Abstract
Objective: To investigate the effects of respiratory muscle training (RMT) combined with the abdominal drawing-in maneuver (ADIM) on the pulmonary function in patients with chronic spinal cord injury (SCI).
Methods: Thirty-seven subjects with SCI (level of injury: C4–T6, time since injury: 4–5 years) were randomly allocated to three groups; the integrated training group (ITG), the RMT group (RMTG), and the control group (CG). The ITG performed RMT using an incentive respiratory spirometer (IRS) and the ADIM using a stabilizer. The RMTG received only RMT using an IRS. Subjects in the CG received alternative and routine physical therapy or usual care. The interventions were conducted over an eight-week period. Pulmonary function was evaluated using spirometry to measure the forced vital capacity (FVC) and forced expiratory volume in 1 second (FEV1).
Results: The differences between the pre- and post-test values for FVC (0.47 ± 0.05 versus 0.15 ± 0.06 versus –0.03 ± 0.01) and FEV1 (0.74 ± 0.07 versus 0.27 ± 0.17 versus 0.02 ± 0.67)were significant among the groups. Post-test, in the ITG, the FVC and FEV1 values showed significant differences from those in the RMTG and CG (F = 11.48 and 11.49, P = 0.002 and 0.001). Furthermore, following the 8week intervention, the change ratio values of the FVC and FEV1 of the ITG were increased further by an average of 9.75% and 7.91%, respectively, compared with those of the RMTG.
Conclusion: These findings suggest positive evidence that RMT with additional ADIM training can improve pulmonary function in SCI pulmonary rehabilitation.
Keywords: Abdominal drawing-in maneuver, Incentive respiratory spirometer, Pulmonary function, Respiratory muscle training, Spinal cord injury
Introduction
The respiratory system is one of the most important routes for obtaining the elements necessary to sustain life. Respiration consists of inspiration and expiration. Inspiration is an active movement during which respiratory muscles, mainly the diaphragm and external inter-costal muscles, contract. On the other hand, expiration is a passive process that causes the thorax to return back to its original position during relaxation of the muscles involved in inspiration.1
In patients with spinal cord injury (SCI), respiratory function may be affected due to complete or partial paralysis of the respiratory muscles.2 In addition, paralysis of the abdominal muscles can also occur, particularly with injuries higher than T7, leading to a reduction in the ability to breathe.3 Consequently, partial or total weakness of the abdominal muscles, as well as the diaphragm and external inter-costal muscles, have been reported to affect the pulmonary function in patients with SCI.1 This condition can cause patients with SCI, who require intensive rehabilitation, to tire easily during aerobic activities requiring endurance,1,2 thereby restricting their performance of activities of daily living. Moreover, inspiratory capacity is reduced and pneumonia is a frequent complication.1 Therefore, the improvement of respiratory and abdominal muscles, and thus pulmonary function, is an important issue in the preservation of life in patients with SCI.
As mentioned above, a decrease in the strength of respiratory and lower abdominal muscles have been shown during the respiratory cycle of subjects with SCI.1,3 Moreover, weakened muscles drastically lower the capacity for coughing and sputum expectoration, and respiratory muscle weakness is attributed to the impairment of the muscles involved in inspiration.1 It has already been shown that respiratory muscle training (RMT) aids treatment and increases respiratory muscle strength in patients with SCI.4,5 The American Thoracic Society/European Respiratory Society pulmonary rehabilitation guidelines recommend respiratory muscle training (RMT) for suspected or confirmed respiratory muscle weakness.6 It is also possible to perform repetitive breathing resistance training using simple instruments to strengthen the respiratory muscles, and thus improve cardiopulmonary function.7 Based on this fact, one of the current techniques used to train respiratory muscles in clinical settings is incentive respiratory spirometry (IRS).8 However, no single standard intervention has yet been identified to be effective in the recovery of pulmonary function following SCI. In other words, abdominal muscle training affecting respiration, as well as training to improve respiratory muscles is also needed.
The thoracic and abdominal muscles used for inspiration include both major and auxiliary muscles. The auxiliary muscles include the scalene, sternocleidomastoid, pectoralis major, and the abdominal muscles.1 Four abdominal muscles are involved in pulling up the abdominal wall and increasing the pressure within the abdominal cavity. A previous study presented conclusive evidence that abdominal muscles are the most crucial muscles forinspiration.9 Most importantly, the activation of the upper and lower fibers of the transverse abdominal (TA) muscle are essential for changes in abdominal pressure. Thus, the diaphragm helps to enhance spinal stability, and its contraction, together with the contraction of the abdominal muscles, increases abdominal pressure10 and continuously contributes to respiration and posture adjustment.11 The abdominal drawing-in maneuver (ADIM) is commonly used in lumbar stabilization training programs.12 This maneuver is designed to activate the TA muscle. The abdominal muscles, including the TA, are also the most powerful muscles involved in expiration.13 Stabilizer is a tool that was developed for ADIM training in order to increase the intra-abdominal pressure and stabilize the lumbar spine. This device also provides visual feedback through a pressure sensor.
