Abstract
Nanosecond pulsed electric fields (nsPEFs) characterized by high voltage, low energy and non-thermal effects, have been broadly investigated as a potential tumor therapy; however, little is known about their effects on somatic cells. In this current study, we evaluated effects of nsPEFs on the phenotype of chondrocytes (morphology, glycosaminoglycan (GAG) content, proliferation and gene expression) and explored the mechanisms involved. Our results demonstrated that exposing chondrocytes to nsPEFs led to enhanced proliferation and dedifferentiation, evidenced by the upregulated gene expression of collagen type I (COL I) and downregulated gene expression of Sox9, collagen type II (COL II) and aggrecan (AGG) with activation of the wnt/β-catenin signaling pathway. Inhibition of the wnt/β-catenin pathway partially blocked these effects. Thus we concluded that nsPEFs induce dedifferentiation of chondrocytes partially through transient activation of the wnt/β-catenin signaling pathway.
Millisecond or microsecond pulsed electric fields (PEFs) have been shown to facilitate delivery of drugs and transfer of genes into cells1,2. PEFs induce a transient transmembrane potential of approximately 200 mV–1 V with limited thermal effects1,3. Nanosecond pulsed electric fields (nsPEFs) have shown some advantages over millisecond or microsecond PEFs, as they can achieve similar membrane potentials with higher voltage differentials, lower energy and negligible thermal effects4. nsPEFs generate nanopores in cell membranes smaller than those induced by traditional PEFs, which only allow transfer of small molecules such as water, chloride ions and alkali metal cations5. Furthermore, nsPEFs allow for selective and operable cell electrofusion independent of the size of the cell6. In addition to changes in the cell membrane, nsPEFs also lead to a series of changes that occur subsequently within the cells, such as transfer of phosphatidylserine from the interior to the exterior of lipid membranes7, sparkler morphology of intracellular granules with cytoplasmic free calcein staining8, release of cellular calcium ions from the endoplasmic reticulum9, and activation of mitogen-activated protein kinases (MAPK) pathways10. Finally, nsPEFs induce tumor cell apoptosis both in vivo and in vitro through release of cytochrome c11,12. Although the function of nsPEFs has been explored in multiple cell lines, including T-lymphocytes cell lines, hematologic cell lines and pancreatic cancer cell lines11,13, limited research has been conducted on primary mammalian somatic cells.
Chondrocytes are critical for maintenance and regeneration of cartilage, and several signaling pathways regulate phenotypes of differentiation, hypertrophy, proliferation, and dedifferentiation. Transforming growth factor-beta (TGF-β) has been shown to promote the differentiation of chondrocyte14. Bone morphogenetic proteins (BMPs) stimulate chondrocyte maturation, while parathyroid hormone (PTH) and parathyroid hormone-related peptide (PTHrP) inhibit the rate of maturation15,16. The wnt/β-catenin pathway causes dedifferentiation of chondrocytes, and promotes cell proliferation17,18. The phenotypes of chondrocytes are tightly regulated by physical factors, including electrical stimuli19. Traditional PEFs have shown to increase protein synthesis and subsequently promote the healing response in osteochondral defects through upregulation of aggrecan (AGG) and collagen type II (COL II) in chondrocytes20,21,22. Low voltage (less than 100 mV/cm) treatments were used in the aforementioned research due to the thermal effects of PEFs. Although the biological effects induced by nsPEFs are largely unknown, it has been reported that MAPK pathways, general control non-depressible-2 (GCN2) pathways and double-stranded RNA-dependent protein kinase-like endoplasmic reticulum kinase (PERK) pathways are involved10,23.
In this study, we evaluated the phenotypic effects of nsPEFs on chondrocytes and found that nsPEFs enhanced cell proliferation while causing dedifferentiation by upregulating gene expression of type I collagen (COL I) and downregulating gene expression of COL II. We then explored whether activation of the wnt/β-catenin signaling pathway was involved in these phenotypic changes (Fig. 1).
Figure 1. Strategic map exploring the effects of nsPEFs on chondrocytes.
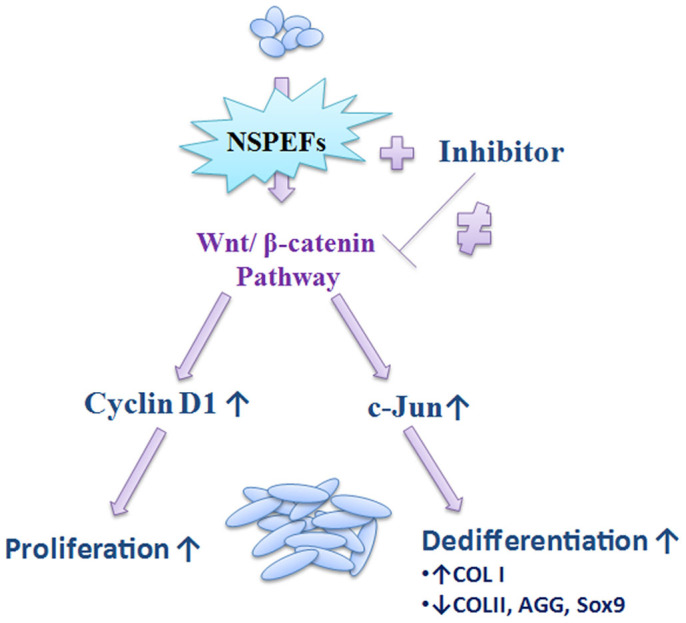
Results
nsPEFs enhance proliferation of chondrocytes
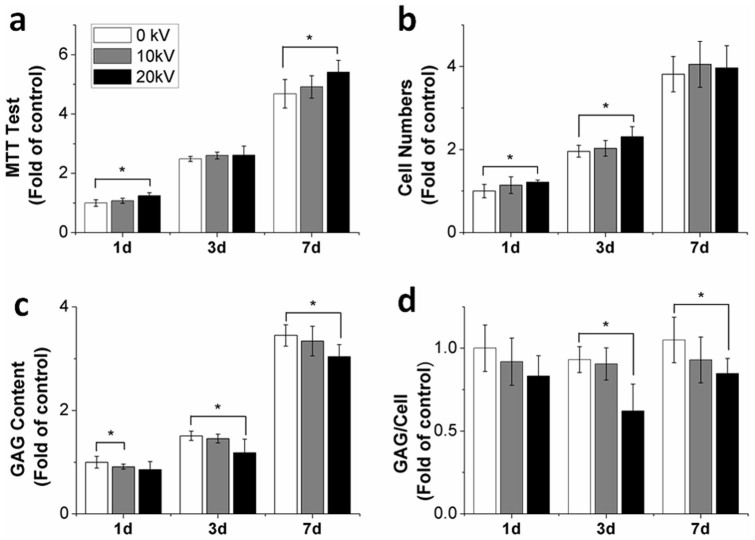
Based on favorable results obtained in previous reports and our pilot study, 5 pulses of 100 ns nsPEFs at 10 or 20 kV/cm were used in this current study10. Cytotoxicity of nsPEFs on chondrocytes was evaluated with 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay24. Exposing chondrocytes to nsPEFs at 10 kV/cm increased absorbance values obtained from MTT assay to 1.07-fold, 1.05-fold and 1.05-fold, while nsPEFs at 20 kV/cm caused a 1.24-fold (p = 0.002), 1.04-fold and 1.16-fold (p = 0.018) increase at days 1, 3 and 7, respectively (Fig. 2a). nsPEFs appeared to have no significant effect on chondrocyte morphology (Supplementary Fig. S1). nsPEFs at 10 kV/cm revealed a slight increase in proliferation of chondrocytes at day 1 (1.14-fold), day 3 (1.03-fold) and day 7 (1.06-fold), whereas nsPEFs at 20 kV/cm significantly increased cell proliferation at day 1 (1.21-fold, p = 0.02), day 3 (1.18-fold, p = 0.02) and day 7 (1.04-fold) (Fig. 2b).
