Abstract
Rationale
Leucocyte telomere length (LTL) is a biological marker of aging, and shorter LTL is associated with adverse cardiovascular outcomes. Reduced regenerative capacity has been proposed as a mechanism. Bone marrow-derived circulating progenitor cells (PCs) are involved in tissue repair and regeneration.
Objective
To examine the relationship between LTL and PCs, and their impact on adverse cardiovascular outcomes.
Methods and Results
We measured LTL by quantitative PCR in 566 outpatients (age 63±9 years, 76% male) with coronary artery disease (CAD). Circulating PCs were enumerated by flow cytometry. After adjustment for age, gender, race, BMI, smoking and previous myocardial infarction, a shorter LTL was associated with a lower CD34+ cell count: for each 10% shorter LTL, CD34+ levels were 5.2% lower (p<0.001). After adjustment for the aforementioned factors, both short LTL (<Q1) and low CD34+ levels (<Q1) predicted adverse cardiovascular outcomes (death, myocardial infarction, coronary revascularization or cerebrovascular events) independently of each other, with a hazards ratio (HR) of 1.8, 95% confidence interval (CI), 1.1–2.0, and a HR of 2.1, 95% CI, 1.3–3.0, respectively, comparing Q1 to Q2–4. Patients who had both short LTL (<Q1) and low CD34+ cell count (<Q1), had the greatest risk of adverse outcomes (HR=3.5, 95% CI, 1.7–7.1).
Conclusion
Although shorter LTL is associated with decreased regenerative capacity, both LTL and circulating PC levels are independent and additive predictors of adverse cardiovascular outcomes in CAD patients. Our results suggest that both biological aging and reduced regenerative capacity contribute to cardiovascular events, independent of conventional risk factors.
Keywords: Telomere length, progenitor cells, CD34, CD133, CXCR4, regenerative capacity, cardiac outcomes, aging, cardiac outcomes, coronary artery disease
INTRODUCTION
Biological aging has been linked to adverse cardiovascular outcomes, yet the underlying mechanisms are unknown.1–3 Telomeres are regions of repetitive nucleotide sequences at each end of a chromosome that protect the chromosome from deterioration or from fusion with neighboring chromosomes during cell replication.4, 5 After each cell division, the length of the telomere shortens, and when a critical shortening is reached, the cell enters senescence or apoptosis.6, 7 Thus, telomere length is considered a marker of cell senescence and replicative capacity.6, 8 Leukocyte telomere length (LTL) represents the average telomere length across a heterogeneous population of leukocytes including monocytes, granulocytes and lymphocytes, and can serve as a biological marker of aging.
Decreased regenerative capacity, estimated by circulating levels of progenitor cells (PCs), has been also linked to adverse cardiovascular outcomes.9–13 Circulating PCs are mononuclear cells that originate primarily from the bone marrow and contribute to vascular repair and regeneration.14–18 CD34+ mononuclear cells from the human bone marrow include distinct lineages of both hematopoietic (CD34+ /CD45med) and non-hematopoietic (mesenchymal) progenitors.19 CD34+ cells have greater myocardial reparative potential than unselected populations.20 CD133 is a 5-transmembrane antigen marker of primitive stem cells that is lost during maturation, and cells expressing both markers (CD34+ /CD133+) are thought to be enriched with a vascular PC phenotype.21, 22 Co-expression of chemokine (C-X-C motif) receptor 4 (CXCR4+), which promotes homing of PCs to stromal derived factor-enriched hypoxic environments, may also further characterize PCs with capacity for vascular repair.23 Although chronological aging is a primary determinant of reduced regenerative capacity, other factors including inflammation, oxidative stress, and genetic predisposition may also influence repair from cellular injury.24, 25
Previous studies that have linked LTL to adverse outcomes have postulated that decreased regenerative capacity is a major driver of this effect. However, prior investigations have not specifically addressed this question. Thus, the aim of our study was to examine the association between LTL and PC counts and their mutual and joint relationship with recurrent cardiovascular events and mortality in CAD patients. Our hypothesis was that shorter LTL are associated with lower PC counts and that PCs are a key determinant of the relationship between LTL and adverse cardiovascular outcomes.
METHODS
Study population
Patients were enrolled into the Mental Stress Ischemia Prognosis Study (MIPS), a prospective study of patients with stable CAD recruited between June 2011 and August 2014 at Emory University affiliated hospitals. Presence of CAD was defined by an abnormal coronary angiogram demonstrating evidence of atherosclerosis with at least luminal irregularities, documented previous percutaneous or surgical coronary revascularization, documented myocardial infarction (MI), or a positive nuclear stress test. Patients with acute coronary syndromes or decompensated heart failure, and unstable psychiatric conditions other than depression were excluded. Clinical information including previous CAD events, CAD risk factors, results of coronary angiography and current medications were documented using standardized questionnaires and chart reviews. The Emory University Institutional Review Board approved the research protocol, and all participants provided written informed consent. Blood samples were collected after a 12-hour fast.26 Angiographic CAD severity was calculated using the Gensini score for 490 patients with a median time between the angiogram and enrolment of 2.1 (1.0 – 4.7) years.27 Adjudicated events (death, MI, coronary revascularization and cerebrovascular accident (CVA)) were ascertained for all subjects after enrollment. Mortality data were collected through follow up clinic visits at 1 and 2 years, phone calls at 3 years, medical records review, and querying the Social Security Death Index. The main outcome of the study was a combined endpoint including death, MI, coronary revascularization and CVA. We also considered a separate endpoint that excluded revascularization to rule out potential bias due to patient referral for coronary procedures.
Leukocyte telomere length measurement
Genomic DNA samples were extracted from peripheral blood leukocytes, standardized and used for LTL assay following the original method by Cawthon.8, 28 LTL, measured as the ratio of telomeric product/single copy gene (T/S), was quantified by a high-throughput LTL assay involving quantitative PCR using a serially diluted standard DNA and the standard curve method,8 as described previously (online Supplement). The T/S ratio reflects the average length of the telomeres across all leukocytes.
