Abstract
Bilingualism is a typical linguistic experience, yet relatively little is known about its impact on children's cognitive and brain development. Theories of bilingualism suggest early dual-language acquisition can improve children's cognitive abilities, specifically those relying on frontal lobe functioning. While behavioral findings present much conflicting evidence, little is known about its effects on children's frontal lobe development. Using functional Near-Infrared Spectroscopy (fNIRS), the findings suggest that Spanish-English bilingual children (n=13, ages 7-13) had greater activation in left prefrontal cortex during a non-verbal attentional control task relative to age-matched English monolinguals. In contrast, monolinguals (n=14) showed greater right prefrontal activation than bilinguals. The present findings suggest early bilingualism yields significant changes to the functional organization of children's prefrontal cortex for attentional control and carry implications for understanding how early life experiences impact cognition and brain development.
Keywords: cognitive development, cognitive neuroscience, bilingualism, attention, fnirs, plasticity, neuroimaging
Children's cognitive and neural development arises partly from their everyday learning experiences, including language acquisition. Over the course of language acquisition, children encounter multiple linguistic and socio-linguistic contexts that require some type of conflict resolution (e.g., adjudicate the meanings for similar sounding words like “I” and “eye”; Mazuka, Jincho, & Oishi, 2009). Theories of bilingual cognitive development further suggest that the doubling of these conflicting contexts that are typical of bilingual language acquisition (e.g., increasing the number of possible homophones), and the unique need to selectively attend to one language while suppressing the other may alter bilinguals' attentional control mechanisms (Bialystok, Craik, & Luk, 2012; Green & Abutalebi, 2013; Dong & Li, 2015; Kroll, Dussias, Bice, & Perrotti, 2015). Yet, inconsistent findings across diverse bilingual populations continue to fuel the debate on whether bilingual experiences do (Kroll & Bialystok, 2013) or do not (Paap & Greenberg, 2013) yield benefits for attentional control (Hilchey & Klein, 2011; Valian, 2015). Hence, researchers have suggested that the traditional approach of measuring and comparing children's accuracy or reaction time during attention tasks is insufficient for revealing the full extent to which language acquisition and brain development processes interact to shape young bilinguals' cognitive development (Kroll, 2015). In the present study, we assessed task performance and brain activation in the prefrontal cortex using functional Near-Infrared Spectroscopy (fNIRS) in early exposed and proficient Spanish-English bilingual and English monolingual children.
Attentional control is the ability to focus and shift attention selectively (Posner, 2012). For instance, during a common word-picture matching task, participants take longer to select a picture when they simultaneously see pictures of similar initial sounds (such as “card” and “cart,” versus “card” and “lion”; Marian & Spivey, 2003). During this task, participants experience linguistic interference that requires them to ignore the competing distractor. Importantly, both within- and cross-language distractors can impact bilingual participants' performance in this task (Marian & Spivey, 2003). Such findings exemplify not only the attentional demands in the context of language processing, but also the general notion that bilinguals' languages are often relatively co-active (Van Hell & Dijkstra, 2002; Hernandez, Li, MacWinney, 2005; Kroll, 2015). Such persistent co-activation of bilinguals' two languages is thought to create an increased demand for attentional control across various contexts of bilingual language use, from word recognition to discourse (Kroll et al., 2015). Thus, theories of bilingual development have suggested that childhood bilingual exposure during periods of rapid brain development may yield early-emerging and lifelong changes to children's attentional control abilities (Kroll & Bialystok, 2013).
Indeed, several studies now point to better performance on attentional control tasks in bilingual infants (Kovács & Mehler, 2009; Singh et al., 2015), children (Bialystok, 1999; Carlson & Meltzoff, 2008; Yang, Yang, & Lust, 2011; Tran, Arredondo, & Yoshida, 2015), and adults (Bialystok, Craik, Klein, & Viswanathan, 2004; Bialystok, Craik, & Freedman, 2007; Costa, Hernández, & Sebastián-Gallés, 2008) relative to monolinguals. However, both the hypothesis of better attentional control in bilinguals and subsequent findings continue to be questioned. First, it is possible that the findings are unrelated to language or bilingual experiences per se, but rather are driven by the concurrent circumstances of a rich and varied multicultural upbringing (Morton & Harper, 2009). Second, a growing body of research offers evidence to refute the hypothesis of better attentional control by bilinguals (Hilchey & Klein, 2011; Paap & Greenberg, 2013; Antón et al., 2014; De Bruin, Treccani, & Della Salra, 2015).
Conflicting evidence has especially emerged using the Attentional Network Task (ANT; Fan, McCandliss, Sommer, Raz, & Posner, 2002), which is a non-verbal visuo-spatial attention task that builds upon a cue alerting and orienting scheme along with a flanker task. ANT requires participants to selectively attend to the directionality of a target arrow in the center of the screen, while ignoring the directionality of surrounding flanker arrows (Fan et al., 2002). The flankers point in the same direction as the target (Congruent trials: →→→→→), or in the opposing direction (Incongruent trials: →→←→→). The participants' task is to indicate the direction of the target as quickly and as accurately as possible by pressing buttons. A study by Kapa and Colombo (2013) using the ANT demonstrated that children (average age 9) with early bilingual exposure (before age 3, n=21) had faster reaction times than monolingual peers (n=22). However, a recent large-sample study with about 200 children per group failed to find any evidence of better attentional control in bilingual children relative to monolinguals using the same task (Antón et al., 2014).
Nevertheless, the bilingual attentional control hypothesis suggests that older children (approximately age 6 and older) and young adults, who are in their peak performance years, might generally perform well at a variety of standard experimental measures of reaction time and thus may not necessarily show any evidence of differences on group performance (Bialystok et al., 2012). The precise impact of bilingualism on attentional control might still be in place and easier to detect through traditional measurements of accuracy and reaction time in younger children (before age 6) or older adult populations, as these groups tend to show more variance in their speed of cognitive processing (Bialystok et al., 2012). Neuroimaging offers an additional method for gathering evidence on mental operations when assessing group differences that may or may not manifest as behavioral differences in experimental task performance (Kroll, 2015).
Thus, research suggests that bilingual experiences during the developmental periods of rapid brain development should yield changes in children's cognitive development (Kroll et al., 2015). If such interaction exists, one should be able to detect it using neuroimaging methods. Green and Abutalebi (2013) have put forth the Adaptive Control hypothesis proposing that individuals' cognitive system and their neural networks dynamically adapt to each individuals' daily demands for working with competing verbal or nonverbal representations. Specific to bilingual individuals is the phenomenon of co-activation of the two linguistic systems (see description of the example by Marian and Spivey, 2003 above) and hence the need to selectively increase activation for one language while reducing the interference from the competing language (Rodriguez-Fornells, Rotte, Heinze, Nosselt, & Munter, 2002). Such demand for language selection could incur a set of changes in bilinguals' cognitive processes and their neural representations, such as change in a brain region's efficiency through the tuning of neuronal populations or alteration to the responsiveness of neuronal populations of a region (Green & Abutalebi, 2013; see also Green, 2011). Importantly, such changes should emerge early in development, given that developmental evidence suggests that one-year old bilinguals already selectively modulate the use of their language starting with the first word milestone (Petitto & Kovelman, 2003).
Left prefrontal cortex might be one possible set of loci that is altered as a function of bilingual experiences (Green & Abutalebi, 2013). Language research with monolinguals suggests that the maturing left prefrontal cortex supports children's improvement in the attentional demands associated with early language acquisition (Novick, Trueswell, & Thompson-Schill, 2005, 2010). Language research with bilinguals suggests that the prefrontal cortex activates bilaterally during language switching relative to non-switching trials (Luk, Green, Abutalebi, & Grady, 2012; see also Abutalebi & Green, 2008). Finally, researchers find that bilingual adults show greater left prefrontal activation, relative to monolinguals, during a non-verbal cognitive control task requiring attention mechanisms (Garbin et al., 2010). While it has been generally hypothesized that early bilingual development may change the functionality of prefrontal cortex, it remains unknown when this neural alteration occurs and whether it is already present in young children.
