL patients and 21 NHL patients. In this study, 1298CC genotype patients presented a lower hepatic toxicity risk comparing to the 1298AA genotype patients. The other 6 studies did not find association between the polymorphism and hepatic toxicity. These results agree with our previous meta-analysis where 8 out of 10 studies neither found significant association. Moreover, the 2 studies that showed association in the previous meta-analysis found the opposite effect for the variant C allele that increased hepatic toxicity.33,34
For MTX pharmacokinetics and A1298C since November 2011, 6 new studies have been performed, but significant results have not been detected in any of them. These results in combination with the results from our previous meta-analysis, where only one study conducted in a population of 37 patients obtained significant results,33 give us a total confirmation of no association between the A1298C polymorphism and MTX pharmacokinetics.
None of the other analyzed toxicities (leukopenia, neutropenia, and mucositis with 4 studies; thrombocytopenia and renal toxicity with 3 studies; and neurotoxicity with 1 study) showed statistically significant results (Table 4).
The results from 26 articles and 3,546 patients (16 studies with 2,323 patients from the previous meta-analysis and 10 studies with 1,223 patients from our actual review) makes us reject the MTHFR A1298C polymorphism as a MTX toxicity marker.
To sum up, in these 13 new studies analyzing C677T and A1298C and MTX-induced toxicities, we have confirmed our previous results. Most of the studies concluded that there was no association between MTHFR polymorphisms and MTX toxicity, and in those few studies with positive associations, opposite effects were often observed. Therefore, we consider that MTHFR C677T and A1298C polymorphisms are not good toxicity markers in pediatric ALL, and we think that no further studies are needed in this line.
Outcome and MTHFR C677T and A1298C polymorphisms
In 2014, He et al35 published the results of a meta-analysis that studied the relationship between the MTHFR C677T and A1298C polymorphisms and ALL relapse risk. They conducted a literature search of PubMed (www.ncbi.nlm.nih.gov/pubmed) and Web of Knowledge (http://isiknowledge.com/) using the following keywords and subject terms: “acute lymphoblastic leukemia and relapse and polymorphism (variant)” and “methylenetetrahydrofolate reductase (MTHFR) and acute lymphoblastic leukemia and relapse”, last updated on September 2013. The association between the two SNPs and ALL relapse was evaluated in childhood ALL patients. In He et al’s35 work, the meta-analysis for childhood ALL was performed with 6 studies for C677T (n=1,553) and with 3 studies for A1298C (n=711). Significant association was only found for C677T. According to their results, the relapse risk in pediatric ALL was higher for the 677TT genotype than for the CT/CC genotypes.
Simultaneously, Ojha and Gurney36 published a systematic review on the association between MTHFR C677T and overall survival in pediatric ALL (n=909). The search included literature through March 2013, and the review was based on 6 studies, 3 of them already included on He et al’ s,35 meta-analysis. As expected, they reached a similar conclusion, individuals with MTHFR 677T variant showed a higher relative risk of pediatric ALL mortality, with greater statistical support for the genotype MTHFR 677TT.
Herein, we performed a new review of the literature published after the mentioned two reviews, from September 2013 to November 2016, to assess the relationship between childhood ALL outcome and MTHFR C677T and A1298C polymorphisms. Keywords “MTHFR” and “acute lymphoblastic leukemia” for PubMed database were used.
The study selection process, previously described for toxicity studies, was the same for outcome in the first steps. Out of the 15 articles selected for complete lecture, 7 were discarded, 2 for being published before 2013 and 5 for not studying outcome. Finally, we analyzed 8 articles that studied the MTHFR polymorphisms in relation to pediatric ALL patients outcome (Figure 2).