Figure 2. Induction of dedifferentiated phenotype of chondrocytes after nsPEF treatment.
(a) The toxicity of nsPEFs at 10 kV/cm and 20 kV/cm was evaluated at days 1, 3 and 7. nsPEFs had a nontoxic effect on chondrocytes (n = 5). (b) Cell proliferation was evaluated at days 1, 3 and 7 (n = 5). Cell proliferation increased after nsPEF treatment. (c) GAG content was evaluated at days 1, 3 and 7 (n = 4). GAG content decreased after nsPEF treatment. (d) GAG content produced per chondrocyte was evaluated by the total GAG content divided by cell numbers; it was repressed after 10 kV/cm and 20 kV/cm nsPEF treatment (n = 4). Data expressed as mean ± s.d. * = p < 0.05.
nsPEFs downregulate glycosaminoglycan (GAG) production
Results obtained demonstrated that nsPEFs at 10 kV/cm decreased GAG production at day 1 (0.91-fold), day 3 (0.96-fold) and day 7 (0.97-fold). Moreover, nsPEFs at 20 kV/cm also further decreased GAG production at day 1 (0.86-fold) and significantly at day 3 (0.78-fold, p = 0.046) and day 7 (0.88-fold, p = 0.041) (Fig. 2c). In addition, nsPEFs at 10 kV/cm decreased the GAG/cell ratio to 0.92-fold, 0.97-fold and 0.88-fold at days 1, 3 and 7, while nsPEFs at 20 kV/cm decreased the GAG/cell ratio at day 1 (0.83-fold), with significant findings at day 3 (0.67-fold, p = 0.012) and day 7 (0.81-fold, p = 0.047) (Fig. 2d).
nsPEFs downregulate expression of functional genes
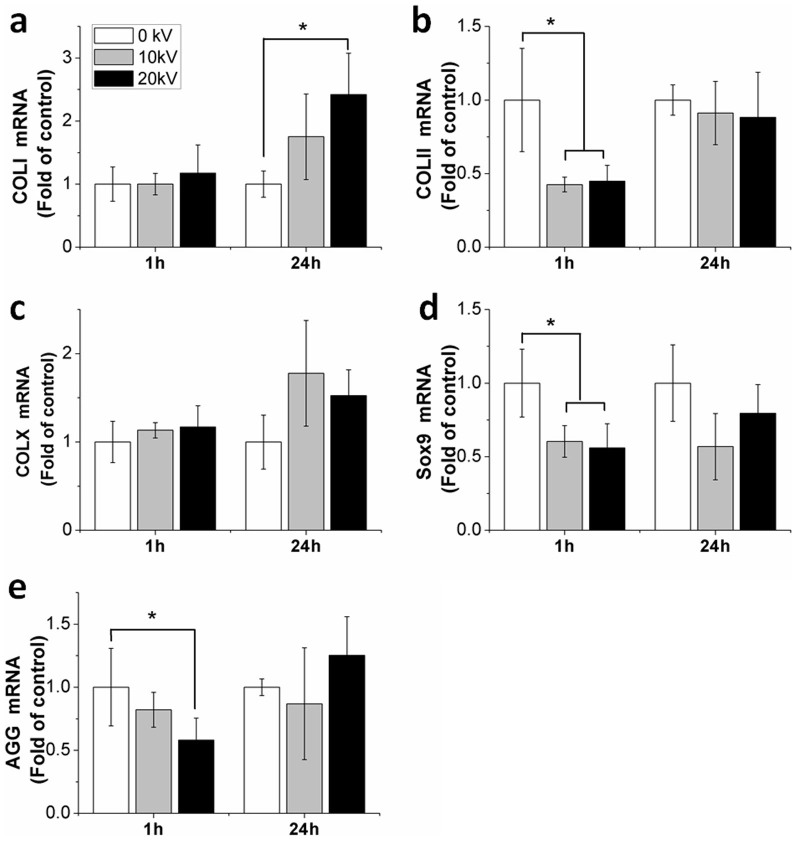
Effects of nsPEFs on gene expression were evaluated at 1 hour and 24 hours after nsPEF treatment. At 1 hour, nsPEFs at 10 kV/cm decreased gene expression of COL II significantly to 0.43-fold (p = 0.017), while nsPEFs at 20 kV/cm decreased COL II gene expression to 0.45-fold (p = 0.02) (Fig. 3b). Gene expression of Sox9 was significantly downregulated to 0.60-fold (p = 0.026) at 10 kV/cm and 0.56-fold (p = 0.014) at 20 kV/cm (Fig. 3d). Similarly, decreased induction of AGG was subsequently detected at 0.82-fold and 0.58-fold (p = 0.047) after 10 kV/cm and 20 kV/cm nsPEF treatment (Fig. 3e). Interestingly, nsPEFs increased gene expression of COL I to 1.02-fold at 10 kV/cm and 1.2-fold at 20 kV/cm. An increase in COL X gene expression to 1.13-fold at 10 kV/cm and 1.17-fold at 20 kV/cm (Fig. 3a, c) was also observed.
