Abstract
Introduction
Subjective cognitive decline (SCD) refers to an at-risk state of Alzheimer's disease and subtle cognitive deficits that have been observed in this condition. Currently, it is unknown whether complex cognitive processes relevant to everyday life, such as future-oriented choice behavior, are also altered in SCD.
Methods
Twenty SCD participants and 24 control (CO) participants took part in a functional magnetic resonance imaging task on intertemporal decisions, with and without simultaneous episodic future imagination.
Results
SCD participants showed reduced future-oriented choices. Future imagination increased future-oriented choices and was associated with increased brain activation in medial frontal polar cortex, right insular cortex, and anterior cingulate cortex in CO only, not SCD. In addition, more future-oriented choices were associated with hippocampal activation during choice processing in CO only.
Discussion
Subtle neuronal network disruptions in SCD may underlie their myopic future decisions and lack of modulation of choice behavior by episodic future imagination.
Keywords: Subjective cognitive decline, Alzheimer's disease, Intertemporal decision making, Delay discounting, Episodic future imagination
1. Introduction
Subjective cognitive decline (SCD) refers to the self-reported experience of worsening cognitive capacity in older adults, who still perform within the normal range on standard neuropsychological tests [1]. SCD has been established as an at-risk condition for Alzheimer's disease (AD) dementia in clinical and epidemiological studies [2], [3], [4]. It has been proposed that SCD may occur before mild cognitive impairment (MCI) in the continuum toward the clinical manifestation of AD [1].
By definition, cognitive impairment in SCD is not typically evident when using standard neuropsychological tests. However, a number of studies have shown SCD to be associated with slight reductions in cognitive performance, which can be detected when sample sizes are sufficiently large [5], [6], or when using challenging cognitive tasks [7], [8], [9]. Nevertheless, this subtle reduction in performance is generally not considered clinically relevant. Thus, it remains unclear to what extent it may interfere with cognitive tasks that have direct impact on individuals' everyday life.
Intertemporal decision making refers to the process of choosing between rewards that differ in the amount and time to delivery. It is the basis for long-term planning of personal matters such as financial issues [10] and health-related behaviors [11]. During intertemporal decision making, people frequently give preference to smaller immediate rewards rather than (even greater) future rewards. This tendency to devalue future rewards as opposed to immediate rewards is termed delay discounting (DD) [12]. Lower DD is associated with better long-term achievements [13]. Individual differences in DD are related to personality traits [14], [15] and cognitive factors such as intelligence, working memory, and episodic memory [16], [17], [18], [19], [20].
Functional magnetic resonance imaging (fMRI) studies have shown multiple brain regions to be involved in DD. These include brain areas associated with valuation processing—such as ventral striatum, medial prefrontal cortex, and posterior cingulate cortex [21]; brain regions associated with cognitive control—such as lateral prefrontal cortex and lateral parietal cortex [22], [23]; and brain regions related to episodic memory and episodic future imagination [19], [24], [25], including the hippocampus. Animal studies have shown that damage to the hippocampus induces an increase in DD. Rats with hippocampus lesions were intolerant of delay and showed a strong bias toward small immediate rewards [26], [27], [28], [29], with such increases in DD showing no relation to deficits in spatial memory [27], [28].
Increased DD has been observed across a broad range of psychiatric disorders (e.g., addiction [30], [31], [32], schizophrenia [33], [34], ADHD [35], etc.) with relationships mainly made to valuation and cognitive control processes [36]. Mild cognitive impairment (MCI) and AD dementia, both of which strongly affect the episodic memory system, are also associated with increased DD [37], [38]. It is currently unknown whether changes in DD occur in SCD and which neuronal systems contribute to an alteration in decision-making process if changes do occur.
The fMRI studies have shown that the association of a future reward combined with the imagination of a personally relevant future event (episodic future imagination) attenuates or modulates DD in healthy participants [19], [20], [25]. The episodic memory system, which provides the recollection of past events, is also crucial when imagining future events. The episodic memory system involves a network that includes medial prefrontal cortex, posterior cingulate cortex, retrosplenial cortex, medial temporal lobe, and lateral parietal cortex [39]. Increasingly, there is evidence in individuals with SCD that components of the episodic memory network are already affected. An fMRI study identified reduced activation in the hippocampus during an episodic memory task [40], whereas magnetic resonance imaging studies have identified slight reductions in structural volume and cortical thickness in medial temporal lobe regions [41], [42], [43]. It is unknown whether this mild episodic memory system dysfunction also impacts on the modulation of DD by episodic future imagination.
In this study, we investigate whether SCD is associated with changes in DD and with changes in the modulation effect of episodic future imagination on DD. We hypothesize that participants with SCD have increased DD and show a reduced modulation effect of future imagination on DD. Furthermore, we hypothesize that behavioral changes in SCD are associated with changes in the episodic memory brain network.
2. Methods
2.1. Participants
Twenty individuals with SCD (11 males) and 24 control (CO) participants (14 males) took part in the study (Table 1). Individuals with SCD were recruited via the memory clinic of the interdisciplinary treatment and research center for neurodegenerative disorders (KBFZ) at the University Hospital of Bonn, and via the interdisciplinary Memory Disorder Center at the University Hospital of Cologne. All SCD participants sought medical help due to experiencing a decline in memory and reported an onset of this decline within the last 5 years. All fulfilled the recently proposed SCD research criteria [1]. Control participants were recruited from the local community via advertisement and reported no decline in cognitive performance within the last 5 years. Psychiatric and neurological diseases were excluded in all participants. No participant used any medication that would influence cognitive function.
Table 1.
