Abstract
Pseudomembranous tracheitis (PMT) is a rare condition most commonly caused by fungal or bacterial infection that is characterized by a pseudomembrane that partially or completely covers the tracheobronchial tree. PMT is most commonly found in immunocompromised patient populations, such as post-chemotherapy, AIDS, post-transplant and hematological malignancies. Due to its rarity, PMT is often not included in the differential diagnosis. This case describes a 65 year old male with persistent fever and refractory cough despite high dose empiric antibiotics. Subsequent bronchoscopy with biopsy revealed pseudomembranous tracheitis due to Aspergillus fumigatus in the setting of T-cell lymphoma. PMT should be considered in the differential diagnosis of refractory cough in the immunocompromised population. However, it has been described in patients with nonspecific respiratory symptoms such as dyspnea, cough, and other airway issues.
Keywords: Pseudomembranous tracheitis, Aspergillus, Bronchoscopy, Immunocompromised, Chronic cough, T-cell lymphoma
1. Introduction
Pseudomembranous tracheitis (PMT) is a rare condition most commonly caused by fungal or bacterial infection that is characterized by a pseudomembrane that partially or completely covers the tracheobronchial tree. PMT is most often found in immunocompromised patient populations, such as post-chemotherapy, AIDS, post-transplant, and hematological malignancies [1], [2]. Fungal infections of the trachea can cause this rare phenomenon which may potentially lead to necrosis [3]. The pathogens known to cause this pseudomembranous infection are: Aspergillus, Candida, Cryptococcus, Rhizopus, and Mucorales [4], [5]. In more rare cases, pseudomembranous tracheitis may be caused by invasive bacterial pathogens such as Bacillus cereus [6]. PMT should be considered in the differential diagnosis of refractory cough in the immunocompromised population. However, it has been described in patients with nonspecific respiratory symptoms such as dyspnea, cough, and other airway issues [7]. Herein, we present a case of pseudomembrane tracheitis in the setting of high grade T-cell lymphoma.
2. Case report
A 65 year old male with a past medical history of non-obstructive coronary artery disease, urothelial cancer (status post resection), abdominal aortic aneurysm (status post repair), hypothyroidism, and 50 pack-year history of smoking, was admitted presenting with recurring fevers and a 30-pound weight loss over the past several months. A Chest x-ray (CXR) revealed a right mid-lung consolidation. Computer tomography (CT) showed a left supraclavicular/lower cervical mass, hilar lymphadenopathy as well as enlargement of the subcarinal and mediastinal lymph nodes. Subsequent lymph node biopsy revealed high grade T-cell lymphoma.
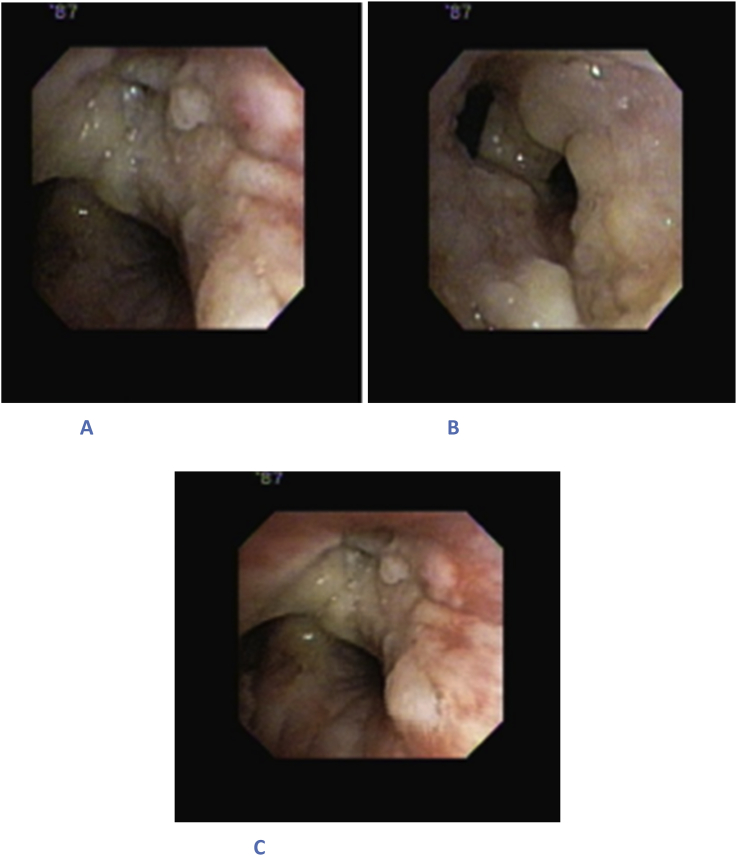
The patient was started up on empiric antibiotic therapy but continued to be febrile. He subsequently underwent bronchoscopy which revealed a pseudomembrane extending from the bronchus intermedius down to the right lower lobe (Fig. 1). Bronchoscopy was negative for any masses, abscesses, erosions or areas of bleeding.