To the best of our knowledge, only one study has investigated the brain bases of attention in bilingual children. Krizman and colleagues (2012) used electroencephalography (EEG) to show that bilingual adolescents exhibited a more rapid and pronounced subcortical response to target auditory stimuli relative to monolinguals,suggesting that top-down attentional control processes influenced bilingual adolescents' subcortical brain-stem responses (Krizman et al., 2012). We employ fNIRS to test whether young Spanish-English bilingual and English monolingual children (ages 7-13) differ in their cortical prefrontal lobe activation during the flanker paradigm of the ANT child-version (Fan et al., 2002; Rueda et al., 2004). The goal of the study is to improve our understanding of how childhood bilingualism, which is one of the most widespread variations in language-learning experiences, impacts cognition and the developing brain.
Method
Participants
Twenty-seven right-handed neurotypical children participated: 14 English monolinguals (8 females; age range = 7.3 – 13.6 years, mean age [Mage] = 9.7 years, standard deviation [SD] = 1.7) and 13 Spanish-English speaking bilinguals (6 females; age range = 8.6 – 13.3 years, Mage = 10.3 years, SD = 1.5), all raised and educated in a Midwestern town in the United States (U.S.). At the time of testing, bilingual children were receiving daily exposure to both languages (Spanish in the home and English outside the home). All bilinguals were first exposed to Spanish at birth and to English between birth and the age of five. Seven bilingual children were born in the U.S., and six of them were born in a Spanish-speaking country (of these 6 children: two were first exposed to English at the age of 1, two at the age of 3 and two at the age of 4). All mothers and most fathers (except two) were native Spanish speakers and reported consistent use of Spanish at home with their child(ren). Seven bilingual children were also attending a local Spanish heritage language-learning school once a week. For monolingual children, English was the only language spoken at home. All children attended English-instruction schools. All families were recruited from the same neighborhoods and were of similar socio-economic status (SES). The children did not differ in English language proficiency or cognitive abilities (p >.05); see Table 1 for more details about the language groups. The study was reviewed and approved by institutional review boards. Families received monetary compensation and a small toy.
Table 1. Participants' mean (and standard deviation) demographics and performance scores for language, literacy, and cognition tasks.
| Measures | Monolingual English N = 14 (8 F) | Bilingual English N = 13 (6 F) | Bilingual Spanish N = 13 (6 F) | T-values Between- Groups | T-values Within (Bilinguals) |
|---|---|---|---|---|---|
| Age | 9.67 (1.67) | 10.31 (1.52) | -- | 1.04 | -- |
| IQ | 114.43 (16.34) | 115.15 (13.15) | -- | 0.13 | -- |
| Demographics a | |||||
| Income | 7.54 (1.76) | 7.92 (1.78) | -- | 0.53 | -- |
| Mother's education | 6.14 (1.41) | 6.27 (2.15) | -- | 0.18 | -- |
| Father's education | 5.86 (1.23) | 5.91 (2.21) | -- | 0.08 | -- |
| Language & Literacy | |||||
| Vocabulary | 121.43 (15.86) | 114.23 (12.39) | 107.85 (19.87) | 1.31 | 1.10 |
| Phonology (%) b | 81.79 (15.89) | 86.15 (7.95) | 87.31 (17.27) | 0.89 | 0.33 |
| Syntax (%)b | 92.41 (5.01) | 94.95 (8.42) | 76.39 (20.25) | 0.96 | 3.25** |
| Reading | 113.11 (13.01) | 116.19 (9.83) | 97.04 (14.26) | 0.69 | 6.82*** |
| Attention & Cognition | |||||
| Naming Speedc | 99.18 (12.72) | 107.46 (11.51) | -- | 1.78 | -- |
| Naming Speed c (seconds) | 31.97 (11.76) | 24.26 (5.91) | 29.50 (14.66) | 2.13* | 1.41 |
| HTKSd | 50.93 (5.15) | 54.0 (5.03) | -- | 1.57 | -- |
| ANT Accuracy (%) | |||||
| Neutral | 98.21 (3.19) | 99.38 (1.50) | -- | 1.20 | -- |
| Congruent | 98.86 (1.87) | 98.38 (2.14) | -- | 0.61 | -- |
| Incongruent | 97.14 (2.91) | 99.38 (1.50) | -- | 2.49* | -- |
| ANT Reaction Time (ms) | |||||
| Neutral | 733.71 (96.66) | 739.04 (140.82) | -- | 0.12 | -- |
| Congruent | 748.53 (90.60) | 736.85 (117.86) | -- | 0.29 | -- |
| Incongruent | 800.75 (97.19) | 789.87 (146.81) | -- | 0.23 | -- |
| Attentional Control (subtractions between Incongruent and control conditions) | |||||
| - Congruent | 52.22 (44.94) | 53.03 (47.31) | -- | 0.05 | -- |
| - Neutral | 67.05 (40.02) | 50.83 (61.73) | -- | 0.82 | -- |
Note.
p < 0.05
p < 0.01
p < 0.001
Options for demographic responses on yearly household income were the following: (1) less than $5,000; (2) $5,000 - $11,999; (3) $12,000 - $15,999; (4) $16,000 - $24,999; (5) $25,000 - $34,999; (6) $35,000 - $49,999; (7) 50,000 - $74,999; (8) $75,000 - $99,999; (9) $100,000 and greater. Options for responses on education were the following: (1) primary school, (2) some secondary school, (3) High school diploma or equivalent (GED), (4) some college, (5) Associate's degree, (6) Bachelor's degree, (7) Master's degree, (8) Doctorate degree [Ph.D], Professional degree [MD, DD, DDS, etc].
Percentage scores are presented due to disproportionate total items in the tasks.
Assessed via the Rapid Automatic Naming (RAN) Numbers subtest, standard scores based on a mean of 100 (SD = 15) are presented first. Below the averaged (and standard deviation) total time taken to complete the naming speed task as measured by seconds are also presented for each group.
HTKS raw score is presented: 30 items, each item's score ranges between 0 as incorrect response, 1 as self-correction, and 2 as correct response.
Much of the prior behavioral and neuroimaging studies on bilingual cognition included between 10-20 participants (e.g. Garbin et al., 2010; Krizman et al., 2012). Aiming to have a similar sample size, we initially invited 25 right-handed, neurotypical bilingual children with early dual-language exposure and high levels of proficiency; of those invited, 22 bilinguals completed the fNIRS imaging portion of the study. Similarly, we invited 28 right-handed, neurotypical monolingual children; of those invited, 27 completed the fNIRS imaging portion. Of these children that completed fNIRS imaging, 18 bilingual and 17 monolingual children were closely matched in age, SES, and were proficient in English with a vocabulary standard score above 85, as assessed using the Kaufman Brief Intelligence Test (KBIT-2) Verbal Knowledge subtest (Kaufman & Kaufman, 2004). Of these matched children, the imaging data for 13 bilingual and 14 monolingual children passed the data artifact rejection procedure (see below for more details); this final set of participants was included in data analyses for the present study.
Procedure
Participants first underwent fNIRS brain imaging and then completed the behavioral assessments outlined below. All participants completed one experimental session in English, and bilingual participants also completed a Spanish session one month later. During the English session, participants completed the ANT flanker neuroimaging paradigm with English-speaking experimenters, as well as two unrelated measures of English morpho-syntax.
Measures of Language, Literacy, and Cognitive Development
Parents completed a detailed Language Background and Use questionnaire (LBU; Kovelman et al., 2008) about their child's cognitive, language and motor development, plus any family history of learning impairments. Parents also completed questions on their educational level and household income from the John D. and Catherine T. MacArthur Foundation Research Network on Socioeconomic Status and Health questionnaire (retrieved from: www.macses.ucsf.edu).
English Vocabulary was assessed using the KBIT-2 Verbal Knowledge subtest (Kaufman & Kaufman, 2004). During testing, the experimenter presented the child with a matrix of 6 images and a word, the participant then pointed to the image that best represented the word. Basal and ceiling levels were established; standard scores were used for analyses (M = 100, SD = 15).
Spanish Vocabulary was assessed using the Receptive One-Word Picture Vocabulary Test Spanish Bilingual Edition (ROWPVT-4; Brownell, 2000), which is a standardized assessment normed with Spanish-English bilinguals. Similar to the English Vocabulary assessment, the experimenter presented the child with a matrix of 4 images and a word, then the participant pointed to the image that best represented the word. Basal and ceiling levels were established; standard scores were used for analyses (M = 100, SD = 15).