Demographic data and cognitive and personality measures of participants
| Control group | SCD group | Comparison | |
|---|---|---|---|
| Mean age, years, n (SD) | 66.49 (7.23) | 68.29 (7.86) | t(42) = −0.79; P = .65 |
| Mean education, years, n (SD) | 14.79 (3.36) | 15.50 (2.69) | t(42) = −0.76; P = .45 |
| MMSE, total score (SD)∗† | 29.54 (0.59) | 29.10 (0.72) | U = 159.00; P = .04 |
| CERAD–word list free recall, n (SD) | 9.04 (0.81) | 8.75 (1.16) | t(42) = 0.98; P = .33 |
| CERAD–word list delayed recall, n (SD) | 8.37 (1.28) | 8.00 (1.84) | t(42) = 0.80; P = .43 |
| CERAD–semantic fluency, n (SD) | 24.88 (4.99) | 24.80 (4.83) | t(42) = 0.05; P = .96 |
| CERAD–phonemic fluency, n (SD) | 14.58 (4.54) | 16.53 (6.16)‡ | t(41) = −1.19; P = .24 |
| CERAD–TMT-A, seconds (SD) | 40.35 (12.80) | 34.63 (14.80)‡ | t(41) = 1.36; P = .18 |
| CERAD–TMT-B, seconds (SD) | 78.04 (18.02) | 77.65 (33.74)‡ | t(41) = 0.05; P = .96 |
| BDI, total score (SD)∗ | 4.46 (3.18) | 7.90 (5.39) | t(42) = −2.63; P = .01 |
| BIS/BAS–drive, mean (SD)∗ | 3.18 (0.48) | 2.79 (0.56) | t(42) = 2.49; P = .02 |
| BIS/BAS–fun seeking, mean (SD) | 3.13 (0.53) | 2.80 (0.58) | t(42) = 1.94; P = .06 |
| BIS/BAS–reward responsive, mean (SD) | 3.47 (0.40) | 3.39 (0.42) | t(42) = 0.62; P = .54 |
| BIS/BAS–behavioral inhibition, mean (SD) | 2.71 (0.49) | 2.96 (0.40) | t(42) = −1.87; P = .07 |
| BIS-11–attention, mean (SD) | 14.08 (3.17) | 15.85 (2.70) | t(42) = −1.97; P = .06 |
| BIS-11–motor impulsivity, mean (SD) | 21.17 (4.07) | 20.55 (3.73) | t(42) = 0.52; P = .61 |
| BIS-11–nonplanning, mean (SD) | 22.33 (5.31) | 23.95 (3.76) | t(42) = −1.14; P = .26 |
| CFC, total score (SD) | 3.50 (0.64) | 3.44 (0.61) | t(42) = 0.33; P = .76 |
| ZTPI–past negative, mean (SD) | 2.52 (0.71) | 2.72 (0.73) | t(42) = −0.92; P = .36 |
| ZTPI–present hedonic, mean (SD) | 3.41 (0.54) | 3.14 (0.52) | t(42) = 1.69; P = .27 |
| ZTPI–future, mean (SD) | 3.68 (0.57) | 3.67 (0.57) | t(42) = 0.06; P = .95 |
| ZTPI–past positive, mean (SD) | 3.68 (0.54) | 3.62 (0.65) | t(42) = 0.36; P = .72 |
| ZTPI–present fatalist, mean (SD) | 2.77 (0.74) | 2.84 (0.65) | t(42) = −0.32; P = .76 |
Abbreviations: SCD, subjective cognitive decline; SD, standard deviation; MMSE, Mini–Mental State Examination; CERAD, Consortium to Establish a Registry for Alzheimer's Disease [44]; TMT-A and -B, Trial Making Test A and B; BDI, Beck Depression Inventory [45]; BIS, Barratt Impulsivity Scale [46], [47]; BIS/BAS, Behavioral Inhibition System/Behavioral Activation System [48]; CFC, Consideration of Future Consequences [49]; ZTPI, Zimbardo Time Perspective Inventory [50].
Indicate significant group differences in this measurement, P < .05.
The group differences in MMSE were tested by the Mann-Whitney U test.
Missing data on one subject.
The cognitive performance of all participants was above −1.5 standard deviations (SDs) of the age-, sex-, and education-adjusted mean on all subtests of the “Consortium to Establish a Registry for Alzheimer's Disease” (CERAD; German version) [44] neuropsychological test battery, which includes the Mini–Mental State Examination (MMSE). Subthreshold depressive symptoms were assessed with the Beck Depression Inventory (BDI) [45].
Self-report questionnaires were administrated for the assessment of personality traits (i.e., impulsivity, reward sensitivity, and time perspectives). These were the Barratt Impulsivity Scale–11 (BIS-11) [47], [48]; the Behavioral Inhibition System and Behavioral Activation System (BIS/BAS) [46]; the consideration of future consequences scale [49]; and the Zimbardo Time Perspective Inventory [50].
Group differences in demographic, cognitive variables, and questionnaires were tested with two sample t-tests, Mann-Whitney U tests, or chi-square tests, as appropriate. The study was approved by the local ethics committees (University of Bonn, Germany; and University of Cologne, Germany) in accordance with the Declaration of Helsinki. All individuals provided written informed consent before participation.
2.2. Procedures
The experiment took place on two separate days. On the first day, a behavioral pretest of the DD task and the neuropsychological assessment were performed. On the second day, an interview for the generation of participants' personally relevant episodic future event and the fMRI experiment were conducted.
For the DD behavioral pretest, participants were required to repeatedly make choices between a 20 € immediate reward and a larger future reward (range: 21 € to 200 €) for six delay intervals (1 week, 2 weeks, 1 month, 3 months, and 1 year; see Supplementary Materials Section 1.1 and Supplementary Fig. 1 for more details). For each delay period, an indifferent point (IP) value that corresponds to the reward amount at which the subject was indifferent to either option was estimated using a binary logistic regression, performed in SPSS (version 22, IBM Corp., NY, USA). For each subject, the IP values were used to adjust the value of the future reward in the fMRI DD task, so that in approximately half of the trials, the future option would be chosen.
On the second day, participants underwent an interview to identify personally relevant future events and to generate a corresponding cue word for later use during the fMRI task (see Supplementary Materials Section 1.2). Thus, the fMRI task was individually tailored with regard to the magnitude of delayed reward and the personalized future events.
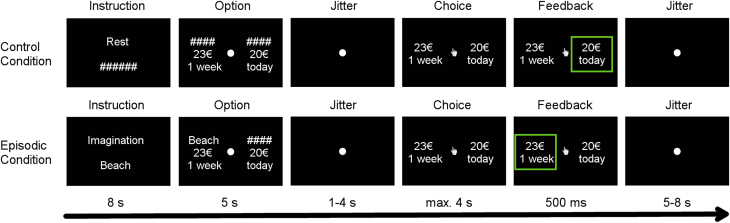
The fMRI task had an event-related design and consisted of three sessions, each with 36 trials. Within each session, 18 trials were randomly assigned as the control condition and the other trials as the episodic condition. The control condition (Fig. 1, upper panel) started with an instruction rest period (8 seconds), followed by an option-viewing period (5 seconds) and a jitter period (range: 1–4 seconds). Participants were asked to press the response button as soon as possible on the display side of which their preferred reward was shown. Choice periods were displayed for a maximum of 4 seconds. After selection, feedback of their choice was shown for 0.5 seconds.
