Abstract
Recent calibrated fMRI techniques using combined hypercapnia and hyperoxia allow the mapping of resting cerebral metabolic rate of oxygen (CMRO2) in absolute units, oxygen extraction fraction (OEF) and calibration parameter M (maximum BOLD). The adoption of such technique necessitates knowledge about the precision and accuracy of the model-derived parameters. One of the factors that may impact the precision and accuracy is the level of oxygen provided during periods of hyperoxia (HO). A high level of oxygen may bring the BOLD responses closer to the maximum M value, and hence reduce the error associated with the M interpolation. However, an increased concentration of paramagnetic oxygen in the inhaled air may result in a larger susceptibility area around the frontal sinuses and nasal cavity. Additionally, a higher O2 level may generate a larger arterial blood T1 shortening, which require a bigger cerebral blood flow (CBF) T1 correction. To evaluate the impact of inspired oxygen levels on M, OEF and CMRO2 estimates, a cohort of six healthy adults underwent two different protocols: one where 60% of O2 was administered during HO (low HO or LHO) and one where 100% O2 was administered (high HO or HHO). The QUantitative O2 (QUO2) MRI approach was employed, where CBF and R2* are simultaneously acquired during periods of hypercapnia (HC) and hyperoxia, using a clinical 3 T scanner. Scan sessions were repeated to assess repeatability of results at the different O2 levels. Our T1 values during periods of hyperoxia were estimated based on an empirical ex-vivo relationship between T1 and the arterial partial pressure of O2. As expected, our T1 estimates revealed a larger T1 shortening in arterial blood when administering 100% O2 relative to 60% O2 (T1LHO = 1.56±0.01 sec vs. T1HHO = 1.47±0.01 sec, P < 4*10−13). In regard to the susceptibility artifacts, the patterns and number of affected voxels were comparable irrespective of the O2 concentration. Finally, the model-derived estimates were consistent regardless of the HO levels, indicating that the different effects are adequately accounted for within the model.
Introduction
Recently, different groups have proposed that resting cerebral metabolic rate of O2 consumption (CMRO2) can be imaged using gas-based fMRI techniques [1–3]. Our team presented an approach, dubbed QUantitative O2 (QUO2) based on respiratory calibration of the BOLD signal, using hypercapnia (HC), and hyperoxia (HO). During the gas manipulation, end-tidal O2 (ETO2) and CO2 (ETCO2) levels are constantly monitored and a dual-echo version of pseudo-continuous Arterial Spin Labeling (de-pCASL) is used to measure BOLD and cerebral blood flow (CBF) simultaneously. ETO2, BOLD and CBF then serve as inputs to the generalized calibration model (GCM) described in Gauthier and Hoge [4], which yields a system of two equations with solutions for the BOLD calibration parameter M, i.e. the maximum BOLD signal increase when venous O2 saturation approaches 100%, and resting oxygen extraction fraction (OEF). The multiplication of OEF by baseline CBF and arterial O2 content (estimated from ETO2 monitoring and, optionally, blood testing) gives the estimated resting CMRO2 in micromoles of oxygen extracted from the cerebral vasculature per minute, per 100g of tissue.
While the initial proof-of-concept of the method produced reliable results when spatially averaged within the brain and over multiple subjects, it suffered from a single-subject instability characterized by large fluctuations in the modeled values and a considerable lack of solution in certain regions [1]. In order to be considered a reliable method for within-subject longitudinal studies, there was a need to improve the single-subject image quality. Additionally, prior to being able to draw conclusion about differences in resting oxidative metabolism between populations or between states of a disease, knowledge about the precision and accuracy of the model-derived estimates was crucial. The breathing circuit and image analysis strategy were updated in previous work [5–6]. The repeatability of the respiratory responses as well as CBF and BOLD responses within gray matter (GM) has also been assessed [7]. Finally, the question of methodological precision was evaluated by assessing the regional intra- and inter-subject variability of QUO2 derived estimates [6].
The choice of O2 and CO2 concentration during respective periods of HO and HC may also have an impact on the accuracy and precision of QUO2 derived estimates, which remains to be assessed. Higher CO2 concentration would have the advantage of increasing the image contrast-to-noise ratio due to higher CBF responses, however it can lead to anxiety and potentially alter brain physiology in ways other than the intended vasodilatory effect [8,9]. In a preliminary phase, it was agreed that the commonly employed 5% CO2 during HC blocks was low enough to preserve participant’s comfort, while high enough to yield significant cerebrovascular responses. As for the O2 concentration, compared to slight HO levels (e.g. 50–60%), more extreme levels of HO may bring the BOLD responses closer to the maximum M value, therefore diminishing the measurement errors while increasing the SNR. However, due to the paramagnetic characteristic of oxygen molecule, the measured signal may be prone to more prominent susceptibility artifacts patterns in vulnerable regions such as the frontal sinuses and nasal cavity, thus yielding inaccurate or non-solution values in those regions. An additional potential impact of the O2 concentration arises when changes in blood flow during HO are encompassed in the model, such as in the generalized calibrated model. Following a low HO level, CBF responses may be smaller than the inherent noise level of ASL acquisitions, making its measurement challenging. Furthermore, a decrease in CBF during periods of HO may reflect a combination of phenomena: a vasoconstrictive effect following a hyperventilation-induced decrease in ETCO2 [10], a vasoconstriction due to increased O2 per se, and an acceleration of arterial blood longitudinal relaxation (T1 shortening) caused by the increase of dissolved molecular oxygen in blood plasma [11–14]. If not taken into account, this T1 decay in arterial blood leads to an overestimation of CBF decrease during HO. As a consequence of those complications, it is common to assume a fixed, pre-determined CBF decrease [2,15–17]. However, assuming a fixed CBF decrease contributes to the systematic errors and can affect the accuracy and repeatability of OEF and CMRO2 estimates as reported in Lajoie et al [6]. Therefore, the application of a T1-correction on the measured CBF during HO is advocated.
Additionally, in theory, the QUO2-derived estimates should not depend on the level of hyperoxia induced, since the model is designed to account for this. In a previous study [6], the within-subject repeatability of the model-derived estimates was assessed based on very small variations of ETO2 during periods of 60% O2 hyperoxia. The effectiveness of the QUO2 model to obtain reproducible M, OEF and CMRO2 despite considerable variations in hyperoxia ETO2 is crucial and remains to be demonstrated.
The present study aims at exploring, in a small cohort of healthy individuals, the impact mentioned above, on QUO2 calibrated fMRI estimates, when providing 100% O2 during periods of HO instead of the previously provided 60% O2, in addition to verifying the reproducibility of results regardless of the inspired oxygen levels.
Materials and methods
From the group of eight healthy adults that underwent the 24 hour QUO2 test-retest study mentioned previously [6], six of them repeated the experiment, but this time, instead of being given 60% O2 during periods of HO (referred to as “lower HO levels protocol” (LHO)), the participants were given 100% O2 (“higher HO levels protocol” (HHO)). Each HO protocol was repeated to assess repeatability of results at the different O2 levels (referred to as “Test A” and “Test B”). To minimize effects of diurnal fluctuation in blood flow [18], all sessions were acquired between 2 PM and 6 PM. The participants were asked to abstain from caffeine 3 hours prior to scanning. All participants (3 females and 3 males, mean age: 30.5 ± 6.7 years) gave written informed consent and the project was approved by the Comité mixte d’éthique de la recherche du Regroupement Neuroimagerie/Québec.
Respiratory paradigm
A gas timing schedule previously described by Bulte et al [2], with a total duration of 18 minutes, was applied, as in [6]. This involves two 2-min periods of hypercapnia (HC) and two 3-min periods of hyperoxia (HO), induced by administering gas mixtures enriched with CO2 and O2 respectively. Hypercapnia was followed by a 1-min normocapnic period and then the 3-min hyperoxic stimulus. Hyperoxia was followed by a 3-min period of normoxia. Periods of normocapnia and normoxia were long enough to ensure a return to baseline as shown by the CBF and BOLD time course in Tancredi et al, figure 3 [7]. Participants inhaled the gas mixtures via a breathing circuit developed in-house [5]. During the first test-retest experiment [6], the hyperoxia periods were induced with the subjects breathing a mixture of 50% pure oxygen balanced with air, yielding a fix inspired O2 concentration of 60% O2. During the second test-retest experiment, the participants were given 100% O2 during periods of HO. Otherwise participants were given medical air to breath. Respiratory gases were continuously monitored using the CO2100C and O2100C modules of a BIOPAC MP150 system (BIOPAC Systems Inc., CA, USA). For additional details, see Lajoie et al. [6].
Image acquisition
Images were acquired on a clinical 3T MRI scanner (Siemens TIM TRIO, Siemens Medical Solutions, Erlangen, Germany) using the vendor’s 32-channel receive-only head coil. The scan session included a 5-minute anatomical acquisition (1 mm3 MPRAGE with TR/TE/flip angle = 2.3 seconds/3 msec/9°, 256x240 matrix, GRAPPA factor = 2), and an 18-minute functional scan using dual-echo pseudo-continuous ASL sequence (de-pCASL) [19] in order to acquire simultaneous measures of BOLD and CBF. The de-pCASL parameters were: TR/TE1/TE2/α = 4.12 seconds/8.4 msec/30 msec/90°, labeling duration = 2 seconds using Hanning window-shaped RF pulse with duration/space = 500 μsec/360 μsec, flip angle = 25°, peak gradient amplitude = 6 mT/m, mean gradient amplitude = 0.6 mT/m, label offset = 100 mm below the center of image slab, nominal and average post-labeling delay (PLD) = 0.9 and 1.44 seconds. The readout consisted of a GRE-EPI with GRAPPA factor = 2, partial sampling of k-space = 7/8, in-plane resolution of 4.5 x 4.5 mm2, 21 slices with 4.5 mm thickness and 0.45 mm gap.
Respiratory data analysis
Analysis of the respiratory data was carried out using an in-house program developed in Matlab (MathWorks, Natick, MA, USA), as in Lajoie et al [6]. An automatic extraction of the end-tidal (ET) and end-inspiratory points from the continuous O2 and CO2 traces was performed. Each ET point was corrected to account for the low-pass filtering effect of the filter placed in series and to account for an expired partial pressure of water of 47 mmHg [20]. More details about the respiratory data analysis can be found in Lajoie et al [6].
The average values of ETO2 at baseline and during both respiratory stimuli were used to compute arterial O2 content (ml O2/ml blood) and change in the venous deoxygenated fraction ([dHb]/[dHb]0) as in Chiarelli et al [14] and Gauthier et al [1]. The latter quantities are needed to obtain the BOLD calibrated value M, resting OEF and CMRO2 as specified below.
Imaging data analysis
Preprocessing
Analysis of functional scans along with exclusion of artifact and non-paranchymal voxels were performed using in-house software implemented in C, as in Lajoie et al [6].
During hyperoxic manipulation, the longitudinal relaxation time (T1) of blood is altered due to an increase in plasma concentration of paramagnetic O2 [13]. To account for this change in blood T1, that would bias the measured CBF changes, a corrective factor using the approach described in Chalela et al [21] and Zaharchuk et al [22] was applied. First, estimates of the arterial blood T1 values during hyperoxic periods were obtained based on the individual ETO2 measurements, used as a surrogate for arterial partial pressure of O2 (PaO2), along with the R1 (1/ T1) and PaO2 relationship in rats’ blood reported in Pilkinton et al [13]. Depending on whether our ETO2 values were within or outside the range of values in Pilkinton et al’s study, the T1 values were either linearly interpolated or extrapolated. Then, the individual blood flow maps during HO were corrected by applying a slice-wise corrective factor based on the quantitative blood flow equation [23], the slice acquisition time and the adjusted T1 value.
Computation of CMRO2
MRI measures of BOLD and CBF acquired during the hypercapnic manipulation, along with the changes in the venous deoxygenated fraction were used as inputs to the generalized calibration model (GCM), described in Gauthier and Hoge [4], yielding a functional curve (the “HC curve”) of possible pairings of M and OEF. Repeating the procedure with the hyperoxia measurements yielded a second curve of possible M and OEF pairings (the “HO curve”). The intersection of these two curves provided the true values of M and OEF at each voxel. Finally, CMRO2 was determined by multiplying OEF by O2 delivery, computed as the product of resting CBF by arterial O2 content. Since the small regional CBF responses to hyperoxia are difficult to measure due to the low SNR of ASL, a uniform change of CBF was assumed throughout the brain, based on the cortical gray matter change after T1 correction. Additional information about the computation of CMRO2 can be found in Lajoie et al [6].
Tissue segmentation
Automated segmentation of GM from the anatomical scans was carried out using the FMRIB Software Library (FSL) [24]. Structural images were extracted from T1-weighted scans using the brain extraction tool (FSL’s BET). Finally, a probability mask of GM was created employing the automated segmentation tool (FSL’s FAST), and was resampled to the resolution of the functional EPI scans.
Regions Of Interest (ROIs)
The model-derived estimates were evaluated throughout cortical GM as well as within six ROIs selected from the ICBM OASIS-TRT-20 atlas [25] and presented in Lajoie et al [6], figure 1: the inferior parietal, superior parietal, precuneus, hippocampus, anterior (caudal and rostral) cingulate and posterior cingulate. Each ICBM three-dimensional ROI was registered to the resolution of the functional EPI scans before being conjoined with the individual’s GM probability mask excluding voxels with a GM probability lower than 50% as well as non-parenchymal voxels previously identified. Additionally, voxels where the QUO2 model could not be solved were excluded when performing the ROI analysis of M, OEF and CMRO2. The resultant ROI probability masks were used to perform weighted averaging of the different measurements and estimates.
Registration
Individual ΔR2*HO, M, OEF and CMRO2 maps were non-linearly registered to the ICBM152 template using the CIVET software package [26] via the CBRAIN tool [27] with 12 degrees of freedom, as in Lajoie et al [6]. Test-averaged maps of ΔR2*HO were computed as arithmetic means using in-house software. Averaged maps of M, OEF and CMRO2 were obtained excluding any voxels where the QUO2 model could not be solved.
Analysis of sensitivity of model-derived QUO2 values to change in O2 concentration
The end-tidal O2, blood flow and R2* measurements during a hyperoxia manipulation depend on the employed O2 concentration. It was discussed that hyperoxia may also perturb the metabolism [28], however, in our model, we consider HO as an isometabolism challenge as assumed in numerous previous calibrated BOLD studies [1–3]. In order to understand the impact of lower and higher levels of HO (respectively LHO and HHO) to QUO2, we performed an analysis of the sensitivity of its model-derived parameters, M, OEF and CMRO2, to changes in ETO2, CBF and ΔR2*. Employing the GM group-average values in Test A during the LHO protocol, we kept constant the parameters not influenced by the O2 concentration, while individually varying ETO2HO, CBFHO and ΔR2*HO within their respective range delimited by GM group-average values in Test A under each HO protocol, to compute the resultant M, OEF and CMRO2.
Statistical analysis
For each model-derived estimate (M, OEF and CMRO2), we carried out a statistical analysis, using Matlab, on three different combinations of tests: 1) comparing Test A and Test B under the LHO protocol; 2) comparing Test A and Test B under the HHO protocol; 3) comparing tests A between both protocols. When needed, a two-tailed paired t-test was performed, considering a P < 0.05 level of significance, to detect any significant difference between tests and protocols. Within each protocol, we also investigated any difference across ROIs by pooling tests values and using family-wise error (FWE) correction for multiple comparisons, set at P < 0.05.
Prior to the analysis, statistical tests were performed on the data to ensure it satisfied the repeatability criteria: each distribution of difference between tests was evaluated for normality using the Shapiro-Wilk W-test, while the independence between the magnitude of difference and mean of measurements was verified using a rank correlation coefficient (Kendall’s τ). If the difference distribution appeared to deviate from a normal distribution, or if the magnitude of difference increased with the mean of measurements, the data were transformed on the log10 scale and the verification was repeated. In cases where the log10 scaled data satisfied the criteria, the repeatability was assessed on these scaled values. Otherwise, assessment of repeatability was based on the original values, as done in previous studies [29–32].
The next metrics were evaluated:
dSD, the standard deviation of the difference between tests measurements.
wsSD, the within-subject standard deviation, equals dSD/√2 considering two measurements.
wsCV, the within-subject (or intra-subject) coefficient of variation, as used in Floyd et al [30] and Chen et al [32]. wsCV = √[mean of the (wsSD/subject mean)2]. wsCV provides an unbiased measure of variability expressed as a percent of the mean with a low wsCV indicating a high reproducibility/repeatability. When data were on the log10 scale, wsCV was approximated by 10^(wsSD)-1 [33].
bsCV, the between-subject (or inter-subject) coefficient of variation as computed in Tjandra et al [34]. bsCV = SDpooledData / meanpooledData * 100.
Results
One participant reported a high level of anxiety during Test A of the LHO protocol, and the measured CBF response to CO2 was found to be twice the standard deviation of the group mean. Data from this participant has been excluded from the present analysis (as in the previous related work [6]).
Gas manipulation
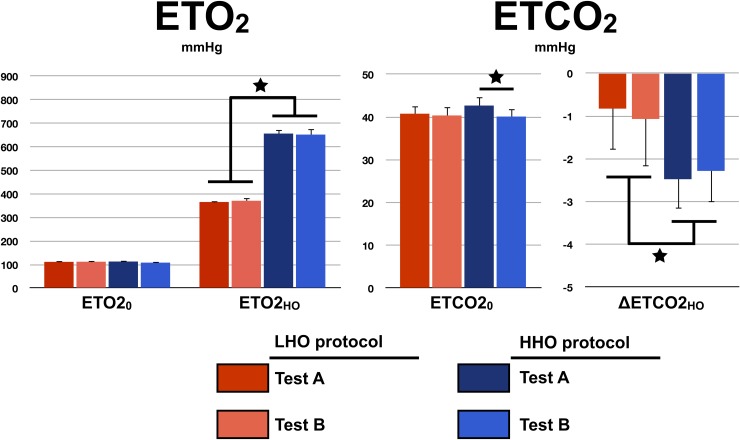
The test-average and standard deviation of end-tidal O2 and CO2 at baseline and during periods of hyperoxia are presented in Fig 1. No difference was found within and between protocols resting ETO2 (within-protocol: TestALHO = 112±7 mmHg vs. TestBLHO = 112±3 mmHg, P = 0.88, TestAHHO = 113±7 mmHg vs. TestBHHO = 108±7 mmHg, P = 0.05; between-protocol: LHO = 112±5 mmHg vs. HHO = 111±7 mmHg, P = 0.7). Within-protocol ETO2HO were identical (TestALHO = 366±6 mmHg vs. TestBLHO = 371±14 mmHg, P = 0.37; TestAHHO = 656±17 mmHg vs. TestBHHO = 652±25 mmHg, P = 0.42), whereas, as expected, between-protocol ETO2HO were found to be significantly different (LHO = 369±10 mmHg vs. HHO = 654±20 mmHg, P < 6*10−12). No difference was detected in between-protocol resting ETCO2 (LHO = 40±2 mmHg vs. HHO = 42±2 mmHg, P = 0.3), nor within the LHO protocol (TestALHO = 41±2 mmHg vs. TestBLHO = 40±2 mmHg, P = 0.57). However a significant difference in resting ETCO2 was observed between Test A and Test B under the HHO protocol (TestAHHO = 43±2 mmHg vs. TestBHHO = 40±2 mmHg, P < 0.002). This difference in resting ETCO2 is in agreement with a lower respiratory rate during Test A compared to Test B (TestAHHO = 6±2 breaths per minute vs. TestBHHO = 8±1 breaths per minute, P = 0.03). The ETCO2 changes observed during periods of hyperoxia were found to be equivalent within protocol. For the LHO protocol, they were: TestALHO = -0.8±1.0 mmHg and TestBLHO = -1.1±1.1 mmHg (P = 0.8), while for the HHO protocol they were: TestAHHO = -2.5±0.7 mmHg and TestBHHO = -2.4±0.7 mmHg (P = 0.5). The averaged decreases in ETCO2 were significantly (P < 0.005) larger in HHO compared to LHO protocol (LHO = -1.0±1.0 mmHg vs. HHO = -2.4±0.7 mmHg).
Fig 1. Gas manipulation.
For each protocol and test, the measured resting (with the subscript ‘0’) and hyperoxic (with the subscript ‘HO’) end-tidal O2 and CO2 are presented. Errors bars indicate standard deviation. A star indicates a significant difference at P < 0.05.
Susceptibility artifacts
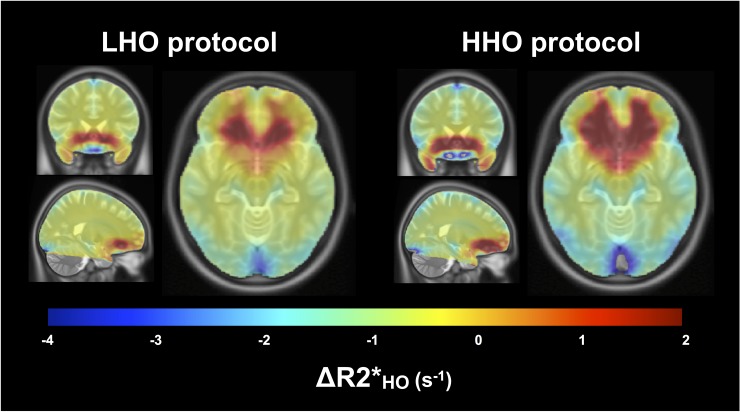
Fig 2 shows a qualitative examination of R2* changes during periods of HO (ΔR2*) through axial, sagittal and coronal views chosen in order to observe regions vulnerable to susceptibility artifacts. No masking, nor median filtering was performed on the functional maps prior to the non-linear registration to the ICBM template and maps average. The contrast window was chosen to facilitate the observation of increase in R2* characterized by orange and red colors. An overall R2* decrease (equivalent to a BOLD increase) in white and gray matter during HO is observed, which is more significant under the more extreme levels of HO. On the other hand, as a repercussion of the presence of paramagnetic oxygen molecules in inhaled air, both protocols presented comparable regions of susceptibility artifacts characterized by positive ΔR2* in voxels surrounding the nasal cavity. Percent of voxels in GM characterized by this increase were found to be the same in both protocols, with 12.8% under the LHO protocol and 11.7% under the HHO protocol (P = 0.25), although the positive values were generally higher under the HHO protocol (shown by darker red color). Any voxel affected by the susceptibility artifacts, later results in a non-solution voxel for M, OEF and CMRO2, and were therefore excluded from the analysis as mentioned in the methodology section.
Fig 2. Susceptibility artifacts.
For each protocol, the averaged maps of ΔR2* during HO are shown in coronal, sagittal and axial views, overlaying the ICBM152 template. The chosen contrast window facilitates the localization of voxels where an increase in R2* is observed (in orange and red). These increases in the transverse relaxation rate are most likely the results of susceptibility artifacts attributable to the presence of paramagnetic O2 in frontal sinuses and nasal cavity.
T1 shortening
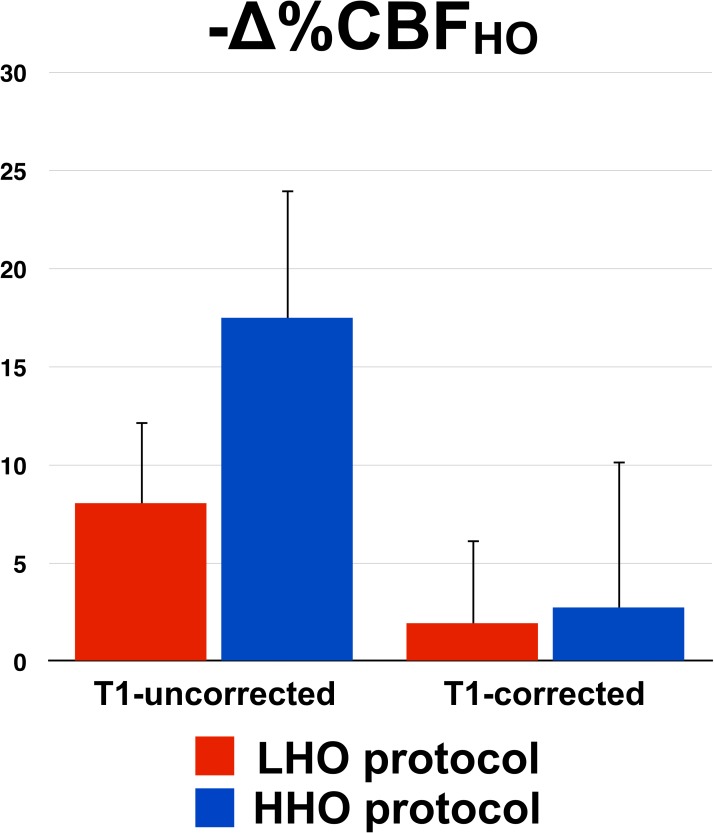
A value of 1.65 sec was assumed for the normoxic arterial blood T1 [35], whereas the estimated blood T1 shortening was larger during the high O2 hyperoxia state than during the low hyperoxia challenge: T1HHO = 1.47±0.01 sec vs. T1LHO = 1.56±0.01 sec, P < 4*10−13. Fig 3 summarizes, in both protocols, the GM tests average and standard deviation of blood flow decrease during HO before and after correction of blood T1. While uncorrected, CBFHO decrease was found to be significantly larger under the HHO protocol (LHO = -8.1±4.2 mmHg, HHO = -17.5±6.6 mmHg, P < 0.002). After T1 correction, CBFHO decreases were less pronounced in both protocols, and were not found significantly different from each other (LHO = -1.9±4.3 mmHg, HHO = -2.8±7.5 mmHg, P = 0.7) nor from zero (PLHO = 0.4, PHHO = 0.3).
Fig 3. T1 shortening.
For each protocol, pre- and post-T1-correction CBF changes during HO, averaged across tests, are presented with the standard deviation as error bars.
Analysis of sensitivity of model-derived QUO2 values to change in O2 concentration
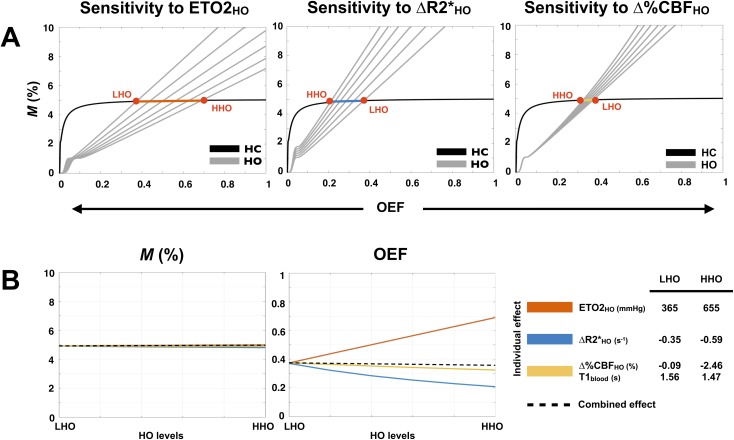
The individual impacts of changes in ETO2HO, ΔR2*HO and Δ%CBFHO, on M and OEF, as a function of the HO levels are examined by numerical simulations. These changes in ETO2HO, ΔR2*HO and Δ%CBFHO are dependent on one another and are examined in order to explain the combined impact on M and OEF. Results are summarized in Fig 4. Fig 4A shows the displacement in the HO curves caused by the respective variation of ETO2HO, ΔR2*HO and Δ%CBFHO, while Fig 4B shows the corresponding OEF and M solutions as a function of the individual (colored solid lines) and combined (dashed black lines) changes. Since the O2 concentration solely modulates the HO curve, which is shifted on the nearly horizontal section of the HC curve, the changes in ETO2HO, ΔR2*HO and Δ%CBFHO, either individual or combined, have virtually no impact on the M estimates. With respect to OEF, the individual impacts appear to cancel each other out, yielding a modest combined effect. The same conclusion stands for CMRO2, since it is the result of multiplying OEF by two measurements that are independent of the hyperoxic stimulus, i.e. the resting CBF and the resting arterial O2 content. Therefore, in principle, one would expect M, OEF and CMRO2 to remain stable, regardless of the O2 concentration used to produce hyperoxia. The following sections explore this assumption using real values computed in different ROIs, but also on a voxel-wise basis.