Fig. 1.
Bronchoscopy shows mucous-like layer in the bronchotrachial tree. A) Right upper lobe apico-posterior B) Bronchus intermedius C) Secondary carina right side.
Both an endobronchial biopsy as well as culture of the bronchioalveolar lavage revealed Aspergillus fumigatus (Fig. 2).
Fig. 2.

Microscopic view of biopsy shows strains of Aspergillus fumigatus with characteristic hyphae.
Patient was initiated on Voriconazole. Repeat bone marrow biopsy was negative for Aspergillus. The patient was discharged on Voriconazole and oxygen. Despite treatment the patient died of progressive pulmonary infiltrates and respiratory failure.
3. Discussion
Pseudomembranous tracheitis (PMT) is commonly caused by fungal or bacterial infection that is characterized by pseudomembrane formation in the large airways [1], [2]. Here we described a case of a 65-year-old male with undiagnosed malignancy that had developed Aspergillus-related PMT. PMT is a rare condition that manifests with different symptoms and etiologic microorganisms. Previously reported cases of PMT have been outlined in Table 1.
Table 1.
Cases of PMT.
| Author | Primary disease | Causes | Organism | Signs/Symptoms | Treatment | Outcomes |
|---|---|---|---|---|---|---|
| Williams et al. [5] | Leukemia | Stem cell transplantation secondary to pancytopenia | Aspergillus | Progressive cough, nausea | Amphotericin B (IV), Amphotericin B (inhaled), caspofungin (IV) | Deceased |
| Strauss et al. [6] | Unknown | Aplastic Anemia | Bacillus cereus | Petechiae, weakness, dyspnea | Broad-spectrum antibiotic, anti-viral, antifungal therapy | Deceased (multiple organ failure) |
| Chang et al. [8] | Pt. 1: Diabetes mellitus Pt. 2: Diabetes |
Diabetic ketoacidosis Diabetic ketoacidosis |
Aspergillus Aspergillus |
Chest pain, cough, dyspnea, wheeze Non-productive cough, right side chest pain, fever |
Parental amphotericin B amphotericin B |
Deceased (septic shock) Improved |
| Tait et al. [7] | Pt. 1:Non-Hodgkin's lymphoma Pt. 2: Systemic lupus erythematosus-like disorder |
Neurtopenia Neurtopenia |
Aspergillus Aspergillus |
Weight loss, anorexia, non-productive cough, and pyrexia Weight loss, polyarthralgia, night sweats, pyrexia |
amphotericin B intravenous amphotericin B (1 mg/kg/day), flucytosine (120 mg/kg/day), and oral itraconazole (600 mg/day) commenced, |
Deceased Deceased (respiratory failure) |
| Hines et al. [9] | Pt.1: COPD Pt.2: Hodgkin's lymphoma Pt. 3 Myelodysplastic syndrome Pt. 4 Hepatic lesions |
Respiratory arrest Neutropenia Bone marrow transplant Neutropenia |
Aspergillus Aspergillus Aspergillus Aspergillus |
Fever, wheezing Fever Fever, hypotension Epigastric and lower back pain |
Vancomycin, Clindamycin, Amikacin Amphotericin B Amphotericin B Broad spectrum antibiotics |
Deceased Deceased (respiratory failure) Deceased (progressive respiratory insufficiency) Deceased |
| Pornsuriyasak et al. [10] | Tuberculous | Tuberculous tracheal stenosis | Aspergillus | Fever, Dyspnea, Chest pain | Oral voriconazole Nebulized amphotericin B | Cured |
