Abstract
Maize (Zea mays) pollen is highly nutritious and can be used by predatory arthropods to supplement or replace a carnivorous diet. We demonstrate that maize pollen can be utilized by larvae of the green lacewing, Chrysoperla carnea (Neuroptera: Chrysopidae) under laboratory conditions. Complete development on maize pollen was not possible, but 25% of neonates reached the third instar. When only one instar was fed with pollen and the other two instars with eggs of Ephestia kuehniella (Lepidoptera: Pyralidae), 58–87% of the larvae reached the pupal stage. The experiments included pollen produced by nine cultivars: three genetically modified (GM) cultivars expressing the Bacillus thuringiensis proteins Cry1Ab or Cry3Bb1, their corresponding non-transformed near-isolines, and three conventional cultivars. Maize cultivars were grown in two batches in a glasshouse. Their pollen differed by up to 59% in total protein content, 25% in C:N ratio, and 14% in grain diameter, but the differences were inconsistent and depended on the batch. Lacewing performance was not affected by maize cultivar. For environmental risk assessment of GM plants, in planta studies must consider the variability among conventional cultivars, individual plants, batches, and environmental conditions when evaluating the ecological significance of differences observed between GM and near-isolines.
During maize anthesis, up to 50 million pollen grains can be produced per tassel and, aided by wind pollination, pollen grains are released from the anthers and drop to the leaves, axils, and the ground1. Over a flowering period of approximately 2 weeks, maize pollen is thus an abundant and easily accessible food source. With a diameter of 90–100 μm, grains of maize pollen are relatively large compared to those of other plants2. Pollen in general represents a highly nutritious food source that can be used by bees and bumblebees but also by a range of other insects, including predators3,4. Maize pollen is rich in carbohydrates (sugars and starch) and nitrogenous compounds (proteins and free amino acids) and contains sterols, lipids, organic acids, vitamins, and minerals1,2. Pollen feeding may allow predators to survive when prey is scarce and the use of pollen as a food supplement can play a key role in the population dynamics of predator–prey systems5. Pollen grains might be ingested either directly or passively when pollen is suspended in nectar, honeydew, or water droplets. In the field, maize pollen ingestion has been reported for ladybird beetles6,7,8, the predatory bug Orius insidiosus9,10, and the spider Araneus diadematus (Araneae: Araneidae)11. Laboratory studies with predatory arthropods demonstrated nutritional benefits from feeding on maize pollen for ladybird beetles (Coleoptera: Coccinellidae), carabid beetles (Coleoptera: Carabidae), Orius spp. predatory bugs (Heteroptera: Anthocoridae), spiders (Araneae), and predatory mites (Acari: Phytoseiidae). A detailed summary of the literature on the utilization of maize pollen by predatory stages of arthropods is provided in the Supporting Information (Table S1).
The common green lacewing, Chrysoperla carnea (Stephens) (Neuroptera: Chrysopidae), is an important natural predator of insect herbivores in many different crop and non-crop habitats12,13,14. Adults are not predacious and live on pollen, nectar, and honeydew15,16,17. Adult C. carnea are present in flowering maize fields, where they ingest and digest large amounts of maize pollen18. Larvae preferentially consume aphids but also other soft-bodied arthropods15. To date, there is little evidence that larvae of C. carnea can also utilize maize pollen as a food source. A previous study showed low survival when neonate C. carnea were fed exclusively with maize pollen19. Consequently, each instar was provided with maize pollen for only 24 h and then eggs of Sitotroga cerealella (Olivier) (Lepidoptera: Gelechiidae) were provided until the next moult. Even though the authors noted that “surviving larvae were observed feeding on the pollen during all developmental stadia”, the contribution of maize pollen to larval development remained unclear. Therefore, the first objective of the present study was to investigate the suitability of maize pollen as a food source for larvae of C. carnea.
Depending on local conditions, farming system, preference of the grower, and market availability, a large number of different maize varieties are grown worldwide. For example, the European common catalogue of seeds contains almost 5000 maize varieties20, although the number of varieties actually grown on a regular basis is likely to be much lower. Nevertheless, in many countries of the world, growers can choose between conventional maize and genetically modified (GM) cultivars producing insecticidal protein from the bacterium Bacillus thuringiensis (Bt maize), between maize for animal feed (grain and silage maize) and maize for human consumption (sweet maize), and among hybrids, varieties, inbred lines, and local landraces. While some cultivars are closely related (such as Bt and non-transformed near-isolines), others have a more distant breeding background. Those genetic differences may influence the suitability of maize pollen as food for arthropods, as demonstrated for the ladybird beetle Coleomegilla maculata (De Geer)21,22. Thus, the second objective of this study was to determine how pollen from different maize varieties affects development of C. carnea larvae.
Little is known about how the nutritional characteristics of maize pollen affect arthropod predators21,22. In general, protein and carbohydrates are major components of insect nutrition23. For our study of lacewings, we thus selected total protein content and the ratio of carbon to nitrogen (C:N) as parameters related to nutrition that are relatively easy to measure. Furthermore, we determined pollen grain size, because preliminary studies revealed that the relationship between mandible size and pollen grain size was critical for the ability of C. carnea larvae to handle maize pollen grains for feeding (unpublished data). The third objective of this study was to determine differences in total protein content, C:N ratio, and pollen diameter among maize cultivars and cultivation batches and to determine whether these differences influence lacewing performance.
Results
Development of lacewing larvae that were fed exclusively with maize pollen (experiment 1)
In the first experiment, neonate C. carnea were fed exclusively with maize pollen from one of seven cultivars used for animal feed (including three Bt maize cultivars and their corresponding non-transformed cultivars) or two conventional cultivars that are grown for human consumption (a sweet maize cultivar and a Swiss landrace). In this experiement, 60% of the larvae across the nine maize treatments and two trials developed into L2. Survival in the second larval stage was 49%. Only one larva in the Rheintaler treatment (Swiss landrace) survived to the pupal stage. Across all maize treatments and both trials, the mean (±SE) development time was 6.0 ± 0.10 days (N = 157) for L1 and 7.4 ± 0.27 days (N = 76) for L2. Mortality from egg hatching to moulting into L3 did not significantly differ among maize cultivars (Table 1). An ANOVA for development time of L1 revealed a significant effect of cultivar (p = 0.008), but the Tukey HSD post hoc test revealed no significant differences among particular cultivars. When analysing development time in the second larval stage, no significant effect of cultivar was evident. Mortality from egg hatching to moulting into L3 and development time in L1 were higher in trial 2 than in trial 1 (p < 0.0001). No C. carnea larva died in the first or second larval stage when fed with Ephestia kuehniella (Lepidoptea: Pyralidae) eggs, and the larvae developed twice as fast to L3 when fed with eggs vs. maize pollen (Table 1). This indicates that the lacewings in our test were in good health and that the experimental setup was suitable for studying lacewing development.