C677T polymorphism analysis
From 2013, eight studies that included 1,353 pediatric ALL patients have analyzed the association between the C677T polymorphism and outcome (relapse, death, or secondary malignancy). None of the new eight studies showed significant association between outcome and C677T variant (Table 5). These results differ from the previously mentioned reviews that found association between increased relapse/mortality risk and 677TT genotype. Nevertheless, in the He et al’s,35 meta-analysis, the significant result is based only in two studies (D’Angelo et al37 and Tantawy et al38) from the 6 studies included. Taking all these results in consideration, we cannot affirm that MTHFR C677T polymorphism is a good outcome marker for pediatric ALL.
Table 5.
List of 8 studies that analyzed association between the MTHFR C677T polymorphism and outcome
|
MTHFR C677T
| |||||
|---|---|---|---|---|---|
| Patient population | MTX dose | Population | Association with outcome | Analyzed event | Reference, year |
| 140 ALLA | High | Argentinian | NA | EFSa | Leonardi et al,51 2016 |
| 109 ALLC | High | Mexican | NA | Relapse | Ramírez-Pacheco et al,24 2016 |
| 106 ALLC | High | Turkish | NA | EFSb | Yazıcıoğlu et al,32 2016 |
| 202 ALLC | High | Argentinian | NA | EFSc | Aráoz et al,26 2015 |
| 94 ALLC/141 ALLC | High | Caucasian/Vietnamese | NA | EFSa | Hoang et al,52 2015 |
| 103 ALL and NHLA | High | Japanese | NA | EFSb | Fukushima et al,28 2013 |
| 499 ALLA | High | German | NA | EFS c | Radtke et al,39 2013 |
| 53 ALLA | Low | Japanese | NA | Relapse | Suzuki et al,30 2015 |
Notes: Type of sample: A, normal; C, unknown. High MTX dose =1.5–5 g m−2; Low MTX dose =25 mg m−2. Identifiers:
event defined as relapse;
event defined as relapse/death;
event defined as relapse/death/secondary malignancies.
Abbreviations: MTHFR, methylenetetrahydrofolate reductase; SNP, single-nucleotide polymorphism; MTX, methotrexate; ALL, acute lymphoblastic leukemia; NHL, non-Hodgkin Lymphoma; NA, no association between the SNP and outcome; EFS, event free survival.
A1298C polymorphism analysis
In the case of A1298C, six new studies that included 1,104 pediatric ALL patients analyzed the association with outcome (relapse, death, or secondary malignancy). From them, 2 studies showed significant results but with opposite effect. Fukushima et al28 demonstrated that individuals with AC-CC genotypes presented increased Event Free Survival (EFS) (versus AA genotype), whereas Radtke et al’s39 study found that individuals with AC or CC genotypes showed decreased EFS (versus AA genotype) (Table 6). The other 4 studies showed no association between A1298C and outcome, which is in line with the previously published meta-analysis of He et al35 and other 3 studies. All these results together suggest that MTHFR A1298C polymorphism is not a good predictor for outcome in childhood ALL.
Table 6.
List of 6 studies that analyzed association between the MTHFR A1298C polymorphism and outcome
|
MTHFR A1298C
| |||||
|---|---|---|---|---|---|
| Patient population | MTX dose | Population | Association with outcome | Analyzed event | Reference, year |
| 106 ALLC | High | Turkish | NA | EFSa | Yazıcıoğlu et al,32 2016 |
| 202 ALLC | High | Argentinian | NA | EFSb | Aráoz et al,26 2015 |
| 94 ALLC/141 ALLC | High | Caucasian/Vietnamese | NA | EFSc | Hoang et al,52 2015 |
| 53 ALLA | Low | Japanese | NA | Relapse | Suzuki et al,30 2015 |
| 499 ALLA | High | German | −AS | EFSc | Radtke et al,39 2013 |
| 103 ALL and NHLA | High | Japanese | +AS | EFSb | Fukushima et al,28 2013 |
Notes: Type of sample: A, normal; C, unknown. High MTX dose =1.5–5 g m−2; Low MTX dose =25 mg m−2. Identifiers:
event defined as relapse/death;
event defined as relapse/death/secondary malignancies;
event defined as relapse.