Fig. 1.
The fMRI intertemporal decision task experiment. Each trial starts with an instruction: either a rest period (control condition) or an imagination period (episodic condition), followed by a choice option–viewing period, a jitter period (1–4 seconds), and reappearance of the choice options: where subjects are then asked to press a button to choose their option as soon as possible, and a choice feedback displayed at the end of each trial. During the control condition, subjects are asked to make the intertemporal choice only; during the episodic condition, participants are asked to imagine their personal future events according to the cue word and to elaborate the imagination as detailed as possible, additional to the choice process. Abbreviation: fMRI, functional magnetic resonance imaging.
The episodic condition (Fig. 1, lower panel) started with an instruction of imagination (8 seconds), during which participants were asked to use imagery to envision future events according to the cue word and to elaborate the events with as much details as possible. The trial is then followed by an option-viewing period (5 seconds), in which the cue word was paired with a delay option. The remainder of the trial was identical to the control condition. The side on which immediate and future rewards were shown was counterbalanced.
Before the fMRI data acquisition, participants completed a short practice session to familiarize with the experiment. The actual reward from the fMRI task was realized by drawing lots after the fMRI session. The fMRI data were acquired using a Siemens Trio–3-Tesla MRI scanner (Supplementary Materials Section 1.3).
2.3. Assessment of behavioral outcomes
The main behavioral outcomes of the fMRI task, the area-under-the-curve (AUC) index, was calculated based on the empirically estimated IP for both conditions (episodic, control) [51], using a binary logistic regression. The normalized discount fraction (the fixed immediate reward value 20 € divided by IP) can be plotted against the normalized delay interval (delay in days divided by 365). The AUC index was calculated by summing up the trapezium areas under the discount fractions. In addition, the discount rate was modeled by a hyperbolic function [52] and indicated by the log-transformed parameter, ln(k) (Supplementary Materials Section 1.4). Only the AUC index was used for the correlational imaging analysis because it is free from theoretical assumptions with regard to the shape of the discount function and is normally distributed [51]. A lower AUC value and a higher ln(k) value indicate a greater (steeper) DD (i.e., a lower tendency to choose future rewards). Behavioral data were analyzed using SPSS 22. A mixed ANOVA with a within-subject factor for condition (episodic, control) and a between-subject factor for group (CO, SCD) were used to compare the mean differences of the behavioral outcomes. To analyze the main effect of group, a second mixed ANOVA with additional covariates of BDI score and the personality measures with significant group differences (BIS/BAS drive, see Table 1) were added. In addition, simple effect analyses were carried out with a general linear model (GLM) by using the estimated marginal means to compare the mean differences between experimental conditions within each group. Effect sizes of the simple effects of condition within each group were calculated by taking the within-subject repeated measurement design into account [53].
2.4. Image processing and analysis
The fMRI data were preprocessed and statistically analyzed with statistical parametric mapping (SPM12; http://www.fil.ion.ucl.ac.uk/spm/) implemented in MATLAB (Supplementary Materials Section 1.5). The fMRI statistics were calculated using the GLM as implemented in SPM12. At the first-level contrast, each subject and each condition were modeled using the instruction-viewing period (imagination, rest), the choice process during the option-viewing period (option/episodic, option/control), and the parametric modulations of the subjective value during the option-viewing period (subjective value/episodic, subjective value/control). In addition, the statistical model also included nuisance regressors to account for the button presses, feedback periods, six realignment parameters and global noises (Supplementary Materials Section 1.5). For each subject, six contrast images representing the instruction-viewing period, choice process, and subjective valuation were created and used in second-level group analyses.
At the group level, three separate flexible factorial designs were created for the contrasts: instruction-viewing period, the choice process, and the subjective valuation. Within each model, differences between conditions were calculated, separately, for each group. To examine brain activations that are common in both groups (episodic_CO > control CO ∩ episodic_SCD > control_SCD), conjunction analyses were performed. To examine the effect of group in brain activation for the task, interaction analyses [(episodic_CO > control_CO) > (episodic_SCD > control_SCD)] were performed. Brain activations at P < .05 familywise corrected at the cluster level for the whole brain were reported for these conjunction and interaction analyses.
Because animal studies have shown increased DD in lesioned hippocampus [26], [27], [28], [29], we hypothesized a significant association between discount rate (AUC) and hippocampal activation. Correlation analyses between AUC and the activation during the choice process were performed, separately, for each group and each condition. For these analyses, we consider bilateral hippocampus as regions of interest, a liberal statistical threshold (P < .005, uncorrected) was applied.
For all second-level analyses, scanner phase (Supplementary Materials Section 1.5) and subthreshold depressive symptoms measured by BDI were added to the models as nuisance covariates. For display purpose, all images are thresholded at P < .005 (uncorrected).
3. Results
3.1. Behavioral data
Table 1 shows demographic and cognitive performance data of the participants. No significant group differences were found in age, sex, and years of education. MMSE scores were significantly different between groups (CO [mean, SD] = 29.54 ± 0.59; SCD [mean, SD] = 29.10 ± 0.72; U = 159.00, P = .036); however, all individual scores range between 28 and 30 points. Groups did not differ in performance on CERAD subtests. Although the SCD group scored slightly higher on the BDI and slightly lower on the BIS/BAS–drive subscale, none of the participants had been diagnosed as having a depressive episode according to ICD-10 during a clinical psychiatric examination before inclusion in the study. No group differences were found for other self-reported measures.
The CO group responded to 99.41 ± 0.95% (mean, SD) and the SCD group to 98.47 ± 3.33% (mean, SD) of the fMRI trials. No group difference in response rates was found [t(42) = 1.32, P = .19]. Response times (RTs) also showed no group difference [F(1,42) = 1.05, P = .31] and no group × condition interaction [F(1,42) = 0.12, P = .73] [CO: RT_control (mean, SD) = 880 ± 234 ms, RT_episodic (mean, SD) = 874 ± 253 ms; SCD: RT_control (mean, SD) = 804 ± 309 ms, RT_episodic (mean, SD) = 786 ± 293 ms].