Based on the above research background, the present study attempted to examine whether RMT and ADIM were conducive to the enhancement of pulmonary function. This study established a hypothesis that RMT and ADIM in pulmonary rehabilitation would increase the abdominal pressure from the abdominal muscles, which would in turn improve pulmonary function following an SCI. It was also predicted that integrating RMT and ADIM into an exercise program would be more beneficial than using either technique alone. Therefore, the purpose of the present study was to examine the effects of integrating exercise with RMT and ADIM, devised for the internal stabilization of the spine, on pulmonary function following an SCI.
Methods
Subjects
Thirty-seven subjects (15 women, 22 men) with SCI, who were receiving inpatient treatment in an SCI rehabilitation center, were enrolled in our randomized controlled trial (RCT), after giving informed consent. The present study was approved by the Human Research Sciences of Local Ethics Committee and registered with the University Clinical Trials Registry. The sample size estimate was based on data collected from previous studies.4,5 In the case of a 20% drop-out rate, a priori power analysis determined that a sample size of at least 11 subjects with SCI was required in each group in order to obtain a statistical power of 0.80 using the General Power Analysis Program 3.1 (University Kiel, Germany).14 This was based on one-way analysis of variance (ANOVA) measurements with a predetermined coefficient of reliability of 0.90, for comparison between the three groups. The subjects were selected if the SCI had occurred at least 3 years prior, and if their injury resulted in motor impairment at the level of T6 or higher based on the clinical assessment of their neurological impairment. The subjects were assessed in accordance with the Spinal Injury Association Impairment Scale.15 Moreover, the subjects had no pre-injury history of pulmonary disease or respiratory symptoms, none reported any recent or active pulmonary infection, and none were receiving medication known to alter airway tone.
Outcome measures
The change in respiratory function of the subjects was evaluated using spirometry measurements, which were administered prior to and following the eight-week intervention period. Three physical therapists who were blinded to the group allocation provided the evaluations. Prior to the spirometry measurement, these blinded evaluators underwent a 24-hour training session for the administration of spirometry, and their competence in the conductance of these measurements was assessed by the primary investigator who possesses 10 years of experience. These evaluators were trained to conduct the spirometry measurements in accordance with the standardized procedures described as follows. The subjects were advised not to disclose their treatment assignment to the evaluators.
Lung function was measured with a computerized spirometer (Chestgraph HI-101, Chest MI Inc., Tokyo, Japan) (Fig. 1A) during maximal voluntary ventilation (MVV) maneuvers. As the maneuvers were being measured, the subjects were asked to sit upright and make sure nothing was restricting chest movement or airflow. The subjects started the test by breathing normally through the mouthpiece, followed by breathing as deeply (recommended depth: 1/2–3/4 of the subject's vital capacity) and rapidly (recommended rate: 70–150 breaths/min) as possible for 20 seconds.16 At the end of the measurement interval, the subjects were asked to resume normal breathing and the mouthpiece was removed. Three trials of the maneuvers were performed, separated by a five-minute rest, and the average of the results was taken. The measured values included forced vital capacity (FVC) and forced expiratory volume in 1 second (FEV1), and the pulmonary function indices were calculated automatically by the computer. FVC measures the vital capacity, which refers to the total capacity of air that can be blown out by maximal forced expiration following maximal inspiration. FEV1 refers to the capacity of air that is blown out for a single second.17 The pulmonary function test was performed with the subjects wearing a nose clip. If the subjects coughed or made a mistake, their numerical values were not recorded.
Figure 1.

The spirometry (A) illustration showing the experimental settings and StabilizerTM pressure biofeedback unit (B) used in this study. (colour online)
Experimental procedures
A total of 37 subjects with SCI were recruited for the present study. Although 49 subjects were initially recruited, seven subjects were excluded because they declined to participate or did not meet the inclusion criteria for this study. In addition, five subjects withdrew due to an unstable medical condition. As a result, the drop-out rate of our study was 11.90%. A flowchart of this study is shown in Figure 2. Group allocation of the subjects was determined using a randomization procedure, in which each subject drew a ball from a box containing three balls marked with 0 (control group; CG), 1 (respiratory muscle training group; RMTG), or 2 (RMT with additional ADIM; integrated training group, ITG). Consequently, the subjects and therapists were not aware of the grouping. The baseline characteristics of all subjects in each group are presented in Table 1. The data indicates that the groups had similar demographic characteristics. Over an eight-week period, all the subjects received functional training in the clinical setting according to the daily routine schedule, which consisted of strengthening and stretching exercises of the limbs, therapist-guided techniques for facilitating the normal movement pattern. The subjects received the above routine therapy for one hour, three times a week. In particular, subjects in the ITG received both RMT, using an incentive spirometer (Tri-ball Incentive Spirometer 600–1200cc, Ark Therapeutic, Lugoff, USA), and ADIM. Subjects in the RMTG received only RMT using the incentive spirometer. All subjects in both groups completed 24 supervised training sessions (3 × 15 minutes per week).