Abbreviations: MTHFR, methylenetetrahydrofolate reductase; SNP, single-nucleotide polymorphism; MTX, methotrexate; ALL, acute lymphoblastic leukemia; NHL, non-Hodgkin Lymphoma; NA, no association between the SNP and outcome; +AS, SNP is associated to increased outcome; −AS, SNP is associated with decreased outcome; EFS, event free survival.
In conclusion, our review indicates that MTHFR C677T and A1298C polymorphisms cannot be considered as outcome markers for childhood ALL.
Conclusion
Numerous studies have been performed analyzing the relationship between the C677T and A1298C polymorphisms of MTHFR and MTX toxicity and/or outcome in pediatric ALL. The majority of these studies does not find association or present opposite effect. As a result, MTHFR C677T and A1298C polymorphisms cannot be considered as toxicity or outcome markers for childhood ALL. Therefore, the efforts should be focused on other genes. For instance, interesting and robust results have been obtained in regards to transporter genes and MTX toxicity. In fact, Treviño et al40 performed a genome-wide association study in patients with ALL and found rs4149081 and rs11045879 in SLCO1B1 strongly associated for the first time with MTX clearance, and this association was widely confirmed by subsequent studies.11,39,41 As a result, other works have focused their interest on the analysis of polymorphism in MTX transporters, finding several SNPs in genes such as SLC19A1, ABCC4, or ABCC2 also associated with MTX levels and other toxicities.42–44
Additionally, miRNA-related SNPs interfering with miRNA levels or function may lead to drug resistance/sensitivity. Since miRNA expression can be exogenously controlled by blocking the expression of upregulated miRNAs or by restoring the expression of downregulated miRNAs, this field seems very promising in pharmacogenetics. Indeed, our group has detected 3 SNPs in miR-5189, miR-595, and miR-453 that might affect SLC46A1, SLC19A1, SLCO1A2, and ABCC4 MTX transport genes regulation and could affect MTX levels in patients with pediatric B-ALL.45,46
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Koppen I, Hermans F, Kaspers G. Folate related gene polymorphisms and susceptibility to develop childhood acute lymphoblastic leukaemia. Br J Haematol. 2009;148:3–14. doi: 10.1111/j.1365-2141.2009.07898.x. [DOI] [PubMed] [Google Scholar]
- 2.Johnston WT, Lightfoot TJ, Simpson J, Roman E. Childhood cancer survival : a report from the United Kingdom Childhood Cancer Study. Cancer Epidemiol. 2010;34(6):659–666. doi: 10.1016/j.canep.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 3.Pui C, Robison L, Look A. Acute lymphoblastic leukaemia. Lancet. 2008;371:1030–1043. doi: 10.1016/S0140-6736(08)60457-2. [DOI] [PubMed] [Google Scholar]
- 4.Gorlick R, Goker E, Trippett R, Waltham M, Banerjee D, Bertino J. Intrinsic and acquired resistance to methotrexate in acute leukemia. N Engl J Med. 1996;335:1041–1048. doi: 10.1056/NEJM199610033351408. [DOI] [PubMed] [Google Scholar]
- 5.Krajinovic M, Moghrabi A. Pharmacogenetics of methotrexate. Pharmacogenomics. 2004;5(7):819–834. doi: 10.1517/14622416.5.7.819. [DOI] [PubMed] [Google Scholar]