English Phonology was assessed using the Comprehensive Test of Phonological Processing (CTOPP) Elision subtest (Wagner, Torgesen, & Rashotte, 1999). During testing, the experimenter asked the child to say a word, and then to repeat it without saying part of it. For example, “Say winter, now say winter without saying /t/,” the correct response would be “winner.” Participants earned 1 point for correct items; 6 practice items and 20 testing items were presented. Testing stopped when ceiling level was reached (3 consecutive errors). Percentage scores are reported and used for analyses.
Spanish Phonology was assessed using the Test of Phonological Processing in Spanish (TOPPS) Elision subtest (Francis et al., 2001). The assessment follows the same format as the English Phonology measure, however it is not a standardized assessment. Participants earned 1 point for correct items; 5 practice items and 20 testing items were presented. Percentage scores are reported and used for analyses.
English and Spanish Syntax were assessed using the Clinical Evaluation of Language Fundamentals (CELF-4) Word Structure subtest (Semel, Wiig, & Secord, 2003, 2006). The assessments measure participants' ability to apply morphology rules and appropriate pronouns. Participants earned 1 point for correct items; a total of 32 testing items were presented for English, and 29 items for Spanish. Percentage scores are reported and used for analyses.
Reading was assessed using the Word-ID subtests on Woodcock Reading Mastery for English (Woodcock, 1998), and on Batería III Woodcock-Muñoz Pruebas de Aprovechamiento for Spanish (Muñoz-Sandoval, Woodcock, McGrew, & Mather, 2005). During testing, the experimenter presented the child with a word to read aloud. Basal and ceiling levels were established; standard scores were used for analyses (M = 100, SD = 15).
Non-verbal Intelligence was assessed using the KBIT-2 Matrices subtest (Kaufman & Kaufman, 2004), which measures the ability to find spatial and abstract relationships among a set of images and patterns by finding the best option out of 4. Basal and ceiling levels were established; standard scores are reported and used for analyses (M = 100, SD = 15).
Naming Speed was assessed using the Rapid Automatized Naming (RAN) Numbers subtest (Wolf & Denckla, 2005). This task is thought to predict reading fluency, resemble executive function abilities, and its performance may be associated to processing speed (Norton & Wolf, 2012). During testing, children were asked to name 50 numbers on a card as fast as possible; the numbers included: 2, 6, 9, 4, and 7. Standard scores (M = 100, SD = 15) and amount of time for completion (seconds) are reported and were used during analyses. A direct translation was used during Spanish testing.
Executive Function was assessed using the Head-Toes-Knees-Shoulders task (HTKS; Ponitz, McClelland, Matthews, & Morrison, 2009). The task includes three portions: in the first part (10 trials), the child is asked to touch their head when the experimenter says “Touch your toes,” and to touch their toes for “Touch your head.” During the second part (10 trials), the child receives additional trials that include a reverse sequence of touching their knees and shoulders. During the last portion (10 trials), the instructions are randomized again; for example, instead of touching their toes during “touch your head,” the child must touch their knees. Participants received scores that range between 0 to 2 for each trial: 0 for touching the incorrect body part, 1 for making a motion towards an incorrect body part but then touching the correct body part, and 2 for touching the correct body part. The sum of the scores for 30 trials are presented and analyzed.
Attentional Control Neuroimaging Measure
Child participants completed the flanker paradigm of the Attentional Network Test (child-version ANT, executive attention network; Fan et al., 2002; Rueda et al., 2004). Participants were presented trials in 3 conditions: Neutral, Congruent, and Incongruent. The task requires participants to monitor their attention and solve trials with conflicting and non-conflicting information. Children were instructed to feed a target “hungry fish” in the center of the screen by pressing buttons as quickly as possible; button presses vary by the directionality of the target fish. The experimental paradigm included two control conditions: Neutral trials presented a single fish with no flankers (→ or ←), and Congruent trials presented a target fish in the middle of the screen along with two flanker fish on each side that faced the same direction as the target (→→→→→ or ←←←←←). The experimental condition was comprised of Incongruent trials in which participants resolved visuo-spatial conflicting information as the target middle fish faced the opposite direction of flanker fish (→→←→→ or ←←→←←).
While Congruent trials are an appropriate control condition, the use of Neutral trials is relatively standard to developmental research as to ensure appropriate levels of experimental control for children (Rueda et al., 2004; Mezzacappa, 2004; Yang et al., 2011; Kapa & Colombo, 2013; Tran et al., 2015). Therefore, having an equal distribution of all condition types allows for the optimal assessment of attentional control in development (Costa, Hernández, Costa-Faidella, & Sebastián-Gallés, 2009; Davidson, Amso, Anderson, & Diamond, 2006) and allows us to validate our findings with previous imaging work using the flanker paradigm with monolingual children (Neutral: Bunge, Dudukovic, Thomason, Vaidya, & Gabrieli, 2002; Congruent: Konrad et al., 2005).
The task had a randomized rapid event-related design with a total of 75 trials, 25 trials per condition (randomized using OptSeq2; Dale, 1999). The entire task contained 25% Neutral trials, 25% Congruent trials, 25% Incongruent trials, and 25% jittered Rest periods (106 seconds randomly distributed during the run). Rest periods were jittered and presented after each trial as a fixation point in the center of the screen. Each trial was displayed for 2.5-seconds and followed by 1.5-seconds of feedback. If the participant did not respond within the first 2.5-seconds of the trial display, the trial was deemed incorrect. Children received visual and auditory feedback: for correct responses, the target fish blew bubbles and a “Woohoo!” sound played; for incorrect responses, no visual feedback was provided and a “Huh!” buzz sound played. Performance was assessed by accuracy and response time. The task lasted ∼7 minutes and was presented using E-Prime 2 (Psychology Software Tools, Inc.) on a 23-inch Philips 230E Wide LCD screen connected to a Dell Optiplex 780 desktop computer; auditory feedback played via two Creative Inspire T12 2.0 multimedia speakers.
Functional NIRS Recordings, Data Processing and Analysis
The study used a TechEN-CW6 system with 690 and 830 nm wavelengths. The set-up included 4 emitters of near-infrared light (sources) and 12 detectors spaced ∼2.7 cm apart, yielding 14 data channels sampled at 10-Hz (7 channels per hemisphere; Figure 1). Sensors were mounted onto a custom-built head cap constructed from polyester cloth with grommets attached to hold the sources and detectors in place during data collection. We examined brain activation in bilateral prefrontal cortex regions, including: inferior (IFG), middle (MFG) and superior frontal gyri (SFG). The probe localization was established and applied consistently for each participant using the international 10-10 transcranial system positioning (Jurcak, Tsuzuki, & Dan, 2007); Fz, Cz and pre-auricular were measured for each participant and the two lower sources were anchored at F7 and F8.
Figure 1.
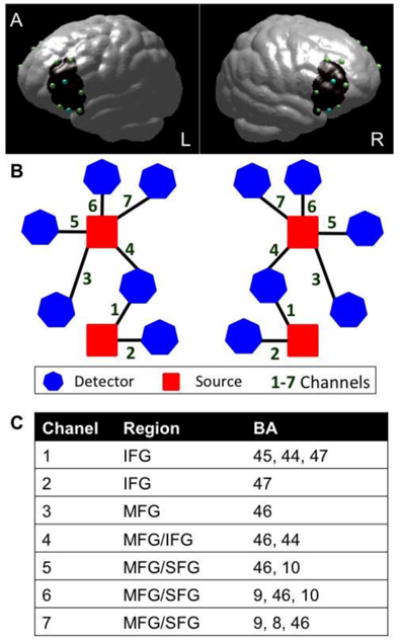
Functional NIRS probe configuration. (A) Dots correspond to optode placements at a distance of ∼2.7 cm, over an average brain template (blue = sources of light; green = detectors; black = approximate area of the brain covered by the fNIRS measurement). (B) Probe-set and channel configuration for right and left hemispheres, respectively. (C) Brain regions maximally overlaid by the probe arrangement in the order of greatest probability for each channel (BA = Brodmann Area).
