Abstract
Background
The effects of body mass index (BMI) and reproductive factors may vary among breast cancer molecular subtypes, evidence of which is lacking in East Asia.
Methods
From 2002 to 2010, 1256 breast cancer patients and 1416 healthy women were recruited. Anthropometric and reproductive factors were collected from medical charts. Breast cancer subtype was defined by ER, PR, and HER2 status. Polytomous logistic regression was used to evaluate associations between risk factors and breast cancer subtypes, with subgroup analysis by menopausal status. A meta-analysis of relevant published studies in East Asia was also performed.
Results
In our case-control study, late menarche was negatively associated with luminal tumor risk (Ptrend = 0.03). Higher BMI was associated with risk of both luminal and triple-negative tumors (Ptrend<0.001). Late age at first live birth was associated with a 1.41- to 2.08-fold increased risk of all subtypes, while late menopause increased risk by 2.62–5.56 times. Heterogeneity of these associations was not detected for different menopausal statuses. The meta-analysis revealed a positive dose-response relationship between BMI and risk of both luminal and ER-PR- subtypes (Ptrend<0.05). Early menarche and nulliparity increased luminal tumor risk by 1.39 and 1.26 times, respectively. Non-breastfeeding also increased the risk of all subtypes.
Conclusions
For East Asian women, overweight, late menopause, and lack of breastfeeding appear to increase risk of both luminal and ER−PR− tumors. Early menarche and nulliparity mainly impacted luminal tumor risk. These associations were not impacted by menopausal status.
Keywords: Breast cancer, East Asia, Body mass index, Reproductive factor, Molecular subtype
Highlights
-
•
For East Asian women, overweight increases risk of both luminal and ER-PR- tumors.
-
•
For East Asian women, early menarche increases luminal tumor risk.
-
•
For East Asian women, nulliparity increases luminal tumor risk.
Introduction
Breast cancer is the most common cancer among women worldwide, with an age-adjusted incidence of 43.3 cases per 100,000 people. Although the incidence of breast cancer is lower in East Asia compared with the West, there has been a rising trend in recent years, with an incidence in 2012 of 27.0 cases per 100,000 people.1
The heterogeneity of breast cancer has been widely recognized in the past decades. Subtypes include luminal, HER2-enriched, and basal-like types, based on global gene expression analyses.2 Breast cancer subtypes carry distinct clinicopathologic characteristics and prognoses, which suggests heterogeneous etiologies.3, 4, 5, 6, 7, 8, 9 Previous studies showed that women's body size10 and reproductive factors, including early menarche, late menopause, nulliparity, late age at first live birth, and no lactation, had a relatively pronounced effect on elevating luminal tumor risk,11 while several recent studies have found a significant association between these factors and triple-negative tumors.10, 11 However, the results of epidemiological studies have not been consistent.
Breast cancer risk factors are known to be distributed differently depending on menopausal status and race.12 Distribution differences in molecular subtypes among women of different menopausal status and ethnicity have also been demonstrated previously.13 Compared with white women, for example, East Asian women have a higher risk of triple-negative and HER2-enriched tumors.14 Luminal tumors are more commonly diagnosed among postmenopausal Caucasian women in Western countries,4 but conversely are more prevalent among premenopausal women in East Asia.15
The difference in subtype distribution between white and black women has been intensively studied in Western countries, while little data are available for East Asian women, especially in China, where the incidence of breast cancer rose from 20.13 to 42.55 cases per 100,000 people from 2005 to 2009.16, 17 The few Chinese studies that have been published reported inconsistent results for associations between common risk factors and different tumor subtypes, and these studies did not evaluate possible associations in groups of women with differing menopausal statuses.15, 18, 19 Furthermore, relevant studies from China were all conducted in the eastern part of the country, while China's western regions have disparate distributions of body size and reproductive factors due to varying levels of economic development.13
Thus, we conducted this population-based case-control study in southwestern China to evaluate associations among BMI, reproductive factors, and breast tumor subtypes. Subgroup analysis was performed by menopausal status. We also conducted a meta-analysis to assess these associations among East Asian women. Our results supply some of the first data on these associations from less developed areas of China, and our meta-analysis is the first to pool the results of relevant studies in East Asia.
Methods
Study subjects
From 2002 to 2010 at Sichuan Cancer Hospital, 1256 pathologically and newly confirmed invasive breast cancer cases with molecular subtypes diagnosed in breast surgery were enrolled in the study. To form the control group, 1416 healthy women undergoing routine physical examinations during the same time period were randomly selected and frequency-matched to the cases by age. The controls were recruited at the Chengdu Children's and Women's Hospital, the leading institution providing medical treatment and health care for women and children in Sichuan Province, which supplies routine health examinations to women in the area. Controls with any known malignancy or mental disorder were excluded. The study protocol was approved by the institutional review boards of Sichuan University, Chengdu Children's and Women's Hospital, and Sichuan Cancer Hospital.
Data collection
Information on breast cancer risk factors was collected from the participants' medical records. Specifically, height and weight were measured, and information on other risk factors, including age at diagnosis, menopausal status, age at menarche, age at menopause, age at first live birth, parity, and breastfeeding were collected during interviews by trained nurses when the participants were admitted to the hospital for the first time or during the physical examination. Women who had lactated for at least 1month were classed as “ever breastfed.” A woman was defined as postmenopausal if she had undergone natural menopause (if she had experienced 12 continuous months without a menstrual cycle before any endocrine treatment or oophorectomy), bilateral oophorectomy, or irradiation of the ovaries before 50 years of age. If a woman was aged 50 or older and no longer experienced menstruation, she was also considered postmenopausal. BMI was calculated as body weight (kg)/height2 (m2) and used as a measure of general obesity. The response rates for both cases and controls were 100%.
Biomarker detection
The ER, PR, and HER2 status of the patients was extracted from medical records. Tumor type was determined using immunohistochemical (IHC) