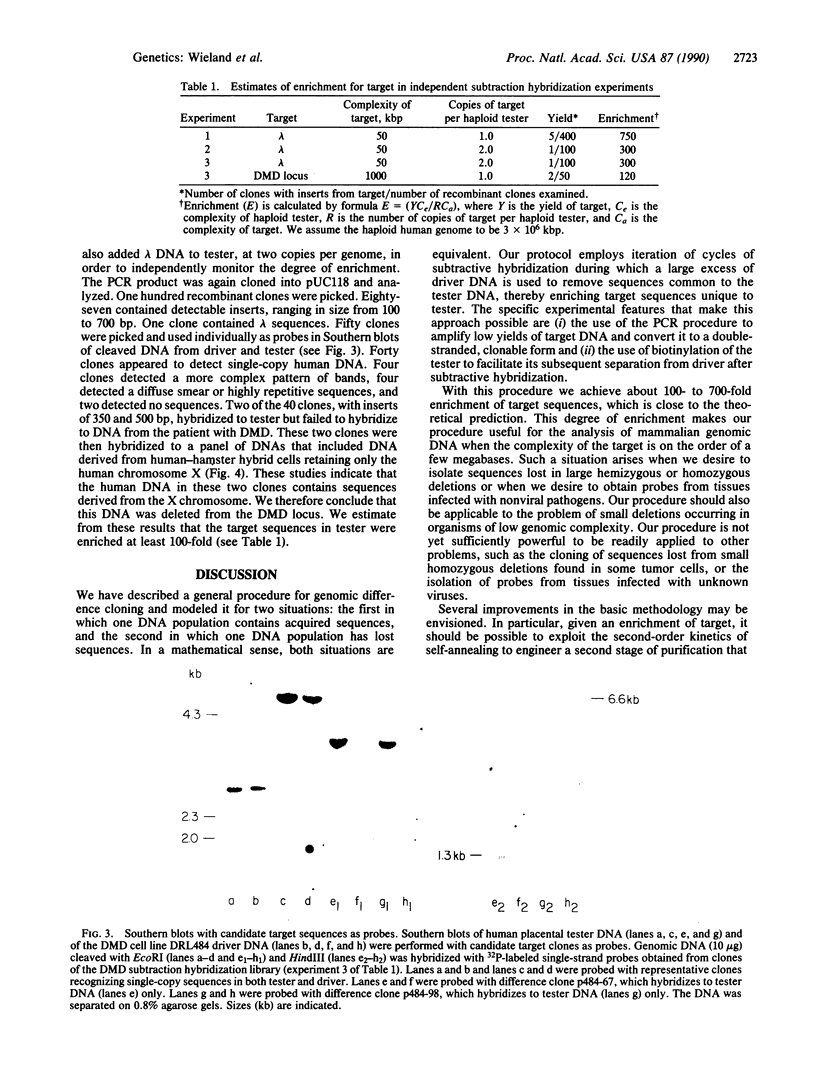
Abstract
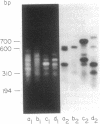
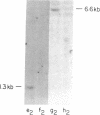
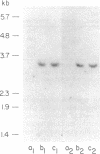
We describe a procedure for genomic difference cloning, a method for isolating sequences present in one genomic DNA population ("tester") that is absent in another ("driver"). By subtractive hybridization, a large excess of driver is used to remove sequences common to a biotinylated tester, enriching the "target" sequences that are unique to the tester. After repeated subtractive hybridization cycles, tester is separated from driver by avidin/biotin affinity chromatography, and single-stranded target is amplified by the polymerase chain reaction, rendering it double-stranded and clonable. We model two situations: the gain of sequences that result from infection with a pathogen and the loss of sequences that result from a large hemizygous deletion. We obtain 100- to 700-fold enrichment of target sequences.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birchmeier C., Birnbaum D., Waitches G., Fasano O., Wigler M. Characterization of an activated human ros gene. Mol Cell Biol. 1986 Sep;6(9):3109–3116. doi: 10.1128/mcb.6.9.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano O., Birnbaum D., Edlund L., Fogh J., Wigler M. New human transforming genes detected by a tumorigenicity assay. Mol Cell Biol. 1984 Sep;4(9):1695–1705. doi: 10.1128/mcb.4.9.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick S. M., Cohen D. I., Nielsen E. A., Davis M. M. Isolation of cDNA clones encoding T cell-specific membrane-associated proteins. Nature. 1984 Mar 8;308(5955):149–153. doi: 10.1038/308149a0. [DOI] [PubMed] [Google Scholar]
- Herman T. M., Lefever E., Shimkus M. Affinity chromatography of DNA labeled with chemically cleavable biotinylated nucleotide analogs. Anal Biochem. 1986 Jul;156(1):48–55. doi: 10.1016/0003-2697(86)90152-1. [DOI] [PubMed] [Google Scholar]
- Kanter P. M., Schwartz H. S. A hydroxylapatite batch assay for quantitation of cellular DNA damage. Anal Biochem. 1979 Aug;97(1):77–84. doi: 10.1016/0003-2697(79)90330-0. [DOI] [PubMed] [Google Scholar]
- Kohne D. E., Levison S. A., Byers M. J. Room temperature method for increasing the rate of DNA reassociation by many thousandfold: the phenol emulsion reassociation technique. Biochemistry. 1977 Nov 29;16(24):5329–5341. doi: 10.1021/bi00643a026. [DOI] [PubMed] [Google Scholar]
- Kunkel L. M., Monaco A. P., Middlesworth W., Ochs H. D., Latt S. A. Specific cloning of DNA fragments absent from the DNA of a male patient with an X chromosome deletion. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4778–4782. doi: 10.1073/pnas.82.14.4778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamar E. E., Palmer E. Y-encoded, species-specific DNA in mice: evidence that the Y chromosome exists in two polymorphic forms in inbred strains. Cell. 1984 May;37(1):171–177. doi: 10.1016/0092-8674(84)90312-x. [DOI] [PubMed] [Google Scholar]