Table 1. Mortality and development time of Chrysoperla carnea larvae during the first two larval stages when fed exclusively with pollen from different Bt and non-Bt maize cultivars or with Ephestia kuehniella eggs (control treatment) (experiment 1). For statistical comparisons, logistic regression on mortality (egg hatching to moulting into L3) and ANOVA on development time (log transformed) of L1 and L2 were performed. Fixed factors were maize cultivar and trial. The model was full factorial. The control treatment was excluded from statistical analyses.
| Treatment | Mortality L1–L2 [%] (N)1 | Development time [days ± SE] (N)2 | |
|---|---|---|---|
| L1 | L2 | ||
| Control treatment | |||
| E. kuehniella eggs | 0 (30) | 3.88 ± 0.17 (30) | 2.60 ± 0.08 (30) |
| Maize pollen treatments | |||
| DKC5143Bt (Bt) | 87 (30) | 6.59 ± 0.64 (11) | 8.13 ± 0.43 (4) |
| DKC5143 | 70 (30) | 6.26 ± 0.28 (17) | 8.06 ± 0.76 (9) |
| DKC3421YG (Bt) | 57 (30) | 6.48 ± 0.32 (22) | 7.92 ± 0.91 (13) |
| DKC3420 | 66 (29) | 6.35 ± 0.29 (17) | 7.50 ± 1.05 (10) |
| Compa CB (Bt) | 70 (30) | 5.80 ± 0.16 (22) | 6.94 ± 0.52 (9) |
| Dracma | 64 (28) | 5.88 ± 0.25 (17) | 6.50 ± 0.41 (10) |
| Radiance | 74 (27) | 5.78 ± 0.32 (16) | 6.86 ± 0.53 (7) |
| Rheintaler | 79 (28) | 5.78 ± 0.35 (16) | 8.67 ± 1.34 (6) |
| Gavott | 72 (29) | 5.55 ± 0.26 (19) | 6.50 ± 0.59 (8) |
| Experimental trials | |||
| Trial 1 | 59 (129) b | 5.21 ± 0.11 (112) a | 5.92 ± 0.30 (68) |
| Trial 2 | 83 (132) a | 6.41 ± 0.18 (75) b | 6.26 ± 0.60 (38) |
1Logistic regression: cultivar n.s., trial p < 0.0001, Wald = 17.4.
2ANOVA, L1: cultivar p = 0.008, F8,139 = 2.71, Tukey HSDcultivar n.s., trial p < 0.0001, F1, 139 = 78.2, trial × cultivar n.s.; L2: cultivar n.s., trial × cultivar n.s., trial not calculated.
Development of lacewing larvae that were fed maize pollen during one larval stage (experiment 2)
When C. carnea larvae were fed maize pollen during one of three larval stages and E. kuehniella eggs during the other two stages, mortality and development time were not significantly affected by maize cultivar (Table 2). Development time was longer in trial 1 than in trial 2 (p < 0.0001) but mortality did not significantly differ between the two trials (Table 2). The stage in which pollen was provided significantly affected both mortality and development time (p < 0.0001). Pollen provided during the first instar resulted in the highest mortality (42%) and longest development time (mean ± SE; 12.1 ± 0.20 days). When second instars were fed with pollen, mortality was low (13%), and development time was reduced to 10.9 ± 0.13 days. When third instars were provided with pollen, mortality and development time were intermediate (24% and 11.4 ± 0.21 days, respectively) (Table 2).
Table 2. Mortality and development time of Chrysoperla carnea larvae when fed maize pollen during one larval stage and Ephestia kuehniella eggs in the other two stages (L1–L3) (experiment 2). Control treatments were no food in larval stage 1, 2, or 3, and E. kuehniella eggs in all stages. For statistical comparisons, logistic regression on mortality and ANOVA on development time (log transformed) were performed. Fixed factors were maize cultivar, trial, and the larval stage that was fed pollen. The model was full factorial. The control treatments were excluded from statistical analyses.
| Treatment | Mortality L1–L3 [%] (N)1 | Development time L1–L3 [days ± SE] (N)2 |
|---|---|---|
| Control treatments | ||
| E. kuehniella eggs | 2 (47) | 8.56 ± 0.11 (46) |
| no food | 97 (87) | 10.00 ± 0.00 (3) |
| Maize pollen treatments | ||
| DKC5143Bt (Bt) | 36 (89) | 11.40 ± 0.24 (57) |
| DKC5143 | 26 (88) | 11.56 ± 0.19 (65) |
| DKC3421YG (Bt) | 21 (87) | 11.27 ± 0.20 (69) |
| DKC3420 | 21 (89) | 11.31 ± 0.21 (70) |
| Experimental trials | ||
| Trial 1 | 23 (177) | 11.85 ± 0.14 (137) a |
| Trial 2 | 30 (176) | 10.87 ± 0.14 (124) b |
| Pollen feeding stage | ||
| Pollen feeding L1 | 42 (115) A | 12.10 ± 0.20 (67) A |
| Pollen feeding L2 | 13 (119) C | 10.94 ± 0.13 (103) B |
| Pollen feeding L3 | 24 (119) B | 11.36 ± 0.21 (91) B |
1Logistic regression: cultivar n.s., trial n.s., pollen-feeding stage p < 0.0001, Wald = 21.8, all interactions n.s.
2ANOVA: cultivar n.s., trial p < 0.0001, F1, 237 = 17.8, pollen-feeding stage p < 0.0001, F2, 237 = 11.5, stage × trial p = 0.0003, other interactions n.s.
When larvae were fed with E. kuehniella eggs for their entire larval development, only one of 47 died (Table 2). The first instar developed in 3.1 ± 0.04 days, the second in 2.6 ± 0.05 days, and the third in 2.8 ± 0.07 days, resulting in 8.6 ± 0.11 days for the complete larval development. This was 25% shorter than the mean development time for all maize treatments combined (11.4 days). When no food was provided during one larval stage, all lacewing larvae died in the respective stage with the exception of three specimens in the L2 stage. Each of those larvae required 10 days to reach the pupal stage.
Total protein and C:N ratio
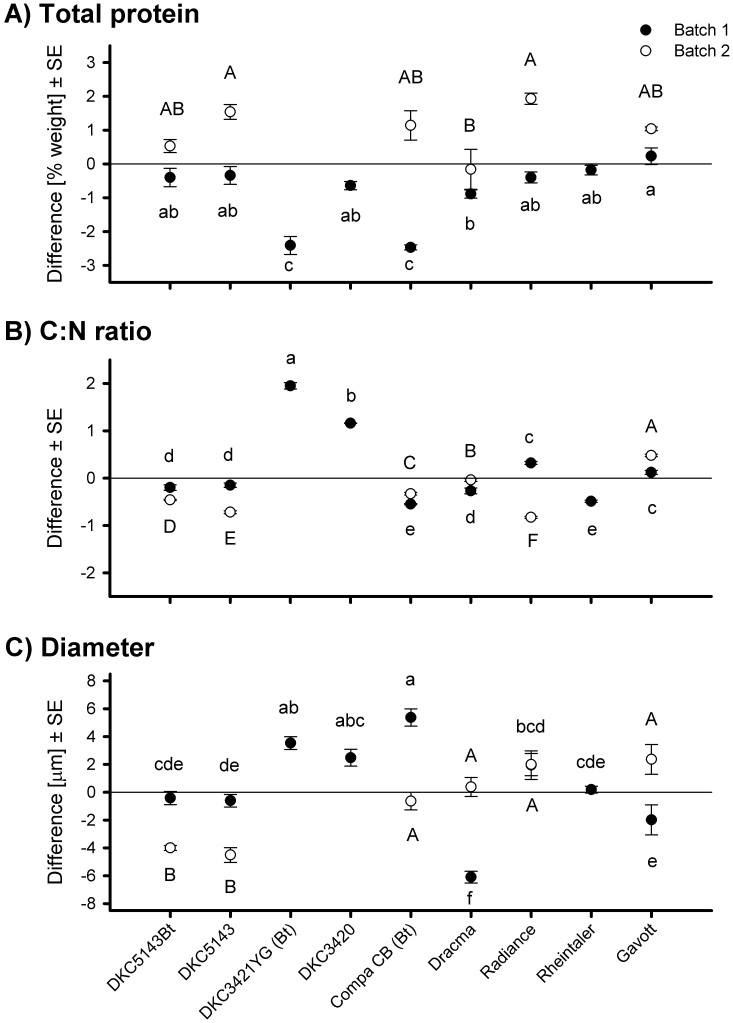
Across the nine cultivars and two batches of pollen, total protein content in pollen was 10.0 ± 0.15% (mean ± SE, N = 72). Total protein content in pollen was highest in the sweet maize cultivar Radiance (11.9 ± 0.17%) of batch 2 and lowest in the GM maize Compa CB (7.5 ± 0.08%) of batch 1; thus, the total protein content was 59% higher in Radiance than in Compa CB (Fig. 1A; Supporting Information Table S2).