Figure 2.
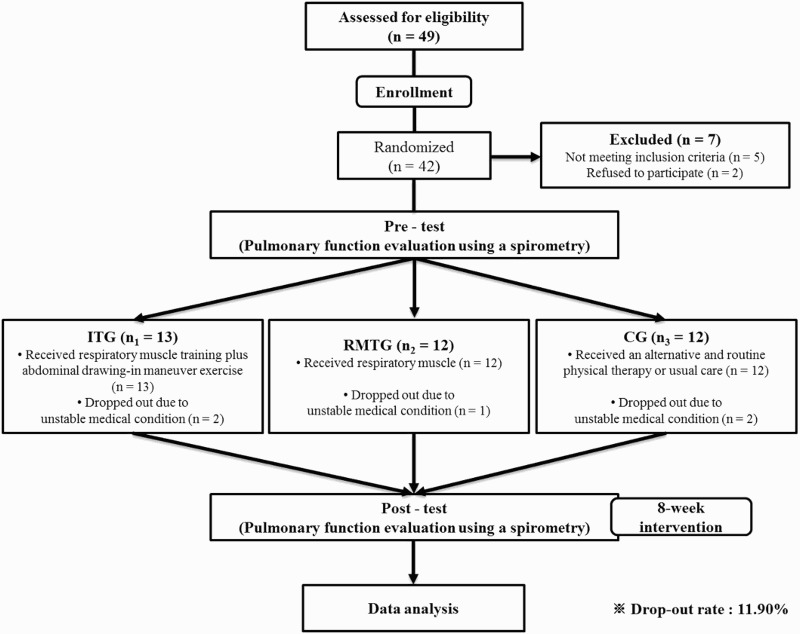
The experimental procedures used in this study. ITG, Integrated training group; RMTG, Respiratory muscle training group; CG, Control group.
Table 1.
Demographic and injury data for each group (N = 37)
| Characteristics | ITG (n1 = 13) | RMTG (n2 = 12) | CG(n3 = 12) | χ2/F (P-Value) |
|---|---|---|---|---|
| Age (years) | 39.98 ± 11.47a | 41.51 ± 10.04 | 40.12 ± 8.73 | 0.27b (0.93) |
| Time since injury (years) | 4.24 ± 0.76 | 4.73 ± 0.84 | 5.04 ± 1.27 | 0.37b (0.94) |
| Education (years) | 11.41 ± 3.49 | 10.18 ± 3.14 | 11.11 ± 3.61 | 0.40b (0.93) |
| Body mass index (kg/m2) | 22.79 ± 3.17 | 21.76 ± 5.80 | 22.37 ± 4.60 | 0.57b (0.92) |
| Sex (Male/Female) | 7 / 6 | 7 / 5 | 8 / 4 | 0.17c (0.98) |
| Smoking (Yes/No) | 4 / 9 | 5 / 7 | 3 / 9 | 0.29c (0.99) |
| Spine fracture (Yes/No) | 9 / 4 | 8 / 4 | 9 / 3 | 0.26c (0.92) |
| Spine surgery (Yes/No) | 10 / 3 | 8 / 4 | 10 / 2 | 0.41c (0.90) |
| Tracheostomy (Yes/No) | 6 / 7 | 7 / 5 | 6 / 6 | 0.25c (0.96) |
| Level of injury (Number) | ||||
| C4-5 (Com / Inc) | 2 (0/2) | 3 (0/3) | 1 (0/1) | 0.29c (0.98) |
| C6-7 (Com / Inc) | 2 (1/1) | 2 (0/2) | 3 (2/1) | |
| T1-2 (Com / Inc) | 2 (0/2) | 2 (1/1) | 2 (0/2) | |
| T3-4 (Com / Inc) | 4 (2/2) | 3 (2/1) | 3 (1/2) | |
| T5-6 (Com / Inc) | 3 (1/2) | 2 (1/1) | 3 (1/2) | |
| Etiology (Number) | ||||
| Motor vehicle collision | 3 | 4 | 2 | 0.24c (0.44) |
| Falls | 3 | 2 | 4 | |
| Diving | 4 | 3 | 4 | |
| Other | 3 | 3 | 2 |
Values are expressed as numbers; aValues are expressed as mean ± standard deviation. bOne-way ANOVA. cχ2 test.
ITG, Integrating training group; RMTG, Respiratory muscle training group; CG, Control group; Com, Complete; Inc, Incomplete.