Figure 3. Functional gene expression of chondrocytes after nsPEF treatment.
Gene expression was analyzed at 1 hour for immediate effects and following a recovery period of 24 hours after 10 kV/cm and 20 kV/cm nsPEF treatment, respectively. (a) COL I; (b) COL II; (c) COL X; (d) Sox9; (e) AGG. Quantitative real-time PCR analysis was performed (n = 3). Untreated chondrocytes (0 kV) served as controls. Data expressed as mean ± s.d. * = p < 0.05.
At 24 hours after nsPEF treatment, gene expression showed an increase compared to the gene expression at 1 hour. An additive effect of COL I was indicated by an increase in the gene expression to 1.75-fold at 10 kV/cm and 2.42-fold (p = 0.02) at 20 kV/cm. Gene expression of COL II, COL X, Sox9 and AGG of nsPEF-altered returned to the levels of untreated chondrocytes, and no significant difference was observed when compared to the untreated cells.
nsPEFs activate wnt/β-catenin signaling pathway
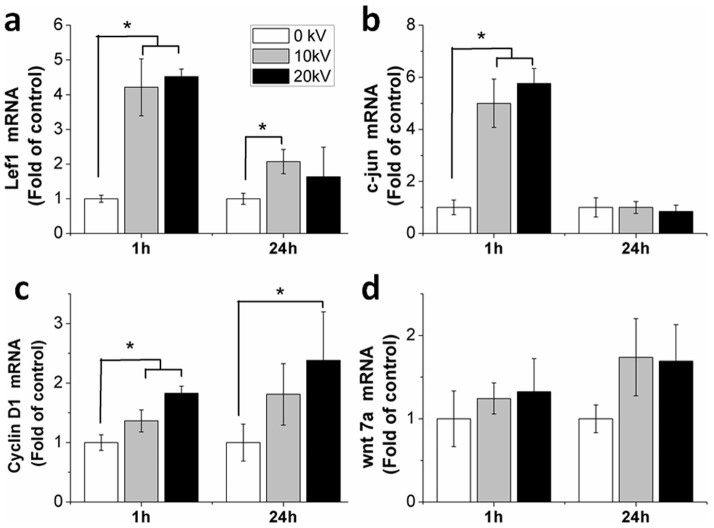
Expression of β-catenin protein increased significantly by 45% (p = 0.005) and 42% (p = 0.006) 1 hour after 10 kV/cm and 20 kV/cm nsPEF treatment, respectively (Fig. 4a, b). As shown, β-catenin accumulated in the nucleus of the cells after nsPEF treatment (Fig. 4c). 10 kV/cm and 20 kV/cm nsPEFs increased the wnt7a gene expression to 1.2-fold and 1.3-fold (Fig. 5d), respectively. Downstream genes of the wnt/β-catenin signaling pathway; Lef1, c-jun and cyclin D1, were also evaluated. Gene expression of Lef1 significantly increased to 4.2-fold (p = 0.001) and 4.5-fold (p = 0.001) after 10 kV/cm and 20 kV/cm nsPEF treatment (Fig. 5a), c-jun increased to 5.1-fold (p = 0.001) at 10 kV/cm and 5.9-fold (p = 0.001) at 20 kV/cm (Fig. 5b) and gene expression of cyclin D1 increased to 1.4-fold (p = 0.04) at 10 kV/cm and 1.8-fold (p = 0.01) at 20 kV/cm (Fig. 5c).
Figure 4. Activated wnt/β-catenin signaling pathway after nsPEF treatment.
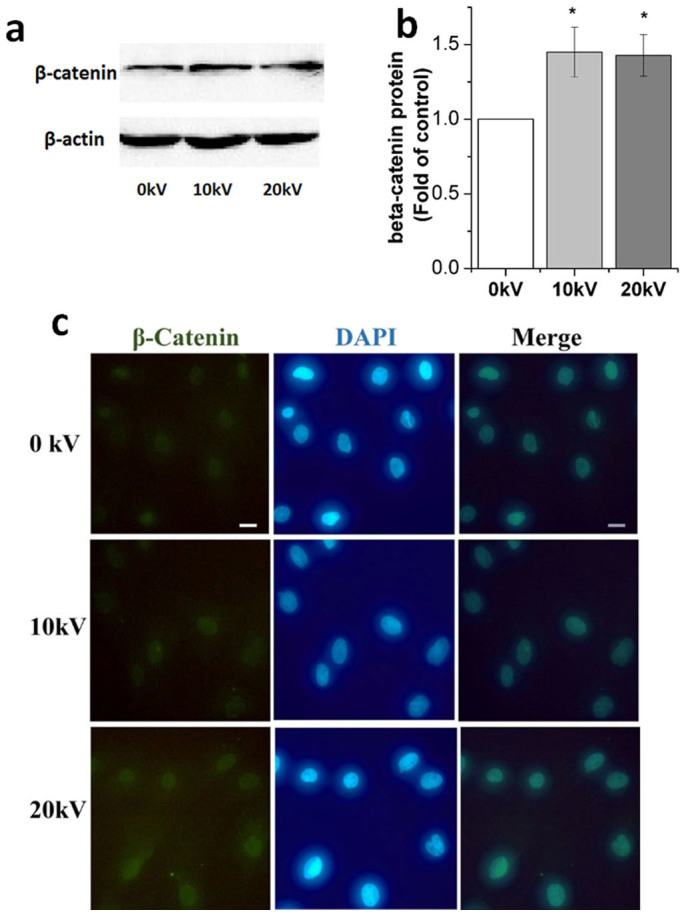
(a) β-catenin protein expression performed with western blotting analysis using specific antibodies for β-catenin at 1 hour after 0, 10 and 20 kV/cm nsPEF treatment. (b) Histogram of quantitative analysis of relative β-catenin expression. (c) Immunofluorescence images of chondrocytes after 0, 10 and 20 kV/cm nsPEF treatment. The β-catenin is labeled with specific antibodies (green). Nucleus is stained with DAPI (blue). Scale bar represents 10 μm. Data expressed as mean ± s.d. * = p < 0.05.
Figure 5. Influence of nsPEFs on gene expression related to wnt/β-catenin signaling.
Gene expression was detected immediately at 1 hour and following a 24 hour recovery after 0, 10 and 20 kV/cm nsPEF treatment, respectively. (a) Lef1; (b) c-jun; (c) cyclin D1; (d) wnt7a. Quantitative real-time PCR analysis was performed (n = 3). Untreated chondrocytes (0 kV) served as controls. Data expressed as mean ± s.d. * = p < 0.05.
Our findings demonstrated that after 24 hours, nsPEFs induced a decrease in Lef1 and c-jun gene expression in comparison to the gene expression observed after 1 hour. nsPEFs increased gene expression of Lef1 to 2.1-fold (p = 0.048) at 10 kV/cm and cyclin D1 to 2.4-fold (p = 0.027) at 20 kV/cm. No significant differences in the gene expression levels of other genes were observed when compared to the untreated cells.
Inhibition of wnt/β-catenin pathway partially blocks effects of nsPEFs
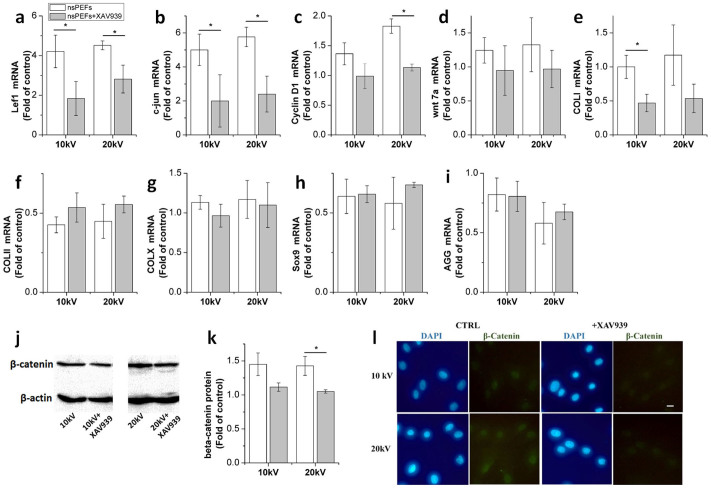
Incubation with XAV939 reverted nsPEF-induced upregulation of β-catenin (Fig. 6j–6l), wnt7a gene (Fig. 6d) and the downstream genes of wnt, Lef1 (Fig. 6a), c-jun (Fig. 6b) and cyclin D1 (Fig. 6c) by inhibition ranging from 30% to 60%. When the effects of inhibition were compared with the untreated chondrocytes, β-catenin, cyclin D1 and wnt7a levels were found to have decreased back to normal levels. Although Lef1 and c-jun levels recorded were higher after inhibition than those in the control group, no significant difference was observed. Results indicated that the wnt/β-catenin pathway played an integral role as a functional pathway after nsPEF treatment of chondrocytes. Functional gene expressions of COL II (Fig. 6f), Sox9 (Fig. 6h) and AGG (Fig. 6i) were elevated by approximately 20%, while COL I (Fig. 6e) was reduced by approximately 50% after XAV939 incubation with nsPEFs. Compared with the control group, functional gene expressions after inhibition were far less than those of untreated chondrocytes.
Figure 6. Influence of inhibition of wnt/β-catenin signaling on gene and protein expression after nsPEF treatment.
Gene expression was detected at 1 hour after 0, 10 and 20 kV/cm nsPEF treatment in the presence or absence of XAV939 (wnt inhibitor) compared with untreated chondrocytes. (a) Lef1; (b) c-jun; (c) cyclin D1; (d) wnt7a; (e) COL I; (f) COL II; (g) COL X; (h) Sox9; (i) AGG. Quantitative real-time PCR analysis was performed (n = 3). Untreated chondrocytes (0 kV) served as controls. (j) β-catenin protein expression was measured in the presence or absence of XAV939 with application of nsPEF treatment. (k) Quantitative analysis of β-catenin expression. (l) Immunofluorescence images of chondrocytes after nsPEF co-treatment with or without XAV939. The β-catenin is labeled with specific antibodies (green). Nucleus is stained with DAPI (blue). Scale bar represents 10 μm. Data expressed as mean ± s.d. * = p < 0.05.
Discussion
nsPEFs have profound effects on multiple organelles of a cell. nsPEFs generate large transmembrane potentials across cellular organelles with limited thermal effects. The most significant characteristic of nsPEFs is their short duration, which is less than the charging time of the plasma membrane with a microsecond range2,25,26. At high pulse duration, the outer plasma membrane with its large capacitance is markedly affected, and the potential across the interior is small. As pulse duration decreases, the outer membrane is likely shorted, and the applied voltage appears mainly across the interior organelles of the cell27. nsPEFs modify the potential and phosphatidylserine externalization of plasma membranes7, induce calcium ion bursts from the endoplasmic reticulum and cytochrome c release from the mitochondria9,11,12. Subsequently, nsPEFs influence nuclear activities by producing DNA speckles and RNA–protein complexes28,29, and produce newly-formed species of oxide, such as H2O230. nsPEFs provide a potential way for cellular interaction in the absence of ligand or receptor by induction of nanopores29,31.
Effects of nsPEFs are usually transient. Typically, the nanopores can be formed within 5 nanoseconds after nsPEF treatment7. Calcium ions efflux from the endoplasmic reticulum within 10 seconds following nsPEF treatment32. nsPEFs induce a cell fusion process within 4 minutes6, and phosphorylate the cellular stress factor eIF2α with a peak at 1 hour post-treatment23. Our study showed, via a time course analysis, that nsPEFs caused rapid effects on gene expression. We monitored the gene expressions of COL II and c-jun mRNA changes at 0.5, 1, 2, 6 and 24 hours. COL II gene expression was found to undergo a rapid and statistically significant decrease after nsPEF treatment, while no significant difference was observed after a recovery period of 1 day (Supplementary Fig. S2a). C-jun gene expression increased immediately to a maximum level at 1 hour, and decreased to the normal level after a recovery period of 1 day (Supplementary Fig. S2b).
Fine-tuned activation of the wnt/β-catenin signaling pathway is essential to regulate the fate of chondrocytes in cartilage. The wnt/β-catenin pathway is involved in dedifferentiation of cultured chondrocytes33. Once activated, wnt ligands combine with frizzled receptors and coreceptors, such as lipoprotein receptors, to facilitate β-catenin accumulation, which subsequently enters the nucleus to regulate the transcription of genes, such as Lef1, c-jun and cyclin D134. Activation of the wnt/β-catenin signaling leads to proliferation of chondrocytes via upregulation of cyclin D1, and dedifferentiation via Lef1, c-jun and Sox934,35,36. Previous studies have illustrated that nsPEFs enhance transcription machinery of the nucleus, mediated through the penetration of intracellular membranes and the induction of nanopores32,37. In addition, Sox9 directly regulates COL II expression by binding the first intron sequences of COL II38, as well as AGG expression39. Although abnormal activation of wnt/β-catenin signaling leads to degradation of cartilage matrix and enhances dedifferentiation of chondrocytes40,41, inhibition of the wnt/β-catenin signaling also leads to apoptosis of chondrocytes in articular cartilage42. nsPEFs induce a transient response of the wnt/β-catenin pathway and can be used to regulate cells in a dose and time-dependent manner43.