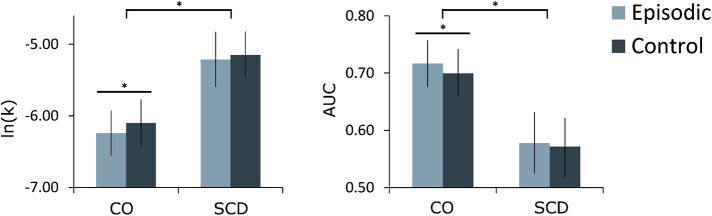
The mixed ANOVA of the DD results [AUC, ln(k)] with the BDI score and the BAS drive subscore of the BIS/BAS scale as covariates showed a main effect for group, AUC: [F(1,1) = 5.52, P = .02; CO (mean, SD) = 0.71 ± 0.20, SCD (mean, SD) = 0.57 ± 0.22, η2 = 0.12; ln(k): F(1,1) = 5.80, P = .02, CO (mean, SD) = −6.17 ± 1.58, SCD (mean, SD) = −5.13 ± 1.54, η2 = 0.13]. Fig. 2 shows that the CO group has higher AUC and lower ln(k) values (or less steep discount rate), indicating a stronger preference for future choices compared to the SCD group. In addition, the mixed ANOVA showed a trend toward a significant main effect of condition [AUC: F(1,42) = 3.35, P = .07, η2 = 0.07; ln(k): F(1,42) = 2.90, P = .09, η2 = 0.07]; but no group × condition interaction [AUC: F(1,42) = 1.17, P = .29, η2 = 0.03; ln(k): F(1,42) = 1.12, P = .30, η2 = 0.03]. Simple effect analyses showed an increased preference for future choices in the episodic than the control condition in the CO group [AUC_control (mean, SD) = 0.697 ± 0.21, AUC_episodic (mean, SD) = 0.717 ± 0.20, F(1,42) = 4.66, P < .05, d = 0.61; ln(k)_control (mean, SD) = −6.09 ± 1.63, ln(k)_episodic (mean, SD) = −6.26 ± 1.55, F(1,42) = 4.19, P < .05, d = 0.51]. No difference between conditions was found in the SCD group [AUC_control (mean, SD) = 0.57 ± 0.22, AUC_episodic (mean, SD) = 0.57 ± 0.24, F(1,42) = 0.26, P = .61, d = 0.00; ln(k)_control (mean, SD) = −5.11 ± 1.43; ln(k)_episodic (mean, SD) = −5.15 ± 1.68, F(1,42) = 0.19, P = .67, d = 0.00].
Fig. 2.
Behavioral performance of the fMRI delay discounting task. Abbreviations: AUC, area-under-the-curve; CO, control; fMRI, functional magnetic resonance imaging; ln(k), logarithm-transformed discount value k estimated by the hyperbolic function; SCD, subjective cognitive decline. *P < .05.
3.2. fMRI data
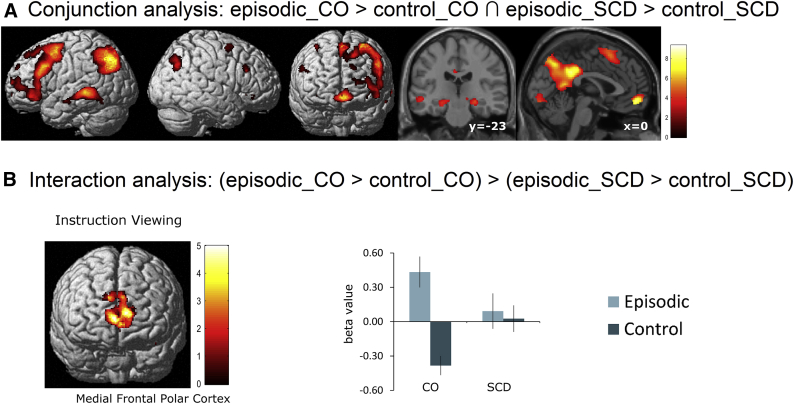
Within the instruction-viewing part of the experiment, stronger activation was observed in both groups during imagination relative to rest period (episodic > control; Supplementary Fig. 2A-B). The conjunction analysis (i.e., episodic_CO > control CO ∩ episodic_SCD > control_SCD) across both groups revealed a network of bilateral prefrontal, temporal, inferior parietal, medial frontal, medial parietal, and medial temporal areas (Fig. 3A; Supplementary Fig. 2C). A significant group × condition interaction [i.e., (episodic_CO > control_CO) > (episodic_SCD > control_SCD)] was found in the medial frontal polar cortex, extending into the left anterior cingulate cortex (Fig. 3B; Supplementary Fig. 2D, Supplementary Table 2). This area was more activated for the episodic condition than the control condition in the CO group (Supplementary Fig. 2A), but not the SCD group (Supplementary Fig. 2B).
Fig. 3.
Brain activations during the instruction-viewing period. (A) Common activations during the episodic future imagination in both CO and SCD groups revealed by conjunction analysis. This network included bilateral prefrontal, temporal, inferior parietal, medial frontal, medial parietal, medial temporal areas (PFWE < .05). (B) Significant brain activations during the instruction-viewing period for the group × condition interaction effect (PFWE < .05). Activations were thresholded at P < .05 (uncorrected) for displaying purposes. Abbreviations: CO, control; SCD, subjective cognitive decline.
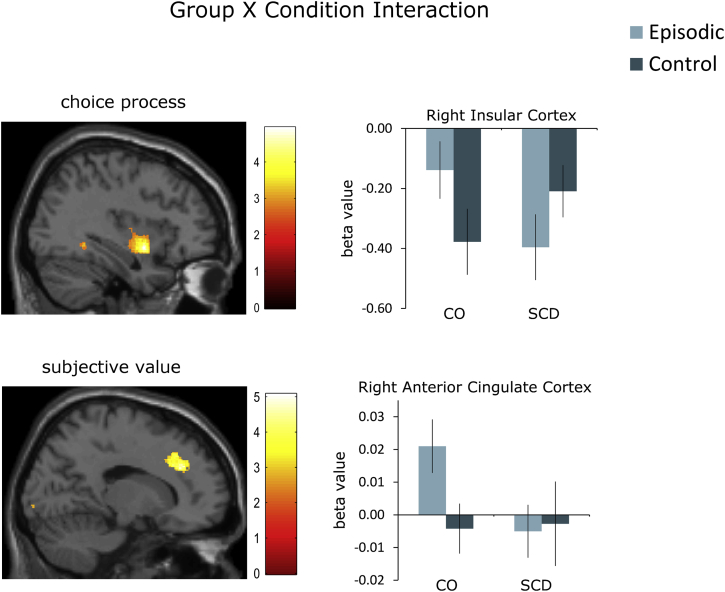
For the choice process during the option-viewing period, a significant group × condition interaction was found in the right insular cortex (Fig. 4, upper panel; Supplementary Fig. 3D, Supplementary Table 2). And the right insular cortex was more activated in the episodic condition than the control condition in the CO group (Supplementary Fig. 3A), but not the SCD group (Supplementary Fig. 3B).