Flow cytometry
Flow cytometry was used for circulating PCs counting as described before.9 Venous blood was collected via a peripheral vein in EDTA tubes after an overnight fast. Blood samples were processed within 4 hours and incubated with fluorochrome labeled monoclonal antihuman mouse antibodies to identify surface markers expressed on mononuclear cells before quantification using flow cytometry using a “lyse no-wash” methodology.9 Mononuclear cells PC that were CD45med and expressed CD34 were enumerated, with subsets of the CD45med, CD34+ cells, that co-expressed CD133 (stem cell marker) and CXCR4 (homing marker that may direct PC to ischemic tissues) measured. Further details are given in the online Supplement.
Statistical analyses
Logarithmic base 2 transformations were used for PC counts and LTL. Simple and multiple linear regression models were used to examine the association between PCs and LTL, and to identify factors that were associated with PCs or LTL among several candidate variables, such as age, gender, race, smoking, body mass index (BMI) and history of MI. The Spearman rank correlation and scatter plots were used to test the association between LTL, and PC and Leukocyte levels, respectively. To study the association between LTL, PCs and cardiovascular events, LTL and PCs were examined both as continuous variables and as quartiles using Kaplan-Meier curves and the log-rank test, as well as Cox proportional hazards regression models. LTL and PC were examined both as independent predictors as well as an interaction term in Cox Proportional hazards regression model. The incidence rate of cardiovascular events was calculated and the association with LTL and PC count was examined using chi-squared tests. Statistical analysis was performed using SPSS statistical software (version 23.0; SPSS Inc.).
RESULTS
Of 695 CAD patients enrolled in MIPS, we had missing PCs for the first 100 patients enrolled, because it was not part of the initial study protocol. This was an ancillary study which begun after MIPS enrollment had already started. Additional patients had missing PCs or LTL because of technical difficulties in sample drawing or processing, or the patient refused. A total of 566 patients had complete data for both LTL and PCs, and were included in this analysis. Patients with missing PC or LTL were less likely to be males or white; there were no other substantial differences in other major demographic or clinical characteristics. The average age was 63 ± 9 years and 76% were male. LTL was normally distributed with a mean ± SD of 0.82 ± 0.14 T/S units (Table 1).
Table 1.
Distribution of LTL, PCs and other characteristics of patients included in the study sample.
| Total | |
|---|---|
| Number of patients | 566 |
| Age, year, mean ± SD | 63 ± 9 |
| Male, % | 76 |
| White, % | 69 |
| BMI, kg/m2, mean ± SD | 30 ± 5 |
| Hypertension, % | 76 |
| Diabetes, % | 32 |
| Dyslipidemia, % | 82 |
| Current/former smoking, % | 60 |
| Previous MI, % | 36 |
| Medication use | |
| ACEI, % | 46 |
| ARBs, % | 17 |
| Aspirin, % | 86 |
| Statins, % | 85 |
| Beta blocker, % | 76 |
| CAD severity, median (IQR) | 24 (9 – 56) |
| LTL, mean ± SD | 0.82 ± 0.14 |
| CIRCULATING PCS (CELLS/µL), MEDIAN (IQR) | |
| CD34+ | 1.6 (1 – 2.4) |
| CD34+/CXCR4+ | 0.7 (0.5 – 1.1) |
| CD34+/CD133+ | 0.7 (0.4 – 1) |
| CD34+/CD133+/CXCR4 | 0.3 (0.2 – 0.5) |
| LEUKOCYTE COUNTS (×103 CELLS/µL), MEDIAN (IQR) | |
| White blood cells | 6 (4.8 – 7.3) |
| Monocytes | 1.8 (1.5 – 2.2) |
| Neutrophils | 0.4 (0.3 – 0.6) |
| Lymphocytes | 3.7 (2.8 – 4.7) |
BMI: Body mass index. ARBs: Angiotensin receptor blockers. ACEI: angiotensin converting enzyme inhibitors; MI: myocardial infarction
Correlates of LTL
In bivariate analysis, increasing age, male gender, white race, history of smoking and BMI were inversely correlated with LTL (Online Table I). All these factors, except BMI, remained inversely correlated with LTL in a multivariable linear regression model that included CAD risk factors, history of MI, medications (beta blockers, aspirin, and statins), and CAD severity (Gensini angiographic score). There was no statistically significant association between CAD severity and LTL; however, history of MI was associated with shorter LTL in multivariable analysis.
Correlates of circulating PCs
In bivariate analysis, younger age, male gender, white race and higher BMI were all associated with higher levels of PCs. These factors remained significant in multivariable regression analysis that included demographic variables, CAD risk factors, history of MI, medications and CAD severity (Online Table II).
Association between LTL and circulating PCs
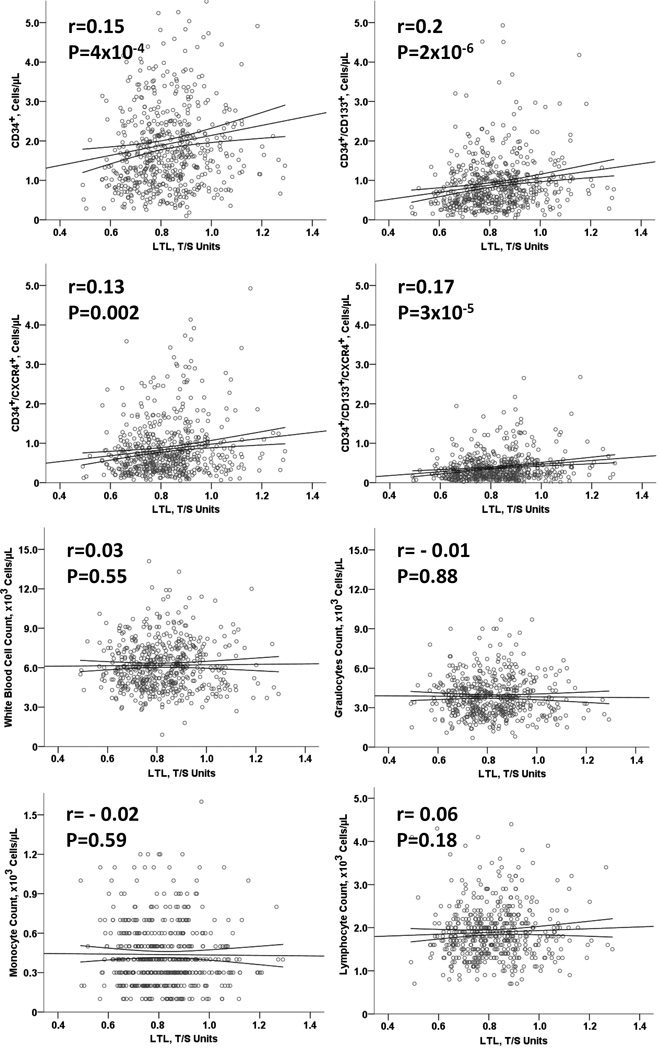
Shorter LTL was independently associated with lower PC counts in all subsets, but not other leukocyte counts, including granulocytes, monocytes or lymphocytes (Figure 1, Table 2). For each 10% shorter LTL, PC counts were lower by 5.2%, 8.6%, 5.3% and 9.3 % for CD34+, CD34+/CD133+, CD34+/CXCR4+ and CD34+/CD133+/CXCR4+, respectively. This association remained statistically significant after adjustment for factors associated with LTL and PC counts (age, gender, race, BMI, smoking and previous MI), total white blood cell counts monocyte, neutrophil and lymphocyte counts (Table 2).