Figure 1. Relative total protein content (A), C:N ratio (B), and diameter (C) of pollen from nine maize cultivars grown in two batches, one after the other, in the same glasshouse.
Pollen was pooled for all plants per batch (3–10 flowering plants). Five subsamples were analyzed per cultivar and batch for each parameter. The difference between the mean of each cultivar and the total mean of all cultivars is plotted on the Y-axis. Statistical comparisons (ANOVA) were performed separately for each pollen batch. Batch 2 did not include all maize cultivars. Different lowercase and uppercase letters indicate significant differences for batch 1 and 2, respectively.
In batch 1, total protein content in pollen did not significantly differ between the GM cultivar DKC5143Bt and its non-transformed counterpart DKC5143 but was significantly lower in the GM cultivars DKC3421YG (19% lower) and Compa CB (17% lower) than in their corresponding conventional cultivars, DKC3420 and Dracma (Fig. 1A). In batch 2, total protein content did not significantly differ between the GM cultivars DKC5143Bt and Compa CB and their non-transformed cultivars DKC5143 and Dracma (Fig. 1A).
Among the conventional cultivars in batch 1, total protein content was significantly greater (12% greater) in Gavott than in Dracma (Tukey HSD, p < 0.05), while all other comparisons were not significant (Fig. 1A). In batch 2, total protein content was significantly greater in Radiance (21% greater) and DKC5143 (17% greater) than in Dracma.
When ANOVA was conducted with batch as a factor and only for those cultivars that were represented in both batches, total protein content in pollen was significantly affected by cultivar, batch, and their interaction (p ≤ 0.0003).
Across all cultivars and both batches, the C:N ratio in pollen was 11.9 ± 0.08 (N = 75). It was highest in DKC3421YG (13.8 ± 0.07) of batch 1 and was lowest in Radiance of batch 2 (11.0 ± 0.02) (Fig. 1B, Table S2). In comparisons of pollen from GM cultivars and their non-Bt counterparts in batch 1, the C:N ratio was significantly higher (6% higher) for DKC3421YG than for DKC3420, was significantly lower (2% lower) for Compa CB than for Dracma, and did not significantly differ between DKC5143Bt and DKC5143. In batch 2, the C:N ratio in pollen was significantly higher (2% higher) for DKC5143Bt and significantly lower (2% lower) for Compa CB than for their corresponding non-Bt cultivars. In batch 1, the C:N ratio significantly differed among the conventional cultivars in the following order: DKC3420 > Radiance and Gavott > DKC5143 and Dracma > Rheintaler. The C:N ratio was 14% higher in DKC3420 than in Rheintaler. In batch 2, the C:N ratio significantly differed among all conventional maize cultivars in the following order: Gavott > Dracma > DKC5143 > Radiance. The C:N ratio was 12% higher in Gavott than in Radiance. In an ANOVA with batch as a factor, cultivar, batch, and their interaction were significant (p < 0.0001).
Regression analyses revealed that total protein content in pollen was negatively correlated with C:N ratio in pollen with marginal significance (p = 0.058, r2 = 0.25). In contrast, no significant relationship was detected between total protein content or C:N ratio and any of the lacewing variables measured in experiments 1 and 2.
Pollen grain size
Across all cultivars and both batches, pollen diameter was 86.1 ± 0.39 μm (N = 75). Pollen diameter was highest for Compa CB (91.5 ± 0.62 μm in batch 1) and lowest for Dracma (80.0 ± 0.42 μm in batch 1), and the 14% difference was significant (p = 0.0001) (Fig. 1C, Table S2). For other comparisons of GM vs. corresponding non-GM cultivars in both batches, differences were not significant. Pollen diameter differed among the conventional varieties in both batches (p < 0.05) (Fig. 1C). In batch 1, pollen diameter was 11% greater for DKC3420 than for Dracma, while values for the other cultivars were intermediate (Fig. 1C). In batch 2, pollen diameter was largest for Gavott and smallest for DKC5143, with values in Gavott being 8% higher than those in DKC5143. Gavott, Radiance, and Dracma had significantly larger pollen grains than DKC5143 (p < 0.05). In an ANOVA with batch as a factor, cultivar and the interaction batch × cultivar were significant (p < 0.0001) but batch was not significant.
Pollen diameter was not correlated with total protein content of pollen, the C:N ratio of pollen, or any of the variables measured in experiments 1 and 2.
Discussion
Pollen utilization by lacewing larvae
Lacewing larvae were unable to complete development on maize pollen alone (with the exception of one larva that was fed pollen from the landrace Rheintaler). Nevertheless, 60% of the neonates developed to the second instar, and 25% developed to the third instar. Furthermore, 58–87% of the lacewing larvae were able to develop to pupa when one instar was fed with maize pollen and the other two instars were fed with an optimal diet of E. kuehniella eggs. The first instar was the most sensitive to pollen feeding, and the second instar was the least sensitive. These results clearly demonstrate that lacewing larvae can utilize maize pollen during their development. Pilcher et al. had previously reported a low survival of C. carnea larvae that were exclusively fed with maize pollen19. When each instar was supplied with maize pollen for the initial 24 h and then with S. cerealella eggs (high nutritional quality) until the next moult, 49% of the larvae completed development19; Pilcher et al. also reported that mortality was highest in the first instar, which was consistent with our results. Patt et al. observed that third instars of C. carnea that were fed a mixture of bee pollen and sucrose solution were able to complete development, while second instars failed to pupate24. Furthermore, lacewing larvae that were fed Drosophila melanogaster Meigen (Diptera: Drosophilidae) larvae (poor nutritional quality) plus a pollen/sucrose mix performed better than lacewings that were fed either fruit flies or pollen/sucrose alone24. Adult lacewings thrive when they are fed with maize pollen alone for a long period of time, i.e., the adults have high fecundity and fertility25, and ingest large amounts of maize pollen in the field18. Our results indicate that lacewing larvae might be well suited to bridge limited periods of prey shortage by consuming maize pollen and probably the pollen of other plants. However, the role of pollen as a food source for lacewing larvae in the field remains to be investigated.
