Abstract
Rationale
Nitrate-rich beetroot juice has been shown to improve exercise capacity in Heart Failure with Preserved Ejection Fraction (HFpEF), but studies using pharmacologic preparations of inorganic nitrate are lacking.
Objectives
To determine: (1) the dose-response effect of potassium nitrate (KNO3) on exercise capacity; (2) the population-specific pharmacokinetic and safety profile of KNO3 in HFpEF.
Methods and Results
We randomized 12 subjects with HFpEF to oral KNO3 (n=9) or potassium chloride (KCl, n=3). Subjects received 6mmol twice-daily during Week-1, followed by 6mmol thrice-daily during Week-2. Supine cycle ergometry was performed at baseline (Visit 1) and after each week (Visits 2&3). Quality of life (QOL) was assessed with the Kansas City Cardiomyopathy Questionnaire (KCCQ). The primary efficacy outcome, peak O2-uptake, did not significantly improve (P=0.13). Exploratory outcomes included exercise duration and quality of life. Exercise duration increased significantly with KNO3 (Visit 1: 9.87 [95%CI=9.31–10.43]; Visit 2: 10.73 [95%CI=10.13–11.33]; Visit 3: 11.61 [95%CI=11.05–12.17] minutes, P=0.002). Improvements in the KCCQ total symptom (Visit 1: 58.0 [95%CI=52.5–63.5], Visit 2: 66.8 [95%CI=61.3–72.3]; Visit 3: 70.8 [95%CI=65.3–76.3], P=0.016) and functional status scores (Visit 1: 62.2 [95%CI=58.5–66.0], Visit 2: 68.6 [95%CI=64.9–72.3], Visit 3: 71.1 [95%CI=67.3–74.8]; P=0.01) were seen after KNO3. Pronounced elevations in trough levels of nitric oxide metabolites (NOm) occurred with KNO3 (Visit 2: 199.5 [95%CI=98.7–300.2]; Visit 3: 471.8 [95%CI=377.8–565.8]) versus baseline (Visit 1: 38.0 [95%CI=0.00–132.0] μM; P<0.001). KNO3 did not lead to clinically-significant hypotension or methemoglobinemia. Following 6 mmol of KNO3, systolic blood pressure was reduced by a maximum of 17.9 (95%CI −28.3-[−7.6]) mmHg 3.75 hours later. Peak NOm concentrations were 259.3 (95%CI 176.2–342.4) μM 3.5 hours after ingestion, and the median half-life was 73.0 (IQR 33.4–232.0) minutes.
Conclusions
KNO3 is potentially well-tolerated and improves exercise duration and QOL in HFpEF. This study reinforces the efficacy of KNO3 and suggests that larger randomized trials are warranted.
ClinicalTrials.gov
NCT02256345; https://www.clinicaltrials.gov/ct2/show/NCT02256345
Subject Terms: Endothelium, Vascular Type, Nitric Oxide, Heart Failure, Exercise
Keywords: Heart failure, exercise capacity, nitric oxide
INTRODUCTION
The prevalence of heart failure with preserved ejection fraction (HFpEF) is rising.1, 2 Recent tissue data demonstrate decreased cyclic GMP (cGMP) concentration, and increased oxidative stress in subjects with HFpEF,3, 4 suggesting impaired nitric oxide (NO) signaling. However, efforts at increasing NO bioavailability, either through phosphodiesterase-5 inhibition or the administration of organic nitrate, have not led to improvements in exercise capacity in HFpEF.5, 6
Traditionally, NO synthesis has been known to occur via the enzymatic oxidation of L-arginine by nitric oxide synthases (NOS), in the presence of molecular oxygen, to yield citrulline and NO.7 Given its dependence on oxygen, NO synthesis from NOS may be inefficient in the setting of hypoxia. Inorganic nitrate, ingested in the diet or via supplementation, is rapidly absorbed in the gastrointestinal tract, concentrated in the salivary glands, and then released into the oral cavity where bacteria facilitate its reduction to nitrite. Nitrite is then reabsorbed into the circulation and can be reduced to NO by deoxyhemoglobin.8 Conversion of nitrite to NO is potentiated by hypoxia and acidosis,9, 10 as would be seen in exercising muscle,11 leading to vasodilation and increased perfusion at sites of greater metabolic need.12 Thus, circulating nitrate/nitrite may serve as an alternative pool for bioactive NO, which can sustain/enhance NO signaling when the traditional pathway for NO generation is less functional.9, 13 Indeed, nitrate/nitrite supplementation has been shown to increase blood flow to exercising muscle in rats with14, 15 and without16, 17 heart failure. NO-induced vasodilation during exercise may facilitate the matching of perfusion to metabolic activity.18
Recently, our group demonstrated that acute supplementation with beetroot juice, which is rich in inorganic nitrate, improves vasodilatory reserve and peak oxygen uptake in HFpEF subjects.19 A more recent study demonstrated improvements in endurance during submaximal exercise following one-week of supplementation with beetroot juice.20 However, studies using pharmacologic preparations of inorganic nitrate are lacking. The current study was an in-depth pharmacodynamic and pharmacokinetic study of oral KNO3 in HFpEF with the specific goals of: (1) Determining the safety profile of short-term (2-week) inorganic nitrate supplementation, administered as potassium nitrate (KNO3) capsules; and (2) Determine the physiologic effects and the dose-response associated with KNO3 administration over 2-weeks on exercise capacity and key physiologic adaptations to exercise; and (3) Assess the pharmacokinetic profile of KNO3 in this population.
METHODS
Participants
Inclusion criteria included symptomatic heart failure in the context of a preserved (≥50%) ejection fraction. Subjects were required to have evidence of elevated filling pressures, demonstrated by at least one of the following: (1) Elevated invasively-measured filling pressures; (2) Septal E/e’>15; (3) Septal E/e’>8 with either elevated natriuretic peptide levels within the past year, left atrial enlargement, or chronic loop diuretic use for control of symptoms. Exclusion criteria included any rhythm other than sinus with native conduction, inability to exercise, ≥ moderate valvular disease, known hypertrophic/infiltrative/inflammatory cardiomyopathy, pericardial disease, current angina, acute coronary syndrome or coronary intervention within the past 2 months, primary pulmonary arteriopathy, clinically-significant lung disease, current ischemia, chronic treatment with organic nitrates or allopurinol, significant liver disease, eGFR<30 mL/min/1.73 m2 or creatinine > 2.5 mg/dL, current smoking, alcohol dependency, Barrett’s esophagus, G6PD deficiency, and a baseline methemoglobin >3%. Background medications were not altered. The study was approved by the Institutional Review Board of the University of Pennsylvania and conducted under an FDA IND (#123,785). Written informed consent was obtained from all subjects. This study was registered on ClinicalTrials.gov (NCT02256345).
Study medication and dosing regimen
This was a randomized trial of KNO3 given in two dosing regimens: 6 mmol twice daily for one week followed by dose-escalation to 6 mmol thrice daily for one week. While a primary goal of the study was to assess the safety of KNO3 and within-group changes in various endpoints in KNO3-treated subjects, a small number of placebo-treated (PB, n=3) subjects were included, only to assess for any potential training-effect on repeated exercise and KCCQ measurements.21 Potassium chloride (KCl), given in equivalent doses, was used as the PB to account for differences in blood pressure or flow that could be attributed to potassium.22
Design
The study was initially designed to be single-blinded, to allow the Principal Investigator to be aware of arm allocation due to potential concerns for methemoglobinemia with drug administration. As described below; however, no clinically-significant elevations in methemoglobin occurred. One investigator (PZ), who was the Primary Investigator responsible for supervising all visits and measurements during the study, remained blinded to treatment allocation throughout the entirety of the study. All physiologic and imaging data were analyzed in a double-blind manner.