Figure 1.
Scatter plots showing the association of LTL with PC and Leukocyte levels, respectively, as continuous variables. The graphs show raw data to ease interpretation, with Spearman correlation coefficients and p values.
Table 2.
Association between LTL and PCs using linear regression models.
| CD34+ | CD34+/CD133+ | CD34+/CXCR4+ | CD34+/CD133+/CXCR4+ | |||||
|---|---|---|---|---|---|---|---|---|
| Δ* | P value† | Δ* | P value† | Δ* | P value† | Δ* | P value† | |
| Unadjusted | −5.2% | 3.9 × 10−4 | −8.6% | 7.2 × 10−7 | −5.3% | 0.002 | −9.3% | 1.1 × 10−5 |
|
Adjusted model 1‡ |
−5.5% | 4.4 × 10−4 | −8.3% | 4.7 × 10−6 | −5.8% | 0.002 | −9.0% | 6.8 × 10−5 |
|
Adjusted model 2§ |
−5.5% | 0.001 | −7.7% | 2.5 × 10−5 | −6.0% | 0.002 | −8.6% | 1.8 × 10−4 |
Δ represents the % change in PCs per 10% decrease in LTL. Linear regression analyses were performed with log base of 2 transformations of LTL and PCs, with PCs as outcomes. ∆ was calculated as (2β − 1) × 0.10.
P values were derived from linear regression models.
Model 1: Adjusted for age, gender, race (white vs non-white), BMI, smoking and previous MI.
Model 2: Adjusted for model 1 + absolute monocyte count. P values remained significant when monocyte count was replaced with total leukocyte, lymphocyte, or granulocyte count.
Abbreviations: LTL: Leukocyte telomere length. PCs: Progenitor Cells.
LTL, circulating PCs and outcomes
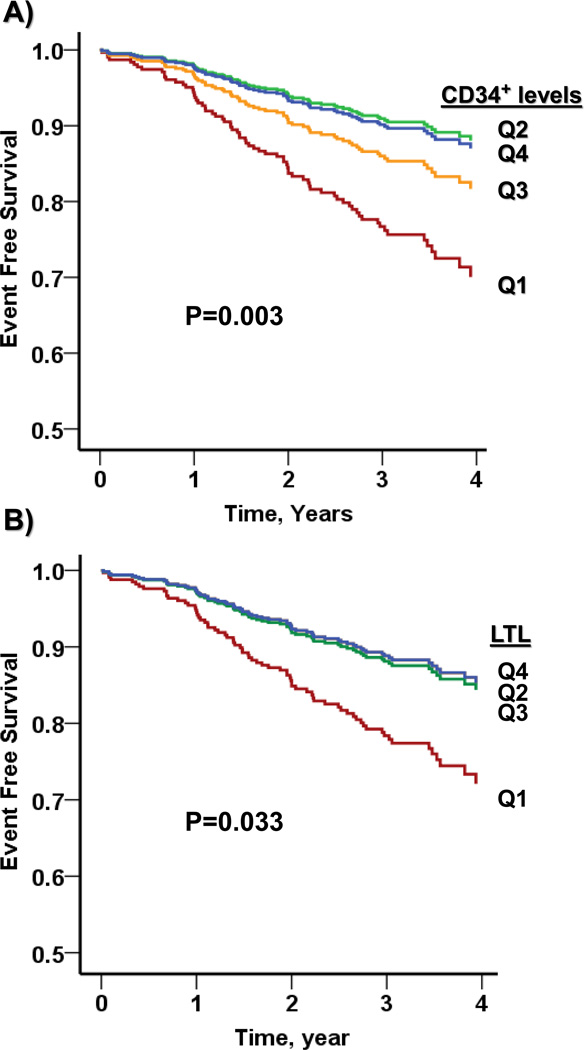
Patients were followed for a median (IQR) period of 3.0 (2.4 – 3.7) years. A total of 71 patients had adverse events, including 13 deaths, 15 MIs, 48 coronary revascularizations, and 8 cerebrovascular events. In Kaplan Meier analysis, both lower levels of LTL and lower PC counts (CD34+, CD34+/CD133+ and CD34+/CXCR4+ cells) were associated with higher rate of adverse cardiovascular events (Figure 2, Online Figure I).
Figure 2.
Association of LTL quartiles and CD34+ cell count quartiles with adverse cardiovascular outcomes. P values were derived from log-rank tests comparing Kaplan Meier survival curves.
To investigate the interaction between LTL and CD34+ cell counts, both were dichotomized by the lowest quartile. In bivariate analysis, both LTL<Q1 and CD34+<Q1 were associated with increased risk of adverse outcomes (Tables 3 and 4). Including both LTL and CD34+ PCs in one model only slightly weakened their associations and both remained independent predictors of outcome. These associations remained significant after adjustment for age, gender, race, BMI, smoking and previous MI (Table 4). Furthermore, there was an additive effect such that patients with short LTL and low CD34+ cell counts had the highest risk of adverse outcomes, approximately the sum of the risk for those two factors alone (Figure 3, Table 3 and 4). In adjusted Cox-proportional hazards modeling, the HR for those with both factors, compared to those with neither, was 4.3 (95% CI, 1.7, 10.8), for death, MI, or CVA, and 3.5 (95% CI, 1,7, 7.1) for death, MI, CVA, or revascularizations. However, the interaction between LTL and CD34+ was not statistically significant (Table 4).