The fact that inhibition of the wnt/β-catenin signaling can only partially block the effects of nsPEFs on chondrocytes hints that there are other mechanisms involved. The functional crosstalk between signaling pathways introduces the possibility that nsPEFs simultaneously activate multiple pathways. A previous study showed that nsPEF-activated gene expression which was inhibited by 30% after co-treatment with a JNK inhibitor, however gene expression was found to be 5-fold higher than the cells without nsPEF treatment44. nsPEFs activate and induce biochemical changes, such as bursts of calcium ions45,26. Calcium ions serve as second messengers and provide important upstream signals for cellular mechanisms to occur such as proliferation, differentiation and apoptosis46. Similar to previous reports, we found calcium ion release after nsPEF treatment (Supplementary Fig. S3). Although a calcium ion chelator effectively blocked calcium efflux, the chelator showed no effect on chondrocyte phenotypes (Supplementary Fig. S4). Previous research has shown that nanopores generated by nsPEF treatment facilitate bursts of calcium ion release, while traditional PEFs cause calcium ion release by altering the transmembrane energy barriers7. nsPEFs also influence nuclear activities and processes. Since DNA is heavily charged due to its folded spiral structure, it is sensitive to nsPEFs and forms speckles after nsPEF treatment28. nsPEFs induce small nuclear ribonucleoprotein particles and RNA-protein complexes, which are important in messenger RNA transcriptional functions29. nsPEFs affect cellular behavior by introducing a mechanical stress through thermoelastic expansion23,47. Therefore, the potential electrochemical influence of electrolysis in high voltage electric fields need to be considered48. Several parameters, such as strength, pulse duration and number of stimulating electric fields, also add complexity to the effects of nsPEFs45. Although the relationship between nsPEFs and cells is not lucid, nsPEFs remain a promising application, as they can potentially alter biochemical, biophysical and electrochemical properties of cells, with tunable parameters.
nsPEFs may have different effects on suspended and attached cells in vitro. Cells in a suspended state were subjected to nsPEFs within electric cuvettes, which provided an instantaneous and consistent distribution of an electric-field49. Similar trends of gene expression were observed in chondrocytes cultured in both an attached and a suspended state. The gene expressions of Lef1, c-jun, cyclin D1, wnt7a and COL I were upregulated, while the expression of COL II, Sox9 and AGG were downregulated (Supplementary Fig. S5). However, the change in gene expression of attached chondrocytes was less than that of suspended chondrocytes, and significant differences could be found in Lef1, c-jun, COLII and Sox9 expression. Results showed that chondrocytes cultured in the attached state were less expressive in comparison with those cultured in a suspended state. Culturing state is an important factor affecting cell physiological function since mechanical properties mainly depend on cellular cytoskeletal structure50. A previous report has shown that cell survival and genotoxic effects of non-adherent cells may be more sensitive than that of adherent cells after nsPEF treatment28. The cytoskeleton, a possible factor affecting cellular viability in both non-adherent and adherent cells, has been demonstrated to be less effective in sustaining cellular viability after nsPEF treatment51. In order to evaluate the cells in an adherent state, a standard culturing system needs to be developed, comparable to the electric cuvette system in the suspended state. One such possibility may be the development of a real-time visual microfluidic system for monitoring adherent cells combined with nsPEFs52.
As nsPEFs do not exist in a natural cellular environment, a comprehensive understanding of nsPEFs as well as the effects of superficial stimuli need to be further explored to determine any difference from previously known cellular functions53. Varied parameters of nsPEFs, such as duration, number of pulses and intensity, may be used to provide versatile tools to regulate different biological processes in the future. The effects of nsPEFs may depend on multiple parameters, such as depth, dose, cell type, cell attachment and tissue type. Understanding nsPEFs influence matrix metabolism and cross-talk signaling pathways may lead to potential methods to regulate genetics. Further research is needed to determine the applicability of nsPEFs on whole tissues.
Methods
Cell culture
Porcine articular cartilage tissue was cut into small pieces by a lancet, and washed with phosphate-buffered saline (PBS). The tissue pieces were collected and digested in 0.1% Collagenase II (17101-015, Gibico) dissolved in Dulbecco's modified Eagle's medium (DMEM, 31600-034, Invitrogen) at 37°C overnight. The isolated chondrocytes were harvested and cultured in monolayer in culture plates with the medium containing 90% DMEM, 10% fetal bovine serum (FBS, SV30087.02, Gibico), and 0.1% penicillin/streptomycin (PS) at 37°C in humidified atmosphere with 5% CO2. Cultured medium was changed every three days. When chondrocytes reached 85% confluency, they were trypsinized with 0.25% trypsin (27250-018, Invitrogen) and frozen in culture medium containing 10% dimethyl sulfoxide (DMSO, Merck). The thawed chondrocytes were prepared for further experimentation. To inhibit the effects of wnt activation, XAV939 (13596, 1 μM, Cayman Chemical) was utilized to pre-treat chondrocytes overnight before nsPEF treatment.
Application of nsPEFs
The nsPEF generator was applied as previously described54. Digital phosphor oscilloscope (DPO4054, Tektronix) with a probe (P6015A, Tektronix) was utilized to monitor the voltage waveform. Chondrocytes were counted with a hemocytometer, and 1.0 × 106 chondrocytes suspended in 500 μL culture medium were added to 0.2 cm gap cuvettes (Biosmith, aluminum plate electrodes, San Diego, CA). The experimental cuvettes were treated with 5 pulses of nsPEFs with 100 ns durations at 10 kV/cm or 20 kV/cm electric fields. Time between each pulse was about 1 s. Cuvettes that did not undergo nsPEF treatment served as the control group.
Celltoxicity
The toxicity of nsPEFs was evaluated by 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT, M2128, Sigma) at days 1, 3 and 7. 5.0 × 103 chondrocytes/well were seeded in 96-well plates. 20 mL of 5 mg/mL MTT solution was added to each well, which were then incubated at 37°C for 3 hours. The solution was emptied and 150 μL of DMSO was added. Optical density was measured at a wavelength of 570 nm with Microplate Reader (680, Bio-rad) to determine viable chondrocytes. The value was expressed as the ratio of the experimental group divided by the control group. Five samples of each group were measured.
