Abstract
Glycosylation is a post-translational modification that is an essential element in cell signaling and neurodevelopmental pathway regulation. Glycan attachment can influence the tertiary structure and molecular interactions of glycosylated substrates, adding an additional layer of regulatory complexity to functional mechanisms underlying central cell biological processes. One type of enzyme-mediated glycan attachment, fucosylation, can mediate glycoprotein and glycolipid cell surface expression, trafficking, secretion, and quality control to modulate a variety of inter- and intracellular signaling cascades. Building on prior reports of glycosylation abnormalities and evidence of dysregulated glycosylation enzyme expression in schizophrenia, we examined the protein expression of 5 key fucose-modifying enzymes: GDP-fucose:protein O-fucosyltransferase 1 (POFUT1), GDP-fucose:protein O-fucosyltransferase 2 (POFUT2), fucosyltransferase 8 (FUT8), fucosyltransferase 11 (FUT11), and plasma α-L-fucosidase (FUCA2) in postmortem superior temporal gyrus of schizophrenia (N = 16) and comparison (N = 14) subjects. We also used the fucose binding protein, Aleuria aurantia lectin (AAL), to assess α-1,6-fucosylated N-glycoprotein abundance in the same subjects. In schizophrenia we found increased expression of POFUT2, a fucosyltransferase uniquely responsible for O-fucosylation of thrombospondin-like repeat domains that is involved in a non-canonical endoplasmic reticulum quality control pathway. We also found decreased expression of FUT8 in schizophrenia. Given that FUT8 is the only α-1,6-fucosyltransferase expressed in mammals, the concurrent decrease in AAL binding in schizophrenia, particularly evident for N-glycoproteins in the ~52–58kDa and ~60–70kDa molecular mass ranges, likely reflects a consequence of abnormal FUT8 expression in the disorder. Dysregulated FUT8 and POFUT2 expression could potentially explain a variety of molecular abnormalities in schizophrenia.
Keywords: FUT8, POFUT2, glycosylation, core fucose, O-fucosylation, neuroglycobiology
1. INTRODUCTION
The role of posttranslational protein modifications in the pathophysiology of schizophrenia has become a target of investigation in this complex neuropsychiatric illness. One modification, glycosylation, has come under study due to the role glycan adornment plays in modulating a wide variety of inter- and intracellular processes. Glycosylation is the enzyme-mediated modification of protein, lipid, or carbohydrate substrates by glycans (sugars) and several unique glycosylation pathways exist with a variety of cellular functions (Varki et al., 2009). Abnormalities of N-linked protein glycosylation (Bauer et al., 2010; Mueller et al., 2014; Stanta et al., 2010; Tucholski et al., 2013a, 2013b), sphingolipid metabolism (Narayan et al., 2009), chondroitin sulfate proteoglycan processing (Berretta, 2012; Pantazopoulos et al., 2013) and polysialylation of cell adhesion molecules in schizophrenia (Barbeau et al., 1995; Gilabert-Juan et al., 2012; Sato and Kitajima, 2013; Varea et al., 2012) have been reported. Our lab recently identified altered transcript levels of 36 carbohydrate active enzymes (CAzymes) in schizophrenia dorsolateral prefrontal cortex (unpublished data). A subset of these enzymes, fucosyltransferases and fucosidases, catalyze the attachment or cleavage of the deoxyhexose monosaccharide fucose on a wide variety of substrates (Luo et al., 2006b; Ma et al., 2006; Miyoshi et al., 2008; Varki et al., 2009), and we found increased mRNA expression of four of the six fucose-modifying CAzymes assessed: GDP-fucose:protein O-fucosyltransferase 1 (POFUT1), fucosyltransferase 8 (FUT8), fucosyltransferase 11 (FUT11), and plasma α-L-fucosidase (FUCA2).
Glycoproteins can be fucosylated either by the direct attachment of fucose to a serine/threonine residue of a growing polypeptide in the endoplasmic reticulum (ER), called O-fucosylation, or by α-fucosylation of glycans on glycoprotein or glycolipid substrates in the Golgi apparatus (Ma et al., 2006; Varki et al., 2009). Types of α-fucose linkages in mammals include α-1,2-fucose on terminal galactose (Gal); α-1,3/4-fucose on N-acetylglucosamine (GlcNAc) within poly-N-acetyllactosamine chains of glycolipids or glycoproteins; or α-1,6-fucose on the proximal GlcNAc of the chitobiose core of protein N-glycans, called “core fucosylation” (for detailed discussion of fucose linkage types, see review by Ma et al., 2006). In addition to being a key determinant of human blood group antigens and facilitating forward trafficking and secretion of fucosylproteins, constellations of fucosylated structures are dynamically regulated by the repertoire of fucosyltransferases expressed to facilitate a variety of cell signaling and developmental processes (Ma et al., 2006; Varki et al., 2009).
To characterize dysregulation of fucosylation in schizophrenia, we measured the protein expression of fucose-modifying enzymes in the superior temporal gyrus (STG) of elderly schizophrenia and comparison subjects. The STG is a brain region of interest in schizophrenia pathophysiology, and abnormalities of N-glycosylation (Mueller et al., 2014) as well as abnormal molecular phenotypes in multiple cell types are reflected in this cortical area (Pietersen et al., 2014a, 2014b; Steffek et al., 2008). In addition to four enzymes we previously found altered at the mRNA level (POFUT1, FUT8, FUT11, and FUCA2), the current study also examines the protein expression of GDP-fucose:protein O-fucosyltransferase 2 (POFUT2). POFUT1 and POFUT2 similarly modify correctly folded cysteine-rich regions of protein and respectively mediate O-linked fucosylation of epidermal growth factor or thrombospondin-like repeat (TSR) domains.(Luo et al., 2006b) We also assayed the binding pattern of the α-1,6-fucose-specific glycan binding protein AAL (Aluria aurantia lectin) in the same subjects (Matsumura et al., 2007; Monzo et al., 2007; Stelck et al., 1999; Wu et al., 2014). FUT8 is an α-1,6-fucosyltransferase that is uniquely responsible for the core fucosylation of N-glycoproteins and is the only α-1,6-fucosyltransferase expressed in mammals (Ihara et al., 2006; Kötzler et al., 2012). Interestingly, a FUT8 knock-out mouse demonstrates a schizophrenia-like behavioral phenotype and has deficits in multiple neurotransmitter pathways (Fukuda et al., 2011; Gu et al., 2015). Using AAL binding as a proxy for α-1,6-fucosylprotein expression, we assessed total levels of core fucosylation as well as the expression of α-1,6-fucosylproteins within specific molecular mass ranges in these same subjects.
2. METHODS AND MATERIALS
2.1 Human Subjects
Postmortem brain tissue was obtained from the Icahn School of Medicine at Mount Sinai NIH Brain and Tissue Repository, and detailed information regarding assessment is available at http://icahn.mssm.edu/research/labs/neuropathology-and-brain-banking/neuropathology-evaluation. Subjects used in this study included patients diagnosed with schizophrenia using DSM-III-R criteria excluding those with death not due to natural causes, previous history of drug/alcohol abuse, or coma longer than six hours prior to death (Mueller et al., 2015). Brains were evaluated micro- and macroscopically using CERAD guidelines. Schizophrenia subjects had no neuropathologies or signs of neurodegenerative disorders, including Alzheimer’s disease, at assessment (Powchik et al., 1993; Purohit et al., 1993). Subjects with no documented history of psychiatric illness obtained from the same collection were assessed similarly and served as a comparison group (Table 1 and Supplementary Table S1).