Fig. 4.
Brain activations during the choice option–viewing period. (Upper panel) Significant group × condition interaction effect of the brain activations related to choice process (PFWE < .05). (Lower panel) Significant group × condition interaction effect of the brain activations related to the subjective valuation process. Activations were thresholded at P < .005 (uncorrected) for displaying purposes. Abbreviations: CO, control; SCD, subjective cognitive decline.
For subjective valuation during the option-viewing period, a significant group × condition interaction was found in the right anterior cingulate cortex (Fig. 4, lower panel; Supplementary Fig. 4C, Supplementary Table 2). In the CO group, the activation related to the subjective value is stronger during the episodic condition in comparison with the control condition (Supplementary Fig. 4A), no such differences were found in the SCD group (Supplementary Fig. 4B).
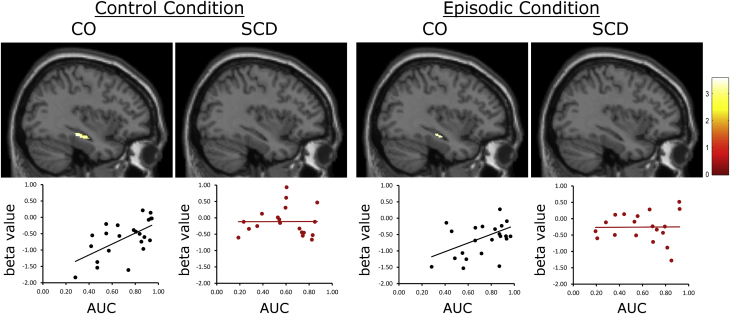
Finally, the correlation analyses showed a significant association between the AUC values and activation in the left hippocampus during the choice process, in the CO group, in both conditions (Fig. 5; Supplementary Table 3). No association between AUC and hippocampal activation was found in the SCD group. The extracted mean beta values, using a 4-mm sphere around the peak coordinates, showed a positive correlation between AUC values and hippocampal activation in the control condition (r = 0.63, P < .005) and in the episodic condition (r = 0.44, P < .05) for the CO group. No correlations were found for the SCD group in either condition (control: r = −0.26, P = .29; episodic: r = 0.08, P = .76).
Fig. 5.
Associations between individual discount rates and parameter beta estimates in the left hippocampus. Significant positive association between discount rate (area-under-the-curve [AUC]) and left hippocampus activity under both episodic and control conditions were found in the CO group only (P < .005, uncorrected), but not in the SCD group. Abbreviations: CO, control; SCD, subjective cognitive decline.
4. Discussion
The present study demonstrates that individuals with SCD show altered choice behavior in an intertemporal choice task with an increased discounting of future rewards. This result extends previous research showing reduced future-oriented choice in individuals with MCI [37]. In addition, the CO group exhibited attenuated discount rates (i.e., increased future-oriented choices) during the episodic future imagination condition, a result that is consistent with findings in young individuals, using similar paradigms [19], [20], [25]. This effect was not observed in the SCD group, indicating a lack of modulated choice behavior when using episodic future imagination. Although the difference in modulation of DD by episodic future imagination between groups was not significant in the interaction analysis, the simple effects analyses in both groups and the effect sizes in each group provide supportive evidence that the modulation of DD works well in the CO group but not in the SCD group. A larger sample with greater statistical power is required to confirm this behavioral effect. Our fMRI data demonstrate subtle changes in the episodic memory system, in the SCD participants, which may underlie myopic future decisions and the lack of modulation in choice behavior by episodic future imagination. In the following, we discuss the nature of these subtle changes in the episodic memory system and the lack of DD modulation effect in SCD, in more detail.
For the fMRI instruction-viewing period, the conjunction analysis revealed that both groups engaged a common network including bilateral prefrontal, temporal, inferior parietal, medial frontal, medial parietal, and medial temporal areas, consistent with the network reported in previous neuroimaging studies on episodic future imagination [39], and very similar to the network activated for future imagination during DD studies in young healthy participants [19], [20], [25]. This analysis indicates that both groups activated the episodic memory system including the medial temporal lobe areas for the episodic future imagination task. However, higher activation in bilateral frontal polar cortex and the adjacent anterior cingulate cortex during imagination relative to the rest period (episodic > control) was only observed in CO and not in SCD, indicating that CO recruited a wider extent of the areas during episodic future imagination. The frontal polar cortex has been found to be active during the elaborated future imagination [54] and to support sustained attention during recollection of past events and imagination of events in the future [55]. Thus, our result suggests that lower engagement of the frontal polar cortex in the SCD may influence episodic future imagination.
We found a significant group × condition interaction during the choice option–viewing period, in which the CO group had higher activation in the episodic condition relative to control conditions, while the SCD group had lower activation in the episodic condition relative to the control condition, in the right insular cortex. The insula has repeatedly been shown to be involved in intertemporal choices [56] and suggested to be a critical component of the decision-making network that integrates the prefrontal cortices and the limbic areas [57]. The insula has also been shown to be engaged in switching between the default mode network, which corresponds to the episodic memory system, and the executive network [58]. In our study, participants need to switch between future imagination that strongly engaged the episodic memory system and intertemporal decision making, which involves a central executive function component [59]. Our data indicate that the CO group may engage the insular cortex more strongly during this switching process, compared to the SCD group.
Regarding the subjective valuation process, a group × condition interaction was found in the right anterior cingulate cortex. The CO group showed increased activation for subjective value–associated activation in the right anterior cingulate cortex, during the episodic condition relative to the control condition, but not in SCD. The valuation-associated signal in the anterior cingulate has been shown to be associated with modulation of DD by future imagination in young healthy participants [19], [20]. Taken together, our result suggests that our elderly CO group uses the same neuronal network for attenuating the discount rate through episodic future imagination as young healthy participants from other studies, something that was not observed in the SCD group.