Study protocol
Subjects presented to the Clinical and Translational Research Center at the University of Pennsylvania in the fasted state. Resting blood pressure was obtained using an automated oscillometric device (Accutorr Plus, Datascope Corp., Paramus, NJ). Blood was obtained for serum nitric oxide metabolites (NOm) and nitrite determination, basic chemistries, NT-pro-BNP determination (Cobas e411 Analyzer, Roche Diagnostics, Indianapolis, IN), and measurement of hemoglobin and methemoglobin (ABL800 Flex Analyzer, Radiometer America, Brea, CA). Subjects refrained from using mouthwash and were provided with a list of nitrate-rich foods to avoid during the study. A registered dietician met with subjects at each study visit to reinforce dietary restrictions. During the baseline visit (Visit 1), subjects proceeded directly to the exercise testing protocol after verification of inclusion/exclusion criteria, a physical examination, echocardiography with arterial tonometry, and laboratory testing. During Visit 2 and Visit 3, study drug was administered with a standardized low-nitrate meal. Two hours later, orthostatic blood pressure measurements were made, followed by echocardiography, arterial tonometry, and the exercise protocol, as described below. The Kansas City Cardiomyopathy Questionnaire was administered at each study visit.23
Echocardiography and arterial tonometry
Subjects underwent resting echocardiography using a GE Vivid I machine (GE Healthcare, Fairfield, CT). Metrics were quantified in triplicate, according to the American Society of Echocardiography guidelines.24 Left atrial volumes were quantified using the Area-Length method and indexed to body-surface area (BSA). Left ventricular outflow tract (LVOT) Doppler velocity-time integrals (VTI) were obtained from the 5-chamber view to obtain stroke volume (LVOT cross-sectional area * VTI) at rest and at peak exercise.
Resting arterial tonometry (Millar Instruments, Houston, TX) was performed at the left radial artery and calibrated using brachial oscillometric blood pressures (Scholar III507EL, Criticare Systems, Inc., Waukesha, WI). Tonometric signals were processed using Sphygmocor software (AtCor Medical, Australia) to derive central pressures. Augmentation index (AIx) was calculated as the ratio of the amplitude of the second peak to the first peak (P2/P1 × 100%).
Exercise studies
As described previously,19 subjects underwent peak effort exercise testing using supine cycle ergometry with gas exchange measurements (Parvo Medics, Sandy, UT). Peak oxygen uptake (VO2,peak) was defined as the average value obtained during the last 30 seconds of exercise. Exercise economy was estimated as the ratio of total work performed (kilojoules) to total oxygen consumed (liters) above baseline during the entirety of the exercise study. Prior work demonstrates an increased benefit for inorganic nitrate supplementation at higher exercise intensities, when Type II muscle fibers are utilized to a greater degree.16, 25–28 In post hoc analysis, we quantified total oxygen uptake and work performed during the last 3- and 6-minutes of exercise, when subjects were exercising at higher intensities, and obtained estimates of exercise economy over those time periods.
After at least 15 minutes of rest, subjects exercised at 25W for 6 minutes with steady-state VO2 measured during the final minute of exercise. Radial tonometry and LVOT VTIs were obtained at rest and during steady-state to determine input impedance, as described previously.29 VO2 recovery kinetics (τ) were modeled according to a mono-exponential decay.
Skeletal muscle mitochondrial oxidative function
We used near infrared spectroscopy (NIRS) to determine skeletal muscle mitochondrial oxidative function after a standardized exercise protocol, as described by Ryan et al.,30 and reported previously by our group.19
Safety and pharmacokinetic determination
On Visit 1 and Visit 2, subjects were given study medications (either 6 mmol KNO3 or 6 mmol KCl) with a standardized nitrate-free meal following exercise testing. For the next 5 hours, blood pressure was measured every 15 minutes, and serum was drawn every 30 minutes for determination of NOm and methemoglobin. The elimination rate (Ke) was the negative of the slope from the linear regression of log concentration versus time over the last 3 time points and used to calculate the half-life (t1/2 = ln 2/Ke).
Serum NOm quantification
Serum levels of NOm were measured as reported previously using vanadium (III)/hydrochloric acid reduction, heated at 95°C, and coupled with chemiluminescence detection using the Sievers-Nitric Oxide Analyzer (Sievers-NOA 280, Nitric Oxide Analyzer, Sievers Instruments, Boulder, CO). This method quantifies nitrate, as well as nitrite, NO-metal and heme complexes, low molecular weight thiol-NO adducts and protein cysteine-NO adducts.19 NOm samples for 3 individuals were repeated in each of the 9 batches performed. The intra-class correlation coefficient for NOm was 0.98 (95%CI 0.93–1.00; P<0.001), demonstrating excellent reproducibility. Serum nitrite levels were quantified using reduction with potassium iodide/crystalline iodide and the same Sievers instrument. Nitrite samples were measured in each of the 6 batches performed. The intra-class correlation coefficient for nitrite was 0.94 (95%CI 0.77–1.00; P<0.001).
Activity monitoring and 24-hour blood pressure measurements
At the conclusion of Visit 1 and Visit 2, subjects were given an ambulatory blood pressure monitor (Mobil-O-Graph PWA, I.E.M. GmbH, Stolberg, Germany) and an activity monitor (wActiSleep-BT Monitor, Actigraph, LLC., Pensacola, FL). Blood pressure measurements were obtained every 30 minutes over a 24-hour period. Actigraphy was used to obtain daily steps taken and estimated kilocalorie expenditure. Because follow-up visits were scheduled during the last 1–2 days of each week, data was abstracted for days 1–5 in all subjects. Values for days 3–5 were averaged to obtain estimates corresponding to steady-state drug effects (SS).
Study outcomes
The primary efficacy endpoint was the change in VO2,peak. Secondary endpoints included changes in vasodilatory reserve (percent reduction in systemic vascular resistance during exercise, compared to the resting measurement), changes in mitochondrial oxidative function using NIRS, and changes in AIx. Exploratory endpoints included other metrics of exercise capacity, and changes in the KCCQ. Pharmacokinetics were assessed via measurement of NOm after single-dose administration of 6 mmol and with trough levels after repeated twice- and thrice-daily administration.
Statistical methods
Demographic data are presented as n(%) or means(SD). Mixed-effects models were generated in STATA (Stata/SE 13.1, StataCorp, College Station, TX), incorporating the correlation between repeated measurements in the same individual. No assumption of linearity was made. As pre-specified in the protocol, only within group comparisons were performed. Marginal means with 95% confidence intervals (CI) were determined from the mixed models and presented in the tables and figures. An overall P-value<0.05 was taken to be significant for each model, with post-hoc comparisons between visits performed subsequently. No adjustment for multiple comparisons was made. The elimination half-life was determined using the PK package in STATA. Linear regression was performed to assess the correlation between changes in serum NOm and nitrite levels and the change in exercise metrics. Regression coefficients are presented as standardized units describing the standard deviation change in the endpoint for each standard deviation change in the predictor variable. In a previous trial, we demonstrated a 1.0±1.2 mL/min/kg improvement in peak VO2 following a single-dose (12.9 mmol) of inorganic nitrate, given in the form of beetroot juice.19 Using a correlation between studies of 0.80, beta=0.80, and alpha=0.05, we estimated that 7 subjects would be needed to demonstrate a similar change. We thus planned for 9 subjects in the active arm to allow for drop-out. Randomization was performed by the University of Pennsylvania Investigational Drug Pharmacy. Randomization tables were constructed using a 3:1 KNO3:PB ratio in blocks of 4 (www.randomization.com).