Table 1.
Summary of subject demographics
| Comparison | Schizophrenia | |
|---|---|---|
| n | 14 | 16 |
| Age | 79.4 ± 9.3 | 75.8 ± 11.9 |
| Sex | 4M/10F | 11M/5F |
| PMI (hours) | 10.0 ± 7.3 | 11.4 ± 4.4 |
| Freezer Storage Time (years) | 15.2 ± 3.4 | 16.3 ± 3.1 |
| Tissue pH | 6.3 ± 0.2 | 6.4 ± 0.3 |
| On/Off Rx | 0/14 | 11/5 |
Abbreviations: Male (M), Female (F), Postmortem interval (PMI), Antipsychotic medication (Rx).
Values are expressed as means ± standard deviation. Off Rx indicates patients that had not received antipsychotic medications for 6 weeks or more at time of death.
2.2 Tissue Preparation
Whole brains were collected at time of autopsy and cut into 1cm slabs. The full thickness of grey matter from the left hemisphere of STG (Brodmann area 22) was dissected, blocked into 1cm cubes, and powdered using small amounts of liquid nitrogen and a mortar and pestle then stored at −80°C. Samples were reconstituted in 1x isotonic extraction buffer (ER0100, Sigma-Aldrich, St Louis, MO) and homogenized on ice in a glass-teflon homogenizer. Homogenate was transferred into a nitrogen cavitation vessel (Parr Instrument Company, Moline, IL) and pressurized for 8min at 450psi, then collected through the vessel outlet during decompression (Mueller et al., 2015). Protein concentrations using a BCA protein assay kit (Thermo Scientific, Waltham, MA) were obtained for each of the homogenates prior to storage at −80°C.
2.3 Antipsychotic Treated Rats
The Institutional Animal Care and Use Committee of the University of Alabama at Birmingham approved all procedures using animals. Male Sprague-Dawley rats were housed in pairs and treated with chronic administration of either 28.5mg/kg haloperidol decanoate (HALO; N = 10) or vehicle (CTRL; N = 10) delivered once every 3 weeks over 9 months via intramuscular injection, for a total of 12 injections. This method of drug delivery, length of treatment, and dose have been previously described and validated (Harte et al., 2005; Kashihara et al., 1986; Kippe et al., 2015). Animals were euthanized by rapid decapitation following CO2 administration; samples of frontal cortex were dissected on ice then snap frozen and stored at −80°C until homogenization. Cortical homogenates were prepared in 320mM sucrose in 5mM Tris-HCL, pH 7.5, with protease and phosphatase inhibitor tablets (Complete Mini, EDTA-free and PhosSTOP, Roche Diagnostics, Indianapolis, IN), and protein concentration determined by BCA Assay (Thermo Scientific) prior to storage at −80°C.
2.4 Western Blot for Protein Expression
Human and rat homogenates were thawed on ice then prepared with 6X reducing buffer (4.5% sodium dodecyl sulfate (SDS), 0.02% bromophenol blue, 15% β-mercaptoethanol, and 36% glycerol in 170 mM Tris-HCl, pH 6.8) to a final 1x buffer concentration and heated at 70°C for 10 min. For each subject, 10μg of prepared sample was loaded in duplicate into NuPAGE Bis-Tris 4–12% gradient gels (Life Technologies, Carlsbad, CA) for SDS-PAGE using NuPAGE MOPS SDS running buffer (Life Technologies) and electrophoresed for 20min at 50V and then 1.5hrs at 150V. Proteins were transferred to nitrocellulose membranes using semi-dry transblotters (Bio-Rad, Hercules, CA) at 16V for 30min. Membranes were then blocked and probed using conditions optimized for each antibody (Table S2). Membranes were washed with Tris-buffered saline with 0.1% Tween-20 (TBST) and probed with the appropriate IR-dye labeled secondary antibody in the same diluent as the primary antibody. Membranes were again washed with TBST, rinsed with sterile water then imaged with a LiCor Odyssey scanner (LiCor, Lincoln, NE). Image Studio Lite Version 4.0.21 (LiCor) was used to measure the signal intensity of each protein band with the median right-left background signal intensity (3 pixels wide) subtracted. Each target was normalized to the intralane signal intensity of valosin-containing protein (VCP), a ubiquitously expressed protein we have previously reported to be unchanged in these same subjects (Mueller et al., 2015). Expression levels were similarly assessed in HALO and CTRL rats for protein measures that were significantly altered in schizophrenia.
2.5 Aleuria aurantia Lectin (AAL) Binding Assay
To measure α-1,6-fucosylated N-glycoprotein expression, human and rat homogenates were prepared, electrophoresed, and western blotted as described above, except 2μg of prepared sample was loaded in duplicate into the gel. Membranes were probed with biotinylated AAL (AAL; Vector Labs, Burlingame, CA) diluted to 1μg/mL in 1x Carbo-Free Blocking Solution (Vector Labs) in TBST. After AAL incubation, membranes were washed with TBST, incubated with IR-dye labeled streptavidin (LiCor) diluted 1:10,000 in TBST, washed again, rinsed with sterile water and imaged. Total α-1,6-fucosylated N-glycoprotein expression was assessed by measuring the signal intensity of the entire protein lane with the median background signal intensity surrounding the lane on all sides (3 pixels wide) subtracted. Specific molecular mass regions of the lane corresponding to distinct protein bands were similarly assessed, except the median right-left background signal intensity (3 pixels wide) was subtracted. Because the pattern of AAL binding is not identical between species, only total α-1,6-fucosylprotein expression was assessed in HALO and CTRL rats.
2.6 Statistical Analysis
For human studies, all dependent measures were normalized to the expression of VCP with median background signal intensity of VCP determined using the same criteria as the dependent measure (3 pixels right-left for bands, 3 pixels on all sides for lanes). Sample size was determined a priori using a power calculation (β = 0.2, π = 0.8) based on expression levels of FUCA2, FUT11, FUT8, and POFUT1 from cortex of 12 non-psychiatrically ill subjects. The investigator who executed experimental protocols was blind to subject diagnosis until study completion and the investigator who performed statistical analyses was blind to diagnosis during data collection. Data analysis was performed using Prism 6.07 (GraphPad Software Inc., La Jolla, CA) and STATISTICA 7.1 (StatSoft Inc., Tulsa, OK). Protein expression levels were calculated as the mean of the VCP-normalized target signal intensity. Calculated values were checked using the D’Agostino & Pearson omnibus normality test and all dependent variables were found normally distributed after removing any outliers identified by the Grubbs’ method. For enzyme protein expression, between group differences were assessed by two-way unpaired Student’s t-tests. For significantly different protein measures, post hoc assessments were performed including simple regression analysis between protein expression and subject age, tissue pH, postmortem interval, and freezer storage time; variables demonstrating any significant associations were further assessed by analysis of covariance (ANCOVA) with the correlated measure as covariate. In the case of multiple continuous covariates, multiple regression analysis was performed. Tests of significant dependent measures grouped by medication status and sex were performed using the non-parametric Kruskal-Wallis test due to small group sizes, and no significant differences were identified. Based on our prediction of decreased α-1,6-fucosylation, measures of AAL binding were assessed by one-way unpaired Student’s t-tests. For rat studies, dependent measures were normalized to VCP and similarly determined to be normally distributed before analysis using two-way unpaired Student’s t-tests. All statistical tests used α = 0.05.