Several animal studies have shown increased DD in lesioned hippocampus [26], [27], [28], [29], and theses deficit in future-oriented choices was not associated with deficits in spatial memory [27], [28]. Based on these animal studies, we investigated further the relationship between discount rates and bilateral hippocampal activation using a region of interest analysis. We observed an association between individual discount rates and left hippocampal activations in the CO group only, not the SCD group. In humans, the hippocampus seems to have a complex role in delay discounting. Increased connectivity between the hippocampus and medial prefrontal cortex has been associated with the increased attenuation of discount rates due to future imagination in young healthy participants [19], [20], [25], indicating that the hippocampus serves to facilitate the desire of future reward. However, patients with hippocampus lesions may [60], or may not [61], display a deficit in this modulation effect. Nevertheless, a clear interpretation from lesion studies in humans can be often hard to achieve due to differences in the accuracy of lesion mapping, the spatial extent of reported lesions, reported symptoms/deficits, and the type and number of statistical controls used [62], [63]. Thus, it is very interesting for future researchers to examine the role of subregions of the hippocampus and their connectivity to other brain areas during DD. Our data suggest that the discount rate in the CO group was associated with increased hippocampus activity, a finding consistent with data from animal-based research, whereas the relationship between the discount rate and hippocampal activation may be more complex due to subtle changes in the episodic memory system (e.g., changes in hippocampal subregions) in SCD participants.
This study has limitations. We did not use biomarkers to determine amyloid or tau pathology in our study. Hence, we attribute our findings to SCD as an at-risk state of AD dementia [4] and not to preclinical AD. Second, the present study used a laboratory measurement of intertemporal choice using monetary reward; for this reason, it is not certain how our findings, if at all, translate to the everyday life decisions for events such as retirement planning, medical treatment planning, or other health-related behavior. Third, we acknowledge that the SCD group has a relatively small sample size that may affect the statistical power of the reported results. A replication of this study with a larger sample size is recommended in the future to confirm the observed behavioral effect.
In summary, the SCD group showed reduced future-oriented decisions and an absence of modulation by episodic future imagination compared to the CO group. The fMRI data indicated subtle neural network disruptions in the SCD group, including the reduced activation within the episodic memory system and reduced activation related to attentional processes and to subjective valuation. A tendency to choose immediate rewards rather than wait for greater future rewards along with a reduced capacity to change decisions using episodic future imagination may combine to have a significant impact on everyday life decisions in individuals with SCD.
Research in context.
-
1.
Systematic review: We reviewed the literature on subjective cognitive decline (SCD) as an at-risk condition for Alzheimer's disease (AD). SCD is characterized by subtle cognitive dysfunctions. In addition, we reviewed the brain areas involved in intertemporal choices, including the hippocampus, along with literature that addressed impaired intertemporal choices in patients with mild cognitive impairment and AD.
-
2.
Interpretation: We found that SCD participants made reduced future-oriented choices and that their choice behaviors were not modulated by episodic future imagination. Functional magnetic resonance imaging data showed that decreased engagement of the episodic memory system, including hippocampus, largely contributed to the myopic decision patterns.
-
3.
Future directions: Future work should evaluate the relationship between the pattern of intertemporal decision making in patients at risk for AD and their everyday life decisions, such as retirement planning, the treatment and prevention planning relating to AD, or other health-related behavior.
Acknowledgments
This research is supported by the German Research Council (DFG), Germany (grant number JE 2707/7-1); Dr. Weber was supported by a Heisenberg Grant from the DFG Germany (We 4427/3-2).
The authors thank Ulrike Broicher and Laura Schinabeck, University Hospital of Bonn for the help with scanning and data acquisition. They did not receive financial compensation for their contributions.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.dadm.2017.02.005.
Supplementary data
References
- 1.Jessen F., Amariglio R.E., van Boxtel M., Breteler M., Ceccaldi M., Chételat G. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimers Dement. 2014;10:844–852. doi: 10.1016/j.jalz.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jessen F., Wiese B., Bachmann C., Eifflaender-Gorfer S., Haller F., Kölsch H. Prediction of dementia by subjective memory impairment. Arch Gen Psychiatry. 2010;67:414–422. doi: 10.1001/archgenpsychiatry.2010.30. [DOI] [PubMed] [Google Scholar]
- 3.van Harten A.C., Visser P.J., Pijnenburg Y.A., Teunissen C.E., Blankenstein M.A., Scheltens P. Cerebrospinal fluid Abeta42 is the best predictor of clinical progression in patients with subjective complaints. Alzheimers Dement. 2013;9:481–487. doi: 10.1016/j.jalz.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell A.J., Beaumont H., Ferguson D., Yadegarfar M., Stubbs B. Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: meta-analysis. Acta Psychiatr Scand. 2014;130:439–451. doi: 10.1111/acps.12336. [DOI] [PubMed] [Google Scholar]
- 5.Amieva H., Le Goff M., Millet X., Orgogozo J.M., Pérès K., Barberger-Gateau P. Prodromal Alzheimer's disease: successive emergence of the clinical symptoms. Ann Neurol. 2008;64:492–498. doi: 10.1002/ana.21509. [DOI] [PubMed] [Google Scholar]
- 6.Jessen F., Wiese B., Cvetanovska G., Fuchs A., Kaduszkiewicz H., Kölsch H. Patterns of subjective memory impairment in the elderly: association with memory performance. Psychol Med. 2007;37:1753–1762. doi: 10.1017/S0033291707001122. [DOI] [PubMed] [Google Scholar]
- 7.Smart C.M., Krawitz A. The impact of subjective cognitive decline on Iowa gambling task performance. Neuropsychology. 2015;29:971–987. doi: 10.1037/neu0000204. [DOI] [PubMed] [Google Scholar]