- 6.De Mattia E, Toffoli G. C677T and A1298C MTHFR polymorphisms, a challenge for antifolate and fluoropyrimidine-based therapy personalisation. Eur J Cancer. 2009;45(8):1333–1351. doi: 10.1016/j.ejca.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Cheok M, Evans W. Acute lymphoblastic leukaemia: a model for the pharmacogenomics of cancer therapy. Nat Rev Cancer. 2006;6(2):117–130. doi: 10.1038/nrc1800. [DOI] [PubMed] [Google Scholar]
- 8.Frosst P, Blom H, Milos R, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995;10(1):111–113. doi: 10.1038/ng0595-111. [DOI] [PubMed] [Google Scholar]
- 9.Weisberg I, Jacques P, Selhub J, et al. The 1298A/C polymorphism in methylenetetrahydrofolate reductase (MTHFR): in vitro expression and association with homocysteine. Atherosclerosis. 2001;156(2):409–415. doi: 10.1016/s0021-9150(00)00671-7. [DOI] [PubMed] [Google Scholar]
- 10.Niedzielska E, Węcławek-Tompol J, Matkowska-Kocjan A, Chybicka A. The influence of genetic RFC1, MS and MTHFR polymorphisms on the risk of acute lymphoblastic leukemia relapse in children and the adverse effects of methotrexate. Adv Clin Exp Med. 2013;22(4):579–584. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23986219. [PubMed] [Google Scholar]
- 11.Lopez-Lopez E, Martin-Guerrero I, Ballesteros J, et al. Polymorphisms of the SLCO1B1 gene predict methotrexate-related toxicity in childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2011;57(4):612–619. doi: 10.1002/pbc.23074. [DOI] [PubMed] [Google Scholar]
- 12.Lopez-Lopez E, Gutierrez-Camino A, Bilbao-Aldaiturriaga N, Pombar-Gomez M, Martin-Guerrero I, Garcia-Orad A. Pharmacogenetics of childhood acute lymphoblastic leukemia. Pharmacogenomics. 2014;15(10):1383–1398. doi: 10.2217/pgs.14.106. [DOI] [PubMed] [Google Scholar]
- 13.Esteller M. Aberrant DNA methylation as a cancer-inducing mechanism. Annu Rev Pharmacol Toxicol. 2005;45:629–656. doi: 10.1146/annurev.pharmtox.45.120403.095832. [DOI] [PubMed] [Google Scholar]
- 14.Abe M, Ohira M, Kaneda A, et al. CpG island methylator phenotype is a strong determinant of poor prognosis in neuroblastomas. Cancer Res. 2005;65(3):828–834. [PubMed] [Google Scholar]
- 15.Lopez-Lopez E, Martin-Guerrero I, Ballesteros J, Garcia-Orad A. A systematic review and meta-analysis of MTHFR polymorphisms in methotrexate toxicity prediction in pediatric acute lymphoblastic leukemia. Pharmacogenomics J. 2013;13(6):498–506. doi: 10.1038/tpj.2012.44. [DOI] [PubMed] [Google Scholar]
- 16.Yang L, Hu X, Xu L. Impact of methylenetetrahydrofolate reductase (MTHFR) polymorphisms on methotrexate-induced toxicities in acute lymphoblastic leukemia: a meta-analysis. Tumor Biol. 2012;677:1445–1454. doi: 10.1007/s13277-012-0395-2. [DOI] [PubMed] [Google Scholar]
- 17.Yanagimachi M, Goto H, Kaneko T, et al. Influence of pre-hydration and pharmacogenetics on plasma methotrexate concentration and renal dysfunction following high-dose methotrexate therapy. Int J Hematol. 2013;98(6):702–707. doi: 10.1007/s12185-013-1464-z. [DOI] [PubMed] [Google Scholar]
- 18.Torun YA, Patiroglu T, Ozdemir MA, Ozkul Y, Ekici A, Karakukcu M. Inherited prothrombotic risk factors in Turkish children with acute lymphoblastic leukemia: significance of concomitant genetic mutation. Clin Appl Thromb Hemost. 2012;18(2):218–221. doi: 10.1177/1076029611412366. [DOI] [PubMed] [Google Scholar]