The list of predatory arthropods that are known to benefit from maize pollen feeding includes predatory mites (14 species), a spider (1 species), carabid and ladybird beetles (13 and 4 species, respectively), and Orius bugs (4 species) (see Table S1). Previously, larvae of C. carnea were regarded as predators feeding exclusively on soft-bodied insects, preferably on aphids15. Our experiments, however, clearly demonstrate that C. carnea larvae can be added to the list of predatory arthropods benefiting from the consumption of maize pollen.
Influence of Bt proteins on lacewing performance
The consumption of maize pollen by C. carnea larvae can explain why Cry protein was detected in larvae collected in Bt (event Bt176 and MON88017) maize fields during flowering26,27. Compared to the low levels of exposure experienced by lacewing larvae in the field25, the larvae in the current study can be assumed to have ingested relatively high doses of Cry1Ab and Cry3Bb1 when they were fed with pollen from maize cultivars Compa CB and DKC5143Bt, respectively. In contrast, DKC3421YG pollen contains two orders of magnitude less Cry1Ab than Compa CB pollen28. In spite of these substantial differences in the Cry protein content of pollen, lacewing performance in the current study did not significantly differ between cultivars producing Bt proteins and their conventional counterparts. This confirms earlier reports that C. carnea is not susceptible to Cry1Ab or the closely related Cry1Ac and Cry3Bb125,29,30,31,32,33. Similarly, data obtained for other Chrysoperla species did not indicate sensitivity to Lepidoptera-active Cry1 and Cry2 proteins. Chrysoperla sinica (Tjeder) was not influenced by Cry1Ab, Cry1Ac, Cry1C, or Cry2Aa34,35,36; Chrysoperla rufilabris (Burmeister) was not affected by Cry1Ac, Cry1F, or Cry2Ab37; and maize pollen containing Cry1Ab and Cry1F did not affect Chrysoperla plorabunda (Fitch)38.
Influence of maize cultivars on lacewing performance
The maize cultivars used in our experiments did not significantly affect lacewing performance, and this was true whether the cultivars were Bt, closely related non-Bt, or more distantly related commercial cultivars including the landrace Rheintaler and the sweet maize Radiance. Because maize pollen represents a relatively poor diet for C. carnea larvae, we assumed that the larvae would be stressed and thus sensitive to rather small differences in pollen quality, but that was not the case in the current study. Although pollen characteristics did differ among cultivars, these differences did not result in differential effects on C. carnea larvae. However, development times of C. carnea larvae that were fed exclusively with pollen (experiment 1) or were fed pollen during one developmental stage (experiment 2) were significantly different in the two trials of both experiments, even though trial 1 and trail 2 in each experiment used the same experimental setup, the same source of lacewings, and the same sources of maize pollen. Like development time, the mortality of larvae that were fed exclusively with pollen (experiment 1) differed between the two trials. This indicates that the variation between trials was greater than the influence of maize cultivars even though total protein differed by up to 36%, C:N ratio differed by up to 22%, and pollen diameter differed by up to 14% among maize cultivars within the first batch of pollen. In many cases, the values for Bt cultivars were similar to those of the near-isogenic, non-Bt cultivars. In some cases, however, the differences between the Bt and corresponding non-Bt cultivar was of the same magnitude as the variation among the wider range of cultivars. Furthermore, a strong batch effect and significant interactions of batch and maize cultivar for total protein and C:N ratio indicate that cultivar differences strongly depended on the batch. For example, pollen diameter for Compa CB and Dracma differed greatly in the first batch (diameter was 14% higher in Compa CB) but not in the second (diameter differed by only 1%). The largest difference between batches for total protein was observed in Compa CB (total protein content was 48% higher in batch 2), for C:N ratio in Radiance (C:N ratio was 10% higher in batch 1), and for pollen diameter in Dracma (diameter was 8% higher in batch 2). Maize was cultivated in the same glasshouse under similar conditions. However, the percentage of plants that were unable to produce pollen as well as the amount of pollen produced per plant also varied among cultivars and batches (Table 3). The differences between batches can probably be explained by differences in natural light between batch 1 (spring) and 2 (summer) and differences in the total number of plants in the glasshouse between batches. Size and other characteristics of pollen can be altered by environmental conditions39. Interestingly, Kurtz et al. also observed high variation in pollen characteristics among maize plants grown in the same environment and attributed the variation to genetic or microclimatic differences39. This demonstrates that pollen characteristics will not only depend on the cultivar but also on the actual batch and the local conditions under which the plants are cultivated.
Table 3. Production of pollen by nine maize cultivars in the glasshouse. Pollen batch 1 and 2 were obtained from plants grown consecutively in the same glasshouse.
| Pollen batch and cultivar | Days to anthesis | Number of plants producing pollen | Pollen per plant [mg] |
|---|---|---|---|
| Batch 1 (sown on 15 February 2012) | |||
| DKC5143Bt (Bt) | 61 | 7 of 10 | 0.93 |
| DKC5143 | 62 | 6 of 10 | 1.25 |
| DKC3421YG (Bt) | 57 | 7 of 9 | 1.21 |
| DKC3420 | 57 | 8 of 10 | 1.31 |
| Compa CB (Bt) | 72 | 7 of 11 | 0.29 |
| Dracma | 75 | 7 of 11 | 0.36 |
| Radiance | 55 | 3 of 10 | 0.83 |
| Rheintaler | 61 | 8 of 9 | 1.13 |
| Gavott | 56 | 4 of 10 | 0.81 |
| Batch 2 (sown on 8 May 2012) | |||
| DKC5143Bt (Bt) | 60 | 5 of 5 | 0.66 |
| DKC5143 | 60 | 5 of 5 | 0.60 |
| Compa CB (Bt) | 73 | 7 of 8 | 0.33 |
| Dracma | 73 | 7 of 8 | 0.33 |
| Radiance | 56 | 10 of 11 | 0.80 |
| Gavott | 56 | 4 of 4 | 0.88 |
Little information is available on the influence of different maize cultivars on natural enemy performance. When pollen of five maize cultivars (including one Bt maize) was fed to another predator, the ladybird C. maculata, adult mortality differed among cultivars, while larval duration and mortality, pupal mortality, adult weight, and fecundity did not21. Adult mortality was correlated with the percentage of organic matter in maize pollen but was not correlated with contents of dry matter, crude protein, quercetin, or any amino acid in maize pollen21. In another study with maize pollen from five cultivars, source of maize pollen affected C. maculata development time, female weight, and female tibial length but not survival to adulthood, preoviposition period, population growth rate, or male tibial length22. Larval development times and intrinsic rates of population increase were correlated with sterol content in maize pollen22. When maize pollen of five cultivars including Bt maize was fed to honey bee larvae, no cultivar effect on mortality or prepupal weight was found40.