- 8.Samieri C., Proust-Lima C., Glymour M.M., Okereke O.I., Amariglio R.E., Sperling R.A. Subjective cognitive concerns, episodic memory, and the APOE epsilon4 allele. Alzheimers Dement. 2014;10:752–759.e1. doi: 10.1016/j.jalz.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koppara A., Frommann I., Polcher A., Parra M.A., Maier W., Jessen F. Feature binding deficits in subjective cognitive decline and in mild cognitive impairment. J Alzheimers Dis. 2015;48 Suppl 1:S161–S170. doi: 10.3233/JAD-150105. [DOI] [PubMed] [Google Scholar]
- 10.Weber E.U. Who's afraid of a poor old-age? Risk perception in risk management decision. In: Mitchell O.S., Utkus S.P., editors. Pension Design and Structure: New Lessons from Behavioral Finance. Oxford University Press; Oxford, England: 2004. pp. 53–66. [Google Scholar]
- 11.Daugherty J.R., Brase G.L. Taking time to be healthy: predicting health behaviors with delay discounting and time perspective. Pers Individ Dif. 2010;48:202–207. [Google Scholar]
- 12.Ainslie G. Specious reward: a behavioral theory of impulsiveness and impulse control. Psychol Bull. 1975;82:463–496. doi: 10.1037/h0076860. [DOI] [PubMed] [Google Scholar]
- 13.Mischel W., Ayduk O., Berman M.G., Casey B.J., Gotlib I.H., Jonides J. ‘Willpower’ over the life span: decomposing self-regulation. Soc Cogn Affect Neurosci. 2011;6:252–256. doi: 10.1093/scan/nsq081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bobova L., Finn P.R., Rickert M.E., Lucas J. Disinhibitory psychopathology and delay discounting in alcohol dependence: personality and cognitive correlates. Exp Clin Psychopharmacol. 2009;17:51–61. doi: 10.1037/a0014503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Odum A.L. Delay discounting: trait variable? Behav Processes. 2011;87:1–9. doi: 10.1016/j.beproc.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bickel W.K., Yi R., Landes R.D., Hill P.F., Baxter C. Remember the future: working memory training decreases delay discounting among stimulant addicts. Biol Psychiatry. 2011;69:260–265. doi: 10.1016/j.biopsych.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shamosh N.A., Gray J.R. Delay discounting and intelligence: a meta-analysis. Intelligence. 2008;36:289–305. [Google Scholar]
- 18.Shamosh N.A., Deyoung C.G., Green A.E., Reis D.L., Johnson M.R., Conway A.R. Individual differences in delay discounting: relation to intelligence, working memory, and anterior prefrontal cortex. Psychol Sci. 2008;19:904–911. doi: 10.1111/j.1467-9280.2008.02175.x. [DOI] [PubMed] [Google Scholar]
- 19.Hu X., Kleinschmidt H., Martin J.A., Thelen M., Meiberth D., Jessen F. A reduction in delay discounting by using episodic future imagination and the association with episodic memory capacity. Front Hum Neurosci. 2017;10:663. doi: 10.3389/fnhum.2016.00663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peters J., Büchel C. Episodic future thinking reduces reward delay discounting through an enhancement of prefrontal-mediotemporal interactions. Neuron. 2010;66:138–148. doi: 10.1016/j.neuron.2010.03.026. [DOI] [PubMed] [Google Scholar]
- 21.Peters J., Büchel C. Overlapping and distinct neural systems code for subjective value during intertemporal and risky decision making. J Neurosci. 2009;29:15727–15734. doi: 10.1523/JNEUROSCI.3489-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McClure S.M., Laibson D.I., Loewenstein G., Cohen J.D. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- 23.Figner B., Knoch D., Johnson E.J., Krosch A.R., Lisanby S.H., Fehr E. Lateral prefrontal cortex and self-control in intertemporal choice. Nat Neurosci. 2010;13:538–539. doi: 10.1038/nn.2516. [DOI] [PubMed] [Google Scholar]
- 24.Peters J., Büchel C. The neural mechanisms of inter-temporal decision-making: understanding variability. Trends Cogn Sci. 2011;15:227–239. doi: 10.1016/j.tics.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Benoit R.G., Gilbert S.J., Burgess P.W. A neural mechanism mediating the impact of episodic prospection on farsighted decisions. J Neurosci. 2011;31:6771–6779. doi: 10.1523/JNEUROSCI.6559-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abela A.R., Chudasama Y. Dissociable contributions of the ventral hippocampus and orbitofrontal cortex to decision-making with a delayed or uncertain outcome. Eur J Neurosci. 2013;37:640–647. doi: 10.1111/ejn.12071. [DOI] [PubMed] [Google Scholar]
- 27.Mariano T.Y., Bannerman D.M., McHugh S.B., Preston T.J., Rudebeck P.H., Rudebeck S.R. Impulsive choice in hippocampal but not orbitofrontal cortex-lesioned rats on a nonspatial decision-making maze task. Eur J Neurosci. 2009;30:472–484. doi: 10.1111/j.1460-9568.2009.06837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McHugh S.B., Campbell T.G., Taylor A.M., Rawlins J.N.P., Bannerman D.M. A role for dorsal and ventral hippocampus in inter-temporal choice cost-benefit decision making. Behav Neurosci. 2008;122:1–8. doi: 10.1037/0735-7044.122.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheung T.H., Cardinal R.N. Hippocampal lesions facilitate instrumental learning with delayed reinforcement but induce impulsive choice in rats. BMC Neurosci. 2005;6:36. doi: 10.1186/1471-2202-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dixon M.R., Marley J., Jacobs E.A. Delay discounting by pathological gamblers. J Appl Behav Anal. 2003;36:449–458. doi: 10.1901/jaba.2003.36-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirby K.N., Petry N.M. Heroin and cocaine abusers have higher discount rates for delayed rewards than alcoholics or non-drug-using controls. Addiction. 2004;99:461–471. doi: 10.1111/j.1360-0443.2003.00669.x. [DOI] [PubMed] [Google Scholar]
- 32.MacKillop J., Anderson E.J., Castelda B.A., Mattson R.E., Donovick P.J. Divergent validity of measures of cognitive distortions, impulsivity, and time perspective in pathological gambling. J Gambl Stud. 2006;22:339–354. doi: 10.1007/s10899-006-9021-9. [DOI] [PubMed] [Google Scholar]
- 33.Heerey E.A., Robinson B.M., McMahon R.P., Gold J.M. Delay discounting in schizophrenia. Cogn Neuropsychiatry. 2007;12:213–221. doi: 10.1080/13546800601005900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heerey E.A., Matveeva T.M., Gold J.M. Imagining the future: degraded representations of future rewards and events in schizophrenia. J Abnorm Psychol. 2011;120:483–489. doi: 10.1037/a0021810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hurst R.M., Kepley H.O., McCalla M.K., Livermore M.K. Internal consistency and discriminant validity of a delay-discounting task with an adult self-reported ADHD sample. J Atten Disord. 2011;15:412–422. doi: 10.1177/1087054710365993. [DOI] [PubMed] [Google Scholar]