In order to visualize and estimate the brain regions maximally covered by the channels, we estimated approximate MNI brain coordinates using the geometric structure of our measurement setting for the 16 optodes (emitters and detectors). We used reference points (F7 and F8) to equally distribute 1000 voxel points along the distance of each channel (between each source and detector pair). The voxel points were the distance partitioned to 1000 sections for a distance of 2.7 cm of channels on a 3D image brain template provided by https://irc.cchmc.org/software/pedbrain.php. Then, the corresponding brain regions and Brodmann areas (BA) were estimated using xjView in MATLAB (http://www.alivelearn.net/xjview). The brain areas covered by the 1000 points distributed along each channel are recognized as the brain areas covered by that channel. If a channel covered more than one area, the area indices were arranged in sequence according to the proportion of the 1000 points falling within the given regions (see Figure 1).
Data preprocessing was completed using Homer2, a MATLAB-based software (Huppert, Diamond, Franceschini, & Boas, 2009) and several customized MATLAB scripts (Hu, Hong, Ge, & Jeong, 2010). We performed the following preprocessing steps in the following order: optical density change data conversion, data examination for all channels (including motion artifact detection), filtering, and concentration change data conversion. First, the raw time course data were converted into units of optical density change (ΔOD). Then, the ΔOD data went through three quality control steps for integrity and presence of signal (and motion) artifacts. Participants who did not complete the entire task or were missing data channels (e.g., due to system error) were excluded from analysis (3 bilinguals). The remaining participants with a signal-to-noise ratio of less than 70 dB for more than 70% of the data (combined across channels) in the 690 nm wavelengths were excluded (1 monolingual, 1 bilingual).
The remaining data were analyzed using a one-sided Dixon's Q-test (p< 0.05) for each channel, which is an additional signal-to-noise ratio analysis that identifies participants with extremely high or low activation values, unlikely to stem from physiological changes (Dean & Dixon, 1951; Rorabacher, 1991). The Dixon's Q method is considered well suited for detecting outliers within small samples (Rorabacher, 1991). Specifically, this method estimates the range of signal change for each channel across the entire time-series and whether it varies for any given participant in relation to the mean of that specific channel across all participants. The output variables were rank-ordered across channels and across participants, and the Dixon's Q-test was applied to reject the outliers. Participants with more than 35% of the data (combined across channels) identified as outliers were excluded (2 monolinguals and 1 bilingual did not pass the Dixon's Q threshold criterion). Our final sample included 14 monolinguals and 13 bilinguals (out of the 17 and 18 children respectively matched) retained for data analysis.
The remaining participants' data were processed using both data corrected by the wavelet artifact correction method (recently deemed one of the most effective methods for fNIRS signal processing and now included in the HomER software; Brigadoi et al., 2014) and the uncorrected data. As the use of either wavelet-corrected or unaltered data resulted in the same overall final pattern of group results, we opted to report the results based on the more conservative approach of unaltered data. Finally, a bandpass filter with cutoff frequency at 0.01-0.8 Hz was applied to the ΔOD data and the hemoglobin concentration change data were calculated using the modified Beer-Lambert law, which yielded HbO (oxygenated hemoglobin) and HbR (deoxygenated hemoglobin) values.
Each participant's hemoglobin concentration data was analyzed using a multiple regression approach with a fixed-effects general linear model (GLM), which assumed the dual-gamma canonical hemodynamic response function (Friston, Ashburner, Kiebel, Nichols, & Penny, 2006; Hu et al., 2010.). The fixed-effects GLM included Incongruent, Congruent, Neutral, and rest (jittered fixation period) conditions as factors. The GLM estimated beta values, which constitute raw scores corresponding to unstandardized difference scores between experimental conditions, for all contrasts. Given that behavioral results revealed that each group performed at above 90% accuracy for each of the ANT conditions, no individual trials associated with occasional incorrect responses were removed from the analyses.
For parsimony, the group analyses are only presented and discussed for the HbO signal. Nevertheless, to ensure that the results were accurate with respect to both the resting baseline as well as the HbR values (which should decrease when HbO increases), we conducted a Wilcoxon signed-rank non-parametric t-tests that included HbO and HbR values for the channels in which children showed significantly greater activation during each ANT condition (Neutral, Congruent, Incongruent) relative to resting baseline. These signal quality analyses results revealed that in the channels in which participants showed significantly greater HbO than resting baseline signal (see the list of these Task > baseline channels listed in Supplementary Table 1), the children also showed significantly greater HbO (M = 1.56, SD = 1.52) than HbR signal (M = -1.06, SD = 2.38; Wilcoxon test Z = 3.89, p < .001). Moreover, Supplementary Figure 1 exemplifies data quality with a canonical HbO signal increase and HbR signal decrease for the Incongruent condition (both groups) and Congruent condition (monolinguals only), as is consistent with the group analyses (see Supplementary Table 1).
Group-level analyses were conducted using statistical parametric mapping procedures (Friston, et al., 2006). The key question of the study was whether dual-language experiences can impact children's brain bases for attentional control, as typically measured by the difference between Incongruent relative to Control conditions. Thus, the first step is to estimate Incongruent > Congruent and Incongruent > Neutral comparisons for each group separately through one-sample t-tests (Figure 2a). The second step is to analyze the outcome of these comparisons between the two groups via independent-samples t-tests (Figure 2b). In the event that we find group differences at the second step, we will follow-up with comparisons for Incongruent> Rest and Congruent> Rest contrasts between the two groups (independent-samples t-tests, Figure 2c).
Figure 2.
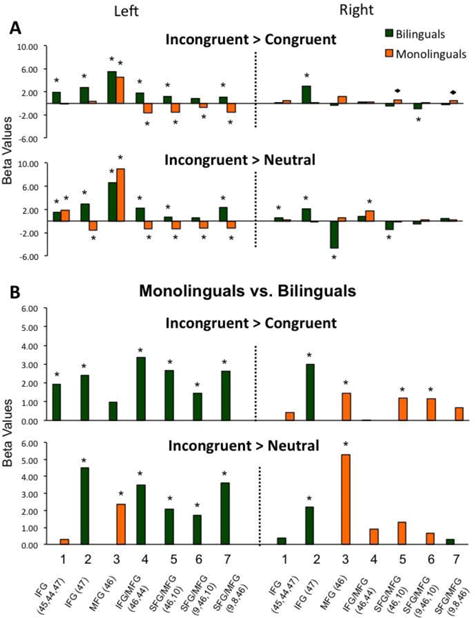
(A) Participants' activation during the Incongruent condition relative to the Congruent and Neutral control conditions. (B) Comparison between bilinguals and monolinguals' brain activation during the Incongruent condition relative to control conditions. (C) Comparison between bilinguals and monolinguals' brain activation during the Incongruent and Congruent conditions condition relative to resting baseline. *p < 0.05, FDR corrected; ◆ p < 0.05, uncorrected.
For each within- and between-group comparison, the statistical analyses were evaluated at a False Discovery Rate (FDR) threshold correction of p< 0.05 (see Benjamini & Hochberg, 1995), which is well-suited for fNIRS analyses (see Lloyd-Fox et al., 2014). To carry out this method, we rank-ordered the channels by their unadjusted p-value. Then, we estimated the FDR-adjusted significance level based on the following equation: (j/m) × δ, where j is the rank-order that the given channel holds, m is the number of channels in the contrast (m = 14), and δ is the unadjusted p-value (δ = 0.05; for more details see Benjamini & Hochberg, 1995; Singh & Dan, 2006). Finally, we used our brain template with interpolated optodes and the “patch” function in MATLAB to generate 3D images to display the results.
To explore the relationship between participants' age, cognition, and language status, we also conducted Pearson correlations between participants' brain activation (as measured with beta values for individual conditions, relative to Rest) in the channels that showed significant modulation for the Incongruent> Control conditions to participants' age, ANT performance, and language competence in English. The correlations were done on the same channels, but separately across the two groups.
Results
T-test comparisons between bilingual and monolingual children's performance on language, literacy, IQ, and HTKS executive function measurements did not reveal any significant differences (Table 1). Bilinguals performed faster in the Naming Speed task (t(25) = 2.13, p = 0.043), but only had marginally better age-adjusted standardized scores (t(25) = 1.78, p = 0.089). Comparisons between bilingual children's English and Spanish language proficiency revealed that bilinguals had comparable vocabulary, phonological, and naming speed abilities in both languages, but better syntax (t(12) = 3.25, p = 0.007) and reading abilities (t(12) = 6.82, p < 0.001) in English.
