Abstract
Objective
The aim of this study was to characterize self-reported sleep quality (SQ) in cases with temporomandibular disorder (TMD) and to compare their results with those of healthy controls.
Methods
The Pittsburgh Sleep Quality Index (PSQI) was used to measure SQ in a convenience sample of 609 TMD cases and 88 controls. The Research Diagnostic Criteria for Temporomandibular Disorders (RDC/TMD) Axis I diagnostic nomenclature was used, but Axis I diagnoses were based on the consensus of two reliable criterion examiners and not the RDC/TMD algorithms. The PSQI scores for TMD cases were calculated also for the RDC/TMD Axis II measures assessing chronic pain and disability, depression, and nonspecific physical symptoms. PSQI scores of the TMD cases were compared with those from controls.
Results
TMD cases with one to five TMD diagnoses (n = 609) had a mean PSQI score of 7.0 [95% confidence interval (CI) = 6.7–7.4]. In comparison, the mean score was 5.2 (95% CI = 4.6–5.9) for control subjects. For the subset of TMD cases with pain-free diagnoses (n = 113), the PSQI score was similar to controls with 5.1 (95% CI = 4.5–5.6), whereas it was significantly different for cases with pain-related diagnoses 7.5 (95% CI = 6.6–8.3; n = 87). Although the number of TMD diagnoses and participant age had some influence on SQ, psychosocial status, and pain-related impairment assessed with RDC/TMD Axis II measures had the strongest association with SQ, in particular, dysfunctional chronic pain.
Conclusion
SQ is impaired in TMD patients with pain-related diagnoses, and even more in those with dysfunctional pain. This relationship between sleep and pain suggests that SQ should be assessed in TMD pain patients, especially in those with significant Axis II involvement.
Keywords: Sleep quality, Temporomandibular disorder, Facial pain, Pittsburgh Sleep Quality Index, Dysfunctional pain, Questionnaire
1. Introduction
An adequate amount of sleep is essential for general healthy functioning. Patients with chronic pain often report poor sleep quality (SQ), which can include impaired initiation or maintenance of sleep, as well as disrupted sleep with frequent arousals, or a combination of these problems [1]. However, the relationship between pain and sleep is not unidirectional; poor sleep also influences the perception of pain [2]. The bidirectional relationship is particularly important when patients experience chronic pain conditions. Studies report sleep problems in 50–89% of patients with some type of chronic pain [3,4]. Up to 90% of patients with temporomandibular disorder (TMD) commonly report poor SQ [5].
TMD is an umbrella term for various musculoskeletal conditions in the stomatognathic system, which mostly affect chewing muscles (ie, masseter muscles, temporal muscles, medial and lateral pterygoid muscles) and/or temporomandibular joints (TMJ) [6]. The typical clinical signs of TMD consist of idiopathic and episodic orofacial musculoskeletal pain and/or TMJ sounds (eg, clicking, popping, and crepitation) and/or limited jaw movements [7]. The TMD is the second most prevalent musculoskeletal pain condition after chronic low back pain [8]. The prevalence of TMD is between 5% and 12% [9] and represents a significant public health issue [8]. Females are affected at least twice as much as males [9].
As in other idiopathic pain disorders (eg, fibromyalgia, irritable bowel syndrome), TMD patients frequently present with overlapping signs and symptoms including psychosocial morbidity, neuroendocrine disorders, and chronic insomnia [10]. Sleep bruxism (ie, sleep-related movement disorder) and/or obstructive sleep apnea (ie, sleep-disordered breathing) are often identified in TMD patients when polysomnography (PSG) sleep studies are performed, but the associations for these two sleep-associated disorders with TMD are still poorly understood [10–12]. Currently, there is also insufficient scientific evidence that sleep bruxism and sleep-disordered breathing are correlated [13]. PSG recordings showed that nearly 36% of TMD patients meet diagnostic criteria for insomnia, and more than 28% meet criteria for obstructive sleep apnea [10].
The Pittsburgh Sleep Quality Index (PSQI) is a valid, reliable, and internationally well-known instrument for assessing self-perceived SQ. Although poor SQ is supposedly substantial in TMD patients [14], the magnitude of the problem is not clearly known because of a number of methodological limitations. To characterize properly the TMD patient population and to measure accurately SQ, psychometrically sound and widely used instruments are needed. Previous SQ studies of TMD patients have been performed [5,15–24], but only a few investigators [17,18,20,22–24] have used a widely accepted TMD diagnostic and classification system such as the Research Diagnostic Criteria for Temporomandibular Disorders (RDC/TMD) [25]. The RDC/TMD Validation Project [26] provided an ideal setting to assess this question. The project had a large number of TMD cases as well as a full spectrum of TMD conditions. Diagnoses were available to characterize TMD with known reliability, and PSQI information was collected. Most importantly, the project put findings into perspective due to the control group providing a framework about the magnitude of SQ in TMD cases.
The RDC/TMD criteria are the dual axis system, in which Axis I comprises only the most common physical TMD diagnoses, such as myofascial pain of the chewing muscles with or without limited mouth opening, internal derangements of the TMJ (eg, disc displacement with or without reduction), TMJ arthralgia, TMJ osteoarthritis, and TMJ osteoarthrosis [25]. Axis II of the RDC/TMD represents a battery of questionnaires to assess the psychosocial impairment due to TMD (eg, depression, somatization, level of chronic pain, and associated disability).
In 2014, the Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) were published as a set of criteria for 12 common TMD diagnoses [8]. Besides the diagnoses from the RDC/TMD, criteria for some new diagnoses were added, such as headache attributed to TMD, and TMJ hypomobility and TMJ hypermobility disorders, among others. In addition, some physical diagnoses from the RDC/TMD criteria, (eg, myofascial pain), were further divided into several new diagnoses (eg, local myalgia, myofascial pain with or without referral) [8]. Nevertheless, from a clinical point of view, it remains unclear whether myofascial pain is clinically seen as a singular disorder, or whether clinically important distinct subtypes exist [27].
When the RDC/TMD was developed, SQ was not included in the comprehensive set of the RDC/TMD Axis II assessment instruments. When the Diagnostic Criteria for TMD (DC/TMD) was developed, assessment of SQ was considered, but was not included as an Axis II instrument because it was not clear whether impaired SQ had a strong relationship with TMD pain and the other Axis II instruments such as depression [8,28]. If a strong relationship was identified, the authors believe that the SQ might be a candidate for an additional Axis II construct.
The aim of this study was to comprehensively characterize SQ in TMD cases. We described SQ between TMD cases and healthy controls, investigated sociodemographic influences, and studied SQ in the full set of diagnostic RDC/TMD subgroups of both Axis I and Axis II.