Training program
For the RMT using an IRS, the method presented by Hall et al., (1996)18 was modified and applied in our study. The subjects maintained a maximal inspiration position for 3 to 4 seconds, and then performed maximal expiration. This exercise was performed for a total of five sets, with 10 repetitions making up one set. After each set, a one-minute rest was allowed. When the subject complained of fatigue or dizziness during the respiratory exercise, they took a short rest and then proceeded with the exercise. If these symptoms were severe, the subjects stopped the exercise.
For the ADIM, a maneuver method presented by Richardson et al., (2004)19 was modified and applied in our study. The subjects laid down in the prone position, and the 3-chamber pressure cell of the StabilizerTM pressure biofeedback unit (STABILIZERTM Pressure Bio-Feedback, CHATTANOOGA Group Inc, Hixson, USA) (Fig. 1B) was placed between the pad of the treatment table and the subjects’ lower abdomen, and inflated to 50 mmHg. The subjects were subsequently asked to draw in the abdomen and hold this position in order for the change in pressure to be measured during a 10-second period. The subjects were then asked to maintain a pressure lower than 6–10 mmHg, based on visual feedback from an analog pressure gauge, in the prone position, followed by a 5-second isometric contraction. Following the five-second maintenance, subjects were allowed to rest for 2 to 3 seconds. This exercise was conducted for a total of five sets (10 repetitions per set).20 In-between sets, the subjects were allowed to rest for one minute. Prior to the exercise, the function of the deep abdominal muscles was explained to the subjects, and each subject was informed about the role and pressure monitoring mechanism of the pressure biofeedback unit. Subjects were asked to perform a preliminary exercise through feedback and reinforcement to ensure that the exercise was performed properly.
Data processing and statistical analysis
Statistical analysis of the data was performed using the SPSS 12.0 software (SPSS Inc., Chicago, IL, USA). The values in each group are expressed as the mean ± standard deviation, number (n), and percentage (%). Since samples involved in the present study were represented by normal distribution curves in the Kolmogorov-Smirnov test, parametric methods were used. The χ2 test (sex, smoking status, spine fracture and surgery, tracheostomy, level of injury, and etiology) and one-way ANOVA (age, duration, education, and body mass index [BMI]) were used to compare demographic characteristics for categorical data of subjects among the three groups. A mixed-model 2 × 3 ANOVA with 1 within-subject factor (time: pre- and post-intervention) and 1 between-subject factor (group: ITG, RMTG, and CG) was used to determine the main effects and the interaction for each FVC and FEV1. Subsequently, comparisons of the differences in pulmonary function among the groups pre- and post-test were investigated using repeated-measures one-way ANOVA for continuous data, followed by Bonferroni's post-hoc test. Consequently, the Bonferroni's post-hoc test was performed to identify the differences among groups for each intervention time. Subsequent post-hoc t-tests with Bonferroni correction were performed to compare the same parameters prior to and following the intervention within each group. To determine the statistical significance of the data collected from the subjects, the significance level was set at P < 0.01.
Results
Comparison of pulmonary functions in accordance with the training methods
The overall dynamics of the changes in the FVC and FEV1 are listed in Table 2. There were significant main effects for the group in all measured values (FVC: F1,36 = 13.85, P = 0.001 and FEV1: F1,36 = 15.20, P = 0.001). Furthermore, there were significant group-by-time interactions in all measured values (FVC: F1,36 = 7.46, P = 0.010 and FEV1: F1,36 = 8.21, P = 0.007). However, there were no significant main effects for time in all measured values (FVC: F1,36 = 3.09, P = 0.090 and FEV1: F1,36 = 3.82, P = 0.062). A comparison of the FVC and FEV1 prior to and following intervention showed a significant increase in the ITG and RMTG (P < 0.01). The FVC and FEV1 following intervention were compared among the three groups; post-hoc testing revealed that the FVC and FEV1 values of the subjects in the ITG (P < 0.001) were significantly different from those in the RMTG and CG post-test; and those in the RMTG (P < 0.01) were significantly different from those in the CG post-test.
Table 2.