Analyses of children's ANT accuracy using a mixed 2 (language group: monolingual, bilingual) × 3 (condition: Neutral, Congruent, Incongruent) ANOVA did not reveal significant main effects of language group or condition, there was, however, a marginally-significant interaction (F(2, 50) = 2.98, p = 0.06, ). The independent-samples t-tests suggested that the interaction stemmed from bilinguals' better accuracy than monolinguals' during the Incongruent condition, t(25) = 2.49, p = 0.02.
A similar mixed 2 × 3 ANOVA for response time (RT) revealed a significant main effect of condition (F(2, 50) = 25.16, p < 0.001, ), as participants responded slower during the Incongruent, relative to the Neutral (t(26) = 6.01, p < 0.001) and Congruent trials (t(26) = 6.05, p < 0.001). The ANOVA did not reveal a main effect of language group, or an interaction between language group and condition. There were no significant group differences in RT for either of the individual conditions, or for the difference in RT between Incongruent and Control trials (which is another standard measure of ANT performance; see Table 1).
Functional NIRS Data Results
Incongruent vs. Congruent, Within-Group Comparisons
Our first step was to examine children's brain response during the ANT task separately across groups; see Figures 2a and 3a. The within-group comparison for the Incongruent > Congruent contrast revealed that monolinguals showed a trend towards greater activation in two right channels (CH 5 and 7) and significant activation in one left channel (CH 3) during the Incongruent relative to the Congruent condition. Bilinguals showed greater activation during Incongruent relative to Congruent trials in six out of seven left channels (CH 1-5, 7) and one right channel (CH 3). The reverse contrast (Congruent > Incongruent) revealed that during the Congruent condition, monolinguals showed greater activation in four left channels (CH 3-7) relative to the Incongruent condition. In contrast, bilinguals showed greater activation during the Congruent relative to the Incongruent condition only in one right channel (CH 7). These within-group results were similar for the Incongruent > Neutral contrast comparisons (see Figure 2a and anatomical correspondences in Figure 1).
Figure 3.
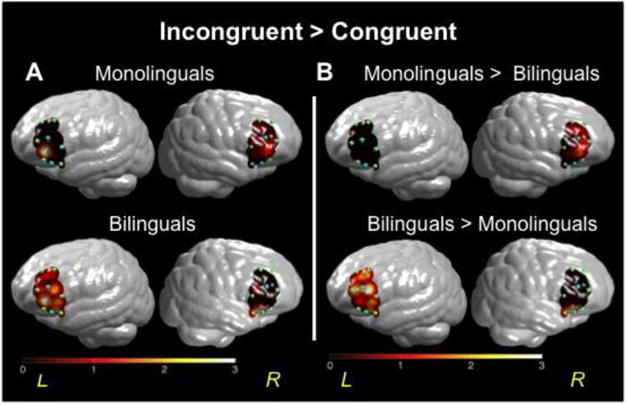
(A) T-values mapped for comparison of brain activation in prefrontal cortex for monolinguals (top row) and bilinguals (bottom row). Higher values on the scale indicate greater brain activity during the Incongruent condition, relative to Congruent trials. (B) T-value map for comparison of brain activation in prefrontal cortex in bilinguals versus monolinguals (the color bar reflects t-values).
Incongruent vs. Congruent, Between-Group Comparisons
The second step was a direct comparison between bilingual and monolingual children's activation for the Incongruent condition relative to Control conditions; see Figures 2b and 3b. The between-group comparison for the Incongruent > Congruent contrast revealed that monolinguals had greater activation in three right hemisphere channels (CH 3, 5-6) relative to bilinguals. In contrast, bilinguals showed greater activation in six left hemisphere channels (CH 1-2, 4-7) and one right hemisphere channel (CH 2) relative to monolinguals. These between-group results were similar for the Incongruent > Neutral contrast comparisons (see Figure 2b).
Incongruent vs. Rest, Between-Group Comparison
Given the significant group differences for the Incongruent > Congruent contrast and to better understand the source of the group variance, we then conducted between-group comparisons for the Incongruent > Rest and Congruent > Rest contrasts. The results for the between-group comparison for the Incongruent > Rest contrast were similar to the Incongruent > Congruent group results reported above: monolinguals had greater activation in right hemisphere channels 3 and 6, while bilinguals had greater activation in left hemisphere channels 2, 4, 5 and 7, as well as right hemisphere channel 2 (see Figure 2c).
Congruent vs. Rest, Between-Group Comparison
A different pattern of results emerged for the between-group comparison during the Congruent > Rest contrast. Monolinguals showed greater activation in left hemisphere channels 1 and 4-7, as well as right hemisphere channel 2. There were no greater activations in bilinguals relative to monolinguals (Figure 2c).
Brain-Behavior Correlations
Pearson correlation analyses revealed only one significant result: bilinguals who had greater competence in English syntax also had reduced activation in left hemisphere channel 7 overlaying left MFG/SFG region (r(12) = -0.62, p = 0.023), as measured with the Incongruent > Rest contrast. There were no other significant correlations with age, ANT, and other English competence measures for either group's brain activity.
Discussion
The goal of the study was to examine the consequences of bilingualism on children's non-verbal attentional control and its functional organization in bilateral prefrontal cortex, specifically when conflict resolution is required. Research suggests that bilinguals commonly access both languages, even when they are speaking or hearing only one of them (Van Hell & Dijkstra, 2002; Kroll et al., 2015). Thus, we hypothesized that the increased demand for selective attention towards competing linguistic information might have an impact on bilingual children's trajectory of brain development, especially with regard to attentional control (Bialystok et al., 2012; Green & Abutalebi, 2013). Indeed, during the Incongruent trials (trials that required greater attention to conflict resolution relative to control trials) bilingual children showed greater left frontal activation, while monolinguals showed greater right hemisphere activation. These novel developmental findings are consistent with those previously reported for adult bilinguals (Garbin et al., 2010), suggesting that early bilingual experiences may influence both the developmental course and the outcomes of the brain's functional specialization for selective attention. The findings therefore offer new insight for better understanding how early life experiences can impact children's functional brain organization in development.
The study's participants were matched on multiple variables that might impact attentional control abilities, including age, parental education, English language proficiency, and IQ (Morton & Harper, 2009; Bialystok et al., 2008). Although both groups performed at ceiling, the bilingual children showed a trend towards better accuracy during the Incongruent condition of the ANT flanker task (a marginally significant task by group interaction) and faster naming speed during the rapid naming task (significant difference for seconds, but only marginally significant for standard scores). Previous research has suggested that an equal distribution between Congruent and Incongruent conditions is best for identifying behavioral differences between bilinguals and monolinguals as the distribution of the conditions may affect target monitoring and thus participants' performance (Costa et al., 2009). The present study included a Neutral control condition with an equal distribution among Neutral, Congruent and Incongruent conditions, as is in line with developmental methodology (e.g., Rueda et al., 2004; Mezzacappa, 2004; Yang et al., 2011; Kapa & Colombo, 2013; Tran et al., 2015), but possibly thus undermining the likelihood of finding stronger group differences. Given the small effect sizes, small sample sizes, and ceiling ANT performance, the present behavioral findings should be treated with caution.
We hypothesized that early bilingual experiences may change the development of bilingual children's prefrontal cortex for non-verbal attentional control, especially within the left hemisphere associated with normative language processing and bilingual language switching (Green & Abutalebi, 2013; Green, 2011). When considering non-verbal attentional control as typically measured by the difference in activation between Incongruent versus Congruent conditions (Konrad et al., 2005), the key findings suggest that bilinguals had greater activation in left prefrontal cortex while monolinguals had greater activation in right homologous regions. This was also the case for the Incongruent versus Neutral and Incongruent versus resting baseline group comparisons.
Moreover, bilingual children's left hemisphere activation (channel 7, MFG/SFG) during Incongruent trials negatively correlated with language competence in English. One possible interpretation is that children with greater English competence might be perceived as English-dominant, hence eliciting more English-only social interactions and reducing bilingual experiences (Bedore et al., 2012). Another possibility is that the correlation reflects emerging left hemisphere selectivity of function in bilinguals. Specifically, the reduced amplitude in channel 7 may reflect the narrowing spatial extent of activation within this region in young bilinguals, with brain activity becoming more focal with better English proficiency (see Durston et al., 2006). Finally, this correlation should be treated with great caution as it was obtained from a small number of children for one experimental task on one language measure.