Table 3.
Event rate of adverse cardiovascular outcomes according to LTL and CD34+ levels.
| Death, MI or CVA (number of events = 36) |
Death, MI, CVA or revascularization (number of events = 84) |
|||
|---|---|---|---|---|
| Event rate | P* value | Event rate | P* value | |
| Bivariate analysis | ||||
| LTL < Q1 vs ≥Q1 | 11.1 % vs 4.5 % | 0.006 | 21.5 % vs 10.6 % | 0.001 |
| CD34+ <Q1 vs ≥Q1 | 11.0 % vs 4.6 % | <0.007 | 22.1 % vs 10.4 % | 0.001 |
| Additive effect | ||||
| LTL ≥ Q1 & CD34+ ≥ Q1 | 3.9 % | 0.001 | 8.5 % | <0.001 |
| LTL ≥ Q1 & CD34+ < Q1 | 6.7 % | 18 % | ||
| LTL < Q1 & CD34+ ≥ Q1 | 6.8 % | 17 % | ||
| LTL < Q1 & CD34+ < Q1 | 19.1 % | 29.8 % | ||
p values were calculated using the Chi Square test.
LTL: leucocyte telomere length. MI: Myocardial infarction, CVA: Cerebrovascular accident. Q1: first quartile
Table 4.
Multivariable Cox regression analysis of the association of LTL and CD34+ with adverse cardiovascular outcomes.
| Death, MI or CVA (number of events = 36) |
Death, MI, CVA or revascularization (number of events = 84) |
|||
|---|---|---|---|---|
| Multivariable Cox regression analyses |
HR (95%CI) | P value | HR (95%CI) | P value |
| Continuous variables* | ||||
| LTL (per 10% increase) | 0.82 (0.71–0.95) | 0.008 | 0.89 (0.80 – 0.98) | 0.018 |
| CD34+ (per 10% increase) | 0.96 (0.93 – 0.99) | 0.027 | 0.97 (0.95–0.99) | 0.011 |
| Dichotomous variables | ||||
| Bivariate analysis* | ||||
| LTL < Q1 vs ≥Q1 | 2.4 (1.2 – 4.7) | 0.013 | 2.0 (1.3 – 3.2) | 0.004 |
| CD34+ <Q1 vs ≥Q1 | 2.5 (1.3 – 5.0) | 0.008 | 2.3 (1.4 – 3.6) | 0.001 |
| Multivariable Model 1† | ||||
| LTL < Q1 vs ≥Q1 | 2.1 (1.1 – 4.3) | 0.032 | 1.8 (1.1 – 2.9) | 0.017 |
| CD34+ <Q1 vs ≥Q1 | 2.3 (1.1 – 4.6) | 0.019 | 2.1 (1.3 – 3.4) | 0.003 |
| Multivariable Model 2‡ | ||||
| LTL < Q1 vs ≥Q1 | 2.0 (0.9 – 4.4) | 0.067 | 1.8 (1.04 – 2.9) | 0.037 |
| CD34+ <Q1 vs ≥Q1 | 2.2 (1.1 – 4.5) | 0.030 | 2.1 (1.3 – 3.5) | 0.003 |
| Multivariable Model 3 § | ||||
| LTL < Q1 vs ≥Q1 | 1.7 (0.6 – 4.6) | 0.306 | 2.5 (1.3 – 4.7) | 0.005 |
| CD34+ <Q1 vs ≥Q1 | 1.6 (0.6 – 4.4) | 0.376 | 2.1 (1.1 – 4) | 0.033 |
| Interaction between LTL and CD34+ |
1.6 (0.4 – 6.9) | 0.519 | 0.7 (0.3 – 1.8) | 0.462 |
LTL and CD34+ in separate models.
Model 1: CD34+ cell counts and LTL in same model
Model 2: Model 1 + age, gender, race (white vs non-white), BMI, smoking and previous MI.
Model 2 + interaction term.
LTL: leucocyte telomere length. MI: Myocardial infarction, CVA: Cerebrovascular accident. Q1: First quartile.
Figure 3.
Additive effect between LTL and CD34+ cell count in predicting cardiovascular outcomes (Death, MI, CVA and revascularization). LTL and CD34+ were dichotomized using the first quartile of the distribution. P values were derived from log-rank tests comparing Kaplan Meier survival curves, and from chi square tests comparing incidence rates.
Similar relationships of LTL and other PC counts (CD34+/CD133+, CD34+/CXCR4+ and CD34+/CD133+/CXCR4+) with cardiovascular events and mortality were observed (Online Figure II).
DISCUSSION
We found that shorter LTL is associated with lower circulating levels of PCs, independent of age and CAD risk factors. All CD34 expressing PCs that are known to be enriched for hematopoietic and endothelial progenitors were lower in patients with shorter LTL, but similar associations were not present with respect to other leukocyte cell counts. Although there was no statistically significant association between severity of coronary atherosclerosis and either LTL or PC counts, the latter were both significantly associated with future cardiovascular events. Both LTL and PC counts were independent predictors of cardiovascular outcomes. No statistically significant interaction was found between LTL and PC as binary predictors in the Cox regression model. Our results suggest that accelerated biological aging, reflected by a shorter LTL, is associated with decreased numbers of circulating PC, a measure of reduced regenerative capacity. However, both accelerated aging and reduced regenerative capacity are independent and additive predictors of future cardiovascular endpoints.
