Abstract
Background
Current guidelines recommend that all sexually active HIV-infected persons be tested at least annually for syphilis, chlamydia, and gonorrhea. We examined temporal trends in syphilis, chlamydia, and gonorrhea testing among sexually active HIV-infected adults receiving medical care in the United States during 2009–2013.
Methods
Using medical record data from the Medical Monitoring Project, a population-based HIV surveillance system, we assessed the proportion of adults in HIV medical care who were tested for syphilis, chlamydia, and gonorrhea in the past 12 months by year and stratified by sex and sexual behavior, age, and race/ethnicity.
Results
During 2009–2013, the proportion of sexually active HIV-infected adults in medical care who were tested in the past year for all three examined STDs increased from 20% to 36% (PTREND < 0.01). Overall testing for syphilis increased from 55% to 65% (PTREND < 0.01), and significant increases were noted for the following subgroups: MSM (58% to 69%), non-Hispanic whites (48% to 64%), and all age groups with the exception of 18–29 year olds. Overall testing for chlamydia and gonorrhea increased from 22% to 42% (PTREND < 0.01) and significant increases were noted for most sub-groups.
Conclusions
STD testing significantly increased among sexually active HIV-infected adults receiving medical care; however, the majority of persons were not tested for all three STDs in 2013. While increased testing indicates progress, testing remained far below recommended guidelines. Our findings suggest enhanced efforts may be warranted to screen all sexually active HIV-infected adults for syphilis, chlamydia, and gonorrhea.
Keywords: Syphilis, chlamydia, gonorrhea, men who have sex with men (MSM), STD, HIV
At the end of 2013, it was estimated that 933,941 persons were living with diagnosed HIV infection in the United States.[1] The Centers for Disease Control and Prevention estimates that nearly 20 million cases of sexually transmitted diseases (STD) occur every year in the United States, and for the first time since 2006, cases of syphilis, chlamydia and gonorrhea increased in 2014. [2] However, national data on HIV and STD co-infection are limited. In 2014, 26 states reported information about the person’s HIV status in STD cases and the sex of their sex partners in at least 70% of primary and secondary syphilis cases.[2] HIV and STD co-infection was high, particularly for men who have sex with men (MSM). Among primary and secondary syphilis cases with known HIV-status, over half of reported cases from MSM were HIV-positive.[2] In addition, among MSM visiting STD Surveillance Network (SSuN) sites, the prevalence of syphilis, gonorrhea, and chlamydia were higher among MSM living with HIV than among HIV-negative MSM.[2] HIV-infected persons who acquire STDs may be at increased risk of developing complications [3] and some STDs increase the likelihood of HIV transmission. [4–6] Therefore, STD diagnosis, treatment and prevention services are an important part of HIV medical care and can have significant public health benefits.[3, 7, 8]
Current guidelines, which have remained largely unchanged since 2004, recommend that HIV-infected persons be screened at the time of HIV care initiation and sexually active persons be screened at least annually for syphilis, chlamydia, and gonorrhea; however, studies suggest that testing is below recommended levels. A recent study that examined STD testing among HIV-infected persons in a large health claims database found that 51% of privately insured persons were tested for syphilis, 22% for chlamydia, and 22% for gonorrhea.[15] A population-based analysis of HIV-infected adults receiving medical care in 2009 found 55% were tested at least once in the past 12 months for syphilis, and 23% and 24% received at least one gonorrhea and chlamydia test, respectively.[16] However, population-based information on trends in STD testing among HIV-infected persons is lacking and of particular importance given the recent increase in STDs. The objective of this analysis is to examine temporal trends in STD testing among sexually active HIV-infected adults receiving medical care in the United States.
METHODS
We analyzed the most recent available data from the Medical Monitoring Project (MMP), (i.e., 2009–2013 cycles) an HIV surveillance system designed to produce nationally representative, cross-sectional estimates of behavioral and clinical characteristics of HIV-infected adults receiving medical care in the United States. MMP methods–including sampling, weighting procedures, and response rates have been described in detail elsewhere [17, 18]. Briefly, during 2009–2013, MMP utilized a three-stage, complex sampling design in which U.S. states and territories were sampled, followed by facilities providing outpatient HIV medical care in those jurisdictions, then HIV-infected adults (aged 18 years and older) receiving care in those facilities. All sampled states and territories participated in MMP. During 2009 to 2013, facility response rates ranged from 76–85%, and patient response rates ranged from 49–55%. Eligible persons were HIV-infected, aged 18 years or older, and had received medical care in participating facilities between January and April in the cycle year for which they were sampled. Data were collected from June 2009 through May 2014 using face-to-face or telephone interviews and medical record abstractions. Data were weighted on the basis of known probabilities of selection at state or territory, facility, and patient levels [19]. In addition, predictors of nonresponse were determined from analysis of data from sampled facilities and patients, and data were then weighted to adjust for non-response, following established methods [20, 21].
In accordance with the federal human subjects protection regulations [22] and guidelines for defining public health research [23], MMP was determined to be a non-research, public health surveillance activity used for disease control program or policy purposes. Participating states or territories and facilities obtained local institutional review board approval to conduct MMP if required locally. Informed consent was obtained from all interviewed participants.
Definitions
We used medical record data to estimate the prevalence of syphilis, chlamydia, and gonorrhea testing in the 12 months prior to interview. Syphilis testing was defined as a result from non-treponemal or treponemal syphilis test, antibody test, or dark-field microscopy. Chlamydia testing was defined as at least one test result from culture, direct fluorescent antibody, enzyme immunoassay or enzyme-linked immunoassay, nucleic acid amplification test (NAAT), or nucleic acid probe. Testing for gonorrhea was defined as documentation of at least one test result from culture, gram stain, NAAT, or the nucleic acid probe. The analysis was limited to persons who reported sexual activity in the past 12 months. We performed stratified analyses according to the sex of participants and their sex partners in the past 12 months (hereafter referred to as sexual behavior). Men who had sex with men only or with men and women were defined as MSM; men who had sex with women only were defined as MSW; women who had sex with men were defined as WSM. Race/ethnicity was defined by self-identification as black or African American, non-Hispanic; Hispanic; or white, non-Hispanic. Due to small sample sizes, people who reported other or multiple race/ethnicities were combined into a single group (hereafter referred to as other race/ethnicity).
We estimated weighted percentages of persons tested for: 1) syphilis and 2) both chlamydia and gonorrhea in the last 12 months. We estimated each of these two outcomes separately by year and stratified by sexual behavior, age group, and race/ethnicity. We also estimated the weighted percentages of persons tested for all three STDs (syphilis, chlamydia, and gonorrhea). We calculated percent change in each outcome from 2009–2013 and used bivariate linear regression to estimate linear trends over time in each outcome, overall and by patient characteristics. Beta coefficients for year represent the average percentage point change (divided by 100) from one year to the next. All analyses accounted for the complex sample design and weights.
RESULTS
Syphilis testing
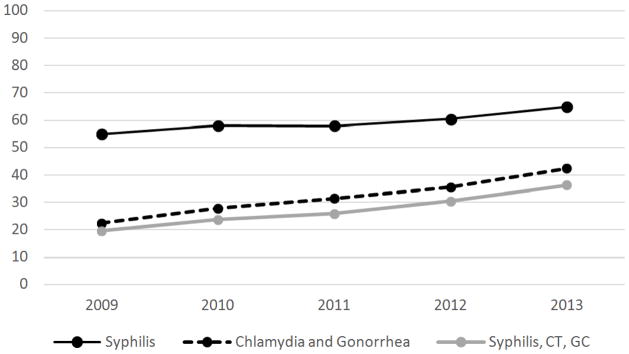
From 2009 to 2013, the proportion of sexually active, HIV-infected adults in medical care tested for syphilis increased from 55% to 65% (β=0.022, PTREND< 0.01). (Figure 1). The significant, increasing linear trend in syphilis testing from 2009–2013 was found for some, but not all subgroups (Table 1). Testing increased significantly among MSM (58% to 69%, β=0.026, PTREND< 0.01) but not among MSW (54% to 61%, β=0.016, PTREND= 0.20) or WSM (50% to 59%, β=0.018, PTREND = 0.15). Significant, increasing linear trends in syphilis testing were found for all age groups with the exception of people aged 18–29 (60% to 66%, β=0.007, PTREND = 0.57). By age, the largest percent change was found among persons aged 30–39 years old (57% to 71%, β= 0.023, PTREND = 0.01). Testing increased significantly among white, non-Hispanics (48% to 64%, β=0.036, PTREND < 0.01), but not among black, non-Hispanics (58% to 64%, β=0.013, PTREND = 0.25), Hispanics (61% to 69%, β=0.014, PTREND = 0.14), or persons of other race/ethnicity (55% to 60%, β=0.021, PTREND = 0.30).
Figure 1.
Testing for syphilis, for chlamydia and gonorrhea, and for all three infections among sexually active HIV-infected adults receiving medical care–United States, Medical Monitoring Project: 2009–2013
Table 1.
Percentage of sexually active HIV-infected adults in medical care tested for syphilis, by patient characteristics—United States, 2009–2013
| 2009 | 2010 | 2011 | 2012 | 2013 | % Δ 2009 – 2013 | β TREND | P-valueTREND | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | 95% CI | n | % | 95% CI | n | % | 95% CI | n | % | 95% CI | n | % | 95% CI | |||||||||
| Sexual orientation/behavior | |||||||||||||||||||||||
| MSM | 827 | 58 | 54 | 62 | 990 | 61 | 57 | 66 | 946 | 63 | 58 | 68 | 1067 | 65 | 60 | 70 | 1626 | 69 | 66 | 72 | 19 | 0.026 | 0.00 |
| MSW | 344 | 54 | 47 | 61 | 376 | 56 | 46 | 65 | 398 | 56 | 48 | 64 | 433 | 58 | 52 | 64 | 416 | 61 | 56 | 66 | 13 | 0.016 | 0.20 |
| WSM | 332 | 50 | 43 | 58 | 340 | 52 | 45 | 60 | 312 | 48 | 42 | 53 | 346 | 52 | 45 | 58 | 419 | 59 | 53 | 65 | 18 | 0.018 | 0.15 |
| Age group (yrs) | |||||||||||||||||||||||
| 18–29 | 172 | 60 | 51 | 69 | 187 | 68 | 60 | 76 | 186 | 67 | 60 | 74 | 206 | 64 | 59 | 69 | 219 | 66 | 59 | 74 | 10 | 0.007 | 0.57 |
| 30–39 | 332 | 57 | 51 | 62 | 366 | 64 | 58 | 70 | 309 | 57 | 52 | 63 | 345 | 58 | 52 | 64 | 421 | 71 | 66 | 75 | 25 | 0.023 | 0.01 |
| 40–49 | 610 | 55 | 49 | 60 | 641 | 56 | 51 | 61 | 626 | 58 | 53 | 63 | 688 | 64 | 58 | 70 | 640 | 64 | 60 | 68 | 18 | 0.027 | <.01 |
| 50+ | 419 | 53 | 47 | 58 | 544 | 54 | 47 | 61 | 557 | 56 | 51 | 60 | 637 | 57 | 52 | 63 | 756 | 62 | 58 | 66 | 18 | 0.023 | 0.01 |
| Race/ethnicity | |||||||||||||||||||||||
| Black, non-Hispanic | 624 | 58 | 51 | 65 | 668 | 59 | 50 | 67 | 649 | 58 | 51 | 64 | 745 | 59 | 55 | 64 | 809 | 64 | 59 | 69 | 11 | 0.013 | 0.25 |
| Hispanic | 365 | 61 | 55 | 68 | 418 | 62 | 56 | 67 | 403 | 61 | 55 | 67 | 424 | 60 | 53 | 68 | 511 | 69 | 64 | 73 | 12 | 0.014 | 0.14 |
| White, non-Hispanic | 469 | 48 | 44 | 52 | 579 | 56 | 52 | 60 | 546 | 57 | 52 | 62 | 619 | 61 | 54 | 68 | 638 | 64 | 60 | 68 | 33 | 0.036 | <.01 |
| Other | 75 | 55 | 41 | 70 | 73 | 54 | 43 | 64 | 80 | 57 | 46 | 67 | 88 | 65 | 57 | 74 | 78 | 60 | 52 | 67 | 8 | 0.021 | 0.30 |
Abbreviations: MSM, men who had sex with men; MSW, men who had sex with women; WSM, women who had sex with men.
Percentages weighted to adjust for unequal selection probabilities and facility and patient non-response
Chlamydia and gonorrhea testing
From 2009 to 2013, the proportion of sexually active, HIV-infected adults in medical care tested for chlamydia and gonorrhea increased from 22% to 42% (β= 0.040, PTREND < 0.01). (Figure 1). There were significant, increasing linear trends in chlamydia and gonorrhea testing from 2009–2013 in every sub-group apart from persons of other race/ethnicity (Table 2). While chlamydia and gonorrhea testing increased for WSM (27% to 45%, β=0.039, PTREND < 0.01), the percent change was larger for MSM (22% to 43%, β=0.051, PTREND < 0.01) and MSW (17% to 38%, β=0.049, PTREND < 0.01). By age, the largest changes in percent tested for chlamydia and gonorrhea were among older age groups. Among 40–49 year olds, testing increased from 21% to 43% (β=0.054, PTREND < 0.01) and among persons 50 or older, testing increased from 18% to 36% (β=0.046, PTREND < 0.01). By race/ethnicity, the largest percent change in chlamydia and gonorrhea testing was among Hispanics (26% to 54%, β=0.062, PTREND < 0.01), but the increase was also significant among white, non-Hispanics (18% to 36%, β=0.044, PTREND < 0.01) and black, non-Hispanics (24% to 42%, β=0.045, PTREND < 0.01). The increase in chlamydia and gonorrhea testing among persons of other race/ethnicity was not significant (29% to 36%, β=0.026, PTREND = 0.11).
Table 2.
Percentage of sexually active HIV-infected adults in medical care tested for gonorrhea and chlamydia, by patient characteristics—United States, 2009–2013
| 2009 | 2010 | 2011 | 2012 | 2013 | % Δ 2009 – 2013 | β TREND | P-value TREND | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | 95% CI | n | % | 95% CI | n | % | 95% CI | n | % | 95% CI | n | % | 95% CI | |||||||||
| Sexual orientation/behavior | |||||||||||||||||||||||
| MSM | 329 | 22 | 18 | 26 | 467 | 27 | 22 | 33 | 478 | 31 | 26 | 36 | 614 | 37 | 32 | 41 | 736 | 43 | 39 | 47 | 94 | 0.051 | <.01 |
| MSW | 116 | 17 | 13 | 22 | 167 | 24 | 18 | 31 | 223 | 33 | 25 | 41 | 236 | 32 | 26 | 38 | 281 | 38 | 22 | 33 | 120 | 0.049 | <.01 |
| WSM | 172 | 27 | 22 | 32 | 204 | 33 | 25 | 41 | 201 | 32 | 26 | 38 | 238 | 36 | 30 | 42 | 314 | 45 | 39 | 50 | 64 | 0.039 | <.01 |
| Age group (yrs) | |||||||||||||||||||||||
| 18–29 | 88 | 31 | 23 | 40 | 116 | 41 | 30 | 51 | 116 | 41 | 34 | 48 | 155 | 47 | 39 | 56 | 168 | 49 | 42 | 56 | 59 | 0.043 | <.01 |
| 30–39 | 153 | 27 | 22 | 31 | 197 | 34 | 26 | 42 | 198 | 37 | 31 | 44 | 214 | 36 | 31 | 41 | 301 | 50 | 45 | 54 | 85 | 0.048 | <.01 |
| 40–49 | 243 | 21 | 18 | 25 | 310 | 26 | 20 | 32 | 335 | 32 | 26 | 38 | 400 | 37 | 31 | 43 | 431 | 43 | 37 | 49 | 101 | 0.054 | <.01 |
| 50+ | 148 | 18 | 14 | 21 | 229 | 22 | 16 | 28 | 262 | 25 | 19 | 30 | 341 | 31 | 26 | 36 | 457 | 36 | 31 | 41 | 107 | 0.046 | <.01 |
| Race/ethnicity | |||||||||||||||||||||||
| Black, non-Hispanic | 246 | 24 | 20 | 28 | 332 | 29 | 22 | 37 | 366 | 33 | 27 | 39 | 472 | 38 | 32 | 43 | 552 | 42 | 37 | 48 | 76 | 0.045 | <.01 |
| Hispanic | 167 | 26 | 22 | 31 | 235 | 35 | 29 | 41 | 250 | 39 | 32 | 46 | 276 | 41 | 36 | 46 | 402 | 54 | 49 | 60 | 105 | 0.062 | <.01 |
| White, non-Hispanic | 181 | 18 | 14 | 21 | 249 | 22 | 17 | 28 | 246 | 24 | 20 | 29 | 311 | 31 | 25 | 36 | 356 | 36 | 29 | 42 | 99 | 0.044 | <.01 |
| Other | 38 | 29 | 19 | 38 | 36 | 25 | 13 | 36 | 49 | 36 | 25 | 46 | 51 | 37 | 25 | 48 | 125 | 36 | 28 | 44 | 25 | 0.026 | 0.11 |
Abbreviations: MSM, men who had sex with men; MSW, men who had sex with women; WSM, women who had sex with men.
Percentages weighted to adjust for unequal selection probabilities and facility and patient non-response
Syphilis, chlamydia, and gonorrhea testing
The proportion of sexually active HIV-infected adults tested for all three STIs (syphilis, chlamydia, and gonorrhea) increased from 20% to 36% (β=0.040, PTREND < 0.01). (Figure 1).
DISCUSSION
We found significant increases in testing for syphilis, for chlamydia and gonorrhea, and for all three infections among sexually active HIV-infected adults receiving medical care in the United States from 2009–2013. However, these increases were not found among all sub-groups. While testing for syphilis increased overall from 55% to 65%, the increase was significant only among MSM, persons aged 30 years and older, and white, non-Hispanics. Notably, the largest percent change occurred among white, non-Hispanics who had the lowest levels of syphilis testing in 2009. In contrast, we found significant increases in chlamydia and gonorrhea testing overall from 22% to 42% and in nearly every sub-group examined. In general, the largest percent change in chlamydia and gonorrhea testing from 2009 to 2013 occurred among groups with low levels of testing in 2009 (i.e., MSW and persons over 30 years of age). However, this was not observed by race/ethnicity. Hispanics had comparatively high levels of chlamydia and gonorrhea testing in 2009, yet still had the largest percent change. We did not identify a significant increase in chlamydia and gonorrhea testing among persons with other race/ethnicity; however, this is likely due to small sample size in this group.
Despite the fact that chlamydia and gonorrhea incidence is substantially higher than incidence of syphilis, we found that the prevalence of syphilis testing was higher than testing for chlamydia and gonorrhea overall, for all sub-groups, and at all time points. In the United States in 2014, reported case rates were 456.1 per 100,000 persons for chlamydia, 110.7 per 100,000 persons for gonorrhea, and 6.3 per 100,000 persons for syphilis.[2] During 2000–2014, there was an increase in reported syphilis cases, which was primarily attributable to increased cases among men, specifically, among MSM.[2] However, during 2013–2014, the rate increased among men and among women.[2] More syphilis testing compared to other STD testing among HIV-infected persons has been noted in several studies [15, 24] and as Hoover et al reported, it is likely attributable to several factors. First, given recent increases in syphilis, especially among MSM, [25, 26] patient and provider awareness of the importance of syphilis screening may contribute to increased testing. In addition, because syphilis infection increases HIV viral load and decreases CD4 cell count in HIV-infected persons, failure to treat syphilis could result in increased risk of HIV transmission.[27–29] Finally, as a measure of quality assurance, syphilis testing is a requirement of Ryan White funding and approximately 73% of MMP facilities received Ryan White funding in 2009–2011.[30]
While increases in STD testing indicate improvement, testing remains far below recommended guidelines, which state that all sexually active HIV-infected persons should be screened at least annually for syphilis, chlamydia and gonorrhea. Possible reasons why providers are not adherent to recommended guidelines include time constraints, the invasiveness of some screening tests, or personal discomfort discussing sexual behavior.[31, 32] However, effective interventions exist to increase provider’s adherence to guidelines as well as patients’ acceptance of screening. Examples of effective tools include electronic alerts for doctors; syphilis serology included with blood tests performed as part of HIV monitoring, educational workshops for clinic staff; internet-based continuing medical education; free sexual health consultations for patients; biannual STD testing, coupled with brief risk-reduction counseling; and provision of a urine specimen cup at the time of patient registration.[33–35] These strategies could be used to increase routine STD screening.
This analysis has limitations. First, our estimates may underestimate STD testing in HIV positive persons in care because we only collected data from the medical record at the persons’ primary HIV care provider, which could miss STD testing that occurred outside of that setting. Second, MMP altered the way STD testing information was captured in 2013 such that it was easier to record tests at multiple anatomical sites on the same day; however, all trends remained significant when we limited the analysis to 2009–2012 data only (data not shown). Third, we were unable to determine if STD tests were administered as part of regular screening, as guidelines recommend, or due to the presence of symptoms. Fourth, while patients were randomly sampled each year, it is possible that a person was selected and participated in more than one data collection cycle. Our analysis did not account for this correlation. However, we replicated the analysis with such patients removed and results of all trend analyses remained unchanged. Lastly, during 2009–2013, MMP included only people who were receiving HIV medical care; therefore, our estimates are unlikely to be generalizable to all HIV-diagnosed persons in the U.S.
From 2009 to 2013, testing for syphilis, chlamydia, and gonorrhea increased among sexually active HIV-infected persons receiving HIV medical care in the United States. However, in 2013 64% of persons did not receive recommended testing for all three STDs. Further, syphilis testing did not increase among MSW, WSM, those aged 18–29, blacks, non-Hispanics or Hispanics. Given that syphilis case rates have increased among MSM, MSW, and women in recent years and the fact that racial disparities exist, the failure to identify increased syphilis testing among all groups indicates a need for improvement. Moreover, these suboptimal testing rates are likely to be even lower among persons not receiving HIV medical care. Given the morbidity associated with STDs and their potential to increase HIV transmission, our findings suggest that enhanced efforts may be warranted to screen all HIV-infected sexually active adults for syphilis, chlamydia, and gonorrhea at all appropriate anatomical sites.
Acknowledgments
We thank participating MMP patients, facilities, project areas, and Provider and Community Advisory Board members. We also acknowledge the contributions of the Clinical Outcomes Team and Behavioral and Clinical Surveillance Branch at CDC and the MMP Project Area staff. (http://www.cdc.gov/hiv/statistics/systems/mmp/resources.html#StudyGroupMembers).
Financial support. This work is supported by the Centers for Disease Control and Prevention.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention (CDC).
Conflicts of interest. The authors declare no conflicts of interest.
References
- 1.CDC. HIV Surveillance Report, 2014. 2015. Nov, [Google Scholar]
- 2.CDC. Sexually Transmitted Disease Surveillance 2014. Atlanta: 2015. [Google Scholar]
- 3.Kaplan JE, Benson C, Holmes KK, et al. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep. 2009;58(RR-4):1–207. quiz CE1-4. [PubMed] [Google Scholar]
- 4.Cohen MS, Hoffman IF, Royce RA, et al. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. AIDSCAP Malawi Research Group Lancet. 1997;349(9069):1868–73. doi: 10.1016/s0140-6736(97)02190-9. [DOI] [PubMed] [Google Scholar]
- 5.Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect. 1999;75(1):3–17. doi: 10.1136/sti.75.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wasserheit JN. Epidemiological synergy. Interrelationships between human immunodeficiency virus infection and other sexually transmitted diseases. Sex Transm Dis. 1992;19(2):61–77. [PubMed] [Google Scholar]
- 7.Aberg JA, Gallant JE, Ghanem KG, et al. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV medicine association of the Infectious Diseases Society of America. Clin Infect Dis. 2014;58(1):e1–34. doi: 10.1093/cid/cit665. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention HRaSA, National Institutes of Health, American Academy of HIV Medicine AoNiAC, International Association of Providers of AIDS Care, the National Minority AIDS Council aUCfHAPS. Recommendations for HIV Prevention with Adults and Adolescents with HIV in the United States, 2014. 2014. [Google Scholar]
- 9.Workowski KA, Bolan GA Centers for Disease C, Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1–137. [PMC free article] [PubMed] [Google Scholar]
- 10.Aberg JA, Kaplan JE, Libman H, et al. Primary care guidelines for the management of persons infected with human immunodeficiency virus: 2009 update by the HIV medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2009;49(5):651–81. doi: 10.1086/605292. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease C, Prevention. Workowski KA, Berman SM. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep. 2006;55(RR-11):1–94. [PubMed] [Google Scholar]
- 12.Workowski KA. Centers for Disease Control and Prevention Sexually Transmitted Diseases Treatment Guidelines. Clin Infect Dis. 2015;61(Suppl 8):S759–62. doi: 10.1093/cid/civ771. [DOI] [PubMed] [Google Scholar]
- 13.Workowski KA, Berman S Centers for Disease C, Prevention. Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59(RR-12):1–110. [PubMed] [Google Scholar]
- 14.Aberg JA, Gallant JE, Anderson J, et al. Primary care guidelines for the management of persons infected with human immunodeficiency virus: recommendations of the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2004;39(5):609–29. doi: 10.1086/423390. [DOI] [PubMed] [Google Scholar]
- 15.Pearson WS, Davis AD, Hoover KW, Gift TL, Owusu-Edusei K, Tao G. Demographic and health services characteristics associated with testing for sexually transmitted infections among a commercially insured population of HIV-positive patients. J Acquir Immune Defic Syndr. 2015;70(3):269–74. doi: 10.1097/QAI.0000000000000709. [DOI] [PubMed] [Google Scholar]
- 16.Flagg EW, Weinstock HS, Frazier EL, Valverde EE, Heffelfinger JD, Skarbinski J. Bacterial sexually transmitted infections among HIV-infected patients in the United States: estimates from the Medical Monitoring Project. Sex Transm Dis. 2015;42(4):171–9. doi: 10.1097/OLQ.0000000000000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bradley H, Frazier E, Huang P, et al. Behavioral and clinical characteristics of persons receiving medical care for HIV infection Medical Monitoring Project United States, 2010. Atlanta, GA: 2014. Oct, [PubMed] [Google Scholar]
- 18.Iachan R, CHJ, RLH, et al. Design and weighting methods for a nationally representative sample of HIV-infected adults receiving medical care in the United States-Medical Monitoring Project. Open AIDS J. 2016;10:164–81. doi: 10.2174/1874613601610010164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harding L, Iachan R, Johnson C, Kyle T, Skarbinski J. Weighting Methods for the 2010 data collection cycle of the Medical Monitoring Project. 2013 Joint Statistical Meeting; Montréal, QC, H2Z 1H2, Canada. 2013. [Google Scholar]
- 20.Särndal C-E, Lundström S. Estimation in Surveys with Nonresponse. Chichester: John Wiley & Sons; 2005. [Google Scholar]
- 21.Heeringa S, West BT, Berglund PA. Applied survey data analysis. Boca Raton, FL: Taylor & Francis; 2010. [Google Scholar]
- 22. [Accessed February 4, 2014];Protection of Human Subjects, US Federal Code Title 45 Part 46. Available at: Available at http://www.hhs.gov/ohrp/humansubjects/guidance/45cfr46.html.
- 23.Centers for Disease Control and Prevention. [Accessed February 4, 2014];Distinguishing Public Health Research and Public Health Nonresearch. Available at: Available at http://www.cdc.gov/od/science/integrity/docs/cdc-policy-distinguishing-public-health-research-nonresearch.pdf.
- 24.Hoover KW, Butler M, Workowski K, et al. STD screening of HIV-infected MSM in HIV clinics. Sex Transm Dis. 2010;37(12):771–6. doi: 10.1097/OLQ.0b013e3181e50058. [DOI] [PubMed] [Google Scholar]
- 25.Peterman TA, Heffelfinger JD, Swint EB, Groseclose SL. The changing epidemiology of syphilis. Sex Transm Dis. 2005;32(10 Suppl):S4–10. doi: 10.1097/01.olq.0000180455.79024.e9. [DOI] [PubMed] [Google Scholar]
- 26.Erbelding E, Rompalo A. Changing epidemiology of syphilis and its persistent relationship with HIV. Curr Infect Dis Rep. 2004;6(2):135–40. doi: 10.1007/s11908-996-0010-z. [DOI] [PubMed] [Google Scholar]
- 27.Buchacz K, Patel P, Taylor M, et al. Syphilis increases HIV viral load and decreases CD4 cell counts in HIV-infected patients with new syphilis infections. AIDS. 2004;18(15):2075–9. doi: 10.1097/00002030-200410210-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kofoed K, Gerstoft J, Mathiesen LR, Benfield T. Syphilis and human immunodeficiency virus (HIV)-1 coinfection: influence on CD4 T-cell count, HIV-1 viral load, and treatment response. Sex Transm Dis. 2006;33(3):143–8. doi: 10.1097/01.olq.0000187262.56820.c0. [DOI] [PubMed] [Google Scholar]
- 29.Palacios R, Jimenez-Onate F, Aguilar M, et al. Impact of syphilis infection on HIV viral load and CD4 cell counts in HIV-infected patients. J Acquir Immune Defic Syndr. 2007;44(3):356–9. doi: 10.1097/QAI.0b013e31802ea4c6. [DOI] [PubMed] [Google Scholar]
- 30.Weiser J, Beer L, Frazier EL, et al. Service delivery and patient outcomes in Ryan White HIV/AIDS program-funded and nonfunded health care facilities in the United States. JAMA Intern Med. 2015;175(10):1650–9. doi: 10.1001/jamainternmed.2015.4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MRV, CBL Prevention with positives: Primary HIV care provider attitudes and practices. National HIV Prevention Conference; Atlanta, GA. 2005. [Google Scholar]
- 32.Tao G, Irwin KL, Kassler WJ. Missed opportunities to assess sexually transmitted diseases in U.S. adults during routine medical checkups. Am J Prev Med. 2000;18(2):109–14. doi: 10.1016/s0749-3797(99)00139-7. [DOI] [PubMed] [Google Scholar]
- 33.Guy RJ, Ali H, Liu B, et al. Efficacy of interventions to increase the uptake of chlamydia screening in primary care: a systematic review. BMC Infect Dis. 2011;11:211. doi: 10.1186/1471-2334-11-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel P, Bush T, Mayer K, et al. Routine brief risk-reduction counseling with biannual STD testing reduces STD incidence among HIV-infected men who have sex with men in care. Sex Transm Dis. 2012;39(6):470–4. doi: 10.1097/OLQ.0b013e31824b3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zou H, Fairley CK, Guy R, Chen MY. The efficacy of clinic-based interventions aimed at increasing screening for bacterial sexually transmitted infections among men who have sex with men: a systematic review. Sex Transm Dis. 2012;39(5):382–7. doi: 10.1097/OLQ.0b013e318248e3ff. [DOI] [PubMed] [Google Scholar]