In summary, the primary findings are that bilinguals showed greater activation during the Incongruent condition as compared to all other control conditions; this difference was especially pronounced in the left hemisphere (Figure 2a). Importantly, the bilinguals showed greater left and lower right hemisphere activation during the Incongruent condition relative to monolinguals (Figure 2b, 2c). In contrast, monolinguals showed greater left hemisphere activation during the Congruent than the Incongruent condition (Figure 2a) and this activation was stronger in monolinguals than for bilinguals (Figure 2b, 2c). We discuss these findings below in terms of neurodevelopmental theoretical perspectives on bilingualism and selective attention.
Theoretical Implications for Frontal Lobe Development in Bilinguals and Monolinguals
The neural “interactive specialization” hypothesis suggests that early in development, a vast number of poorly organized neural networks are simultaneously active and in competition with each other over various cognitive processes. The outcome of such competition is the emergence of neural networks that are most efficient or “specialized” for specific cognitive abilities (Johnson, 2001, 2011). For instance, when learning to read, young readers often show bilateral activation of the occipito-temporal regions when viewing both words and word-like symbols. In contrast, as children become better readers, this activation becomes left lateralized, restricted to focal regions of the fusiform gyrus, and more active for letters relative to symbols (Dehaene & Cohen, 2011). Lifespan developmental perspectives view this neural specificity as integral to both child cognitive development, as well as healthy aging and maintenance of cognitive abilities in old age (Park, Hebrank, Polk, & Park, 2012).
Within this theoretical framework, we suggest that bilingual experiences may alter the developmental course of neural specialization for selective attention. Previous research suggests that adult monolinguals typically show overall greater activation in right frontal regions, than left contralateral regions, during non-verbal visuospatial tasks of attention (Fan, McCandliss, Fossella, Flombaum, & Posner, 2005; Konrad et al., 2005; Wager et al., 2005; Nee, Wager, & Jonides, 2007). Developmental studies suggest two key differences between children and adults: First, children appear to have a left lateralized or a more bilateral response (Bunge et al., 2002; Durston et al., 2002). This is typically explained in terms of the left hemisphere's efficiency at extracting and re-evaluating the rules of a given task, which is especially pertinent early in development (Bunge & Zelazo, 2006; Zelazo, Carlson, & Kesek, 2008; Moriguchi, Sakata, Ishibashi, & Ishikawa, 2015). Second, children tend to have a more similar response across conditions or even a reverse pattern of greater activation to Congruent than Incongruent conditions. This is typically seen as an index of poorly developed neural specificity towards attentional control (Konrad et al., 2005).
An important validation of the present findings is that monolinguals displayed both of these developmental trends, including bilateral activation for the two experimental conditions and greater activation towards the Congruent than Incongruent stimuli in the left hemisphere. It is therefore possible that between the ages of 7-13, monolingual children are still completing the switch from left-to-right in frontal lobe for visuo-spatial attention processing and that they do not yet have adult-like specialization for Incongruent versus Congruent conditions. It might be that for monolinguals, the harder condition is only beginning to gain footing with improved visuo-spatial strategies afforded by right frontal regions, while the left frontal regions continue to support the easier condition, possibly through verbalization and rehearsal strategies.
In contrast, the bilingual children showed adult-like patterns of greater Incongruent than Congruent activations, albeit in the left rather than the right hemisphere. This finding converges with those previously found for bilingual adults who also showed greater left and lesser right prefrontal activation relative to monolingual adults, with both groups showing age-appropriate greater activation for Incongruent than Congruent conditions (Garbin et al., 2010). The Adaptive Control hypothesis suggests that the demands of dual language acquisition should optimize the computational capabilities of bilinguals' left prefrontal cortex towards both verbal and non-verbal tasks of attention. This is possibly aided by both the internal computational capabilities of the prefrontal cortex, as well as its stronger-than-in-monolinguals interconnections with parietal and possibly anterior cingulate regions that also support attentional capabilities (Green & Abutalebi, 2013). It is therefore possible that bilingual exposure accelerates the emergence of adult-like neural specialization for attentional control in bilingual children (yielding greater Incongruent-than-Congruent patterns of activation), but within the left rather than the right hemisphere due to both functional and structural reorganization of bilinguals' left hemisphere's attentional network. The findings are thus consistent with the perspective that during the childhood periods of rapid brain development, an interaction between bilingual experiences and cognitive development may yield changes in children's neurodevelopmental trajectory (Kroll & Bialystok, 2013).
Conclusion
In the contexts of increased migration and growth of multilingual communities, research-based models of bilingual development are vital to addressing the needs of young bilingual learners. Research has shown that even bilingual infants can effectively modulate the use of their two languages (Petitto & Kovelman, 2003), and this in turn might improve young bilinguals' attentional control abilities (Kóvacs & Mehler, 2009). The present findings go further to suggest that early bilingual exposure may change the functionality of children's left prefrontal cortex. The study is limited by a small sample size, measurements restricted to frontal lobe, and the inclusion of only one bilingual group. While further cross-cultural and longitudinal research is warranted, the present findings are nevertheless consistent with previous child behavioral and adult neuroimaging evidence (cf. Kroll & Bialystok, 2013). The present results contribute novel insights to inform models of frontal lobe development for attentional control, while also shedding light on the variability in child brain development as a function of the increasingly common childhood experience of bilingualism.
Supplementary Material
Supplementary Table 1: Oxygenated hemoglobin values (HbO; in beta values) for bilingual and monolingual participants for the three experimental conditions relative to resting baseline (*p < 0.05). Positive values represent increase in HbO signal relative to the resting baseline.
Supplementary Figure: Hemodynamic response (HbO and HbR) group average during 8 seconds for Channel 3 in the left hemisphere, underlying Middle Frontal Gyrus (BA 46). (A) Monolinguals during Neutral, Congruent and Incongruent conditions, and (B) bilinguals during Neutral, Congruent and Incongruent conditions. The time course exemplifies the validity of the statistical analyses: as can be seen in Supplementary Table 1, both groups showed significantly greater Incongruent > Rest HbO signal intensity in Channel 3, while only monolinguals also showed significant increase for Congruent > Rest for HbO signal in this channel.
Research Highlights.
Theories of bilingual development suggest that dual-language exposure can affect children's attention abilities. The present study is the first to offer evidence that bilingualism impacts the functionality of cortical brain regions for attentional control in children.
During a non-verbal attention task, Spanish-English bilingual children showed greater left frontal lobe activation relative to English monolingual children, while monolinguals showed greater right frontal lobe activation than bilinguals.
The findings suggest that bilingualism interacts with early cognitive development to yield changes in the functionality of left prefrontal cortex for children's non-verbal attentional control.
Acknowledgments
The authors thank the University of Michigan Departments of Psychology, Romance Languages and Literatures, and Center for Human Growth and Development. The authors also thank the “En Nuestra Lengua” Literacy and Culture Program, participating families, Lourdes M. Delgado Reyes, Ka I Ip, Erica Seifert, Jaime Muñoz Velazquez, Paola Velosa, Stefanie Younce, and Melanie Armstrong, for their assistance with data collection. Maria Arredondo thanks the National Science Foundation (NSF) Graduate Research Fellowship (Grant No. DGE 1256260) and Ioulia Kovelman thanks the Harrington Fellowship by the University of Texas, Austin. Any opinions, conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of NSF.