Fig 4. Analysis of sensitivity to O2 concentration.
Observed effects, on M and OEF, of changes in ETO2, R2* and CBF following a transition from a low level of hyperoxia (LHO) to a higher level of hyperoxia (HHO) are summarized. Estimates were based on group-averaged Test A measurements during the LHO manipulation, while ETO2, R2* and CBF were varied independently ranging from their respective LHO value to their HHO value (values are specified in the legend, with the corresponding blood T1 below CBFHO). The hypercapnia (HC) and hyperoxia (HO) curves resulting from the use of six different values of ETO2, R2* and CBF are presented (A). Each red dot represents the HC and HO curves intersection (hence one M and OEF solution) when either one of the extremity of the observed range is in use. The remaining M and OEF solutions lie on the colored line connecting both red dots. M and OEF estimates are presented as a function of the individual (colored lines) and combined (dashed black lines) effect of changes in ETO2, R2* and CBF (B).
Protocol-averaged estimates in ROIs
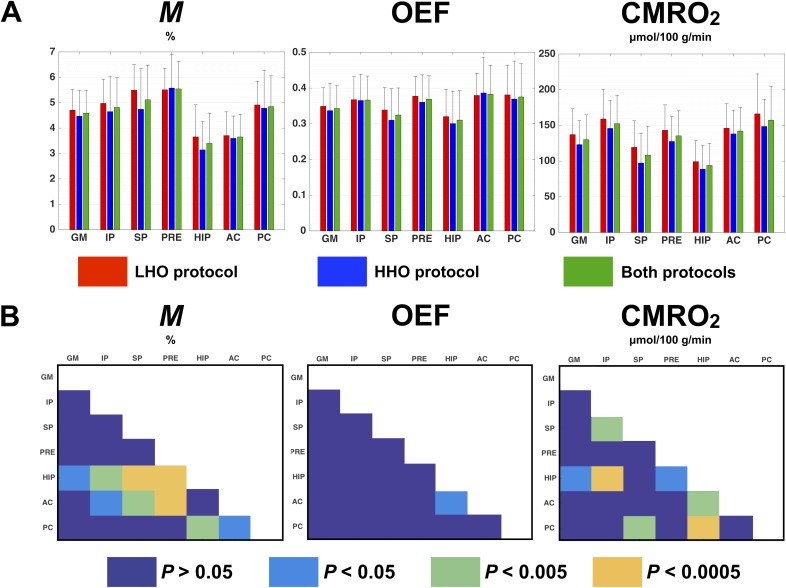
In Fig 5A are shown the ROI-averaged M, OEF and CMRO2 in each protocol (red and blue bars) and over both protocols (green bars). For each combination of model-derived estimate and ROI, we observe a good consistency between protocols with the lowest P values being: P = 0.17 in superior parietal for M, P = 0.37 in superior parietal for OEF and P = 0.06 in GM for CMRO2. Additionally, no apparent divergence was found in variance within each protocol. In Fig 5B are shown, for each estimate, the degree of difference between ROIs, when comparing the estimates averaged over both protocols and correcting for multiple comparisons (FWE set at P < 0.05). OEF estimates were found to be similar across ROIs, with the exception between hippocampus and anterior cingulate where a significant difference was detected (P = 0.04). Values of M and CMRO2 in hippocampus were found to be the smallest compared with the other ROIs, with the exception of anterior cingulate (for M) and superior parietal (for CMRO2).
Fig 5. Protocol-averaged estimates in ROIs.
M, OEF and CMRO2 estimates averaged in different ROIs are compared. Fig 5-A presents the ROI-averaged value and standard deviation obtained under the LHO protocol (red bar), the HHO protocol (blue bar) and in both protocols averaged (green bar). Fig 5-B shows, for each estimate, any significant difference observed between ROIs after correcting for multiple comparisons (FWE, P < 0.05): dark blue indicates an absence of significant difference (P > 0.05), while light blue (P < 0.05), green (P < 0.005) and orange (P < 0.0005) illustrate a significant difference between two ROIs (represented in the X and Y axis). GM = gray matter, IP = inferior parietal, SP = superior parietal, PRE = precuneus, HIP = hippocampus, AC = anterior cingulate, PC = posterior cingulate.
Within-subject variability in ROIs
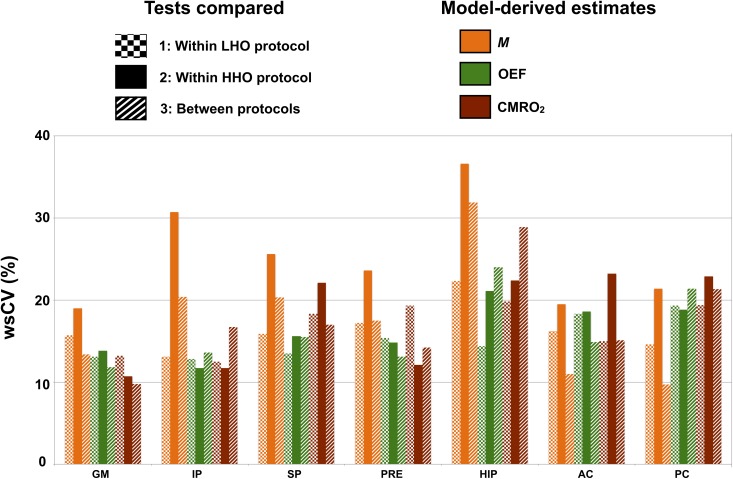
Fig 6 presents the within-subject coefficients of variation (wsCV) in every ROIs for M, OEF and CMRO2. WsCVs were computed for three combinations of tests: 1) test A vs. B under the LHO protocol, 2) test A vs. B under the HHO protocol, 3) tests A between both HO protocols. Across all ROIs, M was found to have a lower within-subject variability under the LHO protocol (mean wsCVLHO = 16%, mean wsCVHHO = 25%, P = 0.006). On the other hand, within-subject variability of OEF and CMRO2 were found unchanged regardless of the HO protocol (OEF: mean wsCVLHO = 15%, mean wsCVHHO = 16%, P = 0.2; CMRO2: mean wsCVLHO = 17%, mean wsCVHHO = 18%, P = 0.6).
Fig 6. Within-subject variability in ROIs.