Comparison of the forced vital capacity (FVC) and forced exploratory volume in 1 second (FEV1), pulmonary function indexes, during maximal voluntary ventilation (MVV) before and after the intervention among groups (N = 37)
| Characteristics | ITG (n1 = 13) | RMTG (n2 = 12) | CG(n3 = 12) | F (P-Valueb) |
|---|---|---|---|---|
| FVC (ℓ) | ||||
| Pre-test | 2.05 ± 0.34 | 2.21 ± 0.54 | 1.97 ± 0.32 | -0.13 (0.721) |
| Post-test | 2.60 ± 0.19†‡ | 2.40 ± 0.69† | 2.02 ± 0.44 | 9.14 (0.003)* |
| t (P-Valuea) | 14.60 (0.000)** | 5.74 (0.005)* | 0.75 (0.762) | |
| Post-pre test differences | 0.47 ± 0.05†‡ | 0.15 ± 0.06† | 0.03 ± 0.01 | 11.48 (0.002)* |
| FEV1 (ℓ) | ||||
| Pre-test | 1.80 ± 0.26 | 1.91 ± 0.44 | 2.07 ± 0.17 | 0.27 (0.679) |
| Post-test | 2.50 ± 0.16†‡ | 2.11 ± 0.13 | 2.09 ± 0.74 | 7.76 (0.004)* |
| t (P-Valuea) | 18.21 (0.000)** | 6.54 (0.004)* | 1.01 (0.463) | |
| Post-pre test differences | 0.74 ± 0.07†‡ | 0.27 ± 0.17† | 0.02 ± 0.67 | 11.49 (0.001)* |
Values are expressed as mean ± standard deviation. aWithin-group comparison. bBetween-group comparison.ITG, Integrating training group; RMTG, Respiratory muscle training group; CG, Control group. *P < 0.01, **P < 0.001.
†: Significantly different compared to the CG.
‡: Significantly different compared to the RMTG.
Comparison of the change ratio of FVC and FEV1 among the three groups
Following intervention, FVC of the ITG and RMTG increased by an average of 19.98% and 10.41%, respectively. On the contrary, that of the CG increased by an average of only 1.78%. In addition, FEV1 of the ITG and RMTG rose by an average of 16.71% and 9.80%, respectively, while that of the CG increased by an average of only 2.41% (Table 3). Following intervention, both FVC and FEV1of the ITG and RMTG significantly increased in comparison with the CG (P < 0.01) (Table 3). Following an 8weekintervention, the FVC and FEV1of the ITG were increased further by an average of 9.75% and 7.01%, compared with those of the RMTG. Post-hoc testing revealed that the FVC and FEV1 values of the subjects in the ITG were significantly different from those in the RMTG (P < 0.01) and CG (P < 0.001) post-test, and those in the RMTG were significantly different from those in the CG (P < 0.01) post-test (Fig. 3).
Table 3.
Comparison of improvement rate of the forced vital capacity (FVC) and forced exploratory volume in 1 second (FEV1) after the intervention among groups (N = 37)
| Characteristics | ITG (n1 = 13) | RMTG (n2 = 12) | CG(n3 = 12) | F (P-Valueb) |
|---|---|---|---|---|
| FVC | ||||
| Improvement rate (%) | 19.98 ± 9.47†‡ | 10.41 ± 5.44† | 1.78 ± 0.87 | 11.74 (0.003)* |
| FEV1 | ||||
| Improvement rate (%) | 16.71 ± 7.89†‡ | 9.80 ± 6.73† | 2.41 ± 0.37 | 12.31 (0.002)* |
Values are expressed as mean ± standard deviation. aWithin-group comparison. bBetween-group comparison.
ITG, Integrating training group; RMTG, Respiratory muscle training group; CG, Control group.*P < 0.01, **P < 0.001.
†: Significantly different compared to the CG.
‡: Significantly different compared to the RMTG.
Figure 3.
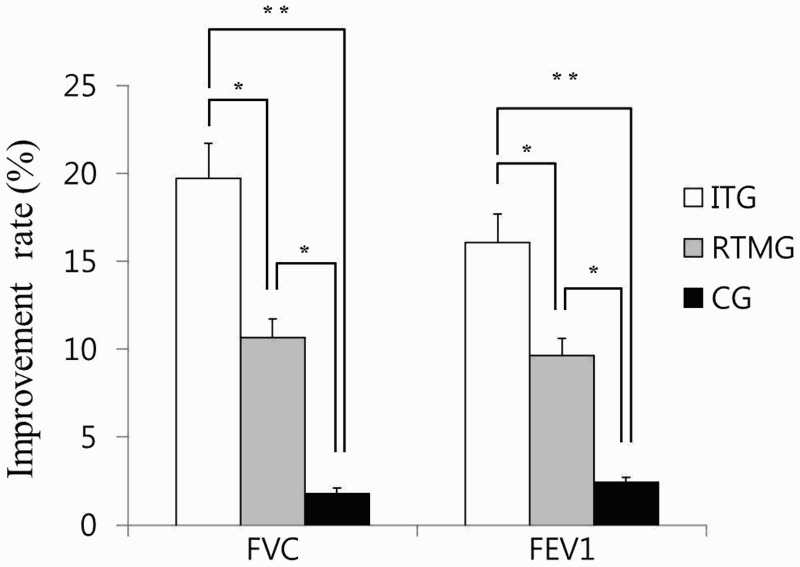
Comparisons of the improvement rate of the forced vital capacity (FVC) and forced expiratory volume in 1 second (FEV1) in accordance with the training methods. ITG, Integrated training group; RMTG, Respiratory muscle training group; CG, Control group. *P < 0.01, **P < 0.001.