References
- Abutalebi J, Green AW. Control mechanisms in bilingual language production: Neural evidence from language switching studies. Language and Cognitive Processes. 2008;23:557–582. [Google Scholar]
- Antón E, Duñabitia JA, Estévez A, Hernández JA, Castillo A, et al. Carreiras M. Is there a bilingual advantage in the ANT task? Evidence from children. Frontiers in Psychology. 2014;5:398. doi: 10.3389/fpsyg.2014.00398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedore LM, Pena ED, Summers CL, Boerger KM, Resendiz MD, Greene K, et al. Gillam RB. The measure matters: Language dominance profiles across measures in Spanish–English bilingual children. Bilingualism: Language and Cognition. 2012;15:616–629. doi: 10.1017/S1366728912000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. 1995;57:289–300. [Google Scholar]
- Bialystok E. Cognitive complexity and attentional control in the bilingual mind. Child Development. 1999;70:636–644. [Google Scholar]
- Bialystok E, Craik FIM, Freedman M. Bilingualism as a protection against the onset of symptoms of dementia. Neuropsychologia. 2007;45:459–464. doi: 10.1016/j.neuropsychologia.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Bialystok E, Craik FIM, Luk G. Bilingualism: consequences for mind and brain. Trends in Cognitive Sciences. 2012;16:240–250. doi: 10.1016/j.tics.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialystok E, Craik FIM, Klein R, Viswanathan M. Bilingualism, aging, and cognitive control: evidence from the simon task. Psychology and Aging. 2004;19:290–303. doi: 10.1037/0882-7974.19.2.290. [DOI] [PubMed] [Google Scholar]
- Brigadoi S, Ceccherini L, Cutini S, Scarpa F, Scatturin P, Selb J, et al. Motion artifacts in functional near-infrared spectroscopy: a comparison of motion correction techniques applied to real cognitive data. NeuroImage. 2014;85:181–191. doi: 10.1016/j.neuroimage.2013.04.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownell R. Receptive One-Word Picture Vocabulary Test (ROWPVT) Spanish-Bilingual Edition. Novato, CA: Academic Therapy Publications; 2000. [Google Scholar]
- Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JDE. Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron. 2002;33:301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA, Zelazo PD. A brain-based account of the development of rule use in childhood. Current Directions in Psychological Science. 2006;15:118–121. [Google Scholar]
- Carlson SM, Meltzoff AN. Bilingual experience and executive functioning in young children. Developmental Science. 2008;11:282–298. doi: 10.1111/j.1467-7687.2008.00675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa A, Hernández M, Sebastián-Gallés N. Bilingualism aids conflict resolution: evidence from the ANT task. Cognition. 2008;106:59–86. doi: 10.1016/j.cognition.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Costa A, Hernández M, Costa-Faidella J, Sebastián-Gallés N. On the bilingual advantage in conflict processing: now you see it, now you don't. Cognition. 2009;113:135–149. doi: 10.1016/j.cognition.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Dale AM. Optimal experimental design for event-related fMRI. Human Brain Mapping. 1999;8:109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson MC, Amso D, Anderson LC, Diamond A. Development of cognitive control and executive functions from 4 to 13 years: evidence from manipulations of memory, inhibition, and task switching. Neuropsychologia. 2006;44:2037–2078. doi: 10.1016/j.neuropsychologia.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bruin A, Treccani B, Della Sala S. Cognitive advantage in bilingualism: an example of publication bias? Psychological Science. 2015;26:99–107. doi: 10.1177/0956797614557866. [DOI] [PubMed] [Google Scholar]
- Dean RB, Dixon WJ. Simplified statistics for small numbers of observations. Analytical Chemistry. 1951;23:636–638. [Google Scholar]
- Dehaene S, Cohen L. The unique role of the visual word form area in reading. Trends in Cognitive Sciences. 2011;15:254–262. doi: 10.1016/j.tics.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Dong Y, Li P. The cognition science of bilingualism. Language and Linguistics Compass. 2015;9:1–13. [Google Scholar]
- Durston S, Davidson MC, Tottenham N, Galvan A, Spicer J, Fossella JA, Casey BJ. A shift from diffuse to focal cortical activity with development. Developmental Science. 2006;9:1–20. doi: 10.1111/j.1467-7687.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- Durston S, Thomas K, Yang Y, Ulug A, Zimmerman R, Casey BJ. A neural basis for development of inhibitory control. Developmental Science. 2002;5:9–16. [Google Scholar]
- Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI. The activation of attentional networks. NeuroImage. 2005;26:471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Sommer T, Raz A, Posner MI. Testing the efficiency and independence of attentional networks. Journal of Cognitive Neuroscience. 2002;14:340–347. doi: 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- Francis D, Carlo M, August D, Kenyon D, Malabonga V, et al. Louguit M. Test of Phonological Processing in Spanish (TOPPS) Washington, DC: Center for Applied Linguistics; 2001. [Google Scholar]
- Friston KJ, Ashburner JT, Kiebel SJ, Nichols TE, Penny WD. Statistical Parametric Mapping: The Analysis of Functional Brain Images. London: Academic Press; 2006. [Google Scholar]
- Garbin G, Sanjuan A, Forn C, Bustamante JC, Rodriguez-Pujadas A, et al. Avila C. Bridging language and attention: Brain basis of the impact of bilingualism on cognitive control. NeuroImage. 2010;53:1272–1278. doi: 10.1016/j.neuroimage.2010.05.078. [DOI] [PubMed] [Google Scholar]
- Green DW. Language control in different contexts: The behavioral ecology of bilingual speakers. Frontiers in Psychology. 2011;2:103. doi: 10.3389/fpsyg.2011.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DW, Abutalebi J. Language control in bilinguals: the adaptive control hypothesis. Journal of Cognitive Psychology. 2013;25:515–530. doi: 10.1080/20445911.2013.796377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez A, Li P, MacWhinney B. The emergence of competing modules in bilingualism. Trends in Cognitive Sciences. 2005;9:220–225. doi: 10.1016/j.tics.2005.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilchey MD, Klein RM. Are there bilingual advantages on nonlinguistic interference tasks? Implications for the plasticity of executive control processes. Psychonomic Bulletin Review. 2011;18:625–658. doi: 10.3758/s13423-011-0116-7. [DOI] [PubMed] [Google Scholar]
- Hu XS, Hong KS, Ge SS, Jeong MY. Kalman estimator- and general linear model-based on-line brain activation mapping by near-infrared spectroscopy. Biomedical Engineering Online. 2010;9 doi: 10.1186/1475-925X-9-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert TJ, Diamond SG, Franceschini MA, Boas DA. HomER: A review of times-series analysis methods for near-infrared spectroscopy of the brain. Applied Optics. 2009;48:D280–298. doi: 10.1364/ao.48.00d280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MH. Functional brain development in humans. Nature Reviews: Neuroscience. 2001;2:475–483. doi: 10.1038/35081509. [DOI] [PubMed] [Google Scholar]
- Johnson MH. Interactive specialization: a domain-general framework for human functional brain development? Developmental Cognitive Neuroscience. 2011;1:7–21. doi: 10.1016/j.dcn.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurcak V, Tsuzuki D, Dan I. 10/20, 10/10, and 10/5 systems revisited: their validity as relative head-surface-based positioning systems. NeuroImage. 2007;34:1600–1611. doi: 10.1016/j.neuroimage.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Kapa LL, Colombo J. Attentional control in early and later bilingual children. Cognitive Development. 2013;28:233–246. doi: 10.1016/j.cogdev.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman A, Kaufman N. Kaufman Brief Intelligence Test Second Edition (KBIT-2) Minneapolis, MN: Pearson; 2004. [Google Scholar]
- Konrad K, Neufang S, Thiel CM, Specht K, Hanisch C, et al. Fink GR. Development of attentional networks: An fMRI study with children and adults. NeuroImage. 2005;28:429–439. doi: 10.1016/j.neuroimage.2005.06.065. [DOI] [PubMed] [Google Scholar]
- Kovács AM, Mehler J. Cognitive gains in 7-month-old bilingual infants. PNAS. 2009;106:6556–6560. doi: 10.1073/pnas.0811323106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovelman I, Baker SA, Petitto LA. Age of bilingual language exposure as a new window into bilingual reading development. Bilingualism: Language & Cognition. 2008;11:203–223. doi: 10.1017/S1366728908003386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizman J, Marian V, Shook A, Skoe E, Kraus N. Subcortical encoding of sound is enhanced in bilinguals and relates to executive function advantages. Proceedings of the National Academy of Sciences. 2012;109:7877–7881. doi: 10.1073/pnas.1201575109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll JF. On the consequences of bilingualism: we need language and the brain to understand cognition. Bilingualism: Language and Cognition. 2015;18:32–34. [Google Scholar]