RESULTS
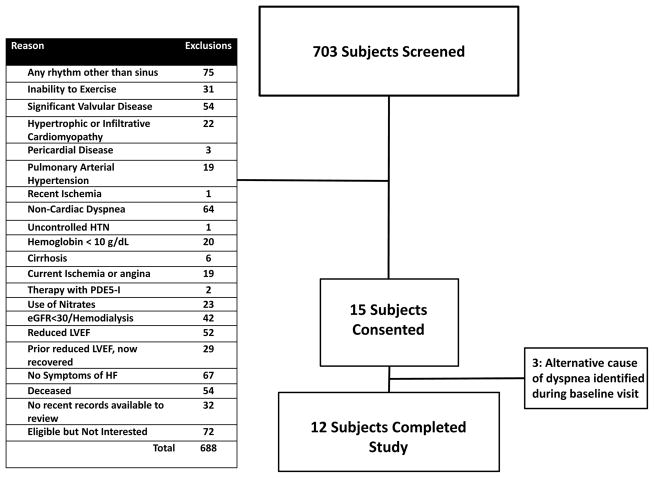
From 703 subjects screened, a total of 15 subjects were enrolled (Figure 1). An alternative explanation for dyspnea was identified during baseline testing in 3 individuals, who were subsequently excluded. Twelve individuals met all inclusion/exclusion criteria and completed the study (Table 1). Of these 12, 7 (58%) previously had elevated invasively-determined intra-cardiac pressures. The remaining individuals had an elevated E/e’ ratio>8 along with at least one of the following: (a) an elevated NT-pro-BNP previously (n=1); (b) left atrial enlargement (n=1); (c) or the need for chronic loop diuretics for control of symptoms (n=3). Mean (SD) age of participants was 62.5 (5.8) years, 4 (33%) were males, and 3 (25%) were African-American. Most participants had comorbidities typical for HFpEF: hypertension (100%), obesity (67%), hyperlipidemia (83%), diabetes (58%), obstructive sleep apnea (50%), coronary disease (33%), and chronic renal insufficiency (33%).
Figure 1. CONSORT diagram.
Summary of the screened subjects, including reasons for exclusions.
Table 1.
Demographic, Laboratory, and Echocardiographic Characteristics of the Study Population at Baseline
| Variable | Overall | KNO3 | Placebo |
|---|---|---|---|
| n=12 | n=9 | n=3 | |
| Age (years), mean (SD) | 62.5 (5.8) | 62.4 (5.5) | 62.7 (8.0) |
| Male, n (%) | 4 (33.3) | 3 (33.3) | 1 (33.3) |
| Race, n (%) | |||
| White | 8 (66.7) | 5 (55.6) | 3 (100) |
| African-American | 3 (25.0) | 3 (33.3) | 0 (0) |
| Pacific Islander | 1 (8.3) | 1 (11.1) | 0 (0) |
| NYHA Class, n (%) | |||
| Class II | 10 (83.3) | 8 (88.9) | 2 (66.7) |
| Class III | 2 (16.7) | 1 (11.1) | 1 (33.3) |
| Body Mass Index (kg/m2), mean (SD) | 34.4 (7.0) | 33.4 (6.3) | 37.4 (9.9) |
| Obese, n (%) | 8 (66.7) | 5 (55.6) | 3 (100) |
| Hypertension, n (%) | 12 (100) | 9 (100) | 3 (100) |
| Hyperlipidemia, n (%) | 10 (83.3) | 7 (77.8) | 3 (100) |
| Coronary Artery Disease, n (%) | 4 (33.3) | 3 (33.3) | 1 (33.3) |
| History of Atrial Fibrillation, n (%) | 1 (8.3) | 0 (0) | 1 (33.3) |
| Diabetes, n (%) | 7 (58.3) | 5 (55.6) | 2 (66.7) |
| Obstructive Sleep Apnea, n (%) | 6 (50.0) | 3 (33.3) | 3 (100) |
| Current CPAP use, n (%) | 5 (41.7) | 2 (22.2) | 3 (100) |
| Obstructive Lung Disease, n (%) | 3 (25.0) | 2 (22.2) | 1 (33.3) |
| Medical Therapy | |||
| Beta-Blockers, n (%) | 9 (75.0) | 7 (77.8) | 2 (66.7) |
| Ace-I/ARB, n (%) | 9 (75.0) | 7 (77.8) | 2 (66.7) |
| Mineralocorticoid Receptor Antagonists, n (%) | 3 (25.0) | 2 (22.2) | 1 (33.3) |
| Calcium-Channel Blockers, n (%) | 4 (33.3) | 3 (33.3) | 1 (33.3) |
| Loop Diuretics, n (%) | 9 (75.0) | 7 (77.8) | 2 (66.7) |
| Thiazide Diuretics, n (%) | 4 (33.3) | 3 (33.3) | 1 (33.3) |
| Statin, n (%) | 7 (58.3) | 5 (55.6) | 2 (66.7) |
| Baseline Laboratories | |||
| eGFR (mL/min/1.73m2), mean (SD) | 69.4 (12.6) | 71.0 (14.1) | 64.7 (5.9) |
| eGFR<60 mL/min/1.73m2, n(%) | 4 (33.3) | 3 (33.3) | 1 (33.3) |
| NT-pro-BNP (pg/mL), mean (SD) | 111.0 (89.0) | 108.3 (94.7) | 119 (87.0) |
| Elevated NT-pro-BNP, n (%) | 5 (41.7) | 3 (33.3) | 2 (66.7) |
| Hemoglobin (g/dL), mean (SD) | 13.6 (0.8) | 13.6 (0.8) | 13.5 (1.0) |
| Methemoglobin (%), mean (SD) | 1.0 (0.3) | 1.0 (0.2) | 1.0 (0.5) |
| Baseline Echocardiographic Data (mean, SD) | |||
| LV Mass (g) | 172.32 (66.36) | 174.43 (77.09) | 165.98 (19.27) |
| LV Mass Index (g/m2), mean (SD) | 78.88 (22.46) | 79.78 (26.01) | 76.18 (7.33) |
| Relative Wall Thickness | 0.47 (0.09) | 0.47 (0.09) | 0.46 (0.09) |
| Mitral Early Inflow Velocity (E; cm/s) | 77.33 (21.91) | 74.53 (24.75) | 85.73 (7.04) |
| Mitral Atrial Inflow Velocity (cm/s) | 75.21 (20.99) | 70.74 (20.06) | 88.61 (21.28) |
| Septal Tissue Doppler Early Velocity (e’; mm/s) | 76.41 (19.26) | 70.64 (12.45) | 93.71 (28.65) |