Cell proliferation
Cell proliferation was assayed with Hoechst 33258 (H6024, Sigma) at days 1, 3 and 7. Chondrocytes in each well were lysed with 100 μL sterile distilled DNAse-free H2O, and the dissolved solution was collected and transferred into 96-well microtiter plates. 100 μL of 0.1 μg/mL Hoechst 33258 was added to each well and detected with the Microplate Reader (CEMINI XS, Molecular Devices). The fluorescence was recorded at 360 nm excitation and 460 nm emission. Five samples of each group were measured.
GAG content
Chondrocytes were digested in 0.5 mg/mL proteinase K at 56°C for 12 hours, and were subsequently examined with dimethylmethylene blue (DMMB, 341088, Sigma). The digestive solution was shaken for 30 minutes, then centrifuged at 10000 × g for 10 minutes. The centrifugal sediment was dissolved in a decomplexation solution and absorbance was detected at 630 nm. The GAG content was calculated according to the standard curve by chondroitin sulfate (27042-10G-F, Sigma). Four samples of each group were measured at days 1, 3 and 7.
Gene expression
Total RNA was extracted and isolated from chondrocytes with Trizol Reagent (206101, New Industry) following the standard protocol, and quantified with Nanodrop spectrophotometer (ND-1000, Thermo). Total RNA (500 ng) was used to perform the reverse transcription reaction with M-MLV reverse transcriptase (C28025, Sigma) and oligo(dT) (FSK-201, TOYOBO) in a PCR thermal cycler (Mycycler, Bio-Rad). Quantitative real-time PCR was performed in the PCR system (Pikoreal 96, Thermo) with RealMasterMix SYBR Green (FP202, Tiangen) following the manufacturer's procedures. The expression of collagen type I (COL I), collagen type II (COL II), collagen type X (COL X), aggrecan (AGG), Sox9, wnt 7a, Lef1, c-jun, cyclin D1, and GAPDH were assessed using PCR with the gene-specific primers listed below: for COL I, 5′- CAG AAC GGC CTC AGG TAC CA-3′ (sense) and 5′- CAG ATC ACG TCA TCG CAC AAC-3′ (antisense); for COL II, 5′- GAG AGG TCT TCC TGG CAA AG-3′ (sense) and 5′- AAG TCC CTG GAA GCC AGAT-3′ (antisense); for COL X, 5′- CAG GTA CCA GAG GTC CCA TC-3′ (sense) and 5′- CAT TGA GGC CCT TAG TTG CT-3′ (antisense); for AGG, 5′- CGA AAC ATC ACC GAG GGT-3′ (sense) and 5′- GCA AAT GTA AAG GGC TCC TC-3′ (antisense); for Sox9 5′-ATC AGT ACC CGC ACC TGC AC-3′ (sense) and 5′-CTT GTA ATC CGG GTG GTC CTT-3′ (antisense); for wnt 7a, 5′- TGC CCG GAC TCT CAT GAA C-3′ (sense) and 5′- GTG TGG TCC AGC AGG TCT TG-3′ (antisense); for Lef1, 5′- CAG TGG ACC CCA AAG GAG AC -3′ (sense) and 5′- CAC AGG TGT GGA TGC AGG AT -3′ (antisense); for c-jun, 5′- CCC CTG TCT CCC ATC GAC ATG-3′ (sense) and 5′- TTG CAA CTG CTG CGT TAG CAT-3′ (antisense); for cyclin D1, 5′- AAC ACG GCT CAC GCT TAC-3′ (sense) and 5′- CCA GAC CCT CAG ACT TGC-3′ (antisense); for GAPDH, 5′- GTC ATC CAT GAC AAC TTC GG-3′ (sense) and 5′- GCC ACA GTT TCC CAG AGG-3′ (antisense). The target genes of each sample were normalized to the values of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as internal control. Three samples of each group were measured.
Western blotting
Chondrocytes were lysed by RIPA lysis buffer (R0020, Solarbio) with fresh protease inhibitor of 0.1% phenylmethanesulfonyl fluoride (PMSF, Solarbio). Total cell lysate was boiled after 4 × SDS loading buffer (P1015, Solarbio) was added. Samples were stored at −80° before SDS polyacrylamide gel electrophoresis. Western blotting was carried out according to standard protocol. Rabbit polyclonal antibody against β-catenin (sc-7199, Santa Cruz Biotechnology) and β-actin (4970, Cell Signaling) was combined with HRP-linked antibody of anti-rabbit IgG (7074, Cell Signaling). The complex of the antigen and the antibody was illuminated by chemiluminescence and detected by ChemiDoc XRS + Molecular Imager (BioRad), then quantified by Quantity One image software (BioRad).
Immunofluorescence
Immunofluorescence was utilized to confirm the location of β-catenin protein. After nsPEF treatment, chondrocytes were fixed with 4% paraformaldehyde for 15 minutes and washed with PBS twice. Cellular permeation was elevated by treatment with 0.5% (v/v) Triton-100, followed by addition of 5% bovine serum albumin (BSA). Chondrocytes were incubated with a primary antibody against β-catenin (sc-7199, Santa Cruz Biotechnology), followed by FITC goat anti-rabbit IgG (0114, Cwbio). The nucleus was stained with DAPI. Relative location between β-catenin and DAPI was observed under the fluorescence microscopy.
Statistical analysis
Analysis was performed using SPSS V13.0 (SPSS Inc.) one-way ANOVA with the least significant difference (LSD) test (data presented as mean ± s.d). The statistical significance was set at 95% confidence interval, with significance level of p < 0.05.
Supplementary Material
Supplementary Information
Acknowledgments
This work was supported by National Basic Research Program of China (973 Program) (2012CB619100), and National Natural Science Foundation of China grant (81271722). The authors would like to thank A. Mujeeb, S. Moran, T. Thote, B. Faught and J. Wang for reviewing this manuscript.
Footnotes
The authors declare no competing financial interests.
Author Contributions Z.G. and J.Z. designed this study, whereas K.Z. and J.G. were involved in the experimentation. K.Z. and Z.G. analyzed data and wrote the manuscript with assistance from J.G. and J.Z. All authors reviewed the manuscript.