| Huang et al. [11] | 16 cases: 56.3% (9/16) Pulmonary malignancies 31.3% (5/16) Bronchial involvement secondary to non-pulmonary tumor 12.5% (2/16) Lung transplant |
62.5% (10/16) Radiotherapy 43.8% (7/16) Repeated chemotherapy 25.0% (4/16) Recurrent intervention therapy by bronchoscope |
Aspergillus | 87.5% (14/16) Progressive dyspnea 75.0% (12/16) Irritable cough |
100% Amphotericin B (inhalation and infusion) | 68.8% (11/16) Deceased |
| Putnam et al. [3] | Leukemia | Bone marrow transplantation secondary to aplastic anemia | Aspergillus | Weakness, fatigue, dyspnea | Amphotericin B (IV) | Deceased |
| Patel et al. [12] | Leukemia | Pancytopenia | Aspergillus | Shortness of breath, cough, pleuritic chest pain | Amphotericin B (IV) | Deceased (progressive leukemia and sepsis) |
| Williams et al. [5] | Type 2 Diabetes and leukemia | allogeneic stem cell trans- plantation. | Rhizopus sp. | Progressive cough, dyspnea, nausea and emesis | intravenous liposomal amphotericin B, inhaled amphotericin B, intravenous caspofungin | Deceased (respiratory failure) |
| Le et al. [13] | Acute lymphoblastic leukemia. | chemotherapy | Aspergillus. | Cough, fever, and hoarseness. | Intravenous voriconazole G-CSF |
Improved |
| Argüder et al. [14] | Diabetes mellitus | Inconsistent use of insulin | Aspergillus | Cough, chest pain, hoarseness, fever, dyspnea | liposomal amphotericin B | Deceased |
| Ramos et al. [15] | Cardiac amyloidosis | Heart transplant | Aspergillus | Fever, dyspnea, wheezing, and a cough | IV voriconazole IV caspofungin |
Improved |
| Shah et al. [16] | Stillbirth | Pulmonary edema | Aspergillus | Dyspnea, stridor | Voriconazole | Improved, then lost to follow up |
Invasive pulmonary aspergillosis (IPA) is the most common form of disease caused by Aspergillus species infection. In addition, a rare form of IPA is an infection of the tracheobronchial tree, called Aspergillus Tracheobronchitis (AT) [17]. Four types of AT: ulcerative tracheobronchitis, obstructive bronchial aspergillosis, aspergillus bronchitis, and pseudomembranous necrotizing bronchial aspergillosis, or PMT have been described [1], [2]. The pseudomembrane is thought to be derived from fibrin, hyphae, and necrotic tissue [12]. Other fungi such as Rhizopus, Cryptococcus and Candida can also form a pseudomembrane via similar mechanisms [4], [5]. Rarely viruses may be implicated in PMT. Known causes of PMT have been outlined in Table 2.
Table 2.
Causes of Psuedomembranous tracheitis.
| Infectious Causes | Noninfectious Causes |
|---|---|
Fungal
|
Smoke inhalation Endotracheal intubation Crohn disease Stevens-Johnson syndrome Agents of bioterrorism Ligneous conjunctivitis Paraquat ingestion |
Adapted from Patel et al. [12].
Patients with pseudomembranous tracheitis typically present symptoms of dyspnea, fever, non-resolving cough, and chest pain. Dyspnea, as one of the presenting symptoms, is usually caused by the pseudomembrane obstructing the airways to the lungs [11]. Colonies of fungi create plaques that line the bronchi which leads to a necrotizing bronchitis. Most common signs and symptoms of PMT are outlined in Table 3.
Table 3.