| Septal E/e’ Ratio | 10.42 (3.40) | 10.70 (3.76) | 9.60 (2.39) |
| Left Atrial Volume Index (mL/m2) | 27.74 (7.15) | 27.20 (7.87) | 29.38 (5.33) |
| Left Ventricular Ejection Fraction (%) | 64.23 (9.27) | 65.84 (7.74) | 59.41 (13.66) |
NYHA = New York Heart Association; CPAP = continuous positive airway pressure; ACE-I = angiotensin converting enzyme inhibitor; ARB = angiotensin receptor blocker; elevated NT-pro-BNP defined as >125 pg/mL; eGFR calculated using the MDRD formula
Maximal effort exercise test
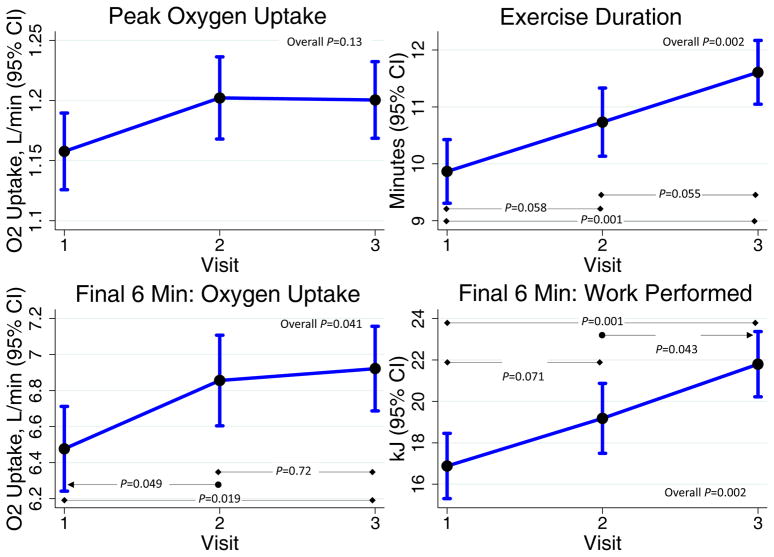
VO2,peak, the primary efficacy endpoint, tended to increase, without reaching statistical significance (P=0.13). Vasodilatory reserve did not change with supplementation (P=0.72). The exploratory endpoint of exercise duration was significantly increased in the KNO3 group (P=0.002; Table 2, Figure 2). Both total work performed (P=0.02) and total oxygen uptake (P=0.019) increased. Additional hemodynamic data from the maximal effort study are presented in Supplemental Table I. Interestingly, the change in VO2,peak from the baseline to final visit correlated with the change in serum NOm levels: Standardized β=0.74; P=0.006. The change in nitrite levels did not correlate with any metrics of exercise capacity.
Table 2.
Peak Effort Gas Exchange Data
| Marginal Mean (95% CI) | KNO3 (n=9) | |||
|---|---|---|---|---|
| Visit 1 | Visit 2 | Visit 3 | P-value | |
| Ventilatory Threshold | ||||
| Ventilatory Threshold (L/min) | 0.75 (0.69–0.81) | 0.68 (0.61–0.74) | 0.74 (0.68–0.80) | 0.23 |
| iVentilatory Threshold (mL/kg/min) | 8.18 (7.65–8.72) | 7.51 (6.94–8.08) | 8.07 (7.53–8.60) | 0.24 |
| Peak Exercise | ||||
| Peak VO2 (L/min) | 1.16 (1.13–1.19) | 1.20 (1.17–1.24) | 1.20 (1.17–1.23) | 0.13 |
| Peak iVO2 (mL/kg/min) | 12.50 (12.18–12.83) | 12.83 (12.49–13.18) | 12.87 (12.55–13.20) | 0.26 |
| Exercise Duration (min) | 9.87 (9.31–10.43) | 10.73 (10.13–11.33) | 11.61† (11.05–12.17) | 0.002 |
| Total Work Performed (kJ) | 23.22 (18.82–27.61) | 26.92 (22.22–31.63) | 33.02† (28.63–37.41) | 0.02 |
| Total Oxygen Consumed (L) | 8.58 (7.72–9.44) | 10.00* (9.08–10.92) | 10.54† (9.67–11.40) | 0.019 |
| Total Exercise Economy (kJ/L O2 Consumed) | 2.52 (2.31–2.72) | 2.61 (2.39–2.83) | 2.85 (2.65–3.06) | 0.10 |
| RER | 1.08 (1.06–1.10) | 1.09 (1.06–1.11) | 1.09 (1.07–1.11) | 0.83 |
| VE/VCO2 Slope | 34.37 (32.75–35.98) | 36.10 (34.38–37.83) | 33.81 (32.20–35.42) | 0.18 |
RER = respiratory exchange ratio; kJ = kilojoules
p<0.05 V1 vs. V2;
p<0.05 V1 vs. V3;
p<0.05 V2 vs. V3
Figure 2.
Maximal Effort Exercise Study Results.
In post hoc analysis, we evaluated oxygen uptake and work performed during the final 3 and final 6 minutes of exercise (i.e. heavy and severe intensity). Work performed in the final 3 minutes increased significantly (P=0.002), as did total O2 uptake in the final 6 minutes (P=0.041) and total work performed in the final 6 minutes (P=0.002; Figure 2). Overall exercise economy in the final 3-minute (P=0.007) and 6-minute (P=0.004) periods increased (Supplemental Table II). The increase in oxygen uptake during the final 3 minutes of exercise correlated with the increase in serum NOm levels (Standardized β=0.64; P=0.024).
Pharmacodynamics following administration of 6 mmol of KNO3
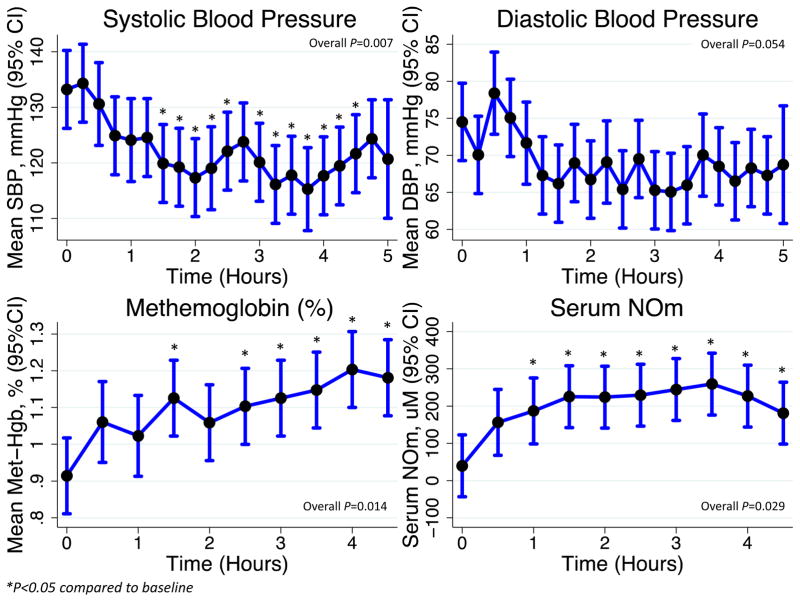
Blood pressure was measured every 15 minutes, and serum collected every 30 minutes, following the administration of 6 mmol of KNO3. The maximal reduction in SBP occurred at 3 hours and 45 minutes (−17.9 [95%CI −28.3-[−7.6]] mmHg; Figure 3). We observed a statistically-significant, though clinically-irrelevant, increase in methemoglobin concurrent with the reduction in SBP (Figure 3). Peak NOm concentrations were 259.3 (95%CI 176.2–342.4) μM occurring 3.5 hours after ingestion. The median half-life was 73.0 (IQR 33.4–232.0) minutes.