3. RESULTS
3.1 POFUT2 protein expression is increased in schizophrenia
POFUT2 protein expression was increased by nearly 30% in schizophrenia relative to non-psychiatrically ill comparison subjects [Figure 1A, Table 2; t (28) 2.46, p = 0.020]. Post hoc regression analysis of POFUT2 expression revealed significant correlations of protein expression with age and PMI. ANCOVAs revealed that the significant difference between diagnostic groups was maintained when covarying for subject age [F (1, 27) = 4.89, p = 0.036] and PMI [F (1, 27) = 5.53, p = 0.026]; multiple regression analysis of POFUT2 expression by diagnosis, age, and PMI also confirmed the effect of diagnosis on POFUT2 expression in the face of multiple covariates [F (3, 26) = 7.08, p = 0.001; tDx (26) = 2.12, p = 0.044; tage (26) = −2.63, p = 0.014; tPMI (26) = 2.29, p = 0.030]. POFUT2 expression levels were not significantly different between rats that had received antipsychotic treatment and those which had not (Figure 1A).
Figure 1. Protein expression of fucosyltransferases POFUT2 and FUT8 is altered in schizophrenia, but not in cortex of rats following chronic antipsychotic treatment.
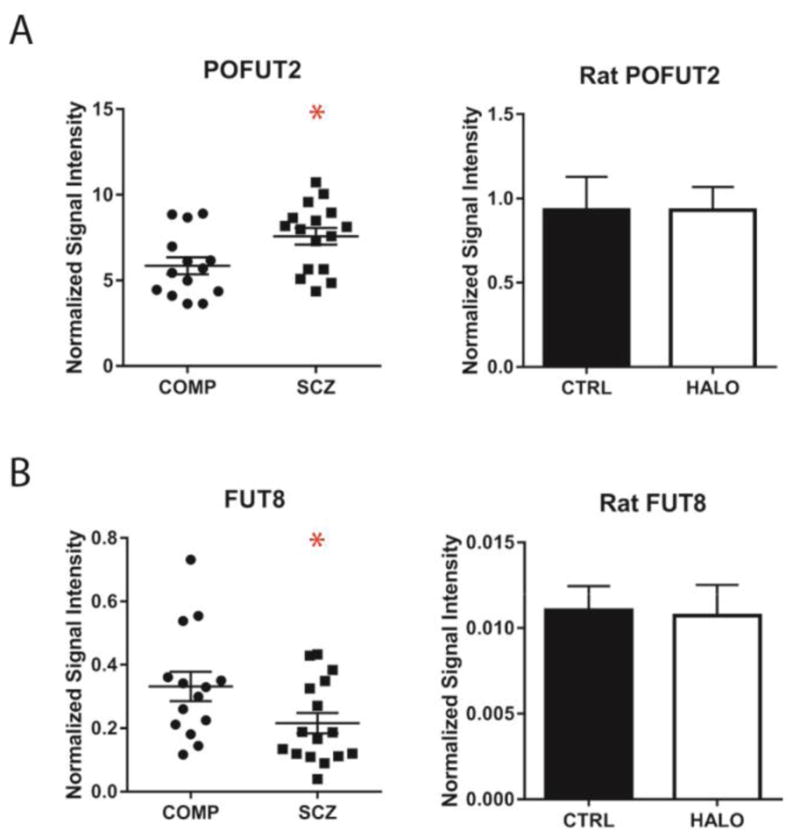
Protein expression levels in STG of comparison subjects (COMP) and patients with schizophrenia (SCZ) and in cortex of rats chronically treated with haloperidol (HALO) or vehicle (CTRL). (A) POFUT2 expression is increased in SCZ relative to COMP subjects, but not different between HALO and CTRL rats. (B) FUT8 expression is decreased in SCZ relative to COMP subjects, but not different between HALO and CTRL rats. Data are expressed as means ± S.E.M. of VCP-normalized average expression from duplicate samples. *p < 0.05.
Table 2.
Protein expression of fucose-modifying enzymes
| Comparison | Schizophrenia | Test Statistic | p-value | |
|---|---|---|---|---|
| FUCA2 | 0.020 ± 0.010 | 0.022 ± 0.012 | t (28) = 0.58 | |
| FUT11 | 2.427 ± 0.405 | 2.305 ± 0.427 | t (28) = 0.80 | |
| FUT8 | 0.332 ± 0.173 | 0.216 ± 0.130 | t (28) = 2.09 | 0.046 |
| POFUT1 | 0.003 ± 0.001 | 0.004 ± 0.001 | t (28) = 0.26 | |
| POFUT2 | 5.861 ± 1.874 | 7.580 ± 1.935 | t (28) = 2.46 | 0.020 |
Values are reported as means ± standard deviation. Data were analyzed using unpaired two-tailed Student’s t-tests. P-values that met the threshold for significance (α = 0.05) are listed.
3.2 FUT8 protein expression is reduced in schizophrenia
FUT8 protein expression was 35% lower in schizophrenia relative to comparison subjects [Figure 1B, Table 2; t (28) = 2.09, p = 0.046]. Post hoc regression analysis revealed a significant association between FUT8 expression and tissue pH, and subsequent ANCOVA revealed the difference of expression between diagnostic groups was maintained when covarying for pH [F(1,27) = 8.83, p = 0.006]. The expression of FUT8 was comparable between HALO and CTRL rats (Figure 1B). There were no differences of protein expression between diagnostic groups for any other fucose-modifying enzymes (FUT11, FUCA2, and POFUT1) assessed in this study (Table 2).
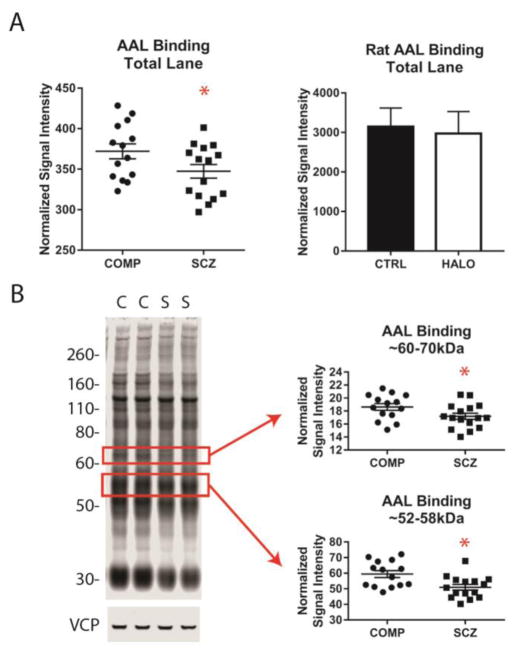
3.3 AAL binding is reduced in the STG in schizophrenia
Based on our finding of reduced FUT8 expression we predicted the enzyme product, core fucose, would also be reduced in schizophrenia. We assessed total AAL binding, as a measure of α-1,6-fucosylated N-glycoprotein expression, in schizophrenia and comparison subjects and found lower levels of AAL binding in schizophrenia [Figure 2; t (27) = 1.98, p = 0.029]. A more detailed analysis revealed AAL binding was specifically reduced for proteins in the ~52–58kDa [t (27) = 2.95, p = 0.003] and ~60–70kDa [t (28) = 2.05, p = 0.025] molecular mass ranges in schizophrenia. No difference in total levels of AAL binding were identified between HALO and CTRL rats (Figure 2).