References
- Chen C., Smye S. W., Robinson M. P. & Evans J. A. Membrane electroporation theories: a review. Med. Biol. Eng. Comput. 44, 5–14 (2006). [DOI] [PubMed] [Google Scholar]
- Awasthi K., Nakabayashi T. & Ohta N. Application of Nanosecond Pulsed Electric Fields into He La Cells Expressing Enhanced Green Fluorescent Protein and Fluorescence Lifetime Microscopy. J. Phys. Chem. B 116, 11159–11165 (2012). [DOI] [PubMed] [Google Scholar]
- Weaver J. C. Electroporation of biological membranes from multicellular to nano scales. IEEE Trns. Dielectr. Electr. Insul. 10, 754–768 (2003). [Google Scholar]
- Schoenbach K. H. et al. Ultrashort electrical pulses open a new gateway into biological cells. Proc. IEEE 92, 1122–1137 (2004). [Google Scholar]
- Silve A., Leray I. & Mir L. M. Demonstration of cell membrane permeabilization to medium-sized molecules caused by a single 10 ns electric pulse. Bioelectrochemistry 87, 260–264 (2012). [DOI] [PubMed] [Google Scholar]
- Rems L. et al. Cell electrofusion using nanosecond electric pulses. Sci. Rep. 3, 3382; 10.1038/srep03382 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q., Joshi R. P. & Schoenbach K. H. Simulations of nanopore formation and phosphatidylserine externalization in lipid membranes subjected to a high-intensity, ultrashort electric pulse. Phys. Rev. E 72 (2005). [DOI] [PubMed] [Google Scholar]
- Schoenbach K. H., Beebe S. J. & Buescher E. S. Intracellular effect of ultrashort electrical pulses. Bioelectromagnetics 22, 440–448 (2001). [DOI] [PubMed] [Google Scholar]
- White J. A., Blackmore P. F., Schoenbach K. H. & Beebe S. J. Stimulation of capacitative calcium entry in HL-60 cells by nanosecond pulsed electric fields. J. Biol. Chem. 279, 22964–22972 (2004). [DOI] [PubMed] [Google Scholar]
- Morotomi-Yano K., Akiyama H. & Yano K. Nanosecond pulsed electric fields activate MAPK pathways in human cells. Arch. Biochem. Biophys. 515, 99–106 (2011). [DOI] [PubMed] [Google Scholar]
- Garon E. B. et al. In vitro and in vivo evaluation and a case report of intense nanosecond pulsed electric field as a local therapy for human malignancies. Int. J. Cancer 121, 675–682 (2007). [DOI] [PubMed] [Google Scholar]
- Ren W., Sain N. M. & Beebe S. J. Nanosecond pulsed electric fields (nsPEFs) activate intrinsic caspase-dependent and caspase-independent cell death in Jurkat cells. Biochem. Biophys. Res. Commun. 421, 808–812 (2012). [DOI] [PubMed] [Google Scholar]
- Basu G., Kalluri B. S., Sabuncu A. C., Osgood C. J. & Stacey M. W. Enhanced Killing Effect of Nanosecond Pulse Electric Fields on PANC1 and Jurkat Cell Lines in the Presence of Tween 80. The Journal of Membrane Biology 245, 611–616 (2012). [DOI] [PubMed] [Google Scholar]
- van der Kraan P. M., Davidson E. N. B., Blom A. & van den Berg W. B. TGF-beta signaling in chondrocyte terminal differentiation and osteoarthritis Modulation and integration of signaling pathways through receptor-Smads. Osteoarthr Cartilage 17, 1539–1545 (2009). [DOI] [PubMed] [Google Scholar]
- Grimsrud C. D. et al. BMP signaling stimulates chondrocyte maturation and the expression of Indian hedgehog. Journal of Orthopaedic Research 19, 18–25 (2001). [DOI] [PubMed] [Google Scholar]
- Riemer S., Gebhard S., Beier F., Poschl E. & von der Mark K. Role of c-fos in the regulation of type X collagen gene expression by PTH and PTHrP: Localization of a PTH/PTHrP-responsive region in the human COL10A1 enhancer. Journal of Cellular Biochemistry 86, 688–699 (2002). [DOI] [PubMed] [Google Scholar]
- Ryu J., Kang S. & Chun J. Regulation of the chondrocyte phenotype by beta-catenin. Mol Biol Cell 13, 118a–118a (2002). [DOI] [PubMed] [Google Scholar]
- Lyu J. & Joo C. K. Wnt-7a up-regulates matrix metalloproteinase-12 expression and promotes cell proliferation in corneal epithelial cells during wound healing. J. Biol. Chem. 280, 21653–21660 (2005). [DOI] [PubMed] [Google Scholar]
- Fini M. et al. Functional Tissue Engineering in Articular Cartilage Repair: Is There a Role for Electromagnetic Biophysical Stimulation? Tissue Eng Part B-Re 19, 353–367 (2013). [DOI] [PubMed] [Google Scholar]
- Macginitie L. A., Gluzband Y. A. & Grodzinsky A. J. Electric-Field Stimulation Can Increase Protein-Synthesis in Articular-Cartilage Explants. Journal of Orthopaedic Research 12, 151–160 (1994). [DOI] [PubMed] [Google Scholar]
- Lippiello L., Chakkalakal D. & Connolly J. F. Pulsing Direct Current-Induced Repair of Articular-Cartilage in Rabbit Osteochondral Defects. Journal of Orthopaedic Research 8, 266–275 (1990). [DOI] [PubMed] [Google Scholar]
- Wang W., Wang Z. Y., Zhang G. H., Clark C. C. & Brighton C. T. Up-regulation of chondrocyte matrix genes and products by electric fields. Clin Orthop Relat R, S163–S173 (2004). [DOI] [PubMed] [Google Scholar]
- Morotomi-Yano K., Oyadomari S., Akiyama H. & Yano K. Nanosecond pulsed electric fields act as a novel cellular stress that induces translational suppression accompanied by eIF2 alpha phosphorylation and 4E-BP1 dephosphorylation. Experimental Cell Research 318, 1733–1744 (2012). [DOI] [PubMed] [Google Scholar]
- Ge Z. G., Baguenard S., Lim L. Y., Wee A. & Khor E. Hydroxyapatite-chitin materials as potential tissue engineered bone substitutes. Biomaterials 25, 1049–1058 (2004). [DOI] [PubMed] [Google Scholar]
- Schoenbach K. H., Katsuki S., Stark R. H., Buescher E. S. & Beebe S. J. Bioelectrics - New applications for pulsed power technology. IEEE Trans. Plasma Sci. 30, 293–300 (2002). [Google Scholar]