Figure 3.
Pharmacokinetics following initial administration of 6 mmol of KNO3.
Tolerability of KNO3
KNO3 was well-tolerated, with all subjects completing the dosing and study protocols. Five individuals experienced side-effects during the study (Supplemental Table III): 4 (44%) in the KNO3 group and 1 (33%) in PB. Gastrointestinal symptoms and headache were the most common side-effect. There were numerically fewer side-effects in the KNO3 group during Week 2 (18 mmol/d of KNO3).
Serum NOm, blood pressure and laboratory testing at each visit
KNO3 significantly increased fasting serum NOm concentration in a dose-dependent manner (Table 3). This primarily reflects an increase in nitrate levels, as nitrite levels did not change (data not shown). We observed a reduction in systolic blood pressure (SBP) with KNO3 (Table 3). Importantly, percent methemoglobin was no different between visits (Table 3). Likely due to the serial blood sampling during the safety/pharmacokinetics portions of Visit 1 and Visit 2, hemoglobin was significantly reduced in the KNO3 group (Table 3).
Table 3.
Vital Signs and Laboratory Data
| Marginal Means (95% CI) | KNO3 (n=9) | |||
|---|---|---|---|---|
| Vital Signs | Visit 1 | Visit 2 | Visit 3 | P |
| Weight (kg) | 95.0 (94.6–95.4) | 95.1 (94.7–95.5) | 95.0 (94.6–95.4) | 0.94 |
| SBP (mmHg) | 127.2 (121.1–133.3) | 118.6 (112.5–124.6) | 115.0† (108.9–121.1) | 0.037 |
| DBP (mmHg) | 72.6 (69.5–75.6) | 68.3 (65.2–71.4) | 69.9 (66.8–73.0) | 0.19 |
| Heart Rate (bpm) | 65.1 (59.9–70.3) | 65.4 (60.2–70.7) | 67.3 (62.1–72.5) | 0.82 |
| Laboratory Data | ||||
| Serum Sodium (mEq/dL) | 137.8 (137.1–138.5) | 137.0 (136.3–137.7) | 136.4 (135.7–137.2) | 0.06 |
| Serum Potassium (mEq/dL) | 3.8 (3.6–4.0) | 4.0 (3.8–4.2) | 4.1 (3.9–4.3) | 0.28 |
| eGFR (mL/min/1.73m2) | 71.0 (66.9–75.1) | 70.5 (66.4–74.6) | 69.6 (65.5–73.7) | 0.89 |
| NT-pro-BNP (pg/mL) | 108.3 (84.0–132.6) | 117.8 (93.5–142.1) | 93.2 (68.9–117.5) | 0.39 |
| Hemoglobin (g/dL) | 13.6 (13.4–13.8) | 12.9* (12.7–13.1) | 12.8† (12.6–13.0) | <0.001 |
| Methemoglobin (%) | 1.04 (0.92–1.17) | 1.19 (1.06–1.32) | 1.18 (1.05–1.31) | 0.25 |
| Serum NOm (μM) | 38.0 (0.00–132.0) | 199.5* (98.7–300.2) | 471.8†,‡ (377.8–565.8) | <0.001 |
p<0.05 V1 vs. V2;
p<0.05 V1 vs. V3;
p<0.05 V2 vs.
Orthostatic blood pressure measurements
Orthostatic measurements were performed 2 hours following dose administration. Inorganic nitrate caused an asymptomatic reduction in SBP following 2 minutes of standing from a supine position, particularly at the highest doses (Visit 1: +1.2 [95%CI −6.1–8.4]; Visit 2: −1.1 [95%CI −7.8–5.6]; Visit 3: −11.6 [95%CI −18.3-[−4.9]] mmHg, P=0.051).
Kansas City cardiomyopathy questionnaire
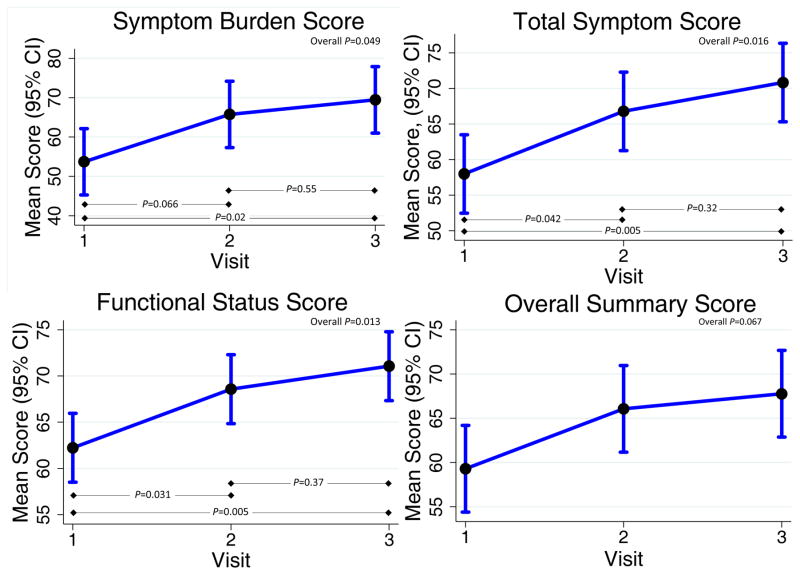
The KNO3 group experienced an improvement in HF symptoms over the 2-week study, as evidenced by higher scores on the KCCQ in several domains: symptom stability (P=0.03), symptom burden (P=0.049), total symptom score (P=0.016), and the functional status score (P=0.01; Figure 4). The overall summary score tended to improve with KNO3 (P=0.067).
Figure 4.
Kansas City Cardiomyopathy Questionnaire Results.
Resting echocardiography
Diastolic function and left-ventricular end-diastolic volumes were quantified at rest. Diastolic function was not impacted by KNO3. Additionally, EDV did not change (P=0.99, Supplemental Table IV).
Oxygen uptake and input impedance during submaximal exercise
Using radial tonometry and LVOT flow, input impedance was determined at rest and while at steady-state during submaximal (25W) exercise. No changes in input impedance were observed (Supplemental Table V). Neither steady-state VO2 (P=0.85) nor economy (P=0.98) were impacted by KNO3 (Supplemental Table VI).
Ambulatory BP
During the 24-hours following Visit 1 (first day of 6 mmol twice daily) and Visit 2 (first day of increase to 6 mmol thrice daily), no differences in 24-hour ambulatory blood pressure were seen (data not shown).
Actigraphy
KNO3 did not alter activity. Specifically, neither average daily steps taken during each week (Week 1: 9244.1 [95%CI 8435.8–10052.4]; Week-2: 9469.8 [95%CI 8661.5–10278.1] steps; P=0.71) nor estimated daily kilocalories expended (Week-1: 1683.3 [95%CI 1555.5–1811.1]; Week-2: 1678.7 [95%CI 1550.9–1806.4]; P=0.96) were altered with KNO3.
Augmentation index and mitochondrial oxidative function
There was no difference in AIx or mitochondrial oxidative function between visits in either the KNO3 or the PB group (P=NS for all).
Lack of training effect in PB-Treated subjects
There were no significant changes in any metric of exercise with PB. Furthermore, KCCQ scores did not change in these subjects during the course of the study, suggesting lack of a training effect on repeated testing. Data on study-endpoints for the PB-treated subjects can be found in the online supplement (Supplemental Table VII).