2. Methods
2.1. Subjects and setting
Of the 705 participants (614 TMD cases and 91 controls) in the multi-center RDC/TMD Validation Project [26], 609 TMD cases (85% female; aged 37.1 ± 13.1 years) with a maximum of one PSQI missing value were included in this study. We used the project’s expert-derived reference standard TMD diagnoses [29] with the RDC/TMD diagnostic nomenclature. Cases had at least one consensus-based TMD diagnosis established by two TMD experts from three study sites. Participants represented a convenience sample and were drawn from two sources: direct referrals from local health care providers to the respective university-based TMD centers (ie, TMD clinic cases), and responses to community advertisements (ie, community controls and TMD cases). For more details of the study subjects’ demographics and the setting, refer to the publication by Schiffman et al. [26]. To provide a frame of reference when interpreting SQ in TMD cases, the Validation Project’s control subjects (88.6% females; aged 36.1 ± 12.7 years) were also included in this study. Study participants were recruited from August 2003 until study closure in September 2006 [26]. Institutional review board ethic approval was obtained at each of the three study sites (the University of Minnesota, the University of Washington, and the University at Buffalo) before initiating the RDC/TMD Validation Project [26].
2.2. Classification of TMD
TMD cases were classified according to eight TMD diagnoses, by consensus of two TMD experts at three research sites, based on a comprehensive history and physical examination that included bilateral TMJ magnetic resonance imaging and computed tomography. According to the RDC/TMD nomenclature, the eight physical diagnoses are as follows: Myofascial pain without limited opening (Ia); Myofascial pain with limited opening (Ib); Disc displacement with reduction (IIa); Disc displacement without reduction, with limited opening (IIb); Disc displacement without reduction, without limited opening (IIc); Arthralgia (IIIa); Osteoarthritis of the TMJ (IIIb); and Osteoarthrosis of the TMJ (IIIc). These diagnoses were further placed into three RDC/TMD Axis I groups: Group I for myofascial pain (diagnoses Ia and Ib), Group II for TMJ disc displacements (diagnoses IIa, IIb, and IIc), and Group III for TMJ arthralgia, TMJ osteoarthritis, and/or TMJ osteoarthrosis (diagnoses IIIa, IIIb, and IIIc). Finally, diagnoses were categorized as pain-related (Ia, Ib, IIIa, IIIb) or pain-free (IIa, IIb, IIc, IIIc). The number of Axis I diagnoses per subject was tabulated.
TMD cases were also classified according to four constructs of Axis II psychological status and pain-related findings: depression (i) was assessed with the SCL-90 depression scale; somatization (ii) was assessed using a Nonspecific Physical Symptoms instrument of the SCL-90 somatization scale; and pain-related disability/chronic pain (iii) was measured with the Graded Chronic Pain Scale (GCPS) [30].
2.3. Sleep quality
Sleep quality was measured using the PSQI questionnaire. It includes 19 self-rated questions (items) and five questions posed to a bed partner or roommate, although only the self-rated items are used in scoring. The self-administered scale contains 15 multiple-choice items that ask about frequency of sleep troubles, medication use, and overall sleep quality during the previous month. Four of the questions are write-in items that ask about typical bedtime, wake-up time, sleep latency, and sleep duration. These 15 PSQI items are combined into seven components: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medications, and daytime dysfunction. Each component has a score that ranges from 0 (no difficulty) to 3 (severe difficulty). All component scores are added to produce a global score ranging from 0 to 21. A PSQI global score greater than 5 is considered to be suggestive of poor SQ. In a previous study, we showed that PSQI is a valid and reliable instrument for assessment of SQ in TMD patient population [31].
2.4. Data analysis
To characterize SQ in all TMD cases, we computed PSQI score mean values and 95% confidence intervals for all the TMD cases and control subjects. Because sociodemographic factors influence TMD pain, PSQI scores were described in groups of TMD cases according to their age (ie, four groups according to age quartiles), sex, race/ ethnicity (ie, white and nonwhite), and education (ie, no college, one or more years of college).
To characterize SQ of the TMD diagnostic subgroups, the PSQI scores were computed for: eight individual RDC/TMD Axis I diagnoses; three RDC/TMD diagnostic groups (Myofascial Pain as group I, TMJ Disc Displacements as group II, and TMJ Arthralgia and/or TMJ Osteoarthritis, and/or TMJ Osteoarthrosis as group III); single versus multiple physical Axis I diagnoses; painful versus pain-free diagnoses; and the four Axis II constructs. Because measures for these constructs were continuous or ordinal in their original metric (eg, the Likert-type scale), subjects were grouped according to RDC/ TMD recommendations for the following: depression and somatization (ie, none, moderate, and severe); and dysfunctional chronic pain (ie, cases with dysfunctional chronic pain with a grade III and IV vs pain-free cases or cases with mild pain having a grade score 0, I or II). For all groups of cases, 95% confidence intervals for the mean PSQI values were calculated. With 95% confidence intervals, effect sizes (ES) or Cohen’s d demonstrated the standardized differences between two groups’ PSQI means. Moreover, if 95% confidence intervals for Cohen’s d contained a zero value, the ES was statistically significant. Common guidelines for interpreting ES suggest that 0.2 is considered small, 0.5 medium, and 0.8 a large effect [32]. The unpaired t test (comparing two groups) or analysis of variance (for comparing three or more groups) was used to test whether differences between/across groups of TMD cases were statistically significant (p < 0.05).
2.5. Missing data and statistical software
Five TMD cases and three healthy controls from the 705 participants (614 TMD cases and 91 controls) had more than one PSQI missing item and were therefore not included in the statistical analysis. One item was missing in datasets from 234 participants, and was imputed using a median of all PSQI items per person. This median represents a typical item severity. Analyses were performed using the statistical software package STATA (Stata Statistical Software: Release 13; StataCorp LP, College Station, TX, USA).
3. Results
3.1. Sleep quality in all TMD cases, in sociodemographic groups, and in control subjects
The severity of impaired SQ was present in 60.3% TMD cases, indicated by a mean PSQI score of 7.0 (95% CI = 6.7–7.4, range 0–19) and a poor SQ prevalence (ie, PSQI > 5). Control subjects had a poor SQ prevalence of 40.9%, indicated by a mean PSQI score of 5.2 (95% CI = 4.6–5.9, range 0–14) (Table 1). The difference in the average PSQI values between TMD cases and control subjects was statistically significant and, when expressed as effect size (ES), was close to medium, or 0.5 (Table 2).