Computed within-subject CVs (wsCV) are shown for M, OEF and CMRO2 within each ROI. The model-derived estimates are represented by different colors, while the three combinations of tests are identified by distinct patterns: 1: Test A vs. B under the LHO protocol (squared pattern), 2: Test A vs. B under the HHO protocol (plain pattern), 3: Tests A between both HO protocols (striped pattern). GM = gray matter, IP = inferior parietal, SP = superior parietal, PRE = precuneus, HIP = hippocampus, AC = anterior cingulate, PC = posterior cingulate.
Parametric maps
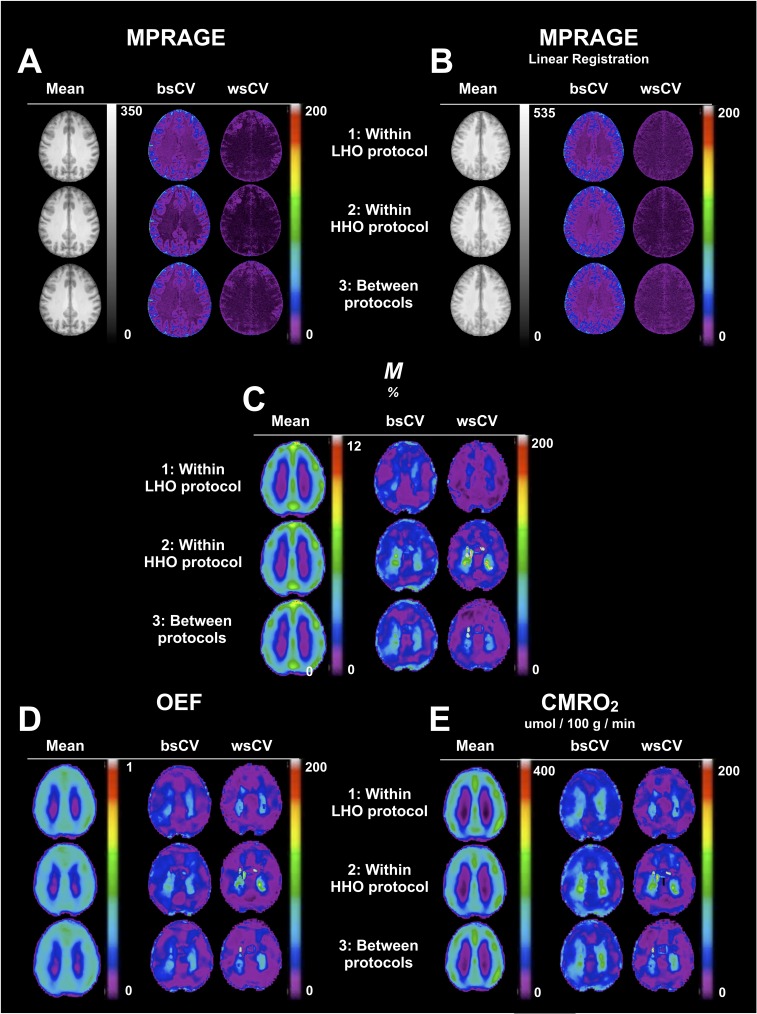
In Fig 7, we present, for each combination of tests (1: Test A vs. B under the LHO protocol, 2: Test A vs. B under the HHO protocol, 3: Tests A between both HO protocols), mean tests, between- and within-subject CV maps of M, OEF and CMRO2. All functional maps were non-linearly registered (NLreg) to the ICBM space. In addition to intrinsic physiological changes, errors in measurements and head movements occurring between the anatomical and the functional scans, a voxel-wise within-subject repeatability may be partly affected by random inaccuracies in registration. In order to evaluate any limitation on the voxel-wise repeatability caused by the registration to the ICBM space, we present the CVs maps for MPRAGE, and verify if any enhancement was possible thanks to the non-linearly registration of our maps (Fig 7A), compared to the linearly registered MPRAGE (Fig 7B). All CVs maps are shown using a window level of 0–200%. At these levels, the passage from 20% to 30% is characterized by the transition from purple to blue, with 30% being an approximate upper limit for what is considered as low variability. Compared to the linearly registered maps (Lreg), the non-linearly registered (NLreg) MPRAGE maps presented a better defined gray matter region, while whole-brain between- and within-subject variability were found to be lower. WsCV values in NLreg were generally found to be <5% in WM, <10% in GM and exceptionally <20% in few small regions, whereas in Lreg wsCV, values were <10% in WM and GM and <20% in with few small regions. Mean maps of M, OEF and CMRO2 (Fig 7C, 7D and 7E) qualitatively exhibited an absence of dependency on the O2 protocol employed. CVs maps of M presented slightly less variability under the LHO than the HHO. All three estimates were found to have low GM within-subject variability for the three combinations of tests (<30%). M and CMRO2 presented a clearer distinction between the population variance and the within-subject variability, whereas OEF was found to have a lower voxel-wise between-subject variability, approaching the within-subject variability.
Fig 7. Parametric maps.
For each combination of tests (1: Test A vs. B under the LHO protocol, 2: Test A vs. B under the HHO protocol, 3: Tests A between both HO protocols), mean tests, between-subject CV and within-subject CV maps for M (C), OEF (D) and CMRO2 (E) are shown in one axial slice. Maps were non-linearly registered to the ICBM152 template. As a reference, the equivalent information is presented for MPRAGE maps non-linearly (A) and linearly registered (B) to the template.
Discussion
Performing an analysis of individual impacts, on M and OEF, of variation in ETO2HO, ΔR2*HO and Δ%CBFHO, we have shown how little M is affected by the O2 concentration in GM, and how the individual impacts on OEF were practically cancelling out, yielding a nearly nonexistent combined impact on OEF and therefore on CMRO2. Exploring the within-subject reproducibility in different ROIs as well as on a voxel-wise basis, we observed an unchanged reproducibility for OEF and CMRO2 regardless of differences in ETO2HO, ΔR2*HO and Δ%CBFHO caused by a distinct O2 concentration in inhaled gas. On the other hand, the M within-subject repeatability was found to be slightly enhanced under the LHO protocol. No significant difference was found between protocol-averaged values.
In certain situations, the differences between subjects’ brain anatomy are such that a linear transformation is insufficient to register their brain maps on to standard spaces. The local deformations produced by the non-linear registration improve the match. The comparison of linearly versus non-linearly registered individual MPRAGE images provides a qualitative example of the improvement brought by the non-linear registration. The method produced sharper group-averaged maps, characterized by more distinct sulci and more accentuated grey/white matter contrast. Quantitatively, the non-linear co-registration afforded lower CV values.
The presence of paramagnetic molecular oxygen in inhaled air produces susceptibility artifacts. We examined regions vulnerable to those artifacts such as the frontal sinuses and nasal cavity of our ΔR2*HO maps. However, no evidence of enlarged patterns of susceptibility artifacts under inhalation of 100% O2 (HHO) compared to 60% (LHO) was found, thus yielding a comparable percent of non-solution voxels in GM for both protocols.
Rather than assuming a fixed value of CBF change during HO, the individual T1-corrected Δ%CBFHO averaged in GM was used, therefore capturing any intra-subject variation between Test A and Test B in blood flow during HO. Our T1 values were extrapolated from experimentally-determined values in animal model, which is a common practice in calibrated fMRI approaches. Human blood constitution is similar to that of bovine and rat blood and is likely to experience comparable T1 shortening during the hyperoxia stimulus [13,36,37]. This is of course an assumption and represents a potential source of confounds in our blood flow changes calculations.