Relative to the limited information concerning the effects of maize cultivar on beneficial insects, more data are available on the influence of maize cultivars on herbivorous arthropods. In the glasshouse, larvae of the cereal leaf beetle, Oulema melanopus (Linnaeus) (Coleoptera: Chrysomelidae), were caged on leaves of some of the maize cultivars used in the present study, i.e., Rheintaler, Radiance, DKC3420, DKC3421YG, DKC5143, and DKC5143Bt41. While larval mortality was higher on the Cry3Bb1-producing Bt maize than on the other cultivars (O. melanopus belongs to the target family of Cry3Bb1), mortality did not significantly differ on the Cry1Ab-producing Bt maize vs. the corresponding non-Bt cultivar. Mortality did differ, however, among the conventional varieties. Field studies revealed that populations of the herbivores Zyginidia scutellaris (Herrich-Schäffer) (Hemiptera: Cicadellidae) and Trigonotylus caelestialium (Kirkaldy) (Heteroptera: Miridae) and natural enemies, i.e., several species of ground beetles (Coleoptera: Carabidae), varied more among conventional cultivars than among DKC5143Bt and its near-isogenic line42,43,44. Zurbrügg et al., who measured the decomposition of leaves of nine maize cultivars in litterbags, reported that Bt cultivars differed from near-isolines in C:N ratios but not in decomposition rate while non-transgenic cultivars differed in C:N ratio; contents of cellulose, hemicellulose, and lignin; and in decomposition rate45. These differences among maize leaves did not affect the invertebrate decomposer communities46.
In our study, differences among the two pollen batches were of the same magnitude as differences between cultivars, and cultivar differences were inconsistent and depended on the batch. From our data and the discussed literature, we conclude that the effect of maize cultivar on arthropods may or may not be evident and that nutrient contents often vary significantly among cultivars. We also conclude that it is very difficult to establish a link between nutritional components of maize pollen and leaves and arthropod performance.
Implications for environmental, non-target risk assessment of GM plants
Early tier laboratory studies that support the risk assessment of insecticidal GM plants are often performed with high doses of the purified insecticidal substances that are mixed into an artificial diet and fed to certain non-target organisms (NTOs)47,48,49. The purpose of these studies is to test the risk hypothesis that the novel insecticidal protein, at concentrations present in the field, does not cause unacceptable adverse effects to valued non-target species. Laboratory studies with purified substances have the power that the obtained results are independent from plant background and are thus generic for the Cry protein as the stressor of concern. In the European Union, the European Food Safety Authority (EFSA) requires additional in planta studies “in which the GM plant–NTO interactions are evaluated at exposure levels likely to occur in the field” with the aim of assessing the impact of unexpected and unintended, transformation-related effects47,50. For such studies, the GM plant is usually compared with the closest related parental line (the so called near-isoline). Interpreting results from such studies, however, is difficult51. Several breeding steps are necessary to generate a stable GM line from the parental line, and these steps are likely to generate differences in the composition of the cultivars. These differences are related to the breeding rather than to the genetic transformation, and the differences are likely to increase when the transgenic event is conventionally crossed into a range of different genetic backgrounds to generate commercial varieties. If a study reveals differences in composition and/or non-target performance between one GM cultivar and its near-isoline, it is very difficult to separate transformation-related effects from breeding/cultivar effects. In addition, the present study shows that differences among batches of pollen can exceed differences among cultivars and that the differences among cultivars depend on pollen batch. This is problematic for risk assessment studies, which must be reliable and reproducible. In any case, it is important to know the natural variation among a range of commercial cultivars grown in different regions of the receiving environment and among different batches of pollen or other plant tissue. This knowledge is important when the observed differences between a GM cultivar and its conventional near isoline are discussed in the context of potential ecological implications. In reality, however, occasional statistical differences between GM and conventional cultivars are often interpreted as evidence for adverse effects of the GM trait. Our study provides one baseline for the variation in some pollen characteristics among nine maize cultivars grown in two batches under glasshouse conditions. With a large number of maize varieties and a wide range of environmental conditions in European maize fields, the natural variation in the field is likely to be much higher than reported here. For in planta studies, we therefore recommend that researchers establish a baseline with several conventional, reference cultivars for a given experimental setup. These reference cultivars will help for the interpretation of the variation in plant characteristics and arthropod performance that is observed when a GM plant is tested against its near-isoline. In addition, results of individual studies should be evaluated in the context of the wider range of environmental conditions in the receiving environment and the potential ecological significance of observed differences.
Methods
Maize plants and pollen collection
In this study three Bt maize cultivars were used: DKC5143Bt (event MON88017, Monsanto, St Louis, USA), DKC3421YG (event MON810, Monsanto), and Compa CB (event Bt176, Syngenta, Stein am Rhein, Switzerland). The study also included the corresponding non-transformed cultivars, which were DKC5143, DKC3420, and Dracma, respectively, and the conventional maize cultivar Gavott (KWS Mais GmbH, Einbeck, Germany). In addition to those cultivars, which are used for animal feed, two conventional cultivars that are grown for human consumption were used: the sweet maize Radiance (Eric Schweizer Samen, Thun, Switzerland) and the Swiss landrace Rheintaler (Verein Rheintaler Ribelmais, Salez, Switzerland).
DKC5143Bt plants express the cry3Bb1 gene from B. thuringiensis ssp. kumamotoensis, targeting corn rootworms (Diabrotica spp., Coleoptera: Chrysomelidae). DKC3421YG and Compa CB plants express the cry1Ab gene from B. thuringiensis ssp. kurstaki HD-1, targeting stem-boring Lepidoptera. Expression of cry genes in DKC5143Bt and DKC3421YG is driven by the constitutive, enhanced CaMV 35 s promoter, while expression in Compa CB is driven by the constitutive PEPC promoter as well as a pollen-specific promoter52,53,54,55.
Maize plants were grown individually in 12-L plastic pots in the glasshouse and were fertilized with 40 g of slow release fertilizer (Osmocote Exact, 16% N, 11% P2O5, 11% K2O, Scotts UK Professional, Bramford, UK) before sowing and weekly with 0.2–0.8 L of 0.2% Vegesan standard (8% N, 7% P2O5, 8% K2O per L, Hauert HBG Dünger AG, Grossaffoltern, Switzerland).
Plants reached anthesis in 55–75 days after sowing (Table 3). Pollen was collected using air-permeable cellophane bags (19.5 × 37.5 cm, Celloclair AG, Liestal, Switzerland), which were clipped over the inflorescences. A small hole was cut in the bottom of each bag to collect pollen. Pollen was collected daily, passed through a mesh (0.2 mm) to remove anthers and contaminants56, and dried at room temperature for 1 day. For each maize cultivar, pollen from several plants and days was pooled and stored in the freezer (−80°C) until used (Table 3). Two batches of maize plants were grown in the same glasshouse: the first was sown on 15 February 2012, and the second was sown on 8 May 2012. In the first batch, 30–89% of the plants produced pollen. In the second batch, the percentages were higher (88–100%), probably because the plants had more space and more sunlight. The mean amount of pollen harvested per plant ranged from 0.20 to 1.31 mg and varied among cultivars and batches (Table 3). The glasshouse was temperature controlled (25°C ± 2°C) and equipped with eight high-pressure sodium growth lights (400 W) providing additional light during 16 h per day. Humidity was not controlled.