Table 1.
Descriptive statistics of Pittsburgh Sleep Quality Index (PSQI) scores for study samples, sociodemographics of temporomandibular disorder (TMD) cases, Axis I physical diagnoses of TMD cases, and Axis II constructs of the RDC/TMD protocol for TMD cases. Values in parentheses represent the TMD cases, who had multiple physical diagnoses of the Axis I.
| Characteristic | n | % of subjects | Mean PSQI | 95% CI for a mean PSQI |
% PSQI >5 |
|---|---|---|---|---|---|
| Study sample | |||||
| Healthy controls | 88 | 5.2 | 4.6–5.9 | 40.9 | |
| TMD patients | 609 | 7.0 | 6.7–7.4 | 60.1 | |
| Sociodemographics of TMD patients | |||||
| Sex | |||||
| Female | 519 | 85.2 | 7.1 | 6.7–7.4 | 59.9 |
| Male | 90 | 14.8 | 6.9 | 6.1–7.6 | 61.1 |
| Age | |||||
| First quartile | 168 | 27.6 | 6.5 | 6.0–7.0 | 61.9 |
| Second quartile | 145 | 23.8 | 7.0 | 6.3–7.7 | 57.9 |
| Third quartile | 146 | 24.0 | 7.1 | 6.4–7.8 | 60.3 |
| Fourth quartile | 150 | 24.6 | 7.7 | 7.0–8.4 | 60.0 |
| Race/ethnicity | |||||
| White | 555 | 91.1 | 7.0 | 6.7–7.4 | 60.2 |
| Nonwhite | 40 | 6.6 | 6.7 | 5.4–7.9 | 50.0 |
| Education | |||||
| No college | 93 | 15.3 | 7.4 | 6.5–8.3 | 54.8 |
| One or more years of college | 502 | 82.4 | 6.9 | 6.6–7.3 | 60.4 |
| RDC/TMD Axis I diagnoses | |||||
| Only pain-free TMD diagnoses | 113 | 56.5 | 5.1 | 4.5–5.6 | 41.6 |
| Only pain-related TMD diagnoses | 87 | 43.5 | 7.5 | 6.6–8.3 | 62.1 |
| RDC/TMD groups of Axis I diagnoses | |||||
| I: Myofascial pain | 14 (491) | 14.1 (80.6) | 8.1 (7.5) | 5.9–10.4 (7.1–7.9) | 64.3 (64.4) |
| II: Disc displacement | 73 (509) | 73.7 (83.6) | 5.4 (7.0) | 4.7–6.1 (6.7–7.4) | 47.9 (59.9) |
| III: Arthralgia, osteoarthritis, and osteoarthrosis | 12 (491) | 12.1 (80.6) | 5.7 (7.2) | 3.4–8.0 (6.9–7.6) | 58.3 (62.3) |
| Number of RDC/TMD Axis I diagnoses per case | |||||
| One | 93 | 15.3 | 5.8 | 5.1–6.5 | 49.5 |
| Two | 131 | 21.5 | 6.6 | 5.9–7.3 | 51.9 |
| Three | 248 | 40.7 | 7.6 | 7.2–8.1 | 69.3 |
| Four | 116 | 19.1 | 7.2 | 6.4–8.0 | 58.6 |
| Five | 21 | 3.4 | 7.5 | 5.4–9.6 | 57.1 |
| Multiple Axis I diagnoses (ie, two or more diagnoses) | 516 | 84.7 | 7.3 | 6.9–7.6 | 62.0 |
| RDC/TMD Axis I diagnoses | |||||
| Ia: Myofascial pain without limited opening | 10 (207) | 1.6 (34.0) | 7.3 (6.7) | 4.1–9.7 (6.2–7.2) | 60.0 (57.0) |
| Ib: Myofascial pain with limited opening | 4 (284) | 0.7 (46.6) | 10.3 (8.1) | 5.0–15.5 (7.6–8.6) | 75.0 (69.7) |
| IIa: Disc displacement with reduction | 61 (357) | 10.0 (58.6) | 5.0 (7.0) | 4.3–5.8 (6.5–7.4) | 42.6 (59.4) |
| IIb: Disc displacement without reduction, with limited opening | 0 (66) | 0 (10.8) | / (7.4) | / (6.2–8.5) | / (60.6) |
| IIc: Disc displacement without reduction, without limited opening | 6 (192) | 1.0 (31.5) | 8.2 (7.1) | 4.3–12.0 (6.5–7.7) | 66.7 (58.3) |
| IIIa: Arthralgia | 1 (303) | 0.2 (49.8) | 8.0 (7.6) | / (7.1–8.1) | 100 (65.3) |
| IIIb: Osteoarthritis of the TMJ | 0 (165) | 0 (27.1) | / (7.5) | / (6.9–8.1) | / (66.7) |
| IIIc: Osteoarthrosis of the TMJ | 11 (94) | 1.8 (15.4) | 5.5 (5.5) | 3.0–7.9 (4.8–6.2) | 54.5 (44.7) |
| RDC/TMD Axis II constructs | |||||
| Depression | |||||
| No depression | 396 | 65.0 | 5.7 | 5.3–6.0 | 47.5 |
| Moderate depression | 149 | 24.5 | 8.7 | 8.1–9.3 | 77.8 |
| Severe depression | 64 | 10.5 | 11.7 | 10.8–12.5 | 96.9 |
| Nonspecific physical symptoms | |||||
| No somatization | 334 | 54.8 | 5.5 | 5.2–5.9 | 46.7 |
| Moderate somatization | 179 | 29.4 | 8.2 | 7.6–8.7 | 71.5 |
| Severe somatization | 96 | 15.8 | 10.2 | 9.3–11.0 | 85.4 |
| Chronic pain level | |||||
| Grade 0 – no chronic pain | 90 | 14.8 | 4.7 | 4.1–5.4 | 37.8 |
| Grade 1 | 294 | 48.3 | 6.5 | 6.1–7.0 | 57.5 |
| Grade 2 | 161 | 26.4 | 7.7 | 7.1–8.3 | 65.8 |
| Grade 3 | 41 | 6.7 | 11.2 | 10.1–12.3 | 95.1 |
| Grade 4 | 23 | 3.8 | 10.5 | 8.7–12.4 | 78.3 |
| Dysfunctional pain | |||||
| No dysfunctional pain | 545 | 89.5 | 6.6 | 6.3–6.9 | 56.7 |
| Present dysfunctional pain | 64 | 10.5 | 11.0 | 10.0–11.9 | 89.1 |
Values in parentheses represent the TMD patients, who had multiple physical diagnoses of the Axis I
Table 2.
Comparing influence of sociodemographic variables, Axis I diagnoses, and Axis II measures on sleep quality using standardized mean effect reported as Cohen’s d.
| Characteristic | Cohen’s d (95% CI) | |
|---|---|---|
| Comparisons of TMD cases and healthy controls | ||
| TMD cases vs controls | +0.45 (+0.23 to +0.68) | |
| TMD cases with pain-related diagnoses vs controls | +0.61 (+0.31 to +0.92) | |
| TMD cases with pain-free diagnoses vs controls | −0.05 (−0.33 to +0.23) | |
| Comparisons between TMD patient subgroups | ||
| Sociodemographics | ||
| Sex | Male TMD cases vs female TMD cases | −0.05 (−0.28 to +0.16) |
| Age | First vs second quartile | −0.13 (−0.36 to +0.09) |
| First vs third quartile | −0.16 (−0.38 to +0.07) | |
| First vs fourth quartile | −0.31 (−0.53 to −0.09) | |
| Second vs third quartile | −0.02 (−0.25 to +0.21) | |
| Second vs fourth quartile | −0.16 (−0.39 to +0.07) | |
| Third vs fourth quartile | −0.14 (−0.37 to +0.09) | |
| Race/ethnicity | White vs nonwhite TMD cases | +0.09 (−0.23 to +0.42) |
| Education | No college vs ≥1 years of college | +0.11 (−0.11 to +0.33) |
| RDC/TMD Axis I diagnoses | ||
| Pain-related TMD vs pain-free TMD cases | +0.68 (+0.39 to +0.97) | |
| Group I: myofascial pain | Group I vs group II | +0.83 (+0.24 to +1.41) |
| Group II: TMS disc displacement | Group II vs group III | −0.08 (−0.69 to +0.54) |
| Group III: TMS arthralgia/osteoarthritis/osteoarthrosis | Group I vs group III | +0.59 (−0.20 to +1.38) |
| Single vs multiple Axis I diagnoses | −0.37 (−0.59 to −0.15) | |
| RDC/TMD Axis II diagnoses | ||
| Depression | Normal vs moderate | −0.88 (−1.08 to −0.69) |
| Moderate vs severe | −0.80 (−1.10 to −0.50) | |
| Nonspecific physical symptoms (ie, somatization) | Normal vs moderate | −0.77 (−0.96 to −0.59) |
| Moderate vs severe | −0.51 (−0.76 to −0.26) | |
| Chronic pain | Grade 0 vs grade 1 | −0.52 (−0.76 to −0.28) |
| Grade 1 vs grade 2 | −0.31 (−0.50 to −0.11) | |
| Grade 2 vs grade 3 | −0.90 (−1.25 to −0.55) | |
| Grade 3 vs grade 4 | +0.17 (−0.34 to +0.69) | |
| Dysfunctional pain | Absent vs present | −1.16 (−1.43 to −0.90) |
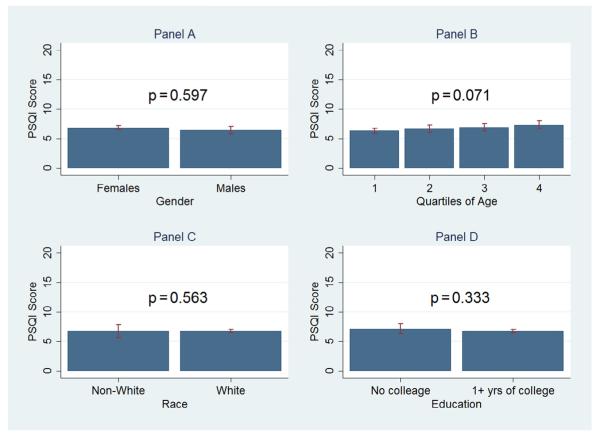
The mean PSQI scores did not differ substantially between females and males (7.1 vs 6.8), between individuals of white ethnicity and those not of white ethnicity (7.0 vs 6.7), or between subjects with or without college education (6.9 vs 7.4) (Table 1). Age had a small influence on SQ, with scores increasing from 6.5 in the first age quartile to 7.7 in the fourth quartile (Table 1). The findings for differences in prevalence of poor SQ were small or absent across sociodemographic groups; any score differences were not statistically significant (Fig. 1) and did not even reach the smallest ES level (Table 2).
Fig. 1.
Bar graphs represent mean Pittsburgh Sleep Quality Index (PSQI) values [±95% confidence intervals (CI)] for sex, age, race/ethnicity, and education. Statistically significant results of independent-samples t tests are displayed for sex, race/ethnicity, and education (A, C, and D, respectively). Statistical significance by analysis of variance is displayed for the age quartiles (B). The p values displayed confirm whether differences between/across groups of TMD cases were statistically significant (p < 0.05).
3.2. Sleep quality in diagnostic groups of TMD cases
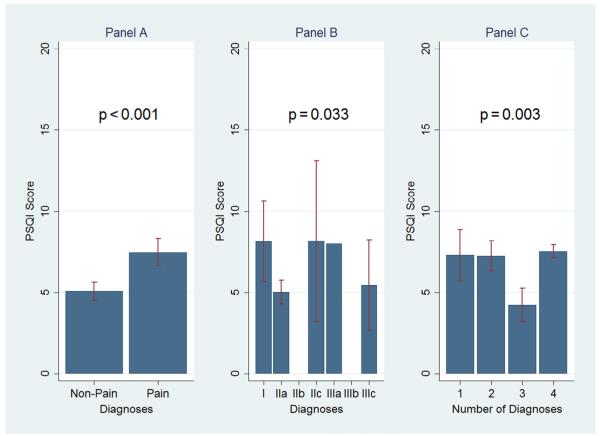
The physical TMD diagnoses had a larger influence on SQ than sociodemographic characteristics. For TMD cases without pain, the PSQI score was statistically different with 5.1 (95% CI = 4.5–5.6) in comparison to 7.5 (95% CI = 6.6–8.3) for TMD cases with pain (Table 1) and ES was found to be medium (Table 2). Other medium or some large effects were observed for differences in SQ between the RDC/ TMD groups I and II, and between groups I and III. Only the number of individual RDC/TMD diagnoses had a small effect on SQ when TMD cases with single versus multiple pain diagnoses were compared (Table 2). All group differences were statistically significant (Fig. 2C).
Fig. 2.