In CBF quantification, so long as the PLD is equal to or higher than the arterial transit time (ATT), the exact ATT value does not matter. In our 2D acquisition, the first and last slices are acquired after a delay of 900 msec and 1986 msec respectively, resulting in a brain-averaged PLD of 1443 msec. Donahue et al. [38] applied a pCASL in a cohort of healthy volunteers (mean age of 30 ± 4 years) and obtained a group-averaged ATT lower than 900 msec within each lobe, including within the occipital lobe with 834 ± 29 msec. We therefore believe that in the large majority of cases, the acquired ASL signal was accurately reflecting CBF and that an increase in our PLD would have resulted in a loss in SNR, especially during hypercapnic where the ATT is known to diminish [38]. Additionally, the ATT increase during HO should be minor as our data indicates that the CBF decreases induced by hyperoxia, even at high O2 concentrations, are not substantial. When using a 2D acquisition in a population of elderly or unhealthy patients, it would be recommended to increase the PLD slightly while also imaging a lower number of thicker slices, as in the study De Vis et al. (2015) where a nominal PLD of 1550 msec and 11 slices with 7 mm slice thickness were employed.
Small cohort sizes like that of the present study have been common in recent years, particularly for complex fMRI protocols with greater physiological specificity than the classic BOLD contrast. Despite the relatively small sample size, which limits confidence in the statistical significance of our findings, the present study provides new information on the impact of inspired oxygen levels on calibrated fMRI technique.
To conclude, it was revealed that the pattern of susceptibility artifacts under hyperoxia was comparable regardless of the HO levels. We also demonstrated that variations in ETO2HO, CBFHO and R2*HO were accounted for within the QUO2 model, resulting in an unchanged ROI-averaged M, OEF and CMRO2 estimates. We observed that the within-subject repeatability was either unchanged (for OEF and CMRO2) or slightly enhanced under the LHO protocol (for M). In summary, the use of a higher hyperoxic challenge revealed no beneficial impact on the calibrated fMRI measurements, while a reduced concentration of 60% O2 was shown to maintain sufficient BOLD contrast and to produce consistent model-derived results.
Acknowledgments
The authors would like to thank Scott Nugent, Marius Tuznik, Bahare Sabouri, Carollyn Hurst and André Cyr for their excellent technical assistance and insightful discussions. Jiongjiong Wang at UCLA is acknowledged for providing the pseudo-continuous arterial spin-labeling sequence.
Data Availability
Our local ethics committee does not allow the deposition of raw imaging data into publicly accessible repositories. Anonymized raw data will nevertheless be made available upon request, subject to review and approval by the Comité d’éthique de la recherche vieillissement-neuroimagerie. Please address requests for data access to data.requests@bic.mni.mcgill.ca.
Funding Statement
This study was supported by the Canadian Institutes for Health Research (http://www.cihr-irsc.gc.ca/e/193.html, MOP 84378), received by RDH; the Canadian Foundation for Innovation, Leaders Opportunity (https://www.innovation.ca/, Fund 17380), received by RDH; the Canadian National Sciences and Engineering Research Council (http://www.nserc-crsng.gc.ca/index_eng.asp, R0018142), received by RDH; the Consortium québécois sur la découverte du médicament (CQDM, http://www.cqdm.org/en/, 8534), received by RDH; and the Fonds Québécois de Recherche Nature et technologies (FQRNT, http://www.frqnt.gouv.qc.ca/accueil), received by IL. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Gauthier CJ, Desjardins-Crépeau L, Madjar C, Bherer L, Hoge RD. Absolute quantification of resting oxygen metabolism and metabolic reactivity during functional activation using QUO2 MRI. NeuroImage. 2012;63:1353–1363. 10.1016/j.neuroimage.2012.07.065 [DOI] [PubMed] [Google Scholar]
- 2.Bulte DP, Kelly M, Germuska M, Xie J, Chappell MA, Okell TW, et al. Quantitative measurement of cerebral physiology using respiratory-calibrated MRI. NeuroImage. 2012;60:582–591. 10.1016/j.neuroimage.2011.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wise RG, Harris AD, Stone AJ, Murphy K. Measurement of OEF and absolute CMRO2: MRI-based methods using interleaved and combined hypercapnia and hyperoxia. NeuroImage. 2013;83:135–147. 10.1016/j.neuroimage.2013.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gauthier CJ, Hoge RD. A generalized procedure for calibrated MRI incorporating hyperoxia and hypercapnia. Hum. Brain Mapp. 2012;34:1053–1069. 10.1002/hbm.21495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tancredi FB, Lajoie I, Hoge RD. A simple breathing circuit allowing precise control of inspiratory gases for experimental respiratory manipulations. BMC Res Notes. 2014;7:235 10.1186/1756-0500-7-235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lajoie I, Tancredi FB, Hoge RD. Regional Reproducibility of BOLD Calibration Parameter M, OEF and Resting-State CMRO2 Measurements with QUO2 MRI. Hendrikse J, editor. PLoS ONE. 2016;11(9):e0163071 10.1371/journal.pone.0163071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tancredi FB, Lajoie I, Hoge RD. Test-retest reliability of cerebral blood flow and blood oxygenation level-dependent responses to hypercapnia and hyperoxia using dual-echo pseudo-continuous arterial spin labeling and step changes in the fractional composition of inspired gases. J. Magn. Reson. Imaging. 2015;42:1144–1157. 10.1002/jmri.24878 [DOI] [PubMed] [Google Scholar]
- 8.Brannan S, Liotti M, Egan G, Shade R, Madden L, Robillard R, et al. Neuroimaging of cerebral activations and deactivations associated with hypercapnia and hunger for air. Proc. Natl. Acad. Sci. U.S.A. 2001. February 13;98(4):2029–2034. 10.1073/pnas.98.4.2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liotti M, Brannan S, Egan G, Shade R, Madden L, Abplanalp B, et al. Brain responses associated with consciousness of breathlessness (air hunger). Proc. Natl. Acad. Sci. U.S.A. 2001. February 13;98(4):2035–2040. 10.1073/pnas.98.4.2035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iscoe S, Fisher JA. Hyperoxia-induced hypocapnia: an underappreciated risk. Chest. 2005;128:430–433. 10.1378/chest.128.1.430 [DOI] [PubMed] [Google Scholar]
- 11.Tadamura E, Hatabu H, Li W, Prasad PV, Edelman RR. Effect of oxygen inhalation on relaxation times in various tissues. J. Magn. Reson. Imaging. 1997;7:220–225. [DOI] [PubMed] [Google Scholar]