Discussion
RMT has already been adopted in SCI rehabilitation to increase respiratory muscle strength and pulmonary function.3–6 However, no study has yet been performed to examine the effect of an integrated training program combining both RMT and ADIM on the improvement of pulmonary function in patients with SCI. Therefore, to the best of our knowledge, we performed the first study with an application protocol. The main purpose of this study was to demonstrate that RMT combined with ADIM is an effective intervention for the enhancement of pulmonary function in patients with SCI. We found that RMT combined with ADIM increases abdominal pressure from the abdominal muscles, thus improving pulmonary function as seen by FVC and FEV1. We also found that an integrated exercise program to conduct RMT and ADIM may be more favorable in the enhancement of pulmonary function in patients with SCI, compared with the use of either technique alone.
Previous research has reported that paralysis of the abdominal muscles appears in SCI patients,2,3 particularly with injuries higher than T7, leading to a reduction in the ability to breathe.3 Therefore, we included subjects with SCI who were impaired higher than T7 in order to measure the recovery of respiratory muscle function. Becklake (1986)22 reported that these test results are affected by technological differences in testing equipment, biological differences unrelated to disease, performance process, examiners and subjects, and diseases themselves. When determining differences in pulmonary function, the most crucial elements are the subject's sex, body size, and age, which affect the results by 30%, 22%, and 8% respectively.22 Race and technological differences affect the results by 20% and 3%, respectively, and the remaining 27% are individual differences that are yet to be explained.22 Based on these facts from a previous study,22 the best strategy when conducting and analyzing a pulmonary function test is to minimize several factors. Therefore, before the measurements, we obtained the BMI data of subjects, which were used to minimize the effect of the confounding factor of body fat and to maintain muscle quality homogeneity. Moreover, we attempted to choose the MVV maneuvers, which are the standardized experimental procedures used to minimize errors in these variables, as suggested by previous studies.16
The evaluation of the pulmonary function of subjects was conducted using spirometry, which may also be used to evaluate pulmonary function indices such as the degree of weakness in respiratory muscles. Usually, in order to evaluate pulmonary function following RMT, measurements are taken of the pulmonary residual volume, FVC, and FEV1.23 The FEV1 and FVC are indices that are used to determine a relative reduction in ventilation capacity, in case the subject has cardiopulmonary ventilation disorders. Therefore, they are measured frequently in the clinical field.23 These indices also have lower variability than others, and are often used to evaluate a prognosis and to observe progression.17 In both the ITG and RMTG, pulmonary function significantly improved following RMT and ADIM. Measurements taken after the 8-week intervention showed significant enhancement. The increase in FEV1 following intervention may be due to the fact that the subjects became accustomed to RMT and ADIM and had their pulmonary functions measured repeatedly. However, the CG did not show a statistically significant difference, suggesting that there was no learning effect from repeated measurement and testing of pulmonary function. The FVC and FEV1 increased significantly in the ITG and RMTG, and pulmonary function in these two intervention groups also improved significantly compared with the CG. Furthermore, the change ratio was higher in the ITG than in the RMTG, and the difference was statistically significant. As a result, improved values of these parameters indicate that the use of RMT combined with ADIM may help to increase pulmonary function in various environmental conditions that patients with SCI will likely experience in their daily life after returning to their community.
An IRS was used in RMT, for which a method presented by Hall et al., (1996)18 was modified. The advantages of using an incentive spirometer are: 1) it is easy to learn how to use the instrument; 2) it is economical; and 3) patients can be motivated to use it, as it produces a visible improvement. Its visual feedback helps to train patients to use the instrument independently and freely,17 and it maximizes their respiratory motivation.17 In the present study, RMT using an IRS was therefore conducive to the enhancement of pulmonary function in patients with SCI, and our results are similar to those seen in a previous study.4 However, it has already been shown that RMT using an IRS aids treatment and increases respiratory muscle strength in patients with SCI,4,5 and no single standard intervention has yet been identified to be effective in the recovery of pulmonary function following SCI. Therefore, abdominal muscle training that affects respiration, as well as training to improve respiratory muscles is also needed. For ADIM, the stabilizer was used to improve factors that were associated with decreased pulmonary function, and the training was performed by modifying a method presented by Richardson et al., (2004).19 According to previous studies, deep abdominal muscles such as the TA and the multifidus muscle contributes not only to stabilization of the spine24,25 and adjustment of posture,11 but also to significant improvements in pulmonary function, when ADIM was applied.19 A similar positive effect on the pulmonary function of patients with high-level SCI was seen in our study.5 Therefore, ADIM reeducates the respiratory muscles, improving muscle strength and endurance, as well as inducing powerful contraction of the respiratory muscles through repetitive afferent stimulation of the abdominal muscles. This increases intra-abdominal pressure, facilitating upward movement of the diaphragm, and decreases pleural pressure and lung volume, improving expiratory and sputum discharge abilities. These abdominal muscles are strong, and they play an important role in activities such as coughing and deep respiration.25 Therefore, activation of deep abdominal muscles, including the TA muscle, would be effective in enhancing respiratory capabilities. For patients with SCI who require intensive rehabilitation, ADIM helps these muscles to perform better during aerobic activities requiring endurance, thereby improving the performance of these patients in activities of daily living. In addition, inspiratory capacity may be increased, meaning that the occurrence of frequent complications such as pneumonia may also be reduced. In conclusion, treatment to strengthen the respiratory and abdominal muscles, resulting in an improvement in pulmonary function, is an important intervention in the successful preservation of life in patients with SCI.