The length of telomeres, although in part genetically determined,29 is thought to be a reflection of the accumulated cellular damage over time, resulting from various mechanical, hemodynamic, metabolic, oxidative and immunological insults.1, 4, 25, 29, 30 These factors may also affect the replicative capacity of stem cells leading to increase cellular activation and possibly exhaustion.31 Consistent with this view, several studies have used LTL as a marker of biological aging and have linked it to age-related diseases including atherosclerosis and its consequences,32 such as peripheral vascular disease,7, 33 CAD,34 and importantly, cardiovascular mortality.2, 32 Since aging is associated with decline in circulating PCs, and atherosclerosis is thought to develop as a result of an imbalance between endogenous repair mechanisms and factors causing cell injury,35, 36 most of the studies have speculated that these observed clinical associations were related to decreased regenerative capacity. Increased inflammation and oxidative stress, two known key factors in the pathophysiology of atherosclerosis,37 may result in increased utilization of circulating PCs, which home to the ischemic tissue and denuded endothelium to contribute to vascular and endothelial repair. In turn, this may trigger bone marrow stimulation and hematopoietic PC division and subsequent shorter LTL. Consistent with this hypothesis, Di Cas et al. reported an association between shorter LTL and lower circulating CD34+ PC counts in a small group of healthy young subjects.38 In our larger study, we have confirmed and expanded this observation in patients with CAD. Although these findings suggest that LTL might be a determinant of the circulating stem cells pool, it predicts adverse outcomes independent of circulating PCs.25 Thus, our data indicate that the relationship between LTL and cardiovascular events is only minimally driven by vascular repair processes. Other mechanisms related to biological aging must be at play.
In this study, LTL was significantly correlated with PCs independent of chorological age, which suggests that it may serve as a biological marker of replicative capacity. Others have shown that LTL largely mirrors the telomere length in human bone marrow hematopoietic stem cells that are also the source for circulating PCs.25, 39 Although LTL represents the average telomere length in a heterogeneous population of leukocytes, there is a robust similarity in the telomere length among different cells. Thus, individuals with relatively short (or long) telomeres in one leukocyte subset have short (or long) telomeres in other leukocyte subsets.40 Previous reports showed a high correlation between LTL and telomere length of CD34+ hematopoietic PCs, in both bone marrow39 and umbilical cord.40, 41 Thus, shorter LTL is a reflection of similar reductions in telomere length of bone marrow progenitors, implying decreased replicative capacity of hematopoietic stem cells. We did not observe any statistically significant association between LTL and other leukocyte counts, suggesting that shorter LTL is a reflection of bone marrow regenerative capacity rather than global circulating cellular reserve, although this issue remains controversial.42–48
Interestingly, we did not find a significant correlation between CAD burden and LTL. This is consistent with findings from the PESA (Progression of Early Subclinical Atherosclerosis) and the Asklepios studies where LTL was not associated with subclinical atherosclerosis.49, 50 In the Brubeck cohort, LTL was also not associated with subclinical atherosclerosis (non-stenotic carotid atherosclerosis), yet it was associated with development of advanced atherosclerosis (stenotic carotid plaques) and adverse cardiovascular outcomes (vascular death, MI, revascularization and stroke).51 Our study suggests that, in terms of cardiovascular risk, LTL is a marker of more downstream processes involved in biological aging, rather than the atherosclerotic process in general. Telomere shortening can cause cell senescence, and once a certain threshold is reached, particularly in the setting of inflammation and oxidative stress, such as in presence of CAD plaque or CAD risk factors, LTL shortening may accelerate the decline in circulating PCs. This may lead to less effective endothelial repair, with subsequent increase in inflammation and oxidative stress at the plaque level, which in turn may lead to plaque destabilization and the triggering of cardiovascular events.52, 53
The lowest quartile of PCs and LTL had highest risk of cardiovascular events, suggesting threshold effects in injury and repair processes, such that repair mechanisms may fail only when critical levels of oxidative damage face a low pool of PCs. The observed findings could also be related to other factors implicated in the pathophysiology of atherosclerosis, including increased inflammation and oxidative stress.37 Whether inflammatory and oxidative pathways trigger both biological aging and decline of regenerative capacity, and whether they explain fully the LTL association with adverse cardiovascular outcomes, need to be further investigated.
Strengths and limitations
Our study is the largest known published study to date investigating the association between LTL and PCs and the only study investigating PCs and LTL in patients with CAD. We used highly accurate assays for both LTL and CD34+ cell count (coefficient of variation of 2.1% and 2.9%, respectively). We did not assess telomere length in different circulating PCs, which would require cellular isolation with apheresis, or large amount of cells obtained usually by either cell culture or bone marrow stimulation.39, 54, 55 However, Sakoff et al has previously shown that LTL correlates with the telomere length of CD34+ cells.39 We assessed regenerative capacity using circulating CD34+ cell subsets; however, there may be other PC pools that would also be informative. Furthermore, we did not assess circulating PCs functionality, which would require cell culture and/or other complex methodology which was not feasible in this study. Whether patients with shorter LTL also have decreased circulating PCs function need to be further investigated. We assessed CAD severity using the closest available coronary angiogram by chart review, with a median time between angiogram and enrollment of 2.1 (1.0 – 4.7) years. Thus, angiogram data may not accurately reflect patients’ current burden of CAD in our study. As in any study, our measurements carry some degree of error, which, however, is expected to be mostly non-systematic error which would bias the estimates towards the null value. However, the analytical methods we used for our main variables of interest, LTL and PCs, have low coefficient of variation (<10%), and our outcomes were independently adjudicated. Finally, our study included patients with stable CAD; whether our findings can be generalized to healthy populations need to be further investigated.
Conclusion and implications
Among patients with stable CAD, LTL is associated with decreased regenerative capacity, independent of age and CAD risk factors. Both LTL and circulating PC levels are independent and incremental predictors of adverse cardiovascular outcomes. Our study suggests that biological aging and regenerative capacity processes, albeit related, index largely independent pathways involved in cardiovascular disease risk.
Supplementary Material
Novelty and Significance.
What Is Known?
Circulating progenitor cells (PC), promote vascular repair and regeneration and in low levels are linked to adverse cardiovascular outcomes.
Leukocyte telomere length (LTL) is a biomarker of biological aging that reflects cumulative inflammatory and oxidative cellular injury. Short LTL has been linked to adverse cardiovascular outcomes.
Previous studies have speculated that the link between biological aging (lower LTL) and adverse cardiovascular outcomes is due to decreased regenerative capacity. However, this hypothesis has not been tested before.
What New Information Does This Article Contribute?
Biological aging, measured as LTL, is associated with decreased regenerative capacity, assessed using circulating PCs.
In relation to adverse cardiovascular events, biological aging and decreased regenerative capacity to be two independent processes, that are additive to each other.