(Left to right) Bar graphs represent mean Pittsburgh Sleep Quality Index (PSQI) ± 95% confidence intervals (CI) for the following: comparison of sleep quality in temporomandibular disorder (TMD) cases with only pain-free Research Diagnostic Criteria for Temporomandibular Disorders (RDC/TMD) Axis I diagnoses versus only pain-related Axis I diagnoses; sleep quality in TMD cases, who had only one Axis I physical diagnosis; and comparison of sleep quality in cases with one to four different physical Axis I diagnoses. According to the RDC/TMD nomenclature, the eight physical diagnoses are as follows: Myofascial pain without limited opening (Ia); Myofascial pain with limited opening (Ib); Disc displacement with reduction (IIa); Disc displacement without reduction, with limited opening (IIb); Disc displacement without reduction, without limited opening (IIc); Arthralgia (IIIa); Osteoarthritis of the TMJ (IIIb); and, Osteoarthrosis of the TMJ (IIIc). Group differences were statistically assessed using a t test (panel A) and analysis of variance (panels B and C) and presented with their p values.
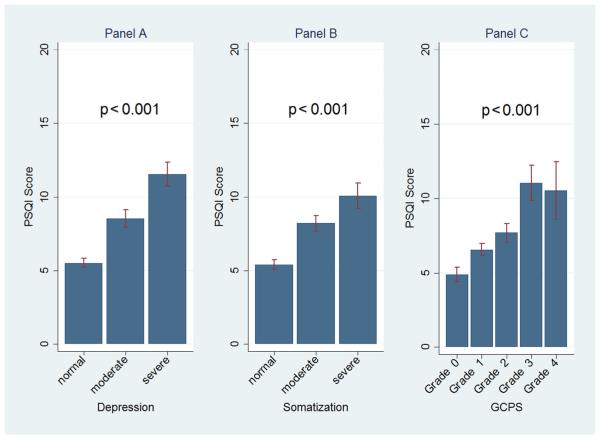
The RDC/TMD’s pain-related disability and psychological status had an even larger influence on SQ. TMD cases with pain-related disability or chronic pain assessed with the GCPS had the lowest PSQI score of 4.7 (Fig. 3C). This score more than doubled in cases with severe depression, the most extreme case group. For all Axis II constructs, dose–response relationships with SQ were present, that is, as the symptoms of depression, somatization, chronic, and dysfunctional pain increased, SQ got worse (Fig. 3). ES or Cohen’s d values, representing the standardized difference of the PSQI means, were of medium to large magnitudes when subgroups of TMD cases according to the Axis II constructs were compared. The largest ES of more than 1.1 was seen in TMD cases with dysfunctional pain in comparison to TMD cases without dysfunctional pain, attesting to the influence of dysfunctional pain on SQ (Table 2).
Fig. 3.
Mean PSQI scores ± 95% CI for TMD cases related to Axis II constructs: depression, nonspecific physical symptoms (ie, somatization), and chronic pain level assessed with the Graded Chronic Pain Scale (GCPS), are displayed. Statistical significances of ANOVA tests are also displayed. The displayed p-values confirmed whether differences between/across subgroups of TMD cases were statistically significant (p < 0.05).
4. Discussion
This is the first study that has aimed to comprehensively characterize and compare the magnitude of SQ for individual TMD physical diagnoses and psychosocial distress based on a comprehensive history and physical examination that included bilateral TMJ magnetic resonance imaging and computed tomography. Although the RDC/TMD diagnostic algorithms have been shown to have poor criterion validity [33], using reliable and valid gold standard (ie, reference) diagnoses from two reliable calibrated examiners [26] overcame this significant validity issue. This study demonstrated that among the cases with TMD, SQ in cases with Axis I pain-related TMD diagnoses was more impaired than in pain-free TMD cases. Among the Axis II constructs, psychosocial distress and pain-related disability, particularly dysfunctional pain, were most strongly associated with SQ.
Although there has been substantial interest in TMD and its psychosocial impact, self-assessed sleep disturbances have been the focus of only a few studies. Yatani et al. [5] assessed SQ, clinical, and psychological characteristics in 137 consecutive TMD patients. Despite higher levels of poor SQ, their subjects’ PSQI scores were almost three points higher than ours were. Similar findings of substantial comorbidity among SQ, perceived pain severity, and psychological distress were observed. This seems to indicate that, regardless of absolute score differences among TMD populations, SQ is substantially correlated with other suffering due to pain and distress. Therefore, as demonstrated in our study, healthy controls, that is, subjects without TMD-related pain and distress, have better SQ than TMD cases. Nevertheless, the healthy controls’ scores are not trivial when a standard threshold of 5 PSQI points is chosen to characterize poor sleep. As much as 40.9% of healthy controls also reported impaired SQ compared to 60.3% of the TMD cases, indicating that impaired SQ is common in general adult population regardless of pain. The evidence that pain and distress are major contributors to SQ is further supported by our findings that TMD cases with Axis I pain-free TMD diagnoses are very similar to control subjects. In contrast to the presence of pain and distress, sociodemographic variables were mostly nonpredictive of SQ; for example, males’ and females’ scores did not differ significantly. This was contrary to literature findings [34,35]. An age-related influence was found in our study. Older TMD cases had higher PSQI scores than younger ones. Although this finding is in line with clinical experience regarding some age-related sleep influence, poor SQ seems to be prevalent already in adolescents with TMD [23]. In that study, dysfunctions, as defined by the RDC/TMD, were associated with poor SQ, and only 17% of those with TMD dysfunctions had good SQ [23].
SQ differences were less pronounced across some specific physical TMD diagnoses. However, our analyses were limited by the number of cases in each category because the majority of cases had more than one clinical (ie, physical) diagnosis. Certain TMD diagnoses were not observed in isolation (eg, disc displacement without reduction, with limited mouth opening or osteoarthritis of the TMJ). Although the number of cases limits the confidence in our findings, a general trend was observed: among the other six physical diagnoses, the mean PSQI score was highest for myofascial pain with limited mouth opening (ie, a pain diagnosis with substantial functional impact), chronic disc displacement without reduction (ie, a disorder with substantial functional impact), and TMJ arthralgia (ie, another pain diagnosis). PSQI scores were lowest (ie, better SQ) for disc displacement with reduction, a diagnosis with only a structural finding related to the articular disc, and osteoarthrosis, a diagnosis with only osseous structural findings. Similar to our study, the substantial influence of myofascial pain to SQ is documented in another study, which assessed differences in SQ between patients with chronic daily headaches and patients with TMD [36]. It was found that SQ was significantly worse in TMD patients with myofascial pain compared to the patients with chronic daily headaches, as well as to the patients with TMJ intracapsular pain [36]. Studies using an instrument other than the PSQI, specifically the Pain Disability Index (PDI), also observed a higher impact in patients with myogenous complaints compared to those with disc disorders [37] and in patients with myofascial pain and dysfunction compared to patients with temporomandibular (joint) pain [38].