- Frey W. et al. Plasma membrane voltage changes during nanosecond pulsed electric field exposure. Biophysical Journal 90, 3608–3615 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellappan P. & Sundararajan R. A simulation study of the electrical model of a biological cell. J. Electrost. 63, 297–307 (2005). [Google Scholar]
- Stacey M. et al. Differential effects in cells exposed to ultra-short, high intensity electric fields: cell survival, DNA damage, and cell cycle analysis. Mutat Res-Gen Tox En 542, 65–75 (2003). [DOI] [PubMed] [Google Scholar]
- Chen N. Y. et al. Nanosecond electric pulses penetrate the nucleus an enhance speckle formation. Biochem. Biophys. Res. Commun. 364, 220–225 (2007). [DOI] [PubMed] [Google Scholar]
- Pakhomova O. N. et al. Oxidative effects of nanosecond pulsed electric field exposure in cells and cell-free media. Arch. Biochem. Biophys. 527, 55–64 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. et al. Nanosecond pulse electric field (nanopulse): A novel non-ligand agonist for platelet activation. Arch. Biochem. Biophys. 471, 240–248 (2008). [DOI] [PubMed] [Google Scholar]
- Beebe S. J., Blackmore P. F., White J., Joshi R. P. & Schoenbach K. H. Nanosecond pulsed electric fields modulate cell function through intracellular signal transduction mechanisms. Physiol Meas 25, 1077–1093 (2004). [DOI] [PubMed] [Google Scholar]
- Hwang S. G., Yu S. S., Poo H. & Chun J. S. c-Jun/activator protein-1 mediates interleukin-1 beta-induced dedifferentiation but not cyclooxygenase-2 expression in articular chondrocytes. J. Biol. Chem. 280, 29780–29787 (2005). [DOI] [PubMed] [Google Scholar]
- Novak A. & Dedhar S. Signaling through beta-catenin and Lef/Tcf. Cellular and Molecular Life Sciences 56, 523–537 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shtutman M. et al. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. P Natl Acad Sci USA 96, 5522–5527 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S. G., Yu S. S., Lee S. W. & Chun J. S. Wnt-3a regulates chondrocyte differentiation via c-Jun/AP-1 pathway. Febs Lett 579, 4837–4842 (2005). [DOI] [PubMed] [Google Scholar]
- Beebe S. J., Fox P. M., Rec L. J., Willis L. K. & Schoenbach K. H. Nanosecond, high-intensity pulsed electric fields induce apoptosis in human cells. Faseb J 17, 1493–+ (2003). [DOI] [PubMed] [Google Scholar]
- Bell D. M. et al. SOX9 directly regulates the type-II collagen gene. Nat Genet 16, 174–178 (1997). [DOI] [PubMed] [Google Scholar]
- de Crombrugghe B. et al. Transcriptional mechanisms of chondrocyte differentiation. Matrix Biol 19, 389–394 (2000). [DOI] [PubMed] [Google Scholar]
- Ryu J. H. et al. Regulation of the chondrocyte phenotype by beta-catenin. Development 129, 5541–5550 (2002). [DOI] [PubMed] [Google Scholar]
- Zhu M. et al. Activation of beta-Catenin Signaling in Articular Chondrocytes Leads to Osteoarthritis-Like Phenotype in Adult beta-Catenin Conditional Activation Mice. J Bone Miner Res 24, 12–21 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M. et al. Inhibition of beta-catenin signaling in articular chondrocytes results in articular cartilage destruction. Arthritis Rheum. 58, 2053–2064 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibey B. L. et al. Dose-Dependent Thresholds of 10-ns Electric Pulse Induced Plasma Membrane Disruption and Cytotoxicity in Multiple Cell Lines. PLoS One 6 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morotomi-Yano K., Uemura Y., Katsuki S., Akiyama H. & Yano K. Activation of the JNK pathway by nanosecond pulsed electric fields. Biochem. Biophys. Res. Commun. 408, 471–476 (2011). [DOI] [PubMed] [Google Scholar]
- Scarlett S. S., White J. A., Blackmore P. F., Schoenbach K. H. & Kolb J. F. Regulation of intracellular calcium concentration by nanosecond pulsed electric fields. Biochim. Biophys. Acta-Biomembr. 1788, 1168–1175 (2009). [DOI] [PubMed] [Google Scholar]
- Nutt L. K. et al. Bax-mediated Ca2+ mobilization promotes cytochrome c release during apoptosis. J. Biol. Chem. 277, 20301–20308 (2002). [DOI] [PubMed] [Google Scholar]
- Lin J. C. Microwave Auditory Phenomenon. Proc. IEEE 68, 67–73 (1980). [Google Scholar]
- Pakhomov A. G. et al. Long-lasting plasma membrane permeabilization in mammalian cells by nanosecond pulsed electric field (nsPEF). Bioelectromagnetics 28, 655–663 (2007). [DOI] [PubMed] [Google Scholar]
- Kirawanich P., Pausawasdi N., Srisawat C., Yakura S. J. & Islam N. E. An FDTD Interaction Scheme of a High-Intensity Nanosecond-Pulsed Electric-Field System for In Vitro Cell Apoptosis Applications. IEEE Trans. Plasma Sci. 38, 2574–2582 (2010). [Google Scholar]
- Wang N., Butler J. P. & Ingber D. E. Mechanotransduction across the Cell-Surface and through the Cytoskeleton. Science 260, 1124–1127 (1993). [DOI] [PubMed] [Google Scholar]
- Thompson G. L., Roth C., Tolstykh G., Kuipers M. & Ibey B. L. Role of Cytoskeleton and Elastic Moduli in Cellular Response to Nanosecond Pulsed Electric Fields. Proc Spie 8585 (2013). [Google Scholar]
- Dalmay C., De Menorval M. A., Francais O., Mir L. M. & Le Pioufle B. A microfluidic device with removable packaging for the real time visualisation of intracellular effects of nanosecond electrical pulses on adherent cells. Lab Chip 12, 4709–4715 (2012). [DOI] [PubMed] [Google Scholar]
- Beebe S. J. Cell responses without receptors and ligands, using nanosecond pulsed electric fields (nsPEFs). Int J Nanomed 8 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. et al. Synergistic Effects of Nanosecond Pulsed Electric Fields Combined with Low Concentration of Gemcitabine on Human Oral Squamous Cell Carcinoma In Vitro. PLoS One 7 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information