Limitations of this study
Although favorable outcomes in the RMT combined with ADIM treatment were demonstrated by many of the inter-and intra-group comparisons, there are some limitations to this study that can be improved in future research. Firstly, the present study did not measure biomechanical parameters such as electromyography. Future studies measuring the biomechanical parameters and electrical recordings from the respiratory muscles such as the diaphragm and external inter-costal muscles would provide direct quantitative parameters during maximal static inspiratory efforts. Secondly, we were unable to analyze more parameters that characterize the changes in symptom severity or quality of life. Therefore, definitive future studies, with a large sample size measuring these parameters in the SCI patients in various circumstances, would provide direct qualitative information. Finally, the present study did not involve a long-term follow-up, and we do not know whether the subjects are actually better in terms of the level of activity that they can undertake, or in terms of reducing the risk of respiratory disease. Thus, our results cannot be recognized as the long-term effect of RMT combined with ADIM in SCI patients. Accordingly, a future RCT to complement the above limitations should be carried out to confirm the findings herein.
Conclusion
Several significant findings were obtained in the present study, showing that pulmonary function results for both ITG and RMTG significantly improved in comparison with the results from the CG. The average change ratio of the pulmonary function of the ITG was larger than the RMTG; and this was statistically significant. Thus, based on our results, we suggest that, in SCI patients, RMT combined with ADIM shows a greater improvement in pulmonary function compared with the application of RMT alone. In conclusion, RMT combined with ADIM may be feasible, with clinical benefits and easy application, as a promising approach to improve the pulmonary function of patients with SCI.
IRB or ethics committee registration
The study was approved by the Human Research Sciences of local ethics committee and registered with University Clinical Trials Registry.
Disclaimer statements
Contributors None.
Funding None.
Conflicts of interest The authors declare no conflict of interest. The roles of the authors in this study are as follows: Chang Yong Kim - primary author, manuscript writing, experimental procedure, interpretation of the results, management of the study; Jung Sun Lee - manuscript writing, experimental procedure, interpretation of the results; Hyeong Dong Kim - critical discussion, corresponding author; Dong-Jin Lee - experimental procedure, critical discussion.
Ethics approval None.
Acknowledgements
Authors are grateful to all subjects involved in this study, as well as authors/publishers/editors of all articles, journals, and books reviewed and discussed for this study. This research received no specific grant from any funding agency in the public, commercial, or profit sectors.
References
- 1.Jardins TD. Cardiopulmonary Anatomy and Physiology: essentials for respiratory care. 4th ed Albany: Delmar Cengage learning; 2002;40–97. [Google Scholar]
- 2.Van den Berg ME, Castellote JM, de Pedro-Cuesta J, Mahillo-Fernandez I. Survival after spinal cord injury: a systematic review. J Neurotrauma 2010;27(8):1517–28. doi: 10.1089/neu.2009.1138 [DOI] [PubMed] [Google Scholar]
- 3.Klebine P, Lindsey L. Understanding and Managing Respiratory Complications After SCI. Birmingham, AL: Office of Research Services; 2007;57–91. [Google Scholar]
- 4.Mueller G, Hopman MT, Perret C. Comparison of respiratory muscle training methods in individuals with motor complete tetraplegia. Top Spinal Cord Inj Rehabil 2012;18(2):118–21. doi: 10.1310/sci1802-118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McBain RA, Boswell-Ruys CL, Lee BB, Gandevia SC, Butler JE. Abdominal muscle training can enhance cough after spinal cord injury. Neurorehabil Neural Repair 2013;27(9):834–43. doi: 10.1177/1545968313496324 [DOI] [PubMed] [Google Scholar]