- 19.Sivaslioglu S, Gursel T, Kocak U, Kaya Z. The risk factors for thrombosis in children with acute lymphoblastic leukemia. Clin Appl Thromb Hemost. 2014;20(6):651–653. doi: 10.1177/1076029612475022. [DOI] [PubMed] [Google Scholar]
- 20.Abdelaziz DH, Elhosseiny NM, Khaleel SA, Sabry NA, Attia AS, El-Sayed MH. Association between combined presence of Hepatitis C Virus and polymorphisms in different genes with toxicities of methotrexate and 6-mercaptopurine in children with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2016;63(9):1539–1545. doi: 10.1002/pbc.26045. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka Y, Manabe A, Nakadate H, et al. Methylenetetrahydrofolate reductase gene haplotypes affect toxicity during maintenance therapy for childhood acute lymphoblastic leukemia in Japanese patients. Leuk Lymphoma. 2014;55(5):1126–1131. doi: 10.3109/10428194.2013.825902. [DOI] [PubMed] [Google Scholar]
- 22.Kaluzna E, Strauss E, Zajac-Spychala O, et al. Functional variants of gene encoding folate metabolizing enzyme and methotrexate-related toxicity in children with acute lymphoblastic leukemia. Eur J Pharmacol. 2015;769:93–99. doi: 10.1016/j.ejphar.2015.10.058. [DOI] [PubMed] [Google Scholar]
- 23.Karas Kuzelicki N, Milek M, Jazbec J, Mlinaric-Rascan I. 5,10-Methylenetetrahydrofolate reductase (MTHFR) low activity genotypes reduce the risk of relapse-related acute lymphoblastic leukemia (ALL) Leuk Res. 2009;33(10):1344–1348. doi: 10.1016/j.leukres.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 24.Ramírez-Pacheco A, Moreno-Guerrero S, Alamillo I, Medina-Sanson A, Lopez B, Moreno-Galvan M. Mexican childhood acute lymphoblastic leukemia: a pilot study of the MDR1 and MTHFR gene polymorphisms and their associations. Genet Test Mol biomrkers. 2016;20(10):597–602. doi: 10.1089/gtmb.2015.0287. [DOI] [PubMed] [Google Scholar]
- 25.Haase R, Elsner K, Stiefel M, Mauz-Korholz C, Kramm C, Korholz D. High dose methotrexate treatment in childhood ALL : pilot study on the impact of the MTHFR 677C > T and 1298A > C polymorphisms on MTX-related toxicity. Klin Pädiatrie. 2012;224(3):156–159. doi: 10.1055/s-0032-1304623. [DOI] [PubMed] [Google Scholar]
- 26.Aráoz H, Aloi K, Foncuberta M, et al. Pharmacogenetic studies in children with acute lymphoblastic leukemia in Argentina Pharmacogenetic studies in children with acute lymphoblastic leukemia in Argentina. Leuk Lymphoma. 2015;56(5):1370–1378. doi: 10.3109/10428194.2014.951844. [DOI] [PubMed] [Google Scholar]
- 27.Zgheib N, Akra-Ismail M, Aridi C, et al. Genetic polymorphisms in candidate genes predict increased toxicity with methotrexate therapy in Lebanese children with acute lymphoblastic leukemia. Pharmacogenet Genomics. 2014;24(8):387–396. doi: 10.1097/FPC.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 28.Fukushima H, Fukushima T, Sakai A, et al. Polymorphisms of MTHFR associated with higher relapse/death ratio and delayed weekly MTX administration in pediatric lymphoid malignancies. Leuk Res Treatment. 2013;2013:1–9. doi: 10.1155/2013/238528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Imanishi H, Okamura N, Yagi M, et al. Genetic polymorphisms associated with adverse events and elimination of methotrexate in childhood acute lymphoblastic leukemia and malignant lymphoma. J Hum Genet. 2007;52(2):166–171. doi: 10.1007/s10038-006-0096-z. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki R, Fukushima H, Noguchi E, et al. Influence of SLCO1B1 polymorphism on maintenance therapy for childhood leukemia. Pediatr Int. 2015;57(4):572–577. doi: 10.1111/ped.12682. [DOI] [PubMed] [Google Scholar]