In comparison with chronological aging, biological aging reflects accumulated burden of individual exposure to injurious factors including oxidative stress, inflammation, toxins and environmental factors. Using peripheral blood, we can detect average leukocyte telomere length, which can serve as a biological marker of aging. Shorter leukocyte telomere length predicts adverse cardiovascular outcomes independent of chronological aging. It has been postulated that this effect is due to decreased regenerative capacity, leading to impaired biological repair processes; however, this was not tested directly before. In a cohort of patients with stable coronary artery disease, found a significant correlation between biological aging, measured as leucocyte telomere length, and decreased regenerative capacity, measured as circulating PCs. However, we found biological aging to be independent and additive to decreased regenerative capacity in predicting adverse cardiovascular outcomes. Thus, mechanisms related to biological aging other than vascular regeneration and repair must be at play.
Acknowledgments
SOURCES OF FUNDING
This work was supported by the NIH (P01 HL101398, P20HL113451-01, P01HL086773-06A1, R56HL126558-01, R01 HL109413, R01HL109413A1S1, R01 HL125246, 5R01NS064162-04, 1DP3DK094346-01, 5R01AG042127-04, 1RF1AG051633-01, 5U10HL110302-04Rev, 1R56HL126558-01, 2P01HL095070-06A1, 1DP3DK108245-01REVI, 5R01HL095479-06, UL1TR000454, KL2TR000455, K24HL07HL77506, K23HL127251 and K24 MH076955). The sponsors of this study had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Dr. Vaccarino and Dr. Quyyumi report research support from NIH. Jue Lin is a co-founder and consultant to Telomere Diagnostics Inc.
Nonstandard Abbreviations and Acronyms
- LTL
Leukocyte telomere length
- PC
circulating progenitor cells
- MI
Myocardial infarction
- CAD
Coronary Artery disease
- BMI
Body mass index
- CVA
Cerebrovascular accident
Footnotes
DISCLOSURES
None of the other authors report conflict of interest relevant to this article.
REFERENCES
- 1.Haycock PC, Heydon EE, Kaptoge S, Butterworth AS, Thompson A, Willeit P. Leucocyte telomere length and risk of cardiovascular disease: systematic review and meta-analysis. BMJ : British Medical Journal. 2014;349:g4227. doi: 10.1136/bmj.g4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rode L, Nordestgaard BG, Bojesen SE. Peripheral Blood Leukocyte Telomere Length and Mortality Among 64 637 Individuals From the General Population. Journal of the National Cancer Institute. 2015;107 doi: 10.1093/jnci/djv074. [DOI] [PubMed] [Google Scholar]
- 3.Svensson J, Karlsson MK, Ljunggren Ö, Tivesten Å, Mellström D, Movérare-Skrtic S. Leukocyte telomere length is not associated with mortality in older men. Experimental Gerontology. 2014;57:6–12. doi: 10.1016/j.exger.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 4.Rota M, Goichberg P, Anversa P, Leri A. Aging Effects on Cardiac Progenitor Cell Physiology. Comprehensive Physiology. 2015;5:1705–1750. doi: 10.1002/cphy.c140082. [DOI] [PubMed] [Google Scholar]
- 5.Blackburn EH, Epel ES. Telomeres and adversity: Too toxic to ignore. Nature. 2012;490:169–171. doi: 10.1038/490169a. [DOI] [PubMed] [Google Scholar]
- 6.Ridout SJ, Ridout KK, Kao HT, Carpenter LL, Philip NS, Tyrka AR, Price LH. Telomeres, early-life stress and mental illness. Adv Psychosom Med. 2015;34:92–108. doi: 10.1159/000369088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baragetti A, Palmen J, Garlaschelli K, Grigore L, Pellegatta F, Tragni E, Catapano AL, Humphries SE, Norata GD, Talmud PJ. Telomere shortening over 6 years is associated with increased subclinical carotid vascular damage and worse cardiovascular prognosis in the general population. Journal of Internal Medicine. 2015;277:478–487. doi: 10.1111/joim.12282. [DOI] [PubMed] [Google Scholar]
- 8.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic acids research. 2002;30:e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel RS, Li Q, Ghasemzadeh N, Eapen DJ, Moss LD, Janjua AU, Manocha P, Al Kassem H, Veledar E, Samady H, Taylor WR, Zafari AM, Sperling L, Vaccarino V, Waller EK, Quyyumi AA. Circulating CD34+ Progenitor Cells and Risk of Mortality in a Population With Coronary Artery Disease. Circulation Research. 2015;116:289–297. doi: 10.1161/CIRCRESAHA.116.304187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, Böhm M, Nickenig G. Circulating Endothelial Progenitor Cells and Cardiovascular Outcomes. New England Journal of Medicine. 2005;353:999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 11.Roura S, Gálvez-Montón C, Fernández MA, Lupón J, Bayes-Genis A. Circulating Endothelial Progenitor Cells: Potential Biomarkers for Idiopathic Dilated Cardiomyopathy. Journal of Cardiovascular Translational Research. 2016;9:80–84. doi: 10.1007/s12265-015-9671-z. [DOI] [PubMed] [Google Scholar]
- 12.Makino H, Miyamoto Y, Kikuchi-Taura A, Soma T, Taguchi A, Kishimoto I. Decreased levels of circulating CD34(+) cells are associated with coronary heart disease in Japanese patients with type 2 diabetes. Journal of Diabetes Investigation. 2015;6:473–478. doi: 10.1111/jdi.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmidt-Lucke C, Rossig L, Fichtlscherer S, Vasa M, Britten M, Kamper U, Dimmeler S, Zeiher AM. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: proof of concept for the clinical importance of endogenous vascular repair. Circulation. 2005;111:2981–2987. doi: 10.1161/CIRCULATIONAHA.104.504340. [DOI] [PubMed] [Google Scholar]