In this study, which assessed the SQ in chronic orofacial musculoskeletal pain conditions, the average PSQI score of pain-related TMD cases was 7.5. In comparison to our findings, studies assessing the SQ in another chronic musculoskeletal pain condition, that is, low-back pain [39,40], reported even a higher degree of compromised SQ. In the study assessing the SQ in 268 patients with chronic low back pain, the average PSQI score was 10.4 [39]. In the French study, a case-control study such as the present study, which compared the SQ of the patients with chronic low back pain (n = 101) to healthy controls (n = 97), the average PSQI scores for the patients and controls were 10.9 and 4.7, respectively [40]. Similarly, in our subsets of TMD cases who had severe depression or severe somatization or grade 4 for the chronic pain level or dysfunctional pain, the average PSQI scores were 11.7, 10.2, 10.5, and 11.0, respectively, and therefore reached magnitudes similar to those in the studies assessing SQ in patients with a chronic low-back pain.
Self-reported SQ was also assessed in patients with sleep bruxism [41–43], a condition that has been regarded as an etiologic, sustaining, and/or exacerbating factor for chronic myofascial TMD for many decades [44]. The average PSQI scores of the sleep bruxers ranged from 6.08 [42] to 10.8 [41], which suggests the poor SQ in patients with sleep bruxism. Although the association between sleep bruxism and TMD has been questioned in recent years [45], we speculate that the sleep bruxism might have influenced the SQ to some extent also in our TMD study cohort. Although self-reported sleep bruxism was documented in the RDC/TMD Validation Project [26], with the use of the Oral Behaviors Checklist questionnaire, these data were not used in the present study, because it was shown that self-reported sleep bruxism underestimates the true prevalence of this condition [44]. Moreover, the validity and utility of the Oral Behaviors Checklist questionnaire have yet to be demonstrated [27].
As opposed to the physical diagnoses of Axis I, which are categorical in nature, the Axis II constructs have ordinal values, with higher scores representing higher levels of severity. All assessed Axis II constructs showed a strong dose–response relationship when measured against the PSQI instrument. A particularly large SQ effect size was present for cases with dysfunctional pain. The concept of dysfunctional pain was introduced by Turk et al. [46,47] and can be described as a construct encompassing pain severity, pain interference, affective disturbance, as well as lowered activity levels. As discovered/determined in many other studies, our results confirmed the association between dysfunctional pain and SQ. The vast majority (90%) of TMD cases who presented with dysfunctional pain also had poor sleep. Our observed pattern of a very strong association of dysfunctional pain, a strong association of pain and distress, and a negligible association of structural TMD with SQ is in line with other studies using the RDC/TMD nomenclature to characterize broad measures such as sleep in TMD patients. Similarly, a Croatian study found that chronic TMD patients and patients with multiple TMD diagnoses had higher rates of depression and somatization [48]. These findings suggest that TMD pain in general, but more so the amount/distribution of the pain (multiple pain diagnoses) and the chronicity/persistency as well as dysfunctional nature of TMD related pain, have a strong influence on broad measures such as sleep. This provides substantial evidence for the need to evaluate routinely the SQ in TMD patients with dysfunctional pain. Depression and nonspecific physical findings (ie, somatization in RDC/ TMD) demonstrating medium to large effect sizes on SQ also support the findings for dysfunctional pain. This suggests that depression and nonspecific physical findings could act as surrogate markers for impaired SQ. Future research should determine whether impaired SQ has a strong independent relationship with TMD pain relative to the other Axis II constructs such as depression.
In conclusion, although the PSQI instrument had a substantial overlap with other measures of Axis II, there may be enough unique information gained from this instrument on its own accord. We believe that SQ is a candidate for an additional Axis II construct, and we advocate its regular assessment in TMD patients with chronic pain-related TMD diagnoses.
This study has strengths and limitations. As the RDC/TMD diagnostic algorithms have shown to have poor criterion validity [33], using reliable and valid gold standard (ie, reference) diagnoses from two reliable calibrated examiners [26] overcame this significant validity issue.
The present study investigated self-perceived SQ in individual Axis I diagnoses. As the vast majority of cases had multiple Axis I diagnoses, their effect on SQ is combined. Therefore, PSQI scores obtained for a particular Axis I diagnosis is often a combined effect of multiple Axis I diagnoses, which needs to be taken into consideration when interpreting this measure. Although we have characterized typical TMD patients who usually have multiple TMD diagnoses, the influence of single TMD diagnosis on SQ remains unclear.
5. Conclusion
TMD patients are a diverse group of patients [49–51]. Nevertheless, assessing the SQ in this patient population may help to better characterize these patients and, most importantly, addressing their sleep impairment may offer another therapeutic approach to reduce pain-related suffering, above and beyond the worthwhile goal of better sleep for TMD patients on its own. In a previous study, which aimed to confirm the adequate psychometric properties of the PSQI in TMD patient population, we confirmed that this questionnaire is a reliable and valid instrument to assess a self-reported SQ in this patient population [31]. Therefore, the assessment of SQ with the use of the PSQI questionnaire must be considered for TMD patients who are chronically distressed by their condition, and is highly recommended in patients with dysfunctional pain.
Acknowledgments
The study was funded by NIDCR grant #U01 DEO13331. The authors thank Mrs. Andrea J. Medina, Executive Administrative Specialist, Division of TMD and Orofacial Pain, School of Dentistry, University of Minnesota, Minneapolis, Minnesota, USA, for proofreading the manuscript.
Footnotes
Conflict of interest
The ICMJE Uniform Disclosure Form for Potential Conflicts of Interest associated with this article can be viewed by clicking on the following link: http://dx.doi.org/10.1016/j.sleep.2016.06.031.