Lacewings
Chrysoperla carnea were obtained from our laboratory colony33. Eggs collected from the colony were separated in small Petri dishes (5 cm diameter, 1 cm high) 1 day before they hatched and were kept in a climatic chamber at 25 ± 1°C, 75 ± 5% RH, and a 16:8 h L:D regime.
Development of lacewing larvae that were fed exclusively with maize pollen (experiment 1)
Neonate C. carnea (<12 h after hatching) were kept individually in Petri dishes (5 cm diameter) with a gauze-covered hole in the lid (2 cm diameter) for ventilation. The larvae were provided either with pollen of one of the nine maize cultivars or with eggs of E. kuehniella (Biotop, Valbonne, France) as a control treatment because Lepidoptera eggs have a high nutritional quality for lacewing larvae57. The first batch of pollen was used. Before pollen was fed to lacewing larvae, it was incubated for at least 3 hours in a plastic box lined with wet tissue paper, which allowed the pollen to rehydrate. Pollen or E. kuehniella eggs were provided ad libitum (at least 3, 6, and 9 mg for first, second, and third instars, respectively). In addition, 50 μl water was provided in a way that a portion of the pollen was wetted. Larval survival and development were recorded twice per day (ca. 9 am and 5 pm), when fresh water was provided. Petri dishes and pollen were changed daily in the afternoon. Dishes with E. kuehniella eggs were changed sporadically as needed. The experiment ended when larvae either reached the pupal stage or died. For each maize treatment and the control treatment, 15 replicates were set up in the first trial of the experiment. The experiment was repeated later in the year (trial 2) with another 15 replicates per treatment and with pollen from the same (first) batch of maize plants.
Development of lacewing larvae that were fed maize pollen during one larval stage (experiment 2)
The same experimental setup described for experiment 1 was used for experiment 2. Larvae were assigned to one of the following “life-stage” treatments: (a) maize pollen in the first instar, E. kuehniella eggs in the second and third instar; (b) maize pollen in the second instar, eggs in the first and third instar, (c) maize pollen in the third instar, eggs in the first and second instar. As a control treatment, larvae were fed eggs in all instars. In addition, “starvation” treatments were included, i.e., larvae received no food in one of the three instars and were fed E. kuehniella eggs in the other two instars. For experiment 2, four pollen sources from the first batch of maize plants were used: DKC3421YG, DKC3420, DKC5143Bt, and DKC5143. In total, this resulted in 12 life-stage × pollen combinations, one eggs-only control, and three starvation treatments. In the first trial, each of these treatments was replicated 15 times, except that the eggs-only treatment was replicated 25 times. The experiment was repeated later in the year (trial 2) with the same number of replicates per treatment and with pollen from the same (first) batch of maize plants.
Total protein, carbon, and nitrogen analyses
All pollen analyses were conducted separately with samples from the two plant batches. Total protein content of pollen was determined according to Bradford58. Protein was extracted from five subsamples of the pollen collected from each maize cultivar and pollen batch. Per sample, 15 mg of maize pollen and a 3-mm tungsten carbide ball were added to 300 μl of a 0.15 M NaCl solution and shaken for 2 min at 30 Hz in a TissueLyser II (Qiagen, Germantown, USA). After centrifugation (5 min at 13,000 × g), the supernatant was diluted 10-fold with NaCl solution. In each well of a 96-well microtiter plate, 10 μl of protein solution was mixed with 190 μl of Bradford reagent (Sigma-Aldrich, Buchs, Switzerland). Bovine serum albumin (BSA) solutions from 0.09 to 1.44 μg/μl served as standards. Absorbance was measured at 595 nm with a Spectrafluor-Plus plate reader (Tecan, Männedorf, Switzerland). Total protein content per weight in pollen was calculated using linear regression analysis.
Carbon and nitrogen contents were measured in five subsamples of lyophilized pollen. For each maize cultivar and batch, 3 to 4 mg of pollen was placed in tin cartridges. Carbon and nitrogen contents were measured with a Euro EA300 elemental analyser (HEKAtech GmbH, Wegberg, Germany) and calculated with Callidus® 2E3 (HEKAtech). To obtain C:N ratios, the proportions of carbon (compared with total weight) were divided by the proportions of nitrogen.
Pollen grain size
For each maize cultivar and pollen batch, the diameter of pollen grains was measured with a high precision M165C stereomicroscope connected to a video camera and image software (Leica Microsystems AG, Heerbrugg, Switzerland). Pollen was measured in a 0.5 M sucrose solution. Pollen diameter was estimated from circles drawn around pollen grains using 3-point measurements. Twenty pollen grains were measured for each of five pollen subsamples per maize cultivar and pollen batch.
Data analyses
Statistical analyses were conducted using the software package STATISTICA 11 (StatSoft Inc., Tulsa, USA). The control treatments (no food or continuously fed with E. kuehniella eggs) served to ensure the quality of the bioassay but were excluded from statistical analyses. In the experiment 1 (exclusive pollen feeding), larval mortality was analyzed from egg hatching to moulting into L3 by logistic regression in the generalized linear models tool. Development time was analyzed for L1 and L2 separately. For the first larval stage, full factorial ANOVA was possible with 3–13 replicates per trial × cultivar interaction. For the second larval stage, DKC5143Bt and DKC3420 in trial 2 were excluded due to zero and one observations, respectively. This resulted in an incomplete ANOVA design with 2–9 replicates per trial × cultivar interaction. Development times were log-transformed and analyzed in the general linear models tool. Fixed factors were maize cultivar and trial.
In experiment 2 (pollen feeding during one larval stage), larval mortality and development time (log-transformed) were analyzed from egg hatching to moulting into pupa. Mortality was analyzed by logistic regression and development time by ANOVA. Fixed factors were maize cultivar, trial, and stage in which pollen was fed to larvae. The model was a full factorial. Significant differences among maize cultivars or developmental stages were further analyzed using Tukey HSD post-hoc tests for ANOVA and pairwise comparisons for logistic regression.
Differences in total protein content, C:N ratio, and pollen diameter among maize cultivars were analyzed by ANOVA. Tukey HSD tests were conducted for significant cultivar effects. Pollen batches one and two were analysed separately because the number of maize cultivars used differed for batch 1 and 2. However, we also conducted ANOVAs including batch as a fixed factor for those varieties that were included in both batches.
Regression analyses were conducted with the variables total protein, C:N ratio, and pollen diameter and the values from batch 1 and 2 to determine whether the pollen characteristics were correlated among each other. In addition, correlations of the pollen characteristics with the experimental variables of mortality and development time (log transformed) were analysed for both experiments.
Supplementary Material
Supporting Information
Acknowledgments
We are grateful to Monsanto Co. for providing seeds. We acknowledge Bruce Jaffee and Steven L. Levine for critical comments on an earlier version of the manuscript. This research was funded by Agroscope.
Footnotes
The authors declare no competing financial interests.
Author Contributions M.M. and J.R. designed the experiments, J.Z., M.W. and M.M. conducted the experiments and biochemical analyses, M.M. and J.R. conducted the statistical analyses, wrote the main manuscript text and prepared the figure and tables. All authors reviewed the manuscript.