- 6.Nici L, Donner C, Wouters E, Zuwallack R, Ambrosino N, Bourbeau J, et al. American Thoracic Society; European Respiratory Society. ATS/ERS statement on pulmonary rehabilitation. Am J Respir Crit Care Med 2006;173(12):1390–413. doi: 10.1164/rccm.200508-1211ST [DOI] [PubMed] [Google Scholar]
- 7.Roth EJ, Stenson KW, Powley S, Oken J, Primack S, Nussbaum SB, et al. Expiratory muscle training in spinal cord injury: a randomized controlled trial. Arch Phys Med Rehabil 2010;91(6):857–61. doi: 10.1016/j.apmr.2010.02.012 [DOI] [PubMed] [Google Scholar]
- 8.Jandt SR, Caballero RM, Junior LA, Dias AS. Correlation between trunk control, respiratory muscle strength and spirometry in patients with stroke: an observational study. Physiother Res Int 2011;16(4):218–24. doi: 10.1002/pri.495 [DOI] [PubMed] [Google Scholar]
- 9.Suzuki S, Juzuki J, Okubo T. Expiratory muscle fatigue in normal subject. J Appl Physiol 1991;70(6):2632–9. [DOI] [PubMed] [Google Scholar]
- 10.Cresswell AG, Grundström H, Thorstensson A. Observations on intra-abdominal pressure and patterns of abdominal intra-muscular activity in man. Acta Physiol Scand 1992;144:409–18. doi: 10.1111/j.1748-1716.1992.tb09314.x [DOI] [PubMed] [Google Scholar]
- 11.Hodges PW, Gadevia SC. Change in intra-abdominal pressure during postural and respiratory activation of the human diaphragm. J Appl Physiol 2000;89(3):967–76. [DOI] [PubMed] [Google Scholar]
- 12.Cairns MC, Harrison K, Wright C. Pressure biofeedback: a useful tool in the quantification of abdominal muscular dysfunction? Phys Ther 2000;86:127–38. [Google Scholar]
- 13.Ishida H, Watanabe S. Changes in lateral abdominal muscles’ thickness immediately after the abdominal drawing-in maneuver and maximum expiration. J Bodyw Mov Ther 2013;17(2):254–8. doi: 10.1016/j.jbmt.2012.12.002 [DOI] [PubMed] [Google Scholar]
- 14.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007;39(2):175–91. doi: 10.3758/BF03193146 [DOI] [PubMed] [Google Scholar]
- 15.Kirshblum SC, Waring W, Biering-Sørensen F, Burns SP, Johansen M, Schmidt-Read M, et al. Reference for the 2011 revision of the International Standards for Neurological Classification of Spinal Cord Injury. J Spinal Cord Med 2011;34(6):547–54. doi: 10.1179/107902611X13186000420242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Thoracic Society/European Respiratory Society ATS/ERS Statement on respiratory muscle testing. Am J Respir Crit Care Med 2002;166(4):518–624. doi: 10.1164/rccm.166.4.518 [DOI] [PubMed] [Google Scholar]
- 17.Westwood K, Griffin M, Roberts K. Incentive spirometer decreases respiratory complications following major abdominal surgery. Surgeon 2007;5(6):339–42. doi: 10.1016/S1479-666X(07)80086-2 [DOI] [PubMed] [Google Scholar]
- 18.Hall LC, Tarala RA, Hall JL. A case control study of postoperative pulmonary complications after laparoscopic and open cholecystectomy. J Lapaloen dosc Surg 1996;6(2):87–92. doi: 10.1089/lps.1996.6.87 [DOI] [PubMed] [Google Scholar]
- 19.Richardson CA, Hodges PW, Hides J. Therapeutic Exercises for Lumbopelvic Stabilization. 2nd ed Churchill Livingstone; Edinburgh: 2004;78–110. [Google Scholar]
- 20.Houge A. Physiotherapy in Respiratory Care. 3rd ed Cheltenham: Nelson Thornes; 2001;59–90. [Google Scholar]
- 21.Wright SP. Adjusted P-values for simultaneous inference. Biometrics 1992;48:1005–13. doi: 10.2307/2532694 [DOI] [Google Scholar]
- 22.Becklake MR. Concepts of normality applied to measurement of lung function. Am J Med 1986;80(6):1158–63. doi: 10.1016/0002-9343(86)90678-9 [DOI] [PubMed] [Google Scholar]
- 23.Roth EJ, Nussbaum SB, Berkowitz M, Primack S, Oken J, Powley S, et al. Pulmonary function testing in spinal cord injury: correlation with vital capacity. Paraplegia 1995;33(8):454–7. doi: 10.1038/sc.1995.99 [DOI] [PubMed] [Google Scholar]
- 24.Wilke HJ, Wolf S, Claes LE, Arand M, Wiesend A. Stability increase of the lumbar spine with different muscle groups. A biomechanical in vitro study . Spine 1995;20(2):192–8. doi: 10.1097/00007632-199501150-00011 [DOI] [PubMed] [Google Scholar]
- 25.De Troyer A, Estenne M. Functional anatomy of the respiratory muscle. Clin Chest Med 1988;9(2):175–93. [PubMed] [Google Scholar]