Figure 2. Total α-1,6-fucosylation and α-1,6-fucosylation of glycoproteins between ~52–58kDa and ~60–70kDa are reduced in schizophrenia.
(A) Total α-1,6-fucosylprotein levels in STG of comparison subjects (COMP) and patients with schizophrenia (SCZ) and in cortex of rats chronically treated with haloperidol (HALO) or vehicle (CTRL). Total α-1,6-fucosylprotein expression is reduced in SCZ relative to COMP subjects, but not different between HALO and CTRL rats. (B) α-1,6-fucosylprotein expression in specific molecular mass ranges with corresponding bands indicated by boxes on a western blot probed with biotinylated Aleuria aurantia lectin (AAL) and IR-dye labeled streptavidin and the same western blot probed for VCP (valosin containing protein). Glycoproteins in the ~52–58kDa and ~60–70kDa molecular mass ranges demonstrate reduced α-1,6-fucosylation in SCZ relative to COMP subjects. Data are expressed as means ± S.E.M. of VCP-normalized average expression from duplicate samples. *p < 0.05.
4. DISCUSSION
Previous studies in schizophrenia brain have identified abnormalities of multiple glycosylation pathways (Bauer et al., 2010; Berretta, 2012; Berretta et al., 2015; Ikemoto, 2014; Mueller et al., 2014; Narayan et al., 2009; Pantazopoulos et al., 2013; Stanta et al., 2010; Telford et al., 2012; Tucholski et al., 2013b, 2013a) and dysregulated expression of glycosylation enzymes has been proposed as a possible mechanism underlying these deficits. Our current report builds on the prior finding of altered transcript levels of CAzymes in schizophrenia cortex by assessing the protein expression of a subset of these, fucosyltransferases and fucosidases. The results of this study establish that protein expression of POFUT2 and FUT8 are altered in schizophrenia STG. Furthermore, we show that the reaction product of FUT8, α-1,6-fucosylated N-glycoproteins, are similarly reduced, demonstrating a functional consequence of decreased FUT8 expression.
4.1 POFUT2 and O-fucosylation in schizophrenia
It is well established that correct protein N-glycosylation in the ER is essential for accurate protein folding and serves as a recognition signal for ER quality control mechanisms (ERQC) (Moremen and Molinari, 2006; Parodi, 2000). O-linked fucose has been identified as an important co-translational modification in a non-canonical ERQC pathway, and acts by stabilizing disulfide bonds in correctly folded cysteine-rich protein domains (Luther and Haltiwanger, 2009; Vasudevan et al., 2015; Vasudevan and Haltiwanger, 2014). This permits O-fucosylproteins to attain the correct tertiary structure and progress forward along the secretory pathway (Luther and Haltiwanger, 2009; Vasudevan et al., 2015; Vasudevan and Haltiwanger, 2014). POFUT1 and POFUT2 are the only enzymes expressed in humans that directly attach fucose to the hydroxyl group of serine/threonine residues of polypeptides, and we found that POFUT2 protein expression is increased in schizophrenia.
POFUT2 has been identified as the sole enzyme responsible for O-fucosylating TSR domains, which have 6 cysteines which form 3 disulfide bonds, between cysteines 1–5, 2–6, and 3–4 (Luo et al., 2006a, 2006b; Luther and Haltiwanger, 2009; Vasudevan et al., 2015; Vasudevan and Haltiwanger, 2014). Based on in vitro protein folding assays, it has been proposed that POFUT2 recognizes when 2 of the 3 disulfide bonds have correctly formed, and catalyzes the addition of fucose to stabilize the TSR domain and accelerate subsequent protein folding reactions (Vasudevan et al., 2015). O-fucose on TSR domains can then be further modified by B3GALTL (also called B3GLCT) to produce a Galβ-1,3Fuc disaccharide which further stabilizes the TSR domain. Interestingly, our prior study of CAzyme gene expression identified increased B3GALTL transcript levels in schizophrenia (unpublished data) and, together with our current finding, suggests that ERQC of disulfide bond formation may be abnormal in schizophrenia. Modification by POFUT2 is also necessary for the secretion of some TSR-domain containing proteins such as the ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs) family of proteins, which cleave chondroitin sulfate proteoglycans (CSPGs) to remodel the ECM and stimulate axonal outgrowth and synaptic plasticity. (Adams and Tucker, 2000; Hall et al., 2003; Kelwick et al., 2015; Luther and Haltiwanger, 2009; Niwa et al., 2015; Ricketts et al., 2007; Vasudevan et al., 2015; Wang et al., 2007). In schizophrenia, CSPG-rich perineuronal nets (PNNs), a specific region of ECM, demonstrate regional reductions in density and atypical CSPG composition (Berretta, 2012; Berretta et al., 2015; Mauney et al., 2013). Future investigations that directly assess effects of increased POFUT2-mediated O-fucosylation on ADAMTS secretion and proteolytic activity may elucidate potential mechanisms underlying CSPG-associated ECM abnormalities in schizophrenia.
4.2 FUT8 and core fucosylation in schizophrenia
FUT8 expression in schizophrenia was lower relative to comparison subjects. Since gene and protein expression levels of CAzymes may not accurately reflect the functional integrity of an expressed enzyme we assessed downstream consequences of reduced FUT8 protein expression using AAL-binding as a measure of α-1,6-fucosylation. After first verifying the binding pattern of AAL in postmortem brain (Supplementary Information and Supplementary Figure S1) was consistent with prior literature reporting that AAL demonstrates binding affinity for α-1,6-fucosylated N-glycans (Matsumura et al., 2007; Monzo et al., 2007; Stelck et al., 1999; Wu et al., 2014), we measured levels of AAL binding in schizophrenia and comparison subjects. We found AAL binding reduced and, in conjunction with our finding of decreased FUT8 expression, we interpret this as evidence of decreased core fucosylation of N-glycoproteins in schizophrenia. The AAL-binding assay indicates both reduced total α-1,6-linked fucose, as well as specific reductions in α-1,6-fucosylation of N-glycoproteins in molecular mass ranges between ~52–58kDa and ~60–70kDa in schizophrenia.