References
- Goss J. A. Development, physiology, and biochemistry of corn and wheat pollen. Bot. Rev. 34, 333–355 (1968). [Google Scholar]
- Stanley R. G. & Linskens H. F. Pollen. Biology, Biochemistry, Management (Springer Verlag, Berlin, 1974). [Google Scholar]
- Lundgren J. G. Relationships of Natural Enemies and Non-Prey Foods (Springer Science + Business Media B. V., 2009). [Google Scholar]
- Wäckers F. L. Suitability of (extra-)floral nectar, pollen and honeydew as insect food sources. in Plant-provided Food for Carnivorous Insects: A Protective Mutualism and its Applications. Wäckers, F. L., van Rijn, P. C. J. & Bruin, J. (eds.) 17–74 (Cambridge University Press, Cambridge, 2005). [Google Scholar]
- Van Rijn P. C. J., van Houten Y. M. & Sabelis M. W. How plants benefit from providing food to predators even when it is also edible to herbivores. Ecology 83, 2664–2679 (2002). [Google Scholar]
- Lundgren J. G., Razzak A. A. & Wiedenmann R. N. Population responses and food consumption by predators Coleomegilla maculata and Harmonia axyridis (Coleoptera: Coccinellidae) during anthesis in an Illinois cornfield. Environ. Entomol. 33, 958–963 (2004). [Google Scholar]
- Lundgren J. G., Huber A. & Wiedenmann R. N. Quantification of consumption of corn pollen by the predator Coleomegilla maculata (Coleoptera: Coccinellidae) during anthesis in an Illinois cornfield. Agric. And Forest Entomol. 7, 53–60 (2005). [Google Scholar]
- Ricci C. & Ponti L. Seasonal food of Ceratomegilla notata (Coleoptera: Coccinellidae) in mountain environments of Northern Italian Alps. Eur. J. Entomol. 102, 527–530 (2005). [Google Scholar]
- Dicke F. F. & Jarvis J. L. The habits and seasonal abundance of Orius insidiosus (Say) (Hemiptera-Heteroptera: Anthocoridae) on corn. J. Kansas Entomol. Soc. 35, 339–344 (1962). [Google Scholar]
- Corey D., Kambhampati S. & Wilde G. Electrophoretic analysis of Orius insidiosus (Hemiptera: Anthocoridae) feeding habits in field corn. J. Kansas Entomol. Soc. 71, 11–17 (1998). [Google Scholar]
- Ludy C. Intentional pollen feeding in the spider Araneus diadematus Clerck, 1757. Newsl. Br. Arachnol. Soc. 101, 4–5 (2004). [Google Scholar]
- McEwen P., New T. R. & Whittington A. E. Lacewings in the Crop Environment (Cambridge University Press, Cambridge, 2001). [Google Scholar]
- Meissle M., Álvarez-Alfageme F., Malone L. A. & Romeis J. Establishing a database of bio-ecological information on non-target arthropod species to support the environmental risk assessment of genetically modified crops in the EU. Supporting Publications 2012:EN-334 (European Food Safety Authority (EFSA), Parma, Italy, 2012) http://www.efsa.europa.eu/en/supporting/pub/334e.htm (Date of access: 07/07/2014).
- Romeis J. et al. Potential use of an arthropod database to support the non-target risk assessment and monitoring of transgenic plants. Transgenic Res. Published online, 10.1007/s11248-014-9791-2 (2014). [DOI] [PubMed] [Google Scholar]
- Principi M. M. & Canard M. Feeding habits. in Biology of Chrysopidae. Canard, M., Séméria, Y. & New, T. R. (eds.) 76–92 (Dr. W. Junk Publishers, The Hague, 1984). [Google Scholar]
- Sheldon J. K. & MacLeod E. G. Studies on the biology of the Chrysopidae. II. The feeding behaviour of the adult of Chrysopa carnea (Neuroptera). Psyche 78, 107–121 (1971). [Google Scholar]
- Villenave J., Thierry D., Mamun A. A., Lodé T. & Rat-Morris E. The pollens consumed by common green lacewings Chrysoperla spp. (Neuroptera: Chrysopidae) in cabbage crop environment in western France. Eur. J. Entomol. 102, 547–552 (2005). [Google Scholar]
- Li Y., Meissle M. & Romeis J. Use of maize pollen by adult Chrysoperla carnea (Neuroptera: Chrysopidae) and fate of Cry proteins in Bt-transgenic varieties. J. Insect Physiol. 56, 157–163 (2010). [DOI] [PubMed] [Google Scholar]
- Pilcher C. D., Obrycki J. J., Rice M. E. & Lewis L. C. Preimaginal development, survival, and field abundance of insect predators on transgenic Bacillus thuringiensis corn. Environ. Entomol. 26, 446–454 (1997). [Google Scholar]
- European Commission (EC). Plant variety database, based on the Common catalogue of varieties of agricultural plant species – 31st complete edition, Official J. Eur. Union C402A, 1–686 (2012).Updated online version: http://ec.europa.eu/food/plant/propagation/catalogues/database/ (Date of access: 07/07/2014). [Google Scholar]
- Lundgren J. G. & Wiedenmann R. N. Nutritional suitability of corn pollen for the predator Coleomegilla maculata (Coleoptera: Coccinellidae). J. Insect Physiol. 50, 567–575 (2004). [DOI] [PubMed] [Google Scholar]
- Pilorget L., Buckner J. & Lundgren J. G. Sterol limitation in a pollen-fed omnivorous lady beetle (Coleoptera: Coccinellidae). J. Insect Physiol. 56, 81–87 (2010). [DOI] [PubMed] [Google Scholar]
- Simpson S. J., Sibly R. M., Pum Lee K., Behmer S. T. & Raubenheimer D. Optimal foraging when regulating intake of multiple nutrients. Anim. Behav. 68, 1299–1311 (2004). [Google Scholar]
- Patt J. M. et al. Assimilation of carbon and nitrogen from pollen and nectar by a predaceous larvae and its effects on growth and development. Ecol. Entomol. 28, 717–728 (2003). [Google Scholar]
- Li Y., Meissle M. & Romeis J. Consumption of Bt maize pollen expressing Cry1Ab or Cry3Bb1 does not harm adult green lacewings, Chrysoperla carnea (Neuroptera: Chrysopidae). PloS ONE 3, e2909 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissle M., Romeis J. The web-building spider Theridion impressum (Araneae: Theridiidae) is not adversely affected by Bt maize resistant to corn rootworms. Plant Biotechnol. J. 7, 645–656 (2009). [Google Scholar]
- Obrist L. B., Dutton A., Albajes R., Bigler F. Exposure of arthropod predators to Cry1Ab toxin in Bt maize fields. Ecol. Entomol. 31, 143–154 (2006). [Google Scholar]
- Nguyen H. T., Jehle J. A. Quantitative analysis of the seasonal and tissue-specific expression of Cry1Ab in transgenic maize Mon810. J. Plant Dis. Protect. 114, 82–87 (2007). [Google Scholar]
- Dutton A., Klein H., Romeis J. & Bigler F. Uptake of Bt-toxin by herbivores feeding on transgenic maize and consequences for the predator Chrysoperla carnea. Ecol. Entomol. 27, 441–447 (2002). [Google Scholar]
