Highlights
-
•
There is no consensus on optimal treatment for GTN and brain metastases.
-
•
Brain metastasis treated with craniotomy and intravenous, EMA-CO chemotherapy
-
•
Intravenous high-dose methotrexate may be adequate to treat brain metastases.
1. Introduction
Choriocarcinoma is the most aggressive gestational trophoblastic neoplasia (GTN) with an incidence of 0.18 per 100,000 women between the ages of 15 and 49 years in the United States (Altieri et al., 2003). Brain metastases are found in 10–20% of choriocarcinoma cases, most often manifesting as an intracerebral or subdural hematoma, and are known to be a poor prognostic factor (Dadlani et al., 2010). Currently, there is not a standardized treatment regimen for patients with brain metastases. We report a case of a patient with choriocarcinoma metastasized to the brain, lungs, and vagina who had a complete response with a craniotomy followed by intravenous EMA-CO (etoposide, methotrexate, actinomycin D, cyclophosphamide, vincristine) chemotherapy including high-dose methotrexate without brain radiation or intrathecal chemotherapy.
2. Case
A 33-year-old (gravida 6, para 5) initially presented to an outside emergency room with vaginal bleeding and abdominal pain. β-Human Chorionic Gonadotropin (β-hCG) was 31,985 mlU/mL and pelvic ultrasound demonstrated features suggestive of a molar pregnancy. Subsequently, she underwent a dilation and curettage (D&C) with final pathology showing choriocarcinoma. She was transferred to our hospital for further oncologic management. On pelvic exam, she was found to have a 6 cm friable mass on the right labia extending to the perineum (Fig. 1). Transvaginal ultrasound at our institution demonstrated heterogeneity of the anterior myometrium with hypervascularity and her β-hCG was found to be 432,000 mlU/mL. CT scan of the chest, abdomen and pelvis showed heterogeneity of the uterus with at least 10 pulmonary nodules and a 3.6 cm cystic lesion at the inferior vaginal canal. The following day, the patient became disoriented and developed focal neurological deficits including the inability to use her left upper extremity. On brain MRI there was a hemorrhagic mass measuring 2.3 × 1.9 × 1.8 associated with vasogenic edema in the left parietal lobe (Fig. 2a). Based on clinical findings and imaging, she had a FIGO stage of IV with a WHO score of 15 (Berkowitz and Goldstein, 2009). She underwent left parieto-occipital craniotomy and complete resection of the mass lesion. Histopathology revealed metastatic choriocarcinoma (Fig. 3). The patient's neurologic symptoms resolved after surgery. She developed secondary hyperthyroidism that was successfully treated with methimazole. Over three weeks from initial diagnosis, β-hCG levels rose to a level of 1,381,600 mlU/mL. Two weeks following craniotomy, she was started on chemotherapy with EMA-CO including high-dose intravenous methotrexate without the use of intrathecal methotrexate (Rustin et al., 1989) (Table 1). She completed a total of five cycles. Due to persistent photophobia, low-dose (100 mg/m2) intravenous methotrexate was given during her last cycle. Over the course of chemotherapy, her β-hCG levels declined rapidly, reaching a negative value (< 5 mlU/mL) after completion of her fourth cycle, and remained negative six months after chemotherapy. Brain MRI after completion of treatment was negative for brain lesions (Fig. 2b), and PET CT scan of the chest, abdomen, and pelvis showed resolution of disease and tiny residual pulmonary nodules within the lung bases with no hypermetabolic activity. On exam there was no evidence of vaginal metastasis (Fig. 1).
Fig. 1.
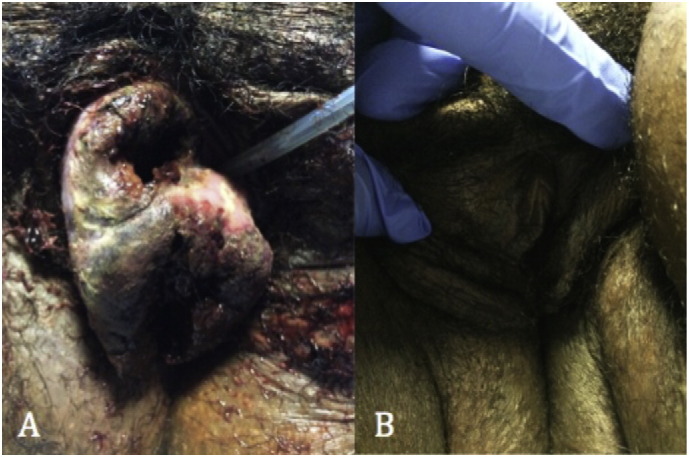
(A) Vaginal metastases prior to initiation of chemotherapy showing 6 cm friable mass on right labia. (B) Post-chemotherapy vaginal image showing complete absence of metastases.
Fig. 2.
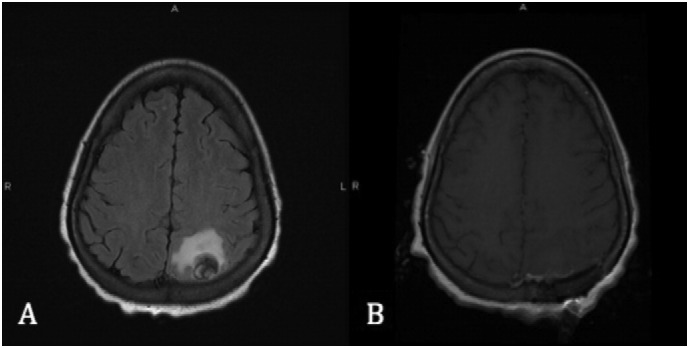
(A) Pre-operative brain MRI without contrast showing a 2.3 × 1.9 × 1.8 extra axial mass associated with vasogenic edema in the left parietal lobe. (B) Post-operative brain MRI without contrast showing post-operative changes of interval left parieto-occipital craniotomy with no mass-like enhancement and stable or slightly improved vasogenic edema, without evidence of infarct.
Fig. 3.
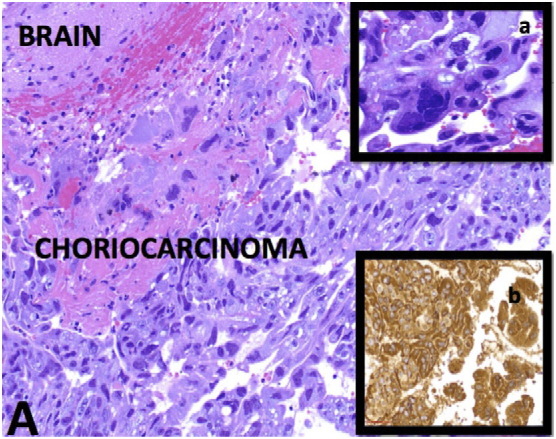
(A) Infiltrating large and pleomorphic tumor cells into the brain parenchyma with extensive hemorrhage and necrosis (H&E stain-Intermediate power). a. Multinucleated syncytiotrophoblasts (H&E stain-High power). b. β-HCG immunoreactivity (IHC stain-High power).
Table 1.
EMA-CO with high dose methotrexate regimen.
| Time pointsa | Day 1 | Day 2 | Day 8 |
|---|---|---|---|
| Chemotherapy agents | Actinomycin 0.5 mg IV bolus | Actinomycin 0.5 mg IV bolus | Vincristine 1.0 mg/m2 IV bolus |
| Etoposide 100 mg/m2 IV infusion (30 min) | Etoposide 100 mg/m2 IV infusion (30 min) | Cyclophosphamide 600 mg/m2 IV infusion | |
| Methotrexate 1000 mg/m2 IV infusion (24 h) | Leucovorin calcium 15 mg orally every 8 h for nine doses starting 32 h after start of methotrexate |
IV = intravenous.
Schedule is based on fourteen-day cycles.
3. Discussion
Management of patients with GTN and brain metastases remains a challenge due to higher morbidity and lack of consensus regarding optimal treatment. Mortality in patients with brain metastases is significant elevated, reported at 29.7% compared to an overall death rate from GTN of 5% (Xiao et al., 2015). Various modalities of multi-agent chemotherapy for high-risk patients have been described including MAC chemotherapy (methotrexate, actinomycin D and cyclophosphamide), CHAMOCA regimen (cyclophosphamide, hydroxyurea, actinomycin D, methotrexate, doxorubicin, melphalan and vincristine) and EMA-CO/EMACE (cyclophosphamide vs. cisplatin) chemotherapy. Cure rates following MAC therapy have been shown to be between 30 and 51% and in contrast, EMA-CO therapy has been showed to have an 88% survival rate in high-risk patients with 75% having no evidence of disease (Hiramatsu et al., 2005). Additionally, EMACE regimen is associated with greater hematologic and ototoxicity and peripheral neuropathy. Certain metastatic sites such as brain metastases require both additional therapy and alternative regimens including high-dose methotrexate by intravenous or intrathecal route in the EMA-CO protocol in order to ensure adequate levels in the cerebrospinal fluid.
Choice of treatment modality for brain metastases is determined by size of tumor, number of metastases, and urgency of treatment. Whole brain radiation, stereotactic radiation, intrathecal chemotherapy and excisional surgery are considered. Brain radiation combined with systemic chemotherapy is successful in controlling brain metastasis with cure rates up to 75% and is generally the standard of care in the US (Mutch et al., 1990). In the United Kingdom, the Charing Cross group has reported comparable cute rates with early surgical resection in combination with EMA-CO chemotherapy including intrathecal methotrexate (Leslie et al., 1996). There have, however, been case reports by Soper et al. documenting successful treatment of brain metastases from choriocarcinoma using systemic intravenous chemotherapy (although alternative regimens to EMA-CO) with surgery or stereotactic brain radiation without the need for whole brain radiation and intrathecal chemotherapy (Soper et al., 2007).
Surgical intervention at the time of presentation of brain metastases has shown to prevent hemorrhage and early mortality. In addition, the majority of brain metastases from GTN are solitary and amenable to surgical resection (Newlands et al., 2002). Due to our patient's focal neurological deficits and concerns for bleeding secondary to hemorrhagic mass as well as the solitary location, craniotomy with complete excision was performed. Although studies show brain radiation reduces mortality it can induce long-term cognitive impairment. Studies from patients with hematologic malignancies, primary brain tumors, and metastatic solid tumors have demonstrated chronic neurologic toxicity of whole brain radiation in survivors including impaired cognitive function, dementia, gait ataxia and behavioral changes (Blay et al., 1998).
The superiority of intrathecal versus intravenous high-dose methotrexate has yet to be established for patients with brain metastases from choriocarcinoma. Initial concerns regarding subtherapeutic CNS methotrexate levels originally led the Charing Cross group to recommend intrathecal methotrexate during EMA-CO treatment, however, most treating physicians do not routinely use intrathecal methotrexate for treatment of brain metastases or prophylaxis (Soper et al., 1994, Gillespie et al., 1999). Therapeutic CSF methotrexate levels have also been documented when > 600 mg/m2 of intravenous moderate/high-dose methotrexate was used to treat CNS metastases (Tetef et al., 2000). Others have hypothesized that disruption of the blood-brain barrier by tumor could allow for delivery of therapeutic levels of cisplatin and etoposide evidenced by patients with CNS metastases who were successfully treated with chemotherapy in the absence of radiation or neurosurgery (Soffietti et al., 2002). Intrathecal chemotherapy is a more invasive approach compared to systemic chemotherapy and can be associated with significant complications such as infection and catheter-related dysfunction.
While our patient's experience is limited and does not establish a standard of care for patients with GTN and brain metastases, it presents an alternative approach to the use of brain radiation and intrathecal chemotherapy.
4. Conclusion
Brain metastases indicate a poor prognosis in patients with choriocarcinoma. Craniotomy followed by EMA-CO with high-dose intravenous methotrexate can be considered as a treatment option for patients with choriocarcinoma and solitary brain metastasis.
Informed consent was obtained from the patient for the publication of this case report and the accompanying images.
Conflict of interest statement
The authors declare that there is no conflict of interest.