Common symptoms of PMT.
| Fever |
| Dyspnea |
| Cough |
| Chest pain |
| Fatigue |
| Unilateral wheeze |
PMT is a rare condition, therefore a strong clinical suspicion is needed to diagnose this condition. Bronchoscopy is essential to discover pseudomembrane in the airways. A pseudomembrane has the potential to form and constrict the airways, thus causing the symptoms that are associated with PMT [7]. Based on pathological tissue, brush smear, and fluid from bronchial that are obtained by a bronchoscopy, the results can lead to a diagnosis of airway aspergillus infection and the type of Aspergillus as well [11]. In our case non resolution of infiltrates despite adequate antibiotic therapy prompted us to perform a bronchoscopy.
Since pseudomembranous tracheitis is mostly caused by fungal infection, a range of antifungal treatments would deem most effective towards the condition. Table 1 suggests that amongst health care providers intravenous Amphotericin B is the initial treatment of choice [11]. Other treatments such as voriconazole, itraconazole, and echinocandins (caspofungin) [5], [12] However recently, Voriconazole has been administered to patients with PMT due to its better prognosis, as shown in Table 1.
PMT has a high morbidity and mortality in immunosuppressed patients. This in itself lends to a high morbidity and mortality that is associated with opportunistic infections. It has been reported that death usually ensues between 1 and 6 weeks after diagnosis [18]. Majority of cases of PMT have resulted in demise as demonstrated in Table 1. Some causes for death include respiratory failure, septic shock, or other organ failure. Respiratory failure in PMT may result from the pseudomembrane constricting the airways and can even dislodge thus creating a ball valve that leads to obstruction [6], [12].
4. Conclusion
PMT is a rare condition that is mostly caused by fungal, and sometimes, bacterial infection. It usually requires a high index of suspicion for diagnosis. The prognosis depends on timely diagnosis and initiation of antifungal therapy.
Funding source
The author(s) received no financial support for the research, authorship and/or publication of this article.
Financial disclosure
The authors have no financial relationships relevant to this case report to disclose.
Conflict of interest
The authors have no potential conflicts of interest to disclose.
Contributor Information
Prashant Malhotra, Email: pmalhotr@northwell.edu.
Karan Singh, Email: ksingh11@northwell.edu.
Paul Gill, Email: paulgill9112@gmail.com.
Sonu Sahni, Email: sahni.sonu@gmail.com.
Mina Makaryus, Email: mmakaryus1@nortwell.edu.
Arunabh Talwar, Email: arunabhtalwar1@gmail.com.
References
- 1.Denning D.W. Invasive aspergillosis. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 1998;26(4) doi: 10.1086/513943. 781-803; quiz 804–5. [DOI] [PubMed] [Google Scholar]
- 2.Kramer M.R., Denning D.W., Marshall S.E., Ross D.J., Berry G., Lewiston N.J., Stevens D.A., Theodore J. Ulcerative tracheobronchitis after lung transplantation. A new form of invasive aspergillosis. Am. Rev. Respir. Dis. 1991;144(3 Pt 1):552–556. doi: 10.1164/ajrccm/144.3_Pt_1.552. [DOI] [PubMed] [Google Scholar]
- 3.Putnam J.B., Jr., Dignani C., Mehra R.C., Anaisse E.J., Morice R.C., Libshitz H.I. Acute airway obstruction and necrotizing tracheobronchitis from invasive mycosis. Chest. 1994;106(4):1265–1267. doi: 10.1378/chest.106.4.1265. [DOI] [PubMed] [Google Scholar]
- 4.Karnak D., Avery R.K., Gildea T.R., Sahoo D., Mehta A.C. Endobronchial fungal disease: an under-recognized entity. Respir. Int. Rev. Thorac. Dis. 2007;74(1):88–104. doi: 10.1159/000094708. [DOI] [PubMed] [Google Scholar]