- 14.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science (New York, NY) 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 15.Lin Y, Weisdorf DJ, Solovey A, Hebbel RP. Origins of circulating endothelial cells and endothelial outgrowth from blood. The Journal of clinical investigation. 2000;105:71–77. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quyyumi AA. Endothelial function in health and disease: new insights into the genesis of cardiovascular disease. The American journal of medicine. 1998;105:32s–39s. doi: 10.1016/s0002-9343(98)00209-5. [DOI] [PubMed] [Google Scholar]
- 17.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 18.Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95:343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 19.Waller EK, Olweus J, Lund-Johansen F, Huang S, Nguyen M, Guo GR, Terstappen L. The "common stem cell" hypothesis reevaluated: human fetal bone marrow contains separate populations of hematopoietic and stromal progenitors. Blood. 1995;85:2422–2435. [PubMed] [Google Scholar]
- 20.Kawamoto A, Iwasaki H, Kusano K, Murayama T, Oyamada A, Silver M, Hulbert C, Gavin M, Hanley A, Ma H, Kearney M, Zak V, Asahara T, Losordo DW. CD34-positive cells exhibit increased potency and safety for therapeutic neovascularization after myocardial infarction compared with total mononuclear cells. Circulation. 2006;114:2163–2169. doi: 10.1161/CIRCULATIONAHA.106.644518. [DOI] [PubMed] [Google Scholar]
- 21.Gehling UM, Ergun S, Schumacher U, Wagener C, Pantel K, Otte M, Schuch G, Schafhausen P, Mende T, Kilic N, Kluge K, Schafer B, Hossfeld DK, Fiedler W. In vitro differentiation of endothelial cells from AC133-positive progenitor cells. Blood. 2000;95:3106–3112. [PubMed] [Google Scholar]
- 22.Yin AH, Miraglia S, Zanjani ED, Almeida-Porada G, Ogawa M, Leary AG, Olweus J, Kearney J, Buck DW. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997;90:5002–5012. [PubMed] [Google Scholar]
- 23.Seeger FH, Rasper T, Koyanagi M, Fox H, Zeiher AM, Dimmeler S. CXCR4 expression determines functional activity of bone marrow-derived mononuclear cells for therapeutic neovascularization in acute ischemia. Arteriosclerosis, thrombosis, and vascular biology. 2009;29:1802–1809. doi: 10.1161/ATVBAHA.109.194688. [DOI] [PubMed] [Google Scholar]
- 24.Goodell MA, Rando TA. Stem cells and healthy aging. Science (New York, NY) 2015;350:1199–1204. doi: 10.1126/science.aab3388. [DOI] [PubMed] [Google Scholar]
- 25.Sidorov I, Kimura M, Yashin A, Aviv A. Leukocyte telomere dynamics and human hematopoietic stem cell kinetics during somatic growth. Experimental hematology. 2009;37:514–524. doi: 10.1016/j.exphem.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 26.Ramadan R, Sheps D, Esteves F, Maziar Zafari A, Douglas Bremner J, Vaccarino V, Quyyumi AA. Myocardial Ischemia During Mental Stress: Role of Coronary Artery Disease Burden and Vasomotion. Journal of the American Heart Association. 2013;2 doi: 10.1161/JAHA.113.000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol. 1983;51:606. doi: 10.1016/s0002-9149(83)80105-2. [DOI] [PubMed] [Google Scholar]
- 28.Lin J, Epel E, Cheon J, Kroenke C, Sinclair E, Bigos M, Wolkowitz O, Mellon S, Blackburn E. Analyses and comparisons of telomerase activity and telomere length in human T and B cells: insights for epidemiology of telomere maintenance. Journal of immunological methods. 2010;352:71–80. doi: 10.1016/j.jim.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang Z, Huang X, Jiang H, Zhang Y, Liu H, Qin C, Eisner GM, Jose PA, Rudolph L, Ju Z. Short telomeres and prognosis of hypertension in a chinese population. Hypertension. 2009;53:639–645. doi: 10.1161/HYPERTENSIONAHA.108.123752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ludke A, Li R-K, Weisel RD. The Rejuvenation of Aged Stem Cells for Cardiac Repair. Canadian Journal of Cardiology. 2014;30:1299–1306. doi: 10.1016/j.cjca.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 31.Cohen KS, Cheng S, Larson MG, Cupples LA, McCabe EL, Wang YA, Ngwa JS, Martin RP, Klein RJ, Hashmi B, Ge Y, O'Donnell CJ, Vasan RS, Shaw SY, Wang TJ. Circulating CD34(+) progenitor cell frequency is associated with clinical and genetic factors. Blood. 2013;121:e50–e56. doi: 10.1182/blood-2012-05-424846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanders JL, Newman AB. Telomere length in epidemiology: a biomarker of aging, age-related disease, both, or neither? Epidemiologic reviews. 2013;35:112–131. doi: 10.1093/epirev/mxs008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen S, Lin J, Matsuguchi T, Blackburn E, Yeh F, Best LG, Devereux RB, Lee ET, Howard BV, Roman MJ, Zhao J. Short leukocyte telomere length predicts incidence and progression of carotid atherosclerosis in American Indians: The Strong Heart Family Study. Aging (Albany NY) 2014;6:414–427. doi: 10.18632/aging.100671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hunt SC, Kimura M, Hopkins PN, Carr JJ, Heiss G, Province MA, Aviv A. Leukocyte Telomere Length and Coronary Artery Calcium. American Journal of Cardiology. 116:214–218. doi: 10.1016/j.amjcard.2015.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vemparala K, Roy A, Bahl VK, Prabhakaran D, Nath N, Sinha S, Nandi P, Pandey RM, Reddy KS, Manhapra A, Lakshmy R. Early accelerated senescence of circulating endothelial progenitor cells in premature coronary artery disease patients in a developing country - a case control study. BMC cardiovascular disorders. 2013;13:104. doi: 10.1186/1471-2261-13-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kovacic JC, Moreno P, Hachinski V, Nabel EG, Fuster V. Cellular senescence, vascular disease, and aging: Part 1 of a 2-part review. Circulation. 2011;123:1650–1660. doi: 10.1161/CIRCULATIONAHA.110.007021. [DOI] [PubMed] [Google Scholar]