- 12.Noseworthy MD, Kim JK, Stainsby JA, Stanisz GJ, Wright GA. Tracking oxygen effects on MR signal in blood and skeletal muscle during hyperoxia exposure. J. Magn. Reson. Imaging. 1999;9:814–820. [DOI] [PubMed] [Google Scholar]
- 13.Pilkinton DT, Hiraki T, Detre JA, Greenberg JH, Reddy R. Absolute cerebral blood flow quantification with pulsed arterial spin labeling during hyperoxia corrected with the simultaneous measurement of the longitudinal relaxation time of arterial blood. Magn. Reson. Med. 2011;67:1556–1565. 10.1002/mrm.23137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma Y, Berman AJL, Pike GB. The effect of dissolved oxygen on the relaxation rates of blood plasma: Implications for hyperoxia calibrated BOLD. Magn. Reson. Med. 2016;76(6):1905–1911. 10.1002/mrm.26069 [DOI] [PubMed] [Google Scholar]
- 15.Chiarelli PA, Bulte DP, Wise R, Gallichan D, Jezzard P. A calibration method for quantitative BOLD fMRI based on hyperoxia. NeuroImage. 2007;37:808–820. [DOI] [PubMed] [Google Scholar]
- 16.Goodwin JA, Vidyasagar R, Balanos GM, Bulte D, Parkes LM. Quantitative fMRI using hyperoxia calibration: Reproducibility during a cognitive Stroop task. NeuroImage. 2009;47:573–580. 10.1016/j.neuroimage.2009.04.064 [DOI] [PubMed] [Google Scholar]
- 17.Bulte DP, Chiarelli PA, Wise RG, Jezzard P. Cerebral perfusion response to hyperoxia. J Cereb Blood Flow Metab. 2006;27:69–75. 10.1038/sj.jcbfm.9600319 [DOI] [PubMed] [Google Scholar]
- 18.Parkes LM, Rashid W, Chard DT, Tofts PS. Normal cerebral perfusion measurements using arterial spin labeling: Reproducibility, stability, and age and gender effects. Magn. Reson. Med. 2004;51:736–743. 10.1002/mrm.20023 [DOI] [PubMed] [Google Scholar]
- 19.Dai W, Garcia D, de Bazelaire C, Alsop DC. Continuous flow-driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magn. Reson. Med. 2008;60:1488–1497. 10.1002/mrm.21790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Severinghaus JW. Water vapor calibration errors in some capnometers: respiratory conventions misunderstood by manufacturers? Anesthesiology. 1989;70:996–998. [DOI] [PubMed] [Google Scholar]
- 21.Chalela JA, Alsop DC, Gonzalez-Atavales JB, Maldjian JA, Kasner SE, Detre JA. Magnetic Resonance Perfusion Imaging in Acute Ischemic Stroke Using Continuous Arterial Spin Labeling. Stroke. 2000;31:680–687. [DOI] [PubMed] [Google Scholar]
- 22.Zaharchuk G, Martin AJ, Dillon WP. Noninvasive Imaging of Quantitative Cerebral Blood Flow Changes during 100% Oxygen Inhalation Using Arterial Spin-Labeling MR Imaging. American Journal of Neuroradiology. 2008;29:663–667. 10.3174/ajnr.A0896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang J, Alsop DC, Song HK, Maldjian JA, Tang K, Salvucci AE, et al. Arterial transit time imaging with flow encoding arterial spin tagging (FEAST). Magn. Reson. Med. 2003;50:599–607. 10.1002/mrm.10559 [DOI] [PubMed] [Google Scholar]
- 24.Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. [DOI] [PubMed] [Google Scholar]
- 25.Klein A, Tourville J. 101 labeled brain images and a consistent human cortical labeling protocol. Front. Neurosci. 2012;6:171 10.3389/fnins.2012.00171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collins DL, Holmes CJ, Peters TM, Evans AC. Automatic 3-D Model-Based Neuroanatomical Segmentation. Hum. Brain Mapp. 1995;3:190–208. [Google Scholar]
- 27.Sherif T, Rioux P, Rousseau M-E, Kassis N, Beck N, Adalat R, et al. CBRAIN: a web-based, distributed computing platform for collaborative neuroimaging research. Front. Integr. Neurosci. 2014;8:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu F, Liu P, Pascual JM, Xiao G, Lu H. Effect of hypoxia and hyperoxia on cerebral blood flow, blood oxygenation, and oxidative metabolism. J Cereb Blood Flow Metab. 2012;32:1909–1918. 10.1038/jcbfm.2012.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Padhani AR, Hayes C, Landau S, Leach MO. Reproducibility of quantitative dynamic MRI of normal human tissues. NMR Biomed. 2002;15:143–153. [DOI] [PubMed] [Google Scholar]
- 30.Floyd TF, Ratcliffe SJ, Wang J, Resch B, Detre JA. Precision of the CASL-perfusion MRI technique for the measurement of cerebral blood flow in whole brain and vascular territories. J. Magn. Reson. Imaging. 2003;18:649–655. 10.1002/jmri.10416 [DOI] [PubMed] [Google Scholar]
- 31.Jain V, Duda J, Avants B, Giannetta M, Xie SX, Roberts T, et al. Longitudinal reproducibility and accuracy of pseudo-continuous arterial spin-labeled perfusion MR imaging in typically developing children. Radiology. 2012;263:527–536. 10.1148/radiol.12111509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Y, Wang DJJ, Detre JA. Test-retest reliability of arterial spin labeling with common labeling strategies. J. Magn. Reson. Imaging. 2011;33:940–949. 10.1002/jmri.22345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bland JM, Altman DG. Measurement error proportional to the mean. BMJ. 1996;313:106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tjandra T, Brooks JCW, Figueiredo P, Wise R, Matthews PM, Tracey I. Quantitative assessment of the reproducibility of functional activation measured with BOLD and MR perfusion imaging: Implications for clinical trial design. NeuroImage. 2005;27:393–401. 10.1016/j.neuroimage.2005.04.021 [DOI] [PubMed] [Google Scholar]
- 35.Lu H, Clingman C, Golay X, van Zijl PCM. Determining the longitudinal relaxation time (T1) of blood at 3.0 Tesla. Magn. Reson. Med. 2004;52(3):679–682. 10.1002/mrm.20178 [DOI] [PubMed] [Google Scholar]
- 36.Benga G, Borza T. Diffusional water permeability of mammalian red blood cells. Comp. Biochem. Physiol. B, Biochem. Mol. Biol. 1995;112(4):653–659. [DOI] [PubMed] [Google Scholar]
- 37.Lin A-L, Qin Q, Zhao X, Duong TQ. Blood longitudinal (T 1) and transverse (T 2) relaxation time constants at 11.7 Tesla. Magn Reson Mater Phy. 2011;25(3):245–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Donahue MJ, Faraco CC, Strother MK, Chappell MA, Rane S, Dethrage LM, et al. Bolus arrival time and cerebral blood flow responses to hypercarbia. J Cereb Blood Flow Metab. 2014;34(7):1243–1252. 10.1038/jcbfm.2014.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Our local ethics committee does not allow the deposition of raw imaging data into publicly accessible repositories. Anonymized raw data will nevertheless be made available upon request, subject to review and approval by the Comité d’éthique de la recherche vieillissement-neuroimagerie. Please address requests for data access to data.requests@bic.mni.mcgill.ca.