- Landegren U., Kaiser R., Caskey C. T., Hood L. DNA diagnostics--molecular techniques and automation. Science. 1988 Oct 14;242(4876):229–237. doi: 10.1126/science.3051381. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Enhanced autoradiographic detection of 32P and 125I using intensifying screens and hypersensitized film. FEBS Lett. 1977 Oct 15;82(2):314–316. doi: 10.1016/0014-5793(77)80609-1. [DOI] [PubMed] [Google Scholar]
- Ley T. J., Anagnou N. P., Pepe G., Nienhuis A. W. RNA processing errors in patients with beta-thalassemia. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4775–4779. doi: 10.1073/pnas.79.15.4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J. B. Molecular genetics: applications to the clinical neurosciences. Science. 1987 Nov 6;238(4828):765–772. doi: 10.1126/science.2890208. [DOI] [PubMed] [Google Scholar]
- Nussbaum R. L., Lesko J. G., Lewis R. A., Ledbetter S. A., Ledbetter D. H. Isolation of anonymous DNA sequences from within a submicroscopic X chromosomal deletion in a patient with choroideremia, deafness, and mental retardation. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6521–6525. doi: 10.1073/pnas.84.18.6521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perucho M., Goldfarb M., Shimizu K., Lama C., Fogh J., Wigler M. Human-tumor-derived cell lines contain common and different transforming genes. Cell. 1981 Dec;27(3 Pt 2):467–476. doi: 10.1016/0092-8674(81)90388-3. [DOI] [PubMed] [Google Scholar]
- Ponder B. Cancer. Gene losses in human tumours. Nature. 1988 Sep 29;335(6189):400–402. doi: 10.1038/335400a0. [DOI] [PubMed] [Google Scholar]
- Rowekamp W., Firtel R. A. Isolation of developmentally regulated genes from Dictyostelium. Dev Biol. 1980 Oct;79(2):409–418. doi: 10.1016/0012-1606(80)90126-8. [DOI] [PubMed] [Google Scholar]
- Sager R. Tumor suppressor genes: the puzzle and the promise. Science. 1989 Dec 15;246(4936):1406–1412. doi: 10.1126/science.2574499. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Schutzbank T., Robinson R., Oren M., Levine A. J. SV40 large tumor antigen can regulate some cellular transcripts in a positive fashion. Cell. 1982 Sep;30(2):481–490. doi: 10.1016/0092-8674(82)90245-8. [DOI] [PubMed] [Google Scholar]
- Scott M. R., Westphal K. H., Rigby P. W. Activation of mouse genes in transformed cells. Cell. 1983 Sep;34(2):557–567. doi: 10.1016/0092-8674(83)90388-4. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]