- 37.Harrison D, Griendling KK, Landmesser U, Hornig B, Drexler H. Role of oxidative stress in atherosclerosis. The American journal of cardiology. 2003;91:7a–11a. doi: 10.1016/s0002-9149(02)03144-2. [DOI] [PubMed] [Google Scholar]
- 38.Dei Cas A, Spigoni V, Franzini L, Preti M, Ardigò D, Derlindati E, Metra M, Monti LD, Dell’Era P, Gnudi L, Zavaroni I. Lower endothelial progenitor cell number, family history of cardiovascular disease and reduced HDL-cholesterol levels are associated with shorter leukocyte telomere length in healthy young adults. Nutrition, Metabolism and Cardiovascular Diseases. 2013;23:272–278. doi: 10.1016/j.numecd.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 39.Sakoff JA, De Waal E, Garg MB, Denham J, Scorgie FE, Enno A, Lincz LF, Ackland SP. Telomere length in haemopoietic stem cells can be determined from that of mononuclear blood cells or whole blood. Leukemia & lymphoma. 2002;43:2017–2020. doi: 10.1080/1042819021000015970. [DOI] [PubMed] [Google Scholar]
- 40.Kimura M, Gazitt Y, Cao X, Zhao X, Lansdorp PM, Aviv A. Synchrony of telomere length among hematopoietic cells. Experimental hematology. 2010;38:854–859. doi: 10.1016/j.exphem.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robertson JD, Gale RE, Wynn RF, Dougal M, Linch DC, Testa NG, Chopra R. Dynamics of telomere shortening in neutrophils and T lymphocytes during ageing and the relationship to skewed X chromosome inactivation patterns. British journal of haematology. 2000;109:272–279. doi: 10.1046/j.1365-2141.2000.01970.x. [DOI] [PubMed] [Google Scholar]
- 42.Neuner B, Lenfers A, Kelsch R, Jager K, Bruggmann N, van der Harst P, Walter M. Telomere Length Is Not Related to Established Cardiovascular Risk Factors but Does Correlate with Red and White Blood Cell Counts in a German Blood Donor Population. PloS one. 2015;10:e0139308. doi: 10.1371/journal.pone.0139308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gutmajster E, Witecka J, Wyskida M, Koscinska-Marczewska J, Szwed M, Owczarz M, Mossakowska M, Milewicz A, Puzianowska-Kuznicka M, Zejda J, Wiecek A, Chudek J, Sieron AL. Telomere length in elderly Caucasians weakly correlates with blood cell counts. TheScientificWorldJournal. 2013;2013:153608. doi: 10.1155/2013/153608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Satoh H, Hiyama K, Takeda M, Awaya Y, Watanabe K, Ihara Y, Maeda H, Ishioka S, Yamakido M. Telomere shortening in peripheral blood cells was related with aging but not with white blood cell count. The Japanese journal of human genetics. 1996;41:413–417. doi: 10.1007/BF01876332. [DOI] [PubMed] [Google Scholar]
- 45.Kozlitina J, Garcia CK. Red Blood Cell Size Is Inversely Associated with Leukocyte Telomere Length in a Large Multi-Ethnic Population. PloS one. 2012;7:e51046. doi: 10.1371/journal.pone.0051046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mollica L, Fleury I, Belisle C, Provost S, Roy DC, Busque L. No association between telomere length and blood cell counts in elderly individuals. The journals of gerontology Series A, Biological sciences and medical sciences. 2009;64:965–967. doi: 10.1093/gerona/glp065. [DOI] [PubMed] [Google Scholar]
- 47.Kaushansky K. Lineage-specific hematopoietic growth factors. The New England journal of medicine. 2006;354:2034–2045. doi: 10.1056/NEJMra052706. [DOI] [PubMed] [Google Scholar]
- 48.Hall JE, Guyton AC. Guyton and Hall textbook of medical physiology. Thirteenth. Philadelphia, PA: Elsevier; 2016. [Google Scholar]
- 49.Fernández-Alvira JM, Fuster V, Dorado B, Soberón N, Flores I, Gallardo M, Pocock S, Blasco MA, Andrés V. Short Telomere Load, Telomere Length, and Subclinical Atherosclerosis The PESA Study. Journal of the American College of Cardiology. 2016;67:2467–2476. doi: 10.1016/j.jacc.2016.03.530. [DOI] [PubMed] [Google Scholar]
- 50.De Meyer T, Rietzschel ER, De Buyzere ML, Langlois MR, De Bacquer D, Segers P, Van Damme P, De Backer GG, Van Oostveldt P, Van Criekinge W, Gillebert TC, Bekaert S. Systemic telomere length and preclinical atherosclerosis: the Asklepios Study. European heart journal. 2009;30:3074–3081. doi: 10.1093/eurheartj/ehp324. [DOI] [PubMed] [Google Scholar]
- 51.Willeit P, Willeit J, Brandstätter A, Ehrlenbach S, Mayr A, Gasperi A, Weger S, Oberhollenzer F, Reindl M, Kronenberg F, Kiechl S. Cellular Aging Reflected by Leukocyte Telomere Length Predicts Advanced Atherosclerosis and Cardiovascular Disease Risk. Arteriosclerosis, thrombosis, and vascular biology. 2010;30:1649–1656. doi: 10.1161/ATVBAHA.110.205492. [DOI] [PubMed] [Google Scholar]
- 52.Rietzschel ER, Bekaert S, De Meyer T. Telomeres and Atherosclerosis: The Attrition of an Attractive Hypothesis. J Am Coll Cardiol. 2016;67:2477–2479. doi: 10.1016/j.jacc.2016.03.541. [DOI] [PubMed] [Google Scholar]
- 53.Al Mheid I, Hayek SS, Ko YA, Akbik F, Li Q, Ghasemzadeh N, Martin GS, Long Q, Hammadah M, Maziar Zafari A, Vaccarino V, Waller EK, Quyyumi AA. Age and Human Regenerative Capacity Impact of Cardiovascular Risk Factors. Circulation research. 2016;119:801–809. doi: 10.1161/CIRCRESAHA.116.308461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baerlocher GM, Vulto I, de Jong G, Lansdorp PM. Flow cytometry and FISH to measure the average length of telomeres (flow FISH) Nat Protocols. 2006;1:2365–2376. doi: 10.1038/nprot.2006.263. [DOI] [PubMed] [Google Scholar]
- 55.Satoh M, Minami Y, Takahashi Y, Tabuchi T, Itoh T, Nakamura M. Effect of intensive lipid-lowering therapy on telomere erosion in endothelial progenitor cells obtained from patients with coronary artery disease. Clinical science (London, England : 1979) 2009;116:827–835. doi: 10.1042/CS20080404. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.