References
- [1].National Institutes of Health National Institutes of Health state of the science conference statement on manifestations and management of chronic insomnia in adults. Sleep. 2005;28:1049–57. doi: 10.1093/sleep/28.9.1049. [DOI] [PubMed] [Google Scholar]
- [2].Edwards RR, Grace E, Peterson S, et al. Sleep continuity and architecture: associations with pain-inhibitory processes in patients with temporomandibular joint disorder. Eur J Pain. 2009;13:1043–7. doi: 10.1016/j.ejpain.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cole JC, Dubois D, Kosinski M. Use of patient-reported sleep measures in clinical trials of pain treatment: a literature review and synthesis of current sleep measures and a conceptual model of sleep disturbance in pain. Clin Ther. 2007;29S:2580–8. doi: 10.1016/j.clinthera.2007.12.005. [DOI] [PubMed] [Google Scholar]
- [4].Merrill RL. Orofacial pain and sleep. Sleep Med Clin. 2010;5:131–44. [Google Scholar]
- [5].Yatani H, Studts J, Cordova M, et al. Comparison of sleep quality and clinical and psychologic characteristics in patients with temporomandibular disorders. J Orofac Pain. 2002;16:221–8. [PubMed] [Google Scholar]
- [6].Rener-Sitar K, Celebić A, Stipetić J, et al. Oral health related quality of life in Slovenian patients with craniomandibular disorders. Coll Antropol. 2008;32:513–17. [PubMed] [Google Scholar]
- [7].Rener-Sitar K, Celebić A, Mehulić K, et al. Factors related to oral health related quality of life in TMD patients. Coll Antropol. 2013;37:407–13. [PubMed] [Google Scholar]
- [8].Schiffman E, Ohrbach R, Truelove E, et al. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for clinical and research applications: recommendations of the International RDC/TMD Consortium Network* and Orofacial Pain Special Interest Group. J Oral Facial Pain Headache. 2014;28:6–27. doi: 10.11607/jop.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].National Institute of Dental and Craniofacial Research [accessed 30.12.15];Prevalence of TMJD and Its Signs and Symptoms. < http://www.nidcr.nih.gov/DataStatistics/FindDataByTopic/FacialPain/PrevalenceTMJD.htm>.
- [10].Smith MT, Wickwire EM, Grace EG, et al. Sleep disorders and their association with laboratory pain sensitivity in temporomandibular joint disorder. Sleep. 2009;32:779–90. doi: 10.1093/sleep/32.6.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sanders AE, Essick GK, Fillingim R, et al. Sleep apnea symptoms and risk of temporomandibular disorder: OPPERA cohort. J Dent Res. 2013;92(7 Suppl):70S–7S. doi: 10.1177/0022034513488140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Dubrovsky B, Raphael KG, Lavigne GJ, et al. Polysomnographic investigation of sleep and respiratory parameters in women with temporomandibular pain disorders. J Clin Sleep Med. 2014;10:195–201. doi: 10.5664/jcsm.3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].De Luca Canto G, Singh V, Gozal D, et al. Sleep bruxism and sleep-disordered breathing: a systematic review. J Oral Facial Pain Headache. 2014;28:299–305. doi: 10.11607/ofph.1294. [DOI] [PubMed] [Google Scholar]
- [14].Sommer I, Lavigne G, Ettlin DA. Review of self-reported instruments that measure sleep dysfunction in patients suffering from temporomandibular disorders and/or orofacial pain. Sleep Med. 2015;16:27–38. doi: 10.1016/j.sleep.2014.07.023. [DOI] [PubMed] [Google Scholar]
- [15].de Leeuw R, Studts JL, Carlson CR. Fatigue and fatigue-related symptoms in an orofacial pain population. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:168–74. doi: 10.1016/j.tripleo.2004.03.001. [DOI] [PubMed] [Google Scholar]
- [16].De Leeuw R, Bertoli E, Schmidt JE, et al. Prevalence of traumatic stressors in patients with temporomandibular disorders. J Oral Maxillofac Surg. 2005;63:42–50. doi: 10.1016/j.joms.2004.04.027. [DOI] [PubMed] [Google Scholar]
- [17].Lindroth JE, Schmidt JE, Carlson CR. A comparison between masticatory muscle pain patients and intracapsular pain patients on behavioral and psychosocial domains. J Orofac Pain. 2002;16:277–83. [PubMed] [Google Scholar]
- [18].Herman CR, Schiffman EL, Look JO, et al. The effectiveness of adding pharmacologic treatment with clonazepam or cyclobenzaprine to patient education and self-care for the treatment of jaw pain upon awakening: a randomized clinical trial. J Orofac Pain. 2002;16:64–70. [PubMed] [Google Scholar]
- [19].Bertoli E, de Leeuw R, Schmidt JE, et al. Prevalence and impact of post-traumatic stress disorder symptoms in patients with masticatory muscle or temporomandibular joint pain: differences and similarities. J Orofac Pain. 2007;21:107–19. [PubMed] [Google Scholar]
- [20].Abrahamsen R, Zachariae R, Svensson P. Effect of hypnosis on oral function and psychological factors in temporomandibular disorders patients. J Oral Rehabil. 2009;36:556–70. doi: 10.1111/j.1365-2842.2009.01974.x. [DOI] [PubMed] [Google Scholar]
- [21].Fillingim RB, Ohrbach R, Greenspan JD, et al. Potential psychosocial risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA patient-control study. J Pain. 2011;12:T46–60. doi: 10.1016/j.jpain.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Porto F, de Leeuw R, Evans DR, et al. Differences in psychosocial functioning and sleep quality between idiopathic continuous orofacial neuropathic pain patients and chronic masticatory muscle pain patients. J Orofac Pain. 2011;25:117–24. [PubMed] [Google Scholar]
- [23].Drabovicz PV, Salles V, Drabovicz PE, et al. Assessment of sleep quality in adolescents with temporomandibular disorders. J Pediatr (Rio J) 2012;88:169–72. doi: 10.2223/JPED.2180. [DOI] [PubMed] [Google Scholar]
- [24].Schmitter M, Kares-Vrincianu A, Kares H, et al. Sleep-associated aspects of myofascial pain in the orofacial area among temporomandibular disorder patients and controls. Sleep Med. 2015;16:1056–61. doi: 10.1016/j.sleep.2015.03.022. [DOI] [PubMed] [Google Scholar]
- [25].Dworkin SF, LeResche L. Research Diagnostic Criteria for Temporomandibular Disorders: review, criteria, examinations and specifications, critique. J Craniomandib Disord. 1992;6:301–55. [PubMed] [Google Scholar]