- 5.Williams K.E., Parish J.M., Lyng P.J., Viggiano R.W., Wesselius L.J., Ocal I.T., Vikram H.R. Pseudomembranous tracheobronchitis caused by Rhizopus sp. After allogeneic stem cell transplantation. J. Bronchol. Intervent. Pulmonol. 2014;21(2):166–169. doi: 10.1097/LBR.0000000000000058. [DOI] [PubMed] [Google Scholar]
- 6.Strauss R., Mueller A., Wehler M., Neureiter D., Fischer E., Gramatzki M., Hahn E.G. Pseudomembranous tracheobronchitis due to Bacillus cereus. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2001;33(5):E39–E41. doi: 10.1086/322674. [DOI] [PubMed] [Google Scholar]
- 7.Tait R.C., O'Driscoll B.R., Denning D.W. Unilateral wheeze caused by pseudomembranous aspergillus tracheobronchitis in the immunocompromised patient. Thorax. 1993;48(12):1285–1287. doi: 10.1136/thx.48.12.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang S.M., Kuo H.T., Lin F.J., Tzen C.Y., Sheu C.Y. Pseudomembranous tracheobronchitis caused by Aspergillus in immunocompromised patients. Scand. J. Infect. Dis. 2005;37(11–12):937–942. doi: 10.1080/00365540510044445. [DOI] [PubMed] [Google Scholar]
- 9.Hines D.W., Haber M.H., Yaremko L., Britton C., McLawhon R.W., Harris A.A. Pseudomembranous tracheobronchitis caused by Aspergillus. Am. Rev. Respir. Dis. 1991;143(6):1408–1411. doi: 10.1164/ajrccm/143.6.1408. [DOI] [PubMed] [Google Scholar]
- 10.Pornsuriyasak P., Murgu S., Colt H. Pseudomembranous aspergillus tracheobronchitis superimposed on post-tuberculosis tracheal stenosis. Respirology. 2009;14(1):144–147. doi: 10.1111/j.1440-1843.2008.01389.x. [DOI] [PubMed] [Google Scholar]
- 11.Huang H.D., Li Q., Huang Y., Bai C., Wu N., Wang Q., Yao X.P., Chen B. Pseudomembranous necrotizing tracheobronchial aspergillosis: an analysis of 16 cases. Chin. Med. J. 2012;125(7):1236–1241. [PubMed] [Google Scholar]
- 12.Patel N., Talwar A., Stanek A., Epstein M. Tracheobronchial pseudomembrane secondary to Aspergillosis. J. Bronchol. 2006;13(3):147–150. [Google Scholar]
- 13.Le X., Jain P., O'Brien S. Aspergillus pseudomembranous necrotizing tracheitis. Am. J. Hematol. 2013;88(3):242. doi: 10.1002/ajh.23332. [DOI] [PubMed] [Google Scholar]
- 14.Arguder E., Senturk A., Hasanoglu H.C., Hasanoglu I., Kanbay A., Dogan H.T. Unique case of pseudomembranous Aspergillus tracheobronchitis: tracheal perforation and Horner's syndrome. Mycopathologia. 2016;181(11–12):885–889. doi: 10.1007/s11046-016-0025-4. [DOI] [PubMed] [Google Scholar]
- 15.Ramos A., Segovia J., Gomez-Bueno M., Salas C., Lazaro M.T., Sanchez I., Pulpon L. Pseudomembranous Aspergillus tracheobronchitis in a heart transplant recipient. Transpl. Infect. Dis. Off. J. Transplant. Soc. 2010;12(1):60–63. doi: 10.1111/j.1399-3062.2009.00444.x. [DOI] [PubMed] [Google Scholar]
- 16.Shah M., Singhal P. Rare case: invasive pseudomembranous Aspergillus tracheobronchitis in a postpartum patient presenting with stridor. J. Bronchol. Intervent. Pulmonol. 2015;22(3):248–250. doi: 10.1097/LBR.0000000000000189. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez-Ruiz M., Silva J.T., San-Juan R., de Dios B., Garcia-Lujan R., Lopez-Medrano F., Lizasoain M., Aguado J.M. Aspergillus tracheobronchitis: report of 8 cases and review of the literature. Medicine. 2012;91(5):261–273. doi: 10.1097/MD.0b013e31826c2ccf. [DOI] [PubMed] [Google Scholar]
- 18.Koh L.P., Goh Y.T., Linn Y.C., Hwang J., Tan P. Pseudomembranous tracheobronchitis caused by Aspergillus in a patient after peripheral blood stem cell transplantation. Ann. Acad. Med. Singap. 2000;29(4):531–533. [PubMed] [Google Scholar]