Deficient core fucosylation has been shown to alter receptor-mediated signaling in neurodevelopmental and cell signaling pathways known to be dysregulated in schizophrenia (Fukuda et al., 2011; Gu et al., 2013; Kurimoto et al., 2014; Shao et al., 2016; Vanhooren et al., 2011; Venkatachalam and Weinberg, 2013; Wang et al., 2006, 2005, 2015; Zhao et al., 2006). Based on findings in FUT8−/− mice, it has been proposed that deficits of AMPAR-mediated signaling in schizophrenia could stem from abnormal α-1,6-fucosylation of glutamate receptor subunits by altering the stoichiometry and localization of intact AMPARs (Gu et al., 2015). Reduced core fucosylation in the hippocampus of FUT8−/− mice enhanced AMPAR subunit heteromerization and increased the membrane expression of GluA1/2 and GluA2/3 AMPAR subtypes (Gu et al., 2015). All four AMPAR subunits are N-glycosylated, but only GluA2-4 demonstrably bind AAL in human cortex, confirming that N-glycans on these subunits are normally core fucosylated (Tucholski et al., 2014). In postmortem schizophrenia brain, the ratio of high mannose to complex N-glycans on GluA2 is reduced (Tucholski et al., 2013a) and expression of GluA1 in early endosomes is increased (Hammond et al., 2010), together providing evidence that GluA1/2 AMPAR trafficking out of the ER may be accelerated in the disorder.
In normal conditions, GluA1/2 receptors are expressed at the synapse in an activity-dependent manner, while GluA2/3 receptors are expressed constitutively (Lynch, 2004; Sans et al., 2001). The N-terminal domain of AMPAR subunits, where most N-glycosylation sites are located, plays an important role in the formation of subunit dimers and intact tetrameric AMPARs (Gan et al., 2014). In humans, AMPAR subunits GluA1-4 each have several N-glycosylation sites that exert regulatory effects on glutamate-signaling. Specific N-glycans expressed on AMPAR subunits modulate neurotransmission by influencing receptor assembly, trafficking, and synaptic stability, as well as by amending interactions between receptors and activity-modulating compounds (Copits et al., 2014; Everts et al., 1997; Kawamoto et al., 1994; Pasternack et al., 2003; Standley et al., 1998; Standley and Baudry, 2000; Takeuchi et al., 2015). N-glycosylation of N370 in GluA2 is necessary for the ER exit of GluA2 as well as the cell surface expression of both GluA2 and GluA1; furthermore, when a specific glyco-epitope (the HNK1 epitope, a unique N-glycan terminal trisaccharide) is expressed on GluA2 N413, both GluA1 and GluA2 demonstrated increased cell surface expression (Takeuchi et al., 2015). This indicates that activity-dependent GluA1/2 subunit intracellular trafficking and synaptic localization is regulated not only by the presence or absence of individual N-glycan(s), but also by the specific carbohydrate structure and composition of attached N-glycans. Furthermore, this shows that the N-glycosylation of GluA2 can impact the trafficking and subcellular localization of GluA1, abnormalities of which have both been reported in schizophrenia (Hammond et al., 2010; Tucholski et al., 2013a).
Reports examining glycan expression in serum and cerebrospinal fluid of schizophrenia patients in early stages of the disorder have found differences in the degree of glycan branching and levels of glycan galactosylation and sialylation following 6 weeks of antipsychotic treatment (Telford et al., 2012). Although specific differences in fucosylation were not observed in those studies, to address the possibility that administration of neuroleptics may result in dysfucosylation in elderly patient brain which may not be reflected in peripheral fluids in earlier stages of the disorder, we evaluated the expression of POFUT2 and FUT8, as well as AAL binding in cortical tissue of rats receiving long-term chronic antipsychotic administration. Our studies in antipsychotic treated rats found no differences in expression of the fucosyltransferases or measures of core fucose, suggesting that changes in these enzymes may be due to the illness rather than a result of antipsychotic exposure. A caveat to this study is that the ratio of male:female subjects differs between diagnostic groups due to the limited availability of postmortem brain samples. Post hoc analyses of subjects grouped by sex were performed to address this potential confound and no sex-associated differences were found. We cannot completely exclude the possibility that other lifestyle or environmental factors may have contributed to the differences we herein report (e.g. lifetime exposure to nicotine, other prescription medications, or over-the-counter drugs). Despite these and other limitations inherent to all studies of postmortem brain (reviewed in McCullumsmith et al., 2014), our report of abnormal fucosyltransferase expression adds to a growing body of literature demonstrating abnormal protein glycosylation in schizophrenia (Bauer et al., 2010; Kippe et al., 2015; McCullumsmith et al., 2014; McCullumsmith and Meador-Woodruff, 2011; Mueller et al., 2014; Narayan et al., 2009; Sato and Kitajima, 2013; Tucholski et al., 2013a).
Our current findings provide evidence for dysregulated fucosyltransferase expression, which in turn may contribute to abnormal protein folding and complex assembly, as well as to the abnormal protein trafficking, secretion, and subcellular localization of substrate proteins in schizophrenia. Increased POFUT2 expression identified in this study could produce deficits in ERQC of TSR-domain containing proteins and suggests a potential mechanism that may contribute to ECM- and CSPG-associated perturbations reported in schizophrenia. We also demonstrate reduced expression of FUT8 and a concurrent reduction of core fucosylated N-glycoproteins in schizophrenia. These changes could underlie a variety of deficits in cellular signaling mechanisms in schizophrenia—from the regulation of neurodevelopmental pathways over the course of a patient’s life to deficits of protein processing in individual neurons. Additional studies of glycosylation and fucosylation of specific substrate molecules in both postmortem brain and patient peripheral fluids may therefore be of clinical significance for the development of novel therapeutics and identification of diagnostic or therapeutic biomarkers.
Supplementary Material
Acknowledgments
ROLE OF FUNDING SOURCES
This work is supported by National Institutes of Health Grant MH53327 (JMW), MH064673 (VH), and MH066392 (VH).
The authors would like to thank Dr. Rosalinda Roberts and the Alabama Brain Collection for postmortem cortical samples used in assay development and tests of AAL binding specificity.