- 36.Bickel W.K., Jarmolowicz D.P., Mueller E.T., Koffarnus M.N., Gatchalian K.M. Excessive discounting of delayed reinforcers as a trans-disease process contributing to addiction and other disease-related vulnerabilities: emerging evidence. Pharmacol Ther. 2012;134:287–297. doi: 10.1016/j.pharmthera.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lindbergh C.A., Puente A.N., Gray J.C., Mackillop J., Miller L.S. Delay and probability discounting as candidate markers for dementia: an initial investigation. Arch Clin Neuropsychol. 2014;29:651–662. doi: 10.1093/arclin/acu043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lebreton M., Bertoux M., Boutet C., Lehericy S., Dubois B., Fossati P. A critical role for the hippocampus in the valuation of imagined outcomes. PLoS Biol. 2013;11:e1001684. doi: 10.1371/journal.pbio.1001684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schacter D.L., Addis D.R., Hassabis D., Martin V.C., Spreng R.N., Szpunar K.K. The future of memory: remembering, imagining, and the brain. Neuron. 2012;76:677–694. doi: 10.1016/j.neuron.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Erk S., Spottke A., Meisen A., Wagner M., Walter H., Jessen F. Evidence of neuronal compensation during episodic memory in subjective memory impairment. Arch Gen Psychiatry. 2011;68:845–852. doi: 10.1001/archgenpsychiatry.2011.80. [DOI] [PubMed] [Google Scholar]
- 41.Meiberth D., Scheef L., Wolfsgruber S., Boecker H., Block W., Träber F. Cortical thinning in individuals with subjective memory impairment. J Alzheimers Dis. 2015;45:139–146. doi: 10.3233/JAD-142322. [DOI] [PubMed] [Google Scholar]
- 42.Scheef L., Spottke A., Daerr M., Joe A., Striepens N., Kölsch H. Glucose metabolism, gray matter structure, and memory decline in subjective memory impairment. Neurology. 2012;79:1332–1339. doi: 10.1212/WNL.0b013e31826c1a8d. [DOI] [PubMed] [Google Scholar]
- 43.Perrotin A., de Flores R., Lamberton F., Poisnel G., La Joie R., de la Sayette V. Hippocampal subfield volumetry and 3D surface mapping in subjective cognitive decline. J Alzheimers Dis. 2015;48 Suppl 1:S141–S150. doi: 10.3233/JAD-150087. [DOI] [PubMed] [Google Scholar]
- 44.Morris J.C., Heyman A., Mohs R.C., Hughes J.P., van Belle G., Fillenbaum G. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 45.Beck A., Steer R.A., Carbin M.G. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clin Psychol Rev. 1988;8:77–100. [Google Scholar]
- 46.Strobel A., Beauducel A., Debener S., Brocke B. Eine deutschsprachige Version des BIS/BAS-Fragebogens von Carver und White. J Individ Differ. 2001;22:216–227. [Google Scholar]
- 47.Patton J.H., Stanford M.S., Barratt E.S. Factor structure of the Barratt impulsivity scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 48.Preuss U.W., Rujescu D., Giegling I., Watzke S., Koller G., Zetzsche T. Psychometric evaluation of the German version of the Barratt Impulsiveness ScaleNervenarzt. 2008;79:305–319. doi: 10.1007/s00115-007-2360-7. [DOI] [PubMed] [Google Scholar]
- 49.Strathman A., Gleicher F., Boninger D.S., Edwards S. The consideration of future consequences: weighing immediate and distant outcomes of behavior. J Pers Soc Psychol. 1994;66:742–752. [Google Scholar]
- 50.Zimbardo P.R., Boyd J.N. Putting time in perspective: a valid, reliable individual-differences metric. J Pers Soc Psychol. 1999;77:1271–1288. [Google Scholar]
- 51.Myerson J., Green L., Warusawitharana M. Area under the curve as a measure of discounting. J Exp Anal Behav. 2001;76:235–243. doi: 10.1901/jeab.2001.76-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mazur J.E. An adjusting procedure for studying delayed reinforcement. In: Commons M.L., Mazur J.E., Nevin J.A., Rachlin H., editors. Vol. 5. Erlbaum; Hilsdale, NJ: 1987. pp. 55–73. (The Effect of the Delayed and of Intervening Events on Reinforcement Value). [Google Scholar]
- 53.Morris S.B., DeShon R.P. Combining effect size estimates in meta-analysis with repeated measures and independent-groups designs. Psychol Methods. 2002;7:105–125. doi: 10.1037/1082-989x.7.1.105. [DOI] [PubMed] [Google Scholar]
- 54.Addis D.R., Wong A.T., Schacter D.L. Remembering the past and imagining the future: common and distinct neural substrates during event construction and elaboration. Neuropsychologia. 2007;45:1363–1377. doi: 10.1016/j.neuropsychologia.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Buckner R.L., Carroll D.C. Self-projection and the brain. Trends Cogn Sci. 2007;11:49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 56.Carter R.M., Meyer J.R., Huettel S.A. Functional neuroimaging of intertemporal choice models: a review. J Neurosci Psychol Econ. 2010;3:27–45. [Google Scholar]
- 57.Newcombe V.F., Outtrim J.G., Chatfield D.A., Manktelow A., Hutchinson P.J., Coles J.P. Parcellating the neuroanatomical basis of impaired decision-making in traumatic brain injury. Brain. 2011;134:759–768. doi: 10.1093/brain/awq388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sridharan D., Levitin D.J., Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bickel W.K., Miller M.L., Yi R., Kowal B.P., Lindquist D.M., Pitcock J.A. Behavioral and neuroeconomics of drug addiction: competing neural systems and temporal discounting processes. Drug Alcohol Depend. 2007;90:S85–S91. doi: 10.1016/j.drugalcdep.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Palombo D.J., Keane M.M., Verfaellie M. The medial temporal lobes are critical for reward-based decision making under conditions that promote episodic future thinking. Hippocampus. 2014;8:1–8. doi: 10.1002/hipo.22376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kwan D., Craver C.F., Green L., Myerson J., Gao F., Black S.E. Cueing the personal future to reduce discounting in intertemporal choice: is episodic prospection necessary? Hippocampus. 2015;443:432–443. doi: 10.1002/hipo.22431. [DOI] [PubMed] [Google Scholar]
- 62.Karnath H.-O., Smith D.V. The next step in modern brain lesion analysis: multivariate pattern analysis. Brain. 2014;137:2405–2407. doi: 10.1093/brain/awu180. [DOI] [PubMed] [Google Scholar]
- 63.Bates E., Wilson S.M., Saygin A.P., Dick F., Sereno M.I., Knight R.T. Voxel-based lesion-symptom mapping. Nat Neurosci. 2003;6:448–450. doi: 10.1038/nn1050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.