References
- Altieri A., Franceschi S., Ferlay J., Smith J., Vecchia C. Epidemiology and aetiology of gestational trophoblastic diseases. Lancet Oncol. 2003;4(11):670–678. doi: 10.1016/s1470-2045(03)01245-2. [DOI] [PubMed] [Google Scholar]
- Berkowitz R., Goldstein D. Current management of gestational trophoblastic diseases. Gynecol. Oncol. 2009;112(3):654–662. doi: 10.1016/j.ygyno.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Blay J., Conroy T., Chevreau C., Thyss A., Quesnel N., Eghbali H. High-dose methotrexate for the treatment of primary cerebral lymphomas: analysis of survival and late neurologic toxicity in a retrospective series. J. Clin. Oncol. 1998;16(3):864–871. doi: 10.1200/JCO.1998.16.3.864. [DOI] [PubMed] [Google Scholar]
- Dadlani R., Furtado S., Ghosal N., Prasanna K., Hegde A. Unusual clinical and radiological presentation of metastatic choriocarcinoma to the brain and long-term remission following emergency craniotomy and adjuvant EMA-CO chemotherapy. J. Cancer Res. Ther. 2010;6(4):552. doi: 10.4103/0973-1482.77069. [DOI] [PubMed] [Google Scholar]
- Gillespie A., Siddiqui N., Coleman R., Hancock B. Gestational trophoblastic disease: does central nervous system chemoprophylaxis have a role? Br. J. Cancer. 1999;79(7/8):1270–1272. doi: 10.1038/sj.bjc.6690203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiramatsu Y., Masuyama H., Ishida M., Murakami K., Sakurai M. Term delivery Choriocarcinoma patient with brain and lung metastases successfully treated by etoposide, methotrexate, actomycin D, cyclophosphamide and vincristine (EMA-CO) chemotherapy. Acta Med. Okayama. 2005;59(5):235–238. doi: 10.18926/AMO/31972. [DOI] [PubMed] [Google Scholar]
- Leslie M., Mangili G., Kemeny A., Newlands E. Gestational choriocarcinoma metastatic to the brain treated successfully by stereotactic radiosurgery and chemotherapy. Clin. Oncol. 1996;8(4):259–260. doi: 10.1016/s0936-6555(05)80668-5. [DOI] [PubMed] [Google Scholar]
- Mutch D., Soper J., Babcock C., Clarke-Pearson D., Hammond C. Recurrent gestational trophoblastic disease. Experience of the southeastern regional trophoblastic disease center. Cancer. 1990;66(5):978–982. doi: 10.1002/1097-0142(19900901)66:5<978::aid-cncr2820660529>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Newlands E., Holden L., Seckl M., McNeish I., Strickland S., Rustin G. Management of brain metastases in patients with high-risk gestational trophoblastic tumors. J. Reprod. Med. 2002;47(6):465–471. [PubMed] [Google Scholar]
- Rustin G., Newlands E., Begent R., Dent J., Bagshawe K. Weekly alternating etoposide, methotrexate, and actinomycin/vincristine and cyclophosphamide chemotherapy for the treatment of CNS metastases of choriocarcinoma. J. Clin. Oncol. 1989;7(7):900–903. doi: 10.1200/JCO.1989.7.7.900. [DOI] [PubMed] [Google Scholar]
- Soffietti R., Ruda R., Mutani R. Management of brain metastases. J. Neurol. 2002;249(10):1357–1369. doi: 10.1007/s00415-002-0870-6. [DOI] [PubMed] [Google Scholar]
- Soper J., Evans A., Clarke-Pearson D., Berchuck A., Rodriguez G., Hammond C. Alternating weekly chemotherapy with etoposide-methotrexate-dactinomycin/cyclophosphamide-vincristine for high-risk gestational trophoblastic disease. Obstet. Gynecol. 1994;83(1):113–117. [PubMed] [Google Scholar]
- Soper J., Spillman M., Sampson J., Kirkpatrick J., Wolf J., Clarke-Pearson D. High-risk gestational trophoblastic neoplasia with brain metastases: individualized multidisciplinary therapy in the management of four patients. Gynecol. Oncol. 2007;104(3):691–694. doi: 10.1016/j.ygyno.2006.10.027. [DOI] [PubMed] [Google Scholar]
- Tetef M., Margolin K., Doroshow J., Akman S., Leong L., Morgan R., Jr. Pharmacokinetics and toxicity of high-dose intravenous methotrexate in the treatment of leptomeningeal carcinomatosis. Cancer Chemother. Pharmacol. 2000;46(1):19–26. doi: 10.1007/s002800000118. [DOI] [PubMed] [Google Scholar]
- Xiao C., Yang J., Zhao J., Ren T., Feng F., Wan X. Management and prognosis of patients with brain metastasis from gestational trophoblastic neoplasia: a 24-year experience in Peking union medical college hospital. BMC Cancer. 2015;15(1) doi: 10.1186/s12885-015-1325-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