Footnotes
CONTRIBUTORS
Authors TMM and JMW and designed the study. Author VH oversaw the collection and characterization of human brain samples, and authors TMM and SDY designed and executed experimental protocols. TMM performed data calculations, statistical analyses, and literature searches, and wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams JC, Tucker RP. The thrombospondin type 1 repeat (TSR) superfamily: diverse proteins with related roles in neuronal development. Dev Dyn. 2000;218:280–99. doi: 10.1002/(SICI)1097-0177(200006)218:2<280::AID-DVDY4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Barbeau D, Liang JJ, Robitalille Y, Quirion R, Srivastava LK. Decreased expression of the embryonic form of the neural cell adhesion molecule in schizophrenic brains. Proc Natl Acad Sci U S A. 1995;92:2785–9. doi: 10.1073/pnas.92.7.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer DE, Haroutunian V, Meador-Woodruff JH, McCullumsmith RE. Abnormal glycosylation of EAAT1 and EAAT2 in prefrontal cortex of elderly patients with schizophrenia. Schizophr Res. 2010;117:92–8. doi: 10.1016/j.schres.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berretta S. Extracellular matrix abnormalities in schizophrenia. Neuropharmacology. 2012;62:1584–1597. doi: 10.1016/j.neuropharm.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berretta S, Pantazopoulos H, Markota M, Brown C, Batzianouli ET. Losing the sugar coating: Potential impact of perineuronal net abnormalities on interneurons in schizophrenia. Schizophr Res. 2015 doi: 10.1016/j.schres.2014.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copits BA, Vernon CG, Sakai R, Swanson GT. Modulation of ionotropic glutamate receptor function by vertebrate galectins. J Physiol. 2014;592:2079–96. doi: 10.1113/jphysiol.2013.269597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everts I, Villmann C, Hollmann M. N-Glycosylation is not a prerequisite for glutamate receptor function but Is essential for lectin modulation. Mol Pharmacol. 1997;52:861–73. doi: 10.1124/mol.52.5.861. [DOI] [PubMed] [Google Scholar]
- Fukuda T, Hashimoto H, Okayasu N, Kameyama A, Onogi H, Nakagawasai O, Nakazawa T, Kurosawa T, Hao Y, Isaji T, Tadano T, Narimatsu H, Taniguchi N, Gu J. alpha-1,6-fucosyltransferase-deficient mice exhibit multiple behavioral abnormalities associated with a schizophrenia-like phenotype: Importance of the balance between the dopamine and serotonin systems. J Biol Chem. 2011;286:18434–18443. doi: 10.1074/jbc.M110.172536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan Q, Salussolia CL, Wollmuth LP, Scientist M, Program T, Brook S. Assembly of AMPA receptors: mechanisms and regulation. J Physiol. 2014;1:39–48. doi: 10.1113/jphysiol.2014.273755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilabert-Juan J, Varea E, Guirado R, Blasco-Ibáñez JM, Crespo C, Nácher J, Blasco-Ib Nez JM, Crespo C, Nácher J. Alterations in the expression of PSA-NCAM and synaptic proteins in the dorsolateral prefrontal cortex of psychiatric disorder patients. Neurosci Lett. 2012;530:97–102. doi: 10.1016/j.neulet.2012.09.032. [DOI] [PubMed] [Google Scholar]
- Gu W, Fukuda T, Isaji T, Hang Q, Lee H, Sakai S, Morise J, Mitoma J, Higashi H, Taniguchi N, Yawo H, Oka S, Gu J. Loss of α1,6-Fucosyltransferase Decreases Hippocampal Long Term Potentiation: IMPLICATIONS FOR CORE FUCOSYLATION IN THE REGULATION OF AMPA RECEPTOR HETEROMERIZATION AND CELLULAR SIGNALING. J Biol Chem. 2015;290:17566–17575. doi: 10.1074/jbc.M114.579938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu W, Fukuda T, Isaji T, Hashimoto H, Wang Y, Gu J. α1,6-Fucosylation regulates neurite formation via the activin/phospho-Smad2 pathway in PC12 cells: the implicated dual effects of Fut8 for TGF-β/activin-mediated signaling. FASEB J. 2013;27:3947–58. doi: 10.1096/fj.12-225805. [DOI] [PubMed] [Google Scholar]
- Hall NG, Klenotic P, Anand-Apte B, Apte SS. ADAMTSL-3/punctin-2, a novel glycoprotein in extracellular matrix related to the ADAMTS family of metalloproteases. Matrix Biol. 2003;22:501–10. doi: 10.1016/S0945-053X(03)00075-1. [DOI] [PubMed] [Google Scholar]
- Hammond JC, McCullumsmith RE, Funk AJ, Haroutunian V, Meador-Woodruff JH. Evidence for abnormal forward trafficking of AMPA receptors in frontal cortex of elderly patients with schizophrenia. Neuropsychopharmacology. 2010;35:2110–9. doi: 10.1038/npp.2010.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harte MK, Bachus SB, Reynolds GP. Increased N-acetylaspartate in rat striatum following long-term administration of haloperidol. Schizophr Res. 2005;75:303–8. doi: 10.1016/j.schres.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Ihara H, Ikeda Y, Taniguchi N. Reaction mechanism and substrate specificity for nucleotide sugar of mammalian alpha1,6-fucosyltransferase--a large-scale preparation and characterization of recombinant human FUT8. Glycobiology. 2006;16:333–42. doi: 10.1093/glycob/cwj068. [DOI] [PubMed] [Google Scholar]
- Ikemoto K. Lectin-Positive Spherical Deposits (SPD) Detected in the Molecular Layer of Hippocampal Dentate Gyrus of Dementia, Down’s Syndrome, and Schizophrenia. J Alzheimer’s Dis Park. 2014;4:4–7. doi: 10.4172/2161-0460.1000169. [DOI] [Google Scholar]
- Kashihara K, Sato M, Fujiwara Y, Harada T, Ogawa T, Otsuki S. Effects of intermittent and continuous haloperidol administration on the dopaminergic system in the rat brain. Biol Psychiatry. 1986;21:650–6. doi: 10.1016/0006-3223(86)90126-5. [DOI] [PubMed] [Google Scholar]
- Kawamoto S, Hattori S, Oiji I, Hamajima K, Mishina M, Okuda K. Ligand-binding properties and N-glycosylation of alpha 1 subunit of the alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionate(AMPA)-selective glutamate receptor channel expressed in a baculovirus system. Eur J Biochem. 1994;223:665–73. doi: 10.1111/j.1432-1033.1994.tb19039.x. [DOI] [PubMed] [Google Scholar]
- Kelwick R, Desanlis I, Wheeler GN, Edwards DR. The ADAMTS (A Disintegrin and Metalloproteinase with Thrombospondin motifs) family. Genome Biol. 2015;16:113. doi: 10.1186/s13059-015-0676-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kippe JM, Mueller TM, Haroutunian V, Meador-Woodruff JH. Abnormal N-acetylglucosaminyltransferase expression in prefrontal cortex in schizophrenia. Schizophr Res. 2015;166:219–24. doi: 10.1016/j.schres.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kötzler MP, Blank S, Bantleon FI, Spillner E, Meyer B. Donor substrate binding and enzymatic mechanism of human core α1,6-fucosyltransferase (FUT8) Biochim Biophys Acta. 2012;1820:1915–25. doi: 10.1016/j.bbagen.2012.08.018. [DOI] [PubMed] [Google Scholar]