- 31.Moulik N, Kumar A, Agrawal S, Mahdi A, Kumar A. Effect of folate status and methylenetetrahydrofolate reductase genotypes on the complications and outcome of high dose methotrexate chemotherapy in north Indian children with acute lymphoblastic leukemia. Indian J Med Paediatr Oncol. 2016;37(2):85–89. doi: 10.4103/0971-5851.180144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yazicioğlu B, Kaya Z, Ergun S, et al. Influence of folate-related gene polymorphisms on high-dose methotrexate-related toxicity and prognosis in Turkish children with Acute Lymphoblastic Leukemia. Turkish J Hematol. 2016:2–19. doi: 10.4274/tjh.2016.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kantar M, Kosova B, Cetingul N, et al. Methylenetetrahydrofolate reductase C677T and A1298C gene polymorphisms and therapy-related toxicity in children treated for acute lymphoblastic leukemia and non-Hodgkin lymphoma. Leuk Lymphoma. 2009;50(6):912–917. doi: 10.1080/10428190902893819. [DOI] [PubMed] [Google Scholar]
- 34.Karathanasis NV, Stiakaki E, Goulielmos GN, Kalmanti M. The role of the methylenetetrahydrofolate reductase 677 and 1298 polymorphisms in Cretan children with acute lymphoblastic leukemia. Genet Test Mol Biomarkers. 2011;15(1–2):5–10. doi: 10.1089/gtmb.2010.0083. [DOI] [PubMed] [Google Scholar]
- 35.He H, Chen S, You H, et al. Association between methylenetetrahydrofolate reductase polymorphisms and the relapse of acute lymphoblastic leukemia: a meta-analysis. Pharmacogenomics J. 2014;14(5):432–438. doi: 10.1038/tpj.2014.10. [DOI] [PubMed] [Google Scholar]
- 36.Ojha RP, Gurney JG. Methylenetetrahydrofolate reductase C677T and overall survival in pediatric acute lymphoblastic leukemia: a systematic review. Leuk Lymphoma. 2014;55(1):67–73. doi: 10.3109/10428194.2013.792336. [DOI] [PubMed] [Google Scholar]
- 37.D´Angelo V, Ramaglia M, Iannotta A, et al. Methotrexate toxicity and efficacy during the consolidation phase in paediatric acute lymphoblastic leukaemia and MTHFR polymorphisms as pharmacogenetic determinants. Cancer Chemother Pharmacol. 2011;68(5):1339–1346. doi: 10.1007/s00280-011-1665-1. [DOI] [PubMed] [Google Scholar]
- 38.Tantawy A, El-Bostany E, Adly A, et al. Methylene tetrahydrofolate reductase gene polymorphism in Egyptian children with acute lymphoblastic leukemia. Blood Coagul Fibrinolysis. 2010;21(1):28–34. doi: 10.1097/MBC.0b013e32833135e9. [DOI] [PubMed] [Google Scholar]
- 39.Radtke S, Zolk O, Renner B, et al. Germline genetic variations in methotrexate candidate genes are associated with pharmacokinetics, toxicity, and outcome in childhood acute lymphoblastic leukemia. Blood J. 2013;121(26):5145–5154. doi: 10.1182/blood-2013-01-480335. [DOI] [PubMed] [Google Scholar]