- Lawo N. C. & Romeis J. Assessing the utilization of a carbohydrate food source and the impact of insecticidal proteins on larvae of the green lacewing, Chrysoperla carnea. Biol. Contr. 44, 389–398 (2008). [Google Scholar]
- Lawo N. C., Wäckers F. L. & Romeis J. Characterizing indirect prey-quality mediated effects of a Bt crop on predatory larvae of the green lacewing, Chrysoperla carnea. J. Insect Physiol. 56, 1702–1710 (2010). [DOI] [PubMed] [Google Scholar]
- Rodrigo-Simón A. et al. Lack of detrimental effects of Bacillus thuringiensis Cry toxins on the insect predator Chrysoperla carnea: a toxicological, histopathological, and biochemical approach. Appl. Environ. Microbiol. 72, 1595–1603 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeis J., Dutton A. & Bigler F. Bacillus thuringiensis toxin (Cry1Ab) has no direct effect on larvae of the green lacewing Chrysoperla carnea (Stephens) (Neuroptera: Chrysopidae). J. Insect Physiol. 50, 175–183 (2004). [DOI] [PubMed] [Google Scholar]
- Li Y. et al. Use of an artificial diet system to study the toxicity of gut-active insecticidal compounds on larvae of the green lacewing Chrysoperla sinica. Biol. Contr. 69, 45–51 (2014). [Google Scholar]
- Li Y., Hu L., Romeis J., Chen X. & Peng Y. Bt rice producing Cry1C protein does not have direct detrimental effects on the green lacewing Chrysoperla sinica Tjeder. Environ. Tox. Chem. 33, 1391–1397 (2014). [DOI] [PubMed] [Google Scholar]
- Wang Y. et al. Consumption of Bt rice pollen expressing Cry2Aa does not cause adverse effects on adult Chrysoperla sinica Tjeder (Neuroptera: Chrysopidae). Biol. Contr. 61, 246–251 (2012). [Google Scholar]
- Tian J. C. et al. Bt crops producing Cry1Ac, Cry2Ab and Cry1F do not harm the green lacewing, Chrysoperla rufilabris. PLoS ONE 8, e60125 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason C. E. et al. Assessment of Chrysoperla plorabunda longevity, fecundity, and egg viability when adults are fed transgenic Bt corn pollen. J. Agric. Urban Entomol. 25, 265–278 (2008). [Google Scholar]
- Kurtz E. B., Liverman J. L. & Tucker H. Some problems concerning fossil and modern corn pollen. Bull. Torrey Bot. Club 87, 85–94 (1960). [Google Scholar]
- Hendriksma H. P., Härtel S. & Steffan-Dewenter I. Testing pollen of single and stacked insect-resistant Bt-maize on in vitro reared honey bee larvae. PLoS ONE 6, e28174 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissle M., Knecht S., Waldburger M. & Romeis J. Sensitivity of the cereal leaf beetle Oulema melanopus (Coleoptera: Chrysomelidae) to Bt maize-expressed Cry3Bb1 and Cry1Ab. Arthropod-Plant Interact. 6, 203–211 (2012). [Google Scholar]
- Priesnitz K. U., Benker U. & Schaarschmidt F. Assessment of the potential impact of a Bt maize hybrid expressing Cry3Bb1 on ground beetles (Carabidae). J. Plant Dis. Protect. 120, 131–140 (2013). [Google Scholar]
- Rauschen S., Schultheis E., Pagel-Wieder S., Schuphan I. & Eber S. Impact of Bt-corn MON88017 in comparison to three conventional lines on Trigonotylus caelestialium (Kirkaldy) (Heteroptera: Miridae) field densities. Transgenic Res. 18, 203–214 (2009). [DOI] [PubMed] [Google Scholar]
- Rauschen S. et al. Diabrotica-resistant Bt-maize DKc5143 event MON88017 has no impact on the field densities of the leafhopper Zyginidia scutellaris. Environ. Biosafety Res. 9, 87–99 (2010). [DOI] [PubMed] [Google Scholar]
- Zurbrügg C., Hönemann L., Meissle M., Romeis J. & Nentwig W. Decomposition dynamics and structural plant components of genetically modified Bt maize leaves do not differ from leaves of conventional hybrids. Transgenic Res. 19, 257–267 (2010). [DOI] [PubMed] [Google Scholar]
- Hönemann L., Zurbrügg C. & Nentwig W. Effects of Bt-corn decomposition on the composition of the soil meso- and macrofauna. Appl. Soil Ecol. 40, 203–209 (2008). [Google Scholar]
- European Food Safety Authority (EFSA). Guidance on the environmental risk assessment of genetically modified plants. EFSA J. 8, 1879 (European Food Safety Authority, Parma, Italy, 2010) http://www.efsa.europa.eu/it/scdocs/doc/1879.pdf (Date of access: 07/07/2014). [Google Scholar]
- Romeis J. et al. Assessment of risk of insect-resistant transgenic crops to nontarget arthropods. Nat. Biotechnol. 26, 203–208 (2008). [DOI] [PubMed] [Google Scholar]
- Romeis J. et al. Recommendations for the design of laboratory studies on non-target arthropods for risk assessment of genetically engineered plants. Transgenic Res. 20, 1–22 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devos Y. et al. EFSA's scientific activities and achievements on the risk assessment of genetically modified organisms (GMOs) during its first decade of existence: looking back and ahead. Transgenic Res. 23, 1–25 (2014). [DOI] [PubMed] [Google Scholar]
- Romeis J., McLean M. A. & Shelton A. M. When bad science makes good headlines: Bt maize and regulatory bans. Nat. Biotechnol. 31, 386–387 (2013). [DOI] [PubMed] [Google Scholar]
- Koziel M. G. et al. Field performance of elite transgenic maize plants expressing an insecticidal protein derived from Bacillus thuringiensis. Bio/Technology 11, 194–200. [Google Scholar]
- US Environmental Protection Agency (USEPA). Biopesticides registration action document – Bacillus thuringiensis plant-incorporated protectants. (US Environmental Protection Agency, 2001) http://www.epa.gov/oppbppd1/biopesticides/pips/bt_brad.htm (Date of access: 07/07/2014).
- US Environmental Protection Agency (USEPA). Biopesticides registration action document – Bacillus thuringiensis Cry3Bb1 corn (US Environmental Protection Agency, 2007) http://www.epa.gov/oppbppd1/biopesticides/ingredients_keep/tech_docs/brad_006484.htm (Date of access: 07/07/2014).
- Vaughn T. et al. A method of controlling corn rootworm feeding using a Bacillus thuringiensis protein expressed in transgenic maize. Crop Sci. 45, 931–938 (2005). [Google Scholar]
- Hellmich R. L. et al. Monarch larvae sensitivity to Bacillus thuringiensis-purified proteins and pollen. Proc. Natl. Acad. Sci. USA 98, 11925–11930 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman M. Z. & Selman B. J. Effect of larval diet on the performance of the predator Chrysoperla carnea Stephens (Neuropt., Chrysopidae). J. Appl. Ent. 120, 115–117 (1996). [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 (1976). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information