- Kurimoto A, Kitazume S, Kizuka Y, Nakajima K, Oka R, Fujinawa R, Korekane H, Yamaguchi Y, Wada Y, Taniguchi N. The Absence of Core Fucose Up-regulates GnT-III and Wnt Target Genes: A POSSIBLE MECHANISM FOR AN ADAPTIVE RESPONSE IN TERMS OF GLYCAN FUNCTION. J Biol Chem. 2014;289:11704–11714. doi: 10.1074/jbc.M113.502542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Koles K, Vorndam W, Haltiwanger RS, Panin VM. Protein O-fucosyltransferase 2 adds O-fucose to thrombospondin type 1 repeats. J Biol Chem. 2006a;281:9393–9. doi: 10.1074/jbc.M511975200. [DOI] [PubMed] [Google Scholar]
- Luo Y, Nita-Lazar A, Haltiwanger RS. Two distinct pathways for O-fucosylation of epidermal growth factor-like or thrombospondin type 1 repeats. J Biol Chem. 2006b;281:9385–9392. doi: 10.1074/jbc.M511974200. [DOI] [PubMed] [Google Scholar]
- Luther KB, Haltiwanger RS. Role of unusual O-glycans in intercellular signaling. Int J Biochem Cell Biol. 2009;41:1011–24. doi: 10.1016/j.biocel.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch MA. Long-Term Potentiation and Memory. Physiol Rev. 2004;84:87–136. doi: 10.1152/physrev.00014.2003. [DOI] [PubMed] [Google Scholar]
- Ma B, Simala-Grant JL, Taylor DE. Fucosylation in prokaryotes and eukaryotes. Glycobiology. 2006;16:158R–184R. doi: 10.1093/glycob/cwl040. [DOI] [PubMed] [Google Scholar]
- Matsumura K, Higashida K, Ishida H, Hata Y, Yamamoto K, Shigeta M, Mizuno-Horikawa Y, Wang X, Miyoshi E, Gu J, Taniguchi N. Carbohydrate binding specificity of a fucose-specific lectin from Aspergillus oryzae: a novel probe for core fucose. J Biol Chem. 2007;282:15700–8. doi: 10.1074/jbc.M701195200. [DOI] [PubMed] [Google Scholar]
- Mauney Sa, Athanas KM, Pantazopoulos H, Shaskan N, Passeri E, Berretta S, Woo TUW. Developmental pattern of perineuronal nets in the human prefrontal cortex and their deficit in schizophrenia. Biol Psychiatry. 2013;74:427–435. doi: 10.1016/j.biopsych.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullumsmith RE, Hammond JH, Shan D, Meador-Woodruff JH. Postmortem Brain: An Underutilized Substrate for Studying Severe Mental Illness. Neuropsychopharmacology. 2014;39:65–87. doi: 10.1038/npp.2013.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullumsmith RE, Meador-Woodruff JH. Novel approaches to the study of postmortem brain in psychiatric illness: old limitations and new challenges. Biol Psychiatry. 2011;69:127–33. doi: 10.1016/j.biopsych.2010.09.035. [DOI] [PubMed] [Google Scholar]
- Miyoshi E, Moriwaki K, Nakagawa T. Biological function of fucosylation in cancer biology. J Biochem. 2008;143:725–9. doi: 10.1093/jb/mvn011. [DOI] [PubMed] [Google Scholar]
- Monzo A, Bonn GK, Guttman A. Lectin-immobilization strategies for affinity purification and separation of glycoconjugates. TrAC Trends Anal Chem. 2007;26:423–432. doi: 10.1016/j.trac.2007.01.018. [DOI] [Google Scholar]
- Moremen KW, Molinari M. N-linked glycan recognition and processing: the molecular basis of endoplasmic reticulum quality control. Curr Opin Struct Biol. 2006;16:592–9. doi: 10.1016/j.sbi.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller TM, Haroutunian V, Meador-Woodruff JH. N-Glycosylation of GABAA receptor subunits is altered in Schizophrenia. Neuropsychopharmacology. 2014;39:528–37. doi: 10.1038/npp.2013.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller TM, Remedies CE, Haroutunian V, Meador-Woodruff JH. Abnormal subcellular localization of GABAA receptor subunits in schizophrenia brain. Transl Psychiatry. 2015;5:e612. doi: 10.1038/tp.2015.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan S, Head SR, Gilmartin TJ, Dean B, Thomas EA. Evidence for disruption of sphingolipid metabolism in schizophrenia. J Neurosci Res. 2009;87:278–288. doi: 10.1002/jnr.21822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa Y, Suzuki T, Dohmae N, Simizu S. O-fucosylation of CCN1 is required for its secretion. FEBS Lett. 2015;589:3287–93. doi: 10.1016/j.febslet.2015.09.012. [DOI] [PubMed] [Google Scholar]
- Pantazopoulos H, Boyer-Boiteau A, Holbrook EH, Jang W, Hahn CG, Arnold SE, Berretta S. Proteoglycan abnormalities in olfactory epithelium tissue from subjects diagnosed with schizophrenia. Schizophr Res. 2013;150:366–372. doi: 10.1016/j.schres.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parodi AJ. Role of N-oligosaccharide endoplasmic reticulum processing reactions in glycoprotein folding and degradation. Biochem J. 2000;348(Pt 1):1–13. doi: 10.1042/0264-6021:3480001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternack A, Coleman SK, Féthière J, Madden DR, LeCaer JPP, Rossier J, Pasternack M, Keinänen K. Characterization of the functional role of the N-glycans in the AMPA receptor ligand-binding domain. J Neurochem. 2003;84:1184–1192. doi: 10.1046/j.1471-4159.2003.01611.x. [DOI] [PubMed] [Google Scholar]
- Pietersen CY, Mauney SA, Kim SS, Lim MP, Rooney RJ, Goldstein JM, Petryshen TL, Seidman LJ, Shenton ME, McCarley RW, Sonntag KC, Woo TUW. Molecular Profiles of Pyramidal Neurons in the Superior Temporal Cortex in Schizophrenia. J Neurogenet. 2014a;28:53–69. doi: 10.3109/01677063.2014.882918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietersen CY, Mauney Sa, Kim SS, Passeri E, Lim MP, Rooney RJ, Goldstein JM, Petreyshen TL, Seidman LJ, Shenton ME, Mccarley RW, Sonntag KC, Woo TUW, Petryshen TL, Seidman LJ, Shenton ME, Mccarley RW, Sonntag KC, Woo TUW. Molecular profiles of parvalbumin-immunoreactive neurons in the superior temporal cortex in schizophrenia. J Neurogenet. 2014b;28:70–85. doi: 10.3109/01677063.2013.878339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powchik P, Davidson M, Nemeroff CB, Haroutunian V, Purohit D, Losonczy M, Bissette G, Perl D, Ghanbari H, Miller B. Alzheimer’s-disease-related protein in geriatric schizophrenic patients with cognitive impairment. Am J Psychiatry. 1993;150:1726–7. doi: 10.1176/ajp.150.11.1726. [DOI] [PubMed] [Google Scholar]
- Purohit DP, Davidson M, Perl DP, Powchik P, Haroutunian VH, Bierer LM, McCrystal J, Losonczy M, Davis KL. Severe cognitive impairment in elderly schizophrenic patients: a clinicopathological study. Biol Psychiatry. 1993;33:255–60. doi: 10.1016/0006-3223(93)90291-k. [DOI] [PubMed] [Google Scholar]
- Ricketts LM, Dlugosz M, Luther KB, Haltiwanger RS, Majerus EM. O-fucosylation is required for ADAMTS13 secretion. J Biol Chem. 2007;282:17014–23. doi: 10.1074/jbc.M700317200. [DOI] [PubMed] [Google Scholar]