- [26].Schiffman EL, Truelove EL, Ohrbach R, et al. The Research Diagnostic Criteria for Temporomandibular Disorders. I: overview and methodology for assessment of validity. J Orofac Pain. 2010;24:7–24. [PMC free article] [PubMed] [Google Scholar]
- [27].Michelotti A, Alstergren P, Goulet JP, et al. Next steps in development of the diagnostic criteria for temporomandibular disorders (DC/TMD): recommendations from the International RDC/TMD Consortium Network workshop. J Oral Rehabil. 2016 doi: 10.1111/joor.12378. [DOI] [PubMed] [Google Scholar]
- [28].Anderson GC, Gonzalez YM, Ohrbach R, et al. The Research Diagnostic Criteria for Temporomandibular Disorders. VI: future directions. J Orofac Pain. 2010;24:79–88. [PMC free article] [PubMed] [Google Scholar]
- [29].Schiffman EL, Ohrbach R, Truelove EL, et al. The Research Diagnostic Criteria for Temporomandibular Disorders. V: methods used to establish and validate revised Axis I diagnostic algorithms. J Orofac Pain. 2010;24:63–78. [PMC free article] [PubMed] [Google Scholar]
- [30].Ohrbach R, Turner JA, Sherman JJ, et al. The Research Diagnostic Criteria for Temporomandibular Disorders. IV: evaluation of psychometric properties of the Axis II measures. J Orofac Pain. 2010;24:48–62. [PMC free article] [PubMed] [Google Scholar]
- [31].Rener-Sitar K, John MT, Bandyopadhyay D, et al. Exploration of dimensionality and psychometric properties of the Pittsburgh Sleep Quality Index in Patients with temporomandibular disorders. Health Qual Life Outcomes. 2014;12:10. doi: 10.1186/1477-7525-12-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed Lawrence Erlbaum Associates; Hillsdale, NJ: 1988. [Google Scholar]
- [33].Truelove E, Pan W, Look JO, et al. The research diagnostic criteria for temporomandibular disorders. III: validity of axis I diagnoses. J Orofac Pain. 2010;24:35–47. [PMC free article] [PubMed] [Google Scholar]
- [34].Bagis B, Ayaz EA, Turgut S, et al. Gender difference in prevalence of signs and symptoms of temporomandibular joint disorders: a retrospective study on 243 consecutive patients. Int J Med Sci. 2012;9:539–44. doi: 10.7150/ijms.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Manfredini D, Chiappe G, Bosco M. Research Diagnostic Criteria for Temporomandibular Disorders (RDC/TMD) Axis I diagnoses in an Italian patient population. J Oral Rehabil. 2006;33:551–8. doi: 10.1111/j.1365-2842.2006.01600.x. [DOI] [PubMed] [Google Scholar]
- [36].Vazquez-Delgado E, Schmidt JE, Carlson CR, et al. Psychological and sleep quality differences between chronic daily headache and temporomandibular disorders patients. Cephalalgia. 2004;24:446–54. doi: 10.1111/j.1468-2982.2004.00698.x. [DOI] [PubMed] [Google Scholar]
- [37].Bush FM, Harkins SW. Pain-related limitation in activities of daily living in patients with chronic orofacial pain: psychometric properties of a disability index. J Orofac Pain. 1995;9:57–63. [PubMed] [Google Scholar]
- [38].Auerbach SM, Laskin DM, Frantsve LM, et al. Depression, pain, exposure to stressful life events, and long-term outcomes in temporomandibular disorder patients. J Oral Maxillofac Surg. 2001;59:628–33. doi: 10.1053/joms.2001.23371. [DOI] [PubMed] [Google Scholar]
- [39].Marin R, Cyhan T, Miklos W. Sleep disturbance in patients with chronic low back pain. Am J Phys Med Rehabil. 2006;85:430–5. doi: 10.1097/01.phm.0000214259.06380.79. [DOI] [PubMed] [Google Scholar]
- [40].Marty M, Rozenberg S, Duplan B, et al. Quality of sleep in patients with chronic low back pain: a case-control study. Eur Spine J. 2008;17:839–44. doi: 10.1007/s00586-008-0660-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Saletu A, Parapatics S, Saletu B, et al. On the pharmacotherapy of sleep bruxism: placebo-controlled polysomnographic and psychometric studies with clonazepam. Neuropsychobiology. 2005;51:214–25. doi: 10.1159/000085917. [DOI] [PubMed] [Google Scholar]
- [42].Serra-Negra JM, Scarpelli AC, Tirsa-Costa D, et al. Sleep bruxism, awake bruxism and sleep quality among Brazilian dental students: a cross-sectional study. Braz Dent J. 2014;25:241–7. doi: 10.1590/0103-6440201302429. [DOI] [PubMed] [Google Scholar]
- [43].Shim YJ, Kang JK, Lee YM, et al. Comparison of clinical and psychological characteristics between self-reported bruxism and clinically detected bruxism by wear facet on splint. J Oral Med Pain. 2015;40:140–5. [Google Scholar]
- [44].Klasser GD, Greene CS, Lavigne GJ. Oral appliances and the management of sleep bruxism in adults: a century of clinical applications and search for mechanisms. Int J Prosthodont. 2010;23:453–62. [PubMed] [Google Scholar]
- [45].Raphael KG, Sirois DA, Janal MN, et al. Sleep bruxism and myofascial temporomandibular disorders: a laboratory-based polysomnographic investigation. J Am Dent Assoc. 2012;143:1223–31. doi: 10.14219/jada.archive.2012.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Turk DC, Rudy TE. Toward an empirically derived taxonomy of chronic pain patients: integration of psychological assessment data. J Consult Clin Psychol. 1988;56:233–8. doi: 10.1037//0022-006x.56.2.233. [DOI] [PubMed] [Google Scholar]
- [47].Rudy TE, Turk DC, Kubinski JA, et al. Differential treatment responses of TMD patients as a function of psychological characteristics. Pain. 1995;61:103–12. doi: 10.1016/0304-3959(94)00151-4. [DOI] [PubMed] [Google Scholar]
- [48].Celić R, Braut V, Petricević N. Influence of depression and somatization on acute and chronic orofacial pain in patients with single or multiple TMD diagnoses. Coll Antropol. 2011;35:709–13. [PubMed] [Google Scholar]
- [49].Dworkin SF, Sherman J, Mancl L, et al. Reliability, validity, and clinical utility of the Research Diagnostic Criteria for Temporomandibular Disorders Axis II Scales: depression, non-specific physical symptoms, and graded chronic pain. J Orofac Pain. 2002;16:207–20. [PubMed] [Google Scholar]
- [50].List T, Dworkin SF. Comparing TMD diagnoses and clinical findings at Swedish and US TMD centers using Research Diagnostic Criteria for Temporomandibular Disorders. J Orofac Pain. 1996;10:240–53. [PubMed] [Google Scholar]
- [51].Yap AU, Dworkin SF, Chua EK, et al. Prevalence of temporomandibular disorder subtypes, psychologic distress, and psychosocial dysfunction in Asian patients. J Orofac Pain. 2003;17:21–8. [PubMed] [Google Scholar]