- 40.Treviño L, Shimasaki N, Yang W, et al. Germline genetic variation in an organic anion transporter polypeptide associated with methotrexate pharmacokinetics and clinical effects. J Clin Oncol. 2009;27(35):5972–5978. doi: 10.1200/JCO.2008.20.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramsey LB, Panetta JC, Smith C, et al. Genome-wide study of methotrexate clearance replicates SLCO1B1. Blood. 2013;121(6):898–904. doi: 10.1182/blood-2012-08-452839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lopez-Lopez E, Ballesteros J, Pinan MA, et al. Polymorphisms in the methotrexate transport pathway: a new tool for MTX plasma level prediction in pediatric acute lymphoblastic leukemia. Pharmacogenet Genomics. 2013;23(2):53–61. doi: 10.1097/FPC.0b013e32835c3b24. [DOI] [PubMed] [Google Scholar]
- 43.den Hoed MA, Lopez-Lopez E, te Winkel ML, et al. Genetic and metabolic determinants of methotrexate-induced mucositis in pediatric acute lymphoblastic leukemia. Pharmacogenomics J. 2015;15(3):248–254. doi: 10.1038/tpj.2014.63. [DOI] [PubMed] [Google Scholar]
- 44.Gutierrez-Camino A, Lopez-Lopez E, Garcia-Orad A. SLC19A1 hot spot for MTX plasma concentration. Med Oncol. 2014;31(10):204. doi: 10.1007/s12032-014-0204-4. [DOI] [PubMed] [Google Scholar]
- 45.Iparraguirre L, Gutierrez-Camino A, Umerez M, et al. MiR-pharmacogenetics of methotrexate in childhood B-cell acute lymphoblastic leukemia. Pharmacogenet Genomics. 2016;26(11):517–525. doi: 10.1097/FPC.0000000000000245. [DOI] [PubMed] [Google Scholar]
- 46.Lopez-Lopez E, Gutierrez-Camino A, Pinan MA, et al. Pharmacogenetics of microRNAs and microRNAs biogenesis machinery in pediatric acute lymphoblastic leukemia. PLoS One. 2014;9(3):e91261. doi: 10.1371/journal.pone.0091261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsujimoto S, Tanaka F, Yanagimachi M, et al. Influence of ADORA2A gene polymorphism on leukoencephalopathy risk in MTX-treated pediatric patients affected by hematological malignancies. Pediatr Blood Cancer. 2016;63:1983–1989. doi: 10.1002/pbc.26090. [DOI] [PubMed] [Google Scholar]
- 48.Wang S, Sun L, Zeng W, Wu W, Zhang G. Influence of genetic polymorphisms of FPGS, GGH, and MTHFR on serum methotrexate levels in Chinese children with acute lymphoblastic leukemia. Cancer Chemother Pharmacol. 2014;74:283–289. doi: 10.1007/s00280-014-2507-8. [DOI] [PubMed] [Google Scholar]
- 49.de Deus D, de Lima E, Seabra Silva R, Leite E, Cartaxo Muniz M. Influence of methylenetetrahydrofolate reductase C677T, A1298C, and G80A polymorphisms on the survival of pediatric patients with acute lymphoblastic leukemia. Leuk Res Treatment. 2012;2012:1–6. doi: 10.1155/2012/292043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim H, Kang H, Kim H, et al. Pharmacogenetic analysis of pediatric patients with acute lymphoblastic leukemia: a possible association between survival rate and ITPA polymorphism. PLoS One. 2012;7(9):1–10. doi: 10.1371/journal.pone.0045558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leonardi D, Abbate M, Riccheri M, et al. Improving risk stratification of patients with childhood acute lymphoblastic leukemia: glutathione-S-transferases polymorphisms are associated with increased risk of relapse. Oncotarget. 2016;5:1–8. doi: 10.18632/oncotarget.8606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hoang P, Ambroise J, Dekairelle A, et al. Comparative pharmacogenetic analysis of risk polymorphisms in Caucasian and Vietnamese children with acute lymphoblastic leukemia: prediction of therapeutic outcome? Br J Clin Pharmacol. 2015;79(3):429–440. doi: 10.1111/bcp.12481. [DOI] [PMC free article] [PubMed] [Google Scholar]