- Sans N, Racca C, Petralia RS, Wang YX, McCallum J, Wenthold RJ. Synapse-associated protein 97 selectively associates with a subset of AMPA receptors early in their biosynthetic pathway. J Neurosci. 2001;21:7506–7516. doi: 10.1523/JNEUROSCI.21-19-07506.2001. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato C, Kitajima K. Impact of structural aberrancy of polysialic acid and its synthetic enzyme ST8SIA2 in schizophrenia. Front Cell Neurosci. 2013;7:61. doi: 10.3389/fncel.2013.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao K, Chen ZY, Gautam S, Deng NH, Zhou Y, Wu XZ. Posttranslational modification of E-cadherin by core fucosylation regulates Src activation and induces epithelial-mesenchymal transition-like process in lung cancer cells. Glycobiology. 2016;26:142–54. doi: 10.1093/glycob/cwv089. [DOI] [PubMed] [Google Scholar]
- Standley S, Baudry M. The role of glycosylation in ionotropic glutamate receptor ligand binding, function, and trafficking. Cell Mol Life Sci. 2000;57:1508–16. doi: 10.1007/PL00000635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standley S, Tocco G, Wagle N, Baudry M. High- and low-affinity alpha-[3H]amino-3-hydroxy-5-methylisoxazole-4-propionic acid ([3H]AMPA) binding sites represent immature and mature forms of AMPA receptors and are composed of differentially glycosylated subunits. J Neurochem. 1998;70:2434–45. doi: 10.1046/j.1471-4159.1998.70062434.x. [DOI] [PubMed] [Google Scholar]
- Stanta JL, Saldova R, Struwe WB, Byrne JC, Leweke FM, Rothermund M, Rahmoune H, Levin Y, Guest PC, Bahn S, Rudd PM. Identification of N-glycosylation changes in the CSF and serum in patients with schizophrenia. J Proteome Res. 2010;9:4476–89. doi: 10.1021/pr1002356. [DOI] [PubMed] [Google Scholar]
- Steffek AE, McCullumsmith RE, Haroutunian V, Meador-Woodruff JH. Cortical expression of glial fibrillary acidic protein and glutamine synthetase is decreased in schizophrenia. Schizophr Res. 2008;103:71–82. doi: 10.1016/j.schres.2008.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelck S, Robitzki A, Willbold E, Layer PG. Fucose in alpha(1–6)-linkage regulates proliferation and histogenesis in reaggregated retinal spheroids of the chick embryo. Glycobiology. 1999;9:1171–9. doi: 10.1093/glycob/9.11.1171. [DOI] [PubMed] [Google Scholar]
- Takeuchi Y, Morise J, Morita I, Takematsu H, Oka S. Role of Site-Specific N-Glycans Expressed on GluA2 in the Regulation of Cell Surface Expression of AMPA-Type Glutamate Receptors. PLoS One. 2015;10:e0135644. doi: 10.1371/journal.pone.0135644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telford JE, Bones J, McManus C, Saldova R, Manning G, Doherty M, Leweke FM, Rothermundt M, Guest PC, Rahmoune H, Bahn S, Rudd PM. Antipsychotic treatment of acute paranoid schizophrenia patients with olanzapine results in altered glycosylation of serum glycoproteins. J Proteome Res. 2012;11:3743–52. doi: 10.1021/pr300218h. [DOI] [PubMed] [Google Scholar]
- Tucholski J, Pinner AL, Simmons MS, Meador-Woodruff JH. Evolutionarily conserved pattern of AMPA receptor subunit glycosylation in Mammalian frontal cortex. PLoS One. 2014;9:e94255. doi: 10.1371/journal.pone.0094255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucholski J, Simmons MS, Pinner AL, Haroutunian V, McCullumsmith RE, Meador-Woodruff JH. Abnormal N-linked glycosylation of cortical AMPA receptor subunits in schizophrenia. Schizophr Res. 2013a;146:177–83. doi: 10.1016/j.schres.2013.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucholski J, Simmons MS, Pinner AL, McMillan LD, Haroutunian V, Meador-Woodruff JH. N-linked glycosylation of cortical N-methyl-D-aspartate and kainate receptor subunits in schizophrenia. Neuroreport. 2013b;24:688–91. doi: 10.1097/WNR.0b013e328363bd8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhooren V, Dewaele S, Kuro-O M, Taniguchi N, Dollé L, van Grunsven LA, Makrantonaki E, Zouboulis CC, Chen CC, Libert C. Alteration in N-glycomics during mouse aging: a role for FUT8. Aging Cell. 2011;10:1056–66. doi: 10.1111/j.1474-9726.2011.00749.x. [DOI] [PubMed] [Google Scholar]
- Varea E, Guirado R, Gilabert-Juan J, Martí U, Castillo-Gomez E, Blasco-Ibáñez JM, Crespo C, Nacher J. Expression of PSA-NCAM and synaptic proteins in the amygdala of psychiatric disorder patients. J Psychiatr Res. 2012;46:189–97. doi: 10.1016/j.jpsychires.2011.10.011. [DOI] [PubMed] [Google Scholar]
- Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME. Essentials of Glycobiology. 2. Cold Spring Harbor Laboratory Press; 2009. Essentials of Glycobiology. [PubMed] [Google Scholar]
- Vasudevan D, Haltiwanger RS. Novel roles for O-linked glycans in protein folding. Glycoconj J. 2014;31:417–26. doi: 10.1007/s10719-014-9556-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan D, Takeuchi H, Johar SS, Majerus E, Haltiwanger RS. Peters plus syndrome mutations disrupt a noncanonical ER quality-control mechanism. Curr Biol. 2015;25:286–95. doi: 10.1016/j.cub.2014.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam MA, Weinberg JM. New wrinkles in old receptors: core fucosylation is yet another target to inhibit TGF-β signaling. Kidney Int. 2013;84:11–4. doi: 10.1038/ki.2013.95. [DOI] [PubMed] [Google Scholar]
- Wang LW, Dlugosz M, Somerville RPT, Raed M, Haltiwanger RS, Apte SS. O-fucosylation of thrombospondin type 1 repeats in ADAMTS-like-1/punctin-1 regulates secretion: implications for the ADAMTS superfamily. J Biol Chem. 2007;282:17024–31. doi: 10.1074/jbc.M701065200. [DOI] [PubMed] [Google Scholar]
- Wang X, Gu J, Ihara H, Miyoshi E, Honke K, Taniguchi N. Core fucosylation regulates epidermal growth factor receptor-mediated intracellular signaling. J Biol Chem. 2006;281:2572–7. doi: 10.1074/jbc.M510893200. [DOI] [PubMed] [Google Scholar]
- Wang X, Inoue S, Gu J, Miyoshi E, Noda K, Li W, Mizuno-Horikawa Y, Nakano M, Asahi M, Takahashi M, Uozumi N, Ihara S, Lee SH, Ikeda Y, Yamaguchi Y, Aze Y, Tomiyama Y, Fujii J, Suzuki K, Kondo A, Shapiro SD, Lopez-Otin C, Kuwaki T, Okabe M, Honke K, Taniguchi N. Dysregulation of TGF-beta1 receptor activation leads to abnormal lung development and emphysema-like phenotype in core fucose-deficient mice. Proc Natl Acad Sci U S A. 2005;102:15791–6. doi: 10.1073/pnas.0507375102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Fukuda T, Isaji T, Lu J, Gu W, Lee HH, Ohkubo Y, Kamada Y, Taniguchi N, Miyoshi E, Gu J. Loss of α1,6-fucosyltransferase suppressed liver regeneration: implication of core fucose in the regulation of growth factor receptor-mediated cellular signaling. Sci Rep. 2015;5:8264. doi: 10.1038/srep08264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Zhu J, Yin H, Buckanovich RJ, Lubman DM. Analysis of glycan variation on glycoproteins from serum by the reverse lectin-based ELISA assay. J Proteome Res. 2014;13:2197–204. doi: 10.1021/pr401061c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Itoh S, Wang X, Isaji T, Miyoshi E, Kariya Y, Miyazaki K, Kawasaki N, Taniguchi N, Gu J. Deletion of core fucosylation on alpha3beta1 integrin down-regulates its functions. J Biol Chem. 2006;281:38343–50. doi: 10.1074/jbc.M608764200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.