DISCUSSION
In subjects with HFpEF, the principal findings of this investigation are that KNO3 (1) appears to be potentially safe at a dose of up to 18 mmol/day; (2) did not significantly improve the primary efficacy endpoint of peak VO2, although a non-significant trend was observed; (3) significantly increased the exploratory endpoint of exercise duration; (3) significantly improved several domains of the Kansas City Cardiomyopathy Questionnaire; (4) did not worsen outpatient activity; and (5) this preparation demonstrates favorable pharmacokinetics, with high trough levels of NO metabolites achieved with doses of 6 mmol twice daily and substantially higher levels with thrice daily dosing, but without significant methemoglobinemia for either dosing interval.
KNO3 appeared to be potentially safe up to a dose of 18 mmol/day, and no subject prematurely discontinued study medications for any reasons. The side-effects experienced were mild and were not more frequent at the higher dose. Similar to other studies, we demonstrate that KNO3 mildly reduced resting blood pressure.20, 31 We did, however, note a greater decrease in orthostatic SBP at the higher dose of 18 mmol/day, suggesting that this approaches the maximum tolerated dose. The reduction of nitrite to NO leads to the generation of methemoglobin, raising the theoretical concern for methemoglobinemia with KNO3 administration. Consistent with this biology, we demonstrate a clinically-insignificant rise in methemoglobin immediately after dose administration. However, there was no elevation in steady-state methemoglobin levels at any study visit, suggesting that the endogenous mechanisms of hemoglobin reduction operate fast enough to prevent any sustained rise in methemoglobin.
In contrast to NO production via NOS, which requires molecular oxygen, KNO3 may provide an alternative source of NO that causes vasodilation and increased perfusion within hypoxic exercising muscle, leading to improved exercise duration. During our progressive ramp protocol, exercise duration increased via improved tolerance to heavy/severe exercise. As the intensity of exercise increases, a greater proportion of Type II fibers is activated.25 Type II fibers, with their lower capillary volume density and increased dependence on glycolytic pathways for ATP production, reside in a relatively more hypoxic and acidotic milieu, particularly during exercise thus providing an ideal environment for the reduction of nitrite to NO.25 Our findings are in line with recent evidence for the preferential activation of nitrite by Type II fibers16 and at higher exercise intensities.26–28
Participants randomized to KNO3 experienced an improvement in HF-related symptoms, as evidenced by higher total symptom, symptom burden, and functional status scores. The total symptom score has previously been demonstrated to be the most responsive metric for the evaluation of HF symptoms, and lower functional status scores have been associated with an increased incidence of death or HF hospitalization.23 Importantly, the magnitude of the changes seen in our study were generally >5 points, suggesting the potential for clinical relevance.32 Given the substantial morbidity associated with HFpEF,33 strategies that improve quality of life and exercise represent important progress for this patient population that has few therapeutic options. Beyond the short-term effects demonstrated in this trial, inorganic nitrate might enable HFpEF patients to better tolerate the initiation/maintenance of an exercise prescription, improving the adherence to, and efficacy of, an exercise regimen.
Recently, a randomized placebo controlled crossover study of an organic nitrate, isosorbide mononitrate (ISMN), was performed in HFpEF patients.5 This study demonstrated a reduction in physical activity with ISMN. In our study, no decrease in activity was identified, suggesting that KNO3 may not have the same harmful impact.
Limitations
There are several limitations to our study. First, the sample size was small because a larger population could not be justified prior to assessing safety. Given the small number of patients exposed to active agent and the very small number of placebo patients, the study had low power to detect safety signals. Gathering more robust safety data is a goal of the ongoing registered larger clinical trial that was supported by our present findings (KNO3CK OUT HFpEF Trial; ClinicalTrials.gov: NCT02840799). Second, the pharmacokinetics portions of our study required serial blood draws, which caused a significant decrease in hemoglobin concentration from 13.6 g/dL to 12.8 g/dL (−5.9%). Because VO2,peak is limited by oxygen delivery, particularly in heart failure subjects,18 the decrease in hemoglobin may have constrained the increase in VO2,peak and reduced the magnitude of the improvement in exercise capacity and quality of life. Indeed, in our current study, we found a 3.4% improvement in VO2,peak. Had the hemoglobin remained constant, mathematically VO2,peak would be expected to improve by ~6%, leading to a similar magnitude of change as demonstrated in our prior study where a single acute dose of 12.9 mmol of inorganic nitrate improved VO2,peak by approximately 8%.19 Differences in patient characteristics may also have contributed to the non-significant increase in VO2,peak. Our prior study included a greater number of African-American males. It is conceivable that hypertensive African-Americans represent a unique population with particularly deranged NOS function,34 and stand to benefit more from interventions that increase NO bioavailability, as demonstrated in HFrEF.35 Furthermore, there may be differences in KNO3 responsiveness between genders, as females demonstrate less reduction in blood pressure following inorganic nitrate supplementation despite greater increases in serum nitrate concentration.36 Given the forced up-titration of study medications, we cannot exclude the possibility that the improvement in exercise duration was due to longer exposure to study medications versus the higher dose administered. That the change in VO2,peak and oxygen uptake in the last 3 minutes of exercise correlated with the change in serum NOm levels suggests that the supplementation dose is also important. Additionally, we did not obtain serial urine and saliva samples during the pharmacokinetics segment of the study, precluding more detailed assessments. Finally, it is possible that more PB-treated subjects were needed to demonstrate a training effect; however, we were limited in the number of subjects to be performed in this initial pilot study. Our group is currently conducting a follow-up cross-over study of KNO3 versus PB in HFpEF subjects, which will be adequately powered to detect most relevant treatment-related differences and will also specifically address possible gender and race differences in the response to KNO3 (KNO3CK OUT HFpEF Trial; ClinicalTrials.gov: NCT02840799).
Conclusions
KNO3 improves exercise duration and QOL in subjects with HFpEF. KNO3, at a dose of 18 mmol/day, appears to be potentially safe with only mild reduction in blood pressure, and no elevation in methemoglobin.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is Known?
Heart Failure with Preserved Ejection Fraction (HFpEF) is associated with marked reductions in quality of life and exercise tolerance.
Although nitric oxide (NO) bioavailability is reduced in HFpEF, prior efforts to increase NO using organic nitrate or phosphodiesterase-5 inhibitors have not been beneficial.
Inorganic nitrate, which is reduced to nitrite by oral anaerobic bacteria, can increase NO signaling via selective reduction of nitrite to NO at the site of exercising muscle, leading to increased muscle blood flow.
What New Information Does This Article Contribute?
Inorganic nitrate supplementation appears to be potentially safe and well-tolerated in HFpEF subjects.
Inorganic nitrate supplementation increases exercise duration and quality of life in HFpEF subjects in a dose-dependent fashion.
The benefits of inorganic nitrate in HFpEF subjects are most manifest at high exercise intensities.
We studied the population-specific safety and efficacy data for inorganic nitrate in HFpEF. We performed detailed phenotypic and pharmacokinetic assessments of inorganic nitrate in HFpEF. We found that a dose of up to 18 mmol/day of inorganic nitrate potentially appears to be safe, with dose-dependent improvements in exercise duration and quality of life. In contrast to organic nitrate therapy, inorganic nitrate did not reduce physical activity over the course of our 2-week study. These findings strengthen the rationale for performing a larger trial of inorganic nitrate in HFpEF patients.
Acknowledgments
The authors thank the Clinical and Translational Research Center at the University of Pennsylvania for the financial, equipment, and personnel support that made this study possible.
SOURCES OF FUNDING
PZ: Institute for Translational Medicine and Therapeutics of the University of Pennsylvania (5UL1TR000003-09 from the National Center for Research Resources), 5-T32-HL007843-17, and 1-K23-HL-130551-01. The project described was supported by Grant Number UL1RR024134 from the National Center for Research Resources and by Grant Number UL1TR000003 from the National Center for Advancing Translational Sciences, National Institutes of Health; the content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
JAC: 5R21AG043802-02, 1R56HL124073-01A1, and 1 R01 HL121510-01A1.
HI: NIH HL054926 and the Gisela and Dennis Alter Research Professor of Pediatrics.
KBM: Research Grant; Modest; Juventis Therapeutics, Celladon Corporation, Thoratec Corporation, and Innolign Biomedical, LLC. Research Grant; Significant; Merck.
Nonstandard Abbreviations and Acronyms
- KNO3,
potassium nitrate
- KCl
potassium chloride
- VTI
velocity time integral
- LVOT
left ventricular outflow tract
- AIx
augmentation index
- VO2
peak, peak oxygen uptake
- NIRS
near infrared spectroscopy
- SS
steady state
- KCCQ
Kansas City Cardiomyopathy Questionnaire
- SBP
systolic blood pressure
- EDV
end-diastolic volume
Footnotes
DISCLOSURES
JAC: Consulting fees from Bristol-Myers Squibb, OPKO Healthcare, Fukuda Denshi, Microsoft Research and Merck. JAC received research grants from National Institutes of Health, American College of Radiology Network, Fukuda Denshi, Bristol-Myers Squibb, Microsoft Research and CVRx Inc, and device loans from Atcor Medical. He is named as inventor in a University of Pennsylvania patent application for the use of inorganic nitrates/nitrites for the treatment of Heart Failure and Preserved Ejection Fraction.
KBM: Consultant/Advisory Board; Modest; Janssen, Merck, Glaxo-Smith-Kline, Pfizer, Ridgetop Research, AstraZeneca, NovoNordisk (unpaid).
TPC: Consultant/Advisory Board; Modest; Novartis.
RRT: Consultant for Medtronic, Fukuda Denshi, Relypsa.
References
- 1.Steinberg BA, Zhao X, Heidenreich PA, Peterson ED, Bhatt DL, Cannon CP, Hernandez AF, Fonarow GC Get With the Guidelines Scientific Advisory C Investigators. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: Prevalence, therapies, and outcomes. Circulation. 2012;126:65–75. doi: 10.1161/CIRCULATIONAHA.111.080770. [DOI] [PubMed] [Google Scholar]
- 2.Tsao CW, Lyass A, Larson MG, Vasan RS. Abstract 23: Divergent temporal trends in the incidence of heart failure with preserved and reduced ejection fraction. Circulation. 2015;131:A23. doi: 10.1016/j.jchf.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Heerebeek L, Hamdani N, Falcao-Pires I, Leite-Moreira AF, Begieneman MP, Bronzwaer JG, van der Velden J, Stienen GJ, Laarman GJ, Somsen A, Verheugt FW, Niessen HW, Paulus WJ. Low myocardial protein kinase g activity in heart failure with preserved ejection fraction. Circulation. 2012;126:830–839. doi: 10.1161/CIRCULATIONAHA.111.076075. [DOI] [PubMed] [Google Scholar]
- 4.Franssen C, Chen S, Unger A, Korkmaz HI, De Keulenaer GW, Tschope C, Leite-Moreira AF, Musters R, Niessen HW, Linke WA, Paulus WJ, Hamdani N. Myocardial microvascular inflammatory endothelial activation in heart failure with preserved ejection fraction. JACC Heart Fail. 2016;4:312–324. doi: 10.1016/j.jchf.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Redfield MM, Anstrom KJ, Levine JA, Koepp GA, Borlaug BA, Chen HH, LeWinter MM, Joseph SM, Shah SJ, Semigran MJ, Felker GM, Cole RT, Reeves GR, Tedford RJ, Tang WH, McNulty SE, Velazquez EJ, Shah MR, Braunwald E Network NHFCR. Isosorbide mononitrate in heart failure with preserved ejection fraction. The New England journal of medicine. 2015;373:2314–2324. doi: 10.1056/NEJMoa1510774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, LeWinter MM, Rouleau JL, Bull DA, Mann DL, Deswal A, Stevenson LW, Givertz MM, Ofili EO, O’Connor CM, Felker GM, Goldsmith SR, Bart BA, McNulty SE, Ibarra JC, Lin G, Oh JK, Patel MR, Kim RJ, Tracy RP, Velazquez EJ, Anstrom KJ, Hernandez AF, Mascette AM, Braunwald E, Trial R. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: A randomized clinical trial. Jama. 2013;309:1268–1277. doi: 10.1001/jama.2013.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nature reviews. Drug discovery. 2008;7:156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 8.Liu C, Wajih N, Liu X, Basu S, Janes J, Marvel M, Keggi C, Helms CC, Lee AN, Belanger AM, Diz DI, Laurienti PJ, Caudell DL, Wang J, Gladwin MT, Kim-Shapiro DB. Mechanisms of human erythrocytic bioactivation of nitrite. The Journal of biological chemistry. 2015;290:1281–1294. doi: 10.1074/jbc.M114.609222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maher AR, Milsom AB, Gunaruwan P, Abozguia K, Ahmed I, Weaver RA, Thomas P, Ashrafian H, Born GV, James PE, Frenneaux MP. Hypoxic modulation of exogenous nitrite-induced vasodilation in humans. Circulation. 2008;117:670–677. doi: 10.1161/CIRCULATIONAHA.107.719591. [DOI] [PubMed] [Google Scholar]
- 10.Modin A, Bjorne H, Herulf M, Alving K, Weitzberg E, Lundberg JO. Nitrite-derived nitric oxide: A possible mediator of ‘acidic-metabolic’ vasodilation. Acta physiologica Scandinavica. 2001;171:9–16. doi: 10.1046/j.1365-201X.2001.00771.x. [DOI] [PubMed] [Google Scholar]
- 11.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO, 3rd, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nature medicine. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 12.Jones AM. Dietary nitrate supplementation and exercise performance. Sports medicine. 2014;44(Suppl 1):S35–45. doi: 10.1007/s40279-014-0149-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferguson SK, Glean AA, Holdsworth CT, Wright JL, Fees AJ, Colburn TD, Stabler T, Allen JD, Jones AM, Musch TI, Poole DC. Skeletal muscle vascular control during exercise: Impact of nitrite infusion during nitric oxide synthase inhibition in healthy rats. J Cardiovasc Pharmacol Ther. 2016;21:201–208. doi: 10.1177/1074248415599061. [DOI] [PubMed] [Google Scholar]
- 14.Glean AA, Ferguson SK, Holdsworth CT, Colburn TD, Wright JL, Fees AJ, Hageman KS, Poole DC, Musch TI. Effects of nitrite infusion on skeletal muscle vascular control during exercise in rats with chronic heart failure. American journal of physiology. Heart and circulatory physiology. 2015;309:H1354–1360. doi: 10.1152/ajpheart.00421.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferguson SK, Holdsworth CT, Colburn TD, Wright JL, Craig JC, Fees A, Jones AM, Allen JD, Musch TI, Poole DC. Dietary nitrate supplementation: Impact on skeletal muscle vascular control in exercising rats with chronic heart failure. Journal of applied physiology. 2016;121:661–669. doi: 10.1152/japplphysiol.00014.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferguson SK, Hirai DM, Copp SW, Holdsworth CT, Allen JD, Jones AM, Musch TI, Poole DC. Impact of dietary nitrate supplementation via beetroot juice on exercising muscle vascular control in rats. The Journal of physiology. 2013;591:547–557. doi: 10.1113/jphysiol.2012.243121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colburn TD, Ferguson SK, Holdsworth CT, Craig JC, Musch TI, Poole DC. Effect of sodium nitrite on local control of contracting skeletal muscle microvascular oxygen pressure in healthy rats. Journal of applied physiology. 2016 doi: 10.1152/japplphysiol.00367.2016. jap 00367 02016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poole DC, Hirai DM, Copp SW, Musch TI. Muscle oxygen transport and utilization in heart failure: Implications for exercise (in)tolerance. American journal of physiology. Heart and circulatory physiology. 2012;302:H1050–1063. doi: 10.1152/ajpheart.00943.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zamani P, Rawat D, Shiva-Kumar P, Geraci S, Bhuva R, Konda P, Doulias PT, Ischiropoulos H, Townsend RR, Margulies KB, Cappola TP, Poole DC, Chirinos JA. Effect of inorganic nitrate on exercise capacity in heart failure with preserved ejection fraction. Circulation. 2015;131:371–380. doi: 10.1161/CIRCULATIONAHA.114.012957. discussion 380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eggebeen J, Kim-Shapiro DB, Haykowsky M, Morgan TM, Basu S, Brubaker P, Rejeski J, Kitzman DW. One week of daily dosing with beetroot juice improves submaximal endurance and blood pressure in older patients with heart failure and preserved ejection fraction. JACC Heart Fail. 2016;4:428–437. doi: 10.1016/j.jchf.2015.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russell SD, McNeer FR, Beere PA, Logan LJ, Higginbotham MB. Improvement in the mechanical efficiency of walking: An explanation for the “placebo effect” seen during repeated exercise testing of patients with heart failure. Duke university clinical cardiology studies (duccs) exercise group. American heart journal. 1998;135:107–114. doi: 10.1016/s0002-8703(98)70350-3. [DOI] [PubMed] [Google Scholar]
- 22.Haddy FJ, Vanhoutte PM, Feletou M. Role of potassium in regulating blood flow and blood pressure. American journal of physiology. Regulatory, integrative and comparative physiology. 2006;290:R546–552. doi: 10.1152/ajpregu.00491.2005. [DOI] [PubMed] [Google Scholar]
- 23.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the kansas city cardiomyopathy questionnaire: A new health status measure for heart failure. Journal of the American College of Cardiology. 2000;35:1245–1255. doi: 10.1016/s0735-1097(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 24.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–133. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 25.Jones AM, Ferguson SK, Bailey SJ, Vanhatalo A, Poole DC. Fiber type-specific effects of dietary nitrate. Exerc Sport Sci Rev. 2016;44:53–60. doi: 10.1249/JES.0000000000000074. [DOI] [PubMed] [Google Scholar]
- 26.Bailey SJ, Varnham RL, DiMenna FJ, Breese BC, Wylie LJ, Jones AM. Inorganic nitrate supplementation improves muscle oxygenation, o(2) uptake kinetics, and exercise tolerance at high but not low pedal rates. Journal of applied physiology. 2015;118:1396–1405. doi: 10.1152/japplphysiol.01141.2014. [DOI] [PubMed] [Google Scholar]
- 27.Coggan AR, Leibowitz JL, Kadkhodayan A, Thomas DP, Ramamurthy S, Spearie CA, Waller S, Farmer M, Peterson LR. Effect of acute dietary nitrate intake on maximal knee extensor speed and power in healthy men and women. Nitric oxide : biology and chemistry / official journal of the Nitric Oxide Society. 2015;48:16–21. doi: 10.1016/j.niox.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coggan AR, Leibowitz JL, Spearie CA, Kadkhodayan A, Thomas DP, Ramamurthy S, Mahmood K, Park S, Waller S, Farmer M, Peterson LR. Acute dietary nitrate intake improves muscle contractile function in patients with heart failure: A double-blind, placebo-controlled, randomized trial. Circulation. Heart failure. 2015;8:914–920. doi: 10.1161/CIRCHEARTFAILURE.115.002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chirinos JA, Segers P. Noninvasive evaluation of left ventricular afterload: Part 2: Arterial pressure-flow and pressure-volume relations in humans. Hypertension. 2010;56:563–570. doi: 10.1161/HYPERTENSIONAHA.110.157339. [DOI] [PubMed] [Google Scholar]
- 30.Ryan TE, Southern WM, Reynolds MA, McCully KK. A cross-validation of near-infrared spectroscopy measurements of skeletal muscle oxidative capacity with phosphorus magnetic resonance spectroscopy. Journal of applied physiology. 2013;115:1757–1766. doi: 10.1152/japplphysiol.00835.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kapil V, Khambata RS, Robertson A, Caulfield MJ, Ahluwalia A. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: A randomized, phase 2, double-blind, placebo-controlled study. Hypertension. 2015;65:320–327. doi: 10.1161/HYPERTENSIONAHA.114.04675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flynn KE, Pina IL, Whellan DJ, Lin L, Blumenthal JA, Ellis SJ, Fine LJ, Howlett JG, Keteyian SJ, Kitzman DW, Kraus WE, Miller NH, Schulman KA, Spertus JA, O’Connor CM, Weinfurt KP Investigators H-A. Effects of exercise training on health status in patients with chronic heart failure: Hf-action randomized controlled trial. Jama. 2009;301:1451–1459. doi: 10.1001/jama.2009.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nolte K, Herrmann-Lingen C, Wachter R, Gelbrich G, Dungen HD, Duvinage A, Hoischen N, von Oehsen K, Schwarz S, Hasenfuss G, Halle M, Pieske B, Edelmann F. Effects of exercise training on different quality of life dimensions in heart failure with preserved ejection fraction: The ex-dhf-p trial. European journal of preventive cardiology. 2015;22:582–593. doi: 10.1177/2047487314526071. [DOI] [PubMed] [Google Scholar]
- 34.Zamani P, French B, Brandimarto JA, Doulias PT, Javaheri A, Chirinos JA, Margulies KB, Townsend RR, Sweitzer NK, Fang JC, Ischiropoulos H, Cappola TP. Effect of heart failure with preserved ejection fraction on nitric oxide metabolites. The American journal of cardiology. 2016 doi: 10.1016/j.amjcard.2016.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gupta D, Georgiopoulou VV, Kalogeropoulos AP, Marti CN, Yancy CW, Gheorghiade M, Fonarow GC, Konstam MA, Butler J. Nitrate therapy for heart failure: Benefits and strategies to overcome tolerance. JACC Heart Fail. 2013;1:183–191. doi: 10.1016/j.jchf.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 36.Kapil V, Milsom AB, Okorie M, Maleki-Toyserkani S, Akram F, Rehman F, Arghandawi S, Pearl V, Benjamin N, Loukogeorgakis S, Macallister R, Hobbs AJ, Webb AJ, Ahluwalia A. Inorganic nitrate supplementation lowers blood pressure in humans: Role for nitrite-derived no. Hypertension. 2010;56:274–281. doi: 10.1161/HYPERTENSIONAHA.110.153536. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.