- Kroll JF, Bialystok E. Understanding the consequences of bilingualism for language processing and cognition. Journal of Cognitive Psychology. 2013;25:497–514. doi: 10.1080/20445911.2013.799170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll JF, Dussias PE, Bice K, Perrotti L. Bilingualism, mind, and brain. Annual Review of Linguistics. 2015;1:377–394. doi: 10.1146/annurev-linguist-030514-124937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Fox S, Papademetriou M, Darboe MK, Everdell NL, Wegmuller R, Prentice AM, et al. Elwell CE. Functional near-infrared spectroscopy (fNIRS) to assess cognitive function in infants in rural Africa. Nature Scientific Reports. 2014;4:4740. doi: 10.1038/srep04740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk G, Green DW, Abutalebi J, Grady C. Cognitive control for language switching in bilinguals: a quantitative meta-analysis of functional neuroimaging studies. Language and Cognitive Processes. 2012;27:1479–1488. doi: 10.1080/01690965.2011.613209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marian V, Spivey M. Bilingual and monolingual processing of competing lexical items. Applied Linguistics. 2003;24:173–193. [Google Scholar]
- Mazuka R, Jincho N, Oishi H. Development of executive control and language processing. Language and Linguistics Compass. 2009;3:59–89. [Google Scholar]
- Mezzacappa E. Alerting, orienting, and executive attention: developmental properties and sociodemographic correlates in an epidemiological sample of young, urban children. Child Development. 2004;75:1373–1386. doi: 10.1111/j.1467-8624.2004.00746.x. [DOI] [PubMed] [Google Scholar]
- Moriguchi Y, Sakata Y, Ishibashi M, Ishikawa Y. Teaching others rule-use improves executive function and prefrontal activations in young children. Frontiers in Psychology. 2015;6:894. doi: 10.3389/fpsyg.2015.00894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton JB, Harper SN. Bilinguals show an advantage in cognitive control—the question is why. Developmental Science. 2009;12:502–503. [Google Scholar]
- Muñoz-Sandoval AF, Woodcock RW, McGrew KS, Mather N. Batería III Woodcock-Muñoz: Pruebas de Aprovechamiento. Rolling Meadows, IL: Riverside Publishing; 2005. [Google Scholar]
- Nee DE, Wager TD, Jonides J. Interference resolution: insights from a meta-analysis of neuroimaging tasks. Cognitive, Affective, & Behavioral Neuroscience. 2007;7:1–17. doi: 10.3758/cabn.7.1.1. [DOI] [PubMed] [Google Scholar]
- Norton ES, Wolf M. Rapid automatized naming (RAN) and reading fluency: implications for understanding and treatment of reading disabilities. Annual Review of Psychology. 2012;63:427–452. doi: 10.1146/annurev-psych-120710-100431. [DOI] [PubMed] [Google Scholar]
- Novick JM, Trueswell JC, Thompson-Schill SL. Cognitive control and parsing: reexamining the role of Broca's area in sentence comprehension. Cognitive, Affective, & Behavioral Neuroscience. 2005;5:263–281. doi: 10.3758/cabn.5.3.263. [DOI] [PubMed] [Google Scholar]
- Novick JM, Trueswell JC, Thompson-Schill SL. Broca's area and language processing: Evidence for the cognitive control connection. Language and Linguistics Compass. 2010;4/10:906–924. [Google Scholar]
- Paap KR, Greenberg ZI. There is no coherent evidence for a bilingual advantage in executive processing. Cognitive Psychology. 2013;66:232–258. doi: 10.1016/j.cogpsych.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Park J, Hebrank A, Polk TA, Park DC. Neural dissociation of number from letter recognition and its relationship to parietal numerical processing. Journal of Cognitive Neuroscience. 2012;24:39–50. doi: 10.1162/jocn_a_00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitto LA, Kovelman I. The bilingual paradox: how signing-speaking bilingual children help us resolve it and teach us about the brain's mechanisms underlying all language acquisition. Learning Languages. 2003;8:5–18. [Google Scholar]
- Posner MI. Attention in a Social World. UK: Oxford University Press; 2012. [Google Scholar]
- Ponitz CC, McClelland MM, Matthews JS, Morrison FJ. Touch your knees! Using a direct observation of behavioral regulation to predict math, literacy, and vocabulary achievement in kindergarten. Developmental Psychology. 2009;45:605–619. doi: 10.1037/a0015365. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Fornells A, Rotte M, Heinze HJ, Nosselt TM, Munte TF. Brain potential and functional MRI evidence for how to handle two languages with one brain. Nature. 2002;415:1026–1029. doi: 10.1038/4151026a. [DOI] [PubMed] [Google Scholar]
- Rorabacher DB. Statistical treatment for rejection of deviant values: critical values of Dixon Q parameter and related subrange ratios at the 95 percent confidence level. Analytical Chemistry. 1991;63:139–146. [Google Scholar]
- Rueda MR, Fan J, McCandliss BD, Halparin JD, Gruber DB, et al. Posner MI. Development of attentional networks in childhood. Neuropsychologia. 2004;42:1029–1040. doi: 10.1016/j.neuropsychologia.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Semel E, Wiig EH, Secord WA. Clinical Evaluation of Language Fundamentals Fourth Edition (CELF-4) Bloomington, MN: Pearson; 2003. [Google Scholar]
- Semel E, Wiig EH, Secord WA. Clinical Evaluation of Language Fundamentals Fourth Edition (CELF-4) Bloomington, MN: Pearson; 2006. [Google Scholar]
- Singh L, Fu CSL, Rahman AA, Hameed WB, Sanmugam S, Agarwal P, et al. Back to basics: a bilingual advantage in infant visual habituation. Child Development. 2015;86:294–302. doi: 10.1111/cdev.12271. [DOI] [PubMed] [Google Scholar]
- Singh AK, Dan I. Exploring the false discovery rate in multichannel NIRS. NeuroImage. 2006;33:542–549. doi: 10.1016/j.neuroimage.2006.06.047. [DOI] [PubMed] [Google Scholar]
- Tran CD, Arredondo MM, Yoshida H. Differential effects of bilingualism and culture on early attention: a longitudinal study in the U.S., Argentina, and Vietnam. Frontiers in Psychology. 2015;6:795. doi: 10.3389/fpsyg.2015.00795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valian V. Bilingualism and cognition. Bilingualism: Language and Cognition. 2015;18:3–24. [Google Scholar]
- Van Hell J, Dijkstra T. Foreign language knowledge can influence native language performance in exclusively native contexts. Psychonomic Bulletin & Review. 2002;9:780–789. doi: 10.3758/bf03196335. [DOI] [PubMed] [Google Scholar]
- Wager TD, Sylvester CYC, Lacey SC, Nee DE, Franklin M, Jonides J. Common and unique components of response inhibition revealed by fMRI. NeuroImage. 2005;27:323–340. doi: 10.1016/j.neuroimage.2005.01.054. [DOI] [PubMed] [Google Scholar]
- Wagner RK, Torgesen JK, Rashotte CA. Comprehensive Test of Phonological Processing (CTOPP) Austin, TX: Pro-Ed; 1999. [Google Scholar]
- Wolf M, Denckla M. The Rapid Automatized Naming and Rapid Alternating Stimulus Tests (RAN/RAS) Austin, TX: Pro-Ed; 2005. [Google Scholar]
- Woodcock RW. Woodcock Reading Mastery Tests—Revised Normative Update. Minneapolis, MN: Pearson Assessments; 1998. [Google Scholar]
- Yang S, Yang H, Lust B. Early childhood bilingualism leads to advances in executive attention: dissociating culture and language. Bilingualism: Language and Cognition. 2011;14:412–422. [Google Scholar]
- Zelazo PD, Carlson SM, Kesek A. The development of executive function in childhood. In: Nelson CA, Carlson M, editors. Handbook of Developmental Cognitive Neuroscience. 2nd. Cambridge, MA: MIT Press; 2008. pp. 553–574. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: Oxygenated hemoglobin values (HbO; in beta values) for bilingual and monolingual participants for the three experimental conditions relative to resting baseline (*p < 0.05). Positive values represent increase in HbO signal relative to the resting baseline.
Supplementary Figure: Hemodynamic response (HbO and HbR) group average during 8 seconds for Channel 3 in the left hemisphere, underlying Middle Frontal Gyrus (BA 46). (A) Monolinguals during Neutral, Congruent and Incongruent conditions, and (B) bilinguals during Neutral, Congruent and Incongruent conditions. The time course exemplifies the validity of the statistical analyses: as can be seen in Supplementary Table 1, both groups showed significantly greater Incongruent > Rest HbO signal intensity in Channel 3, while only monolinguals also showed significant increase for Congruent > Rest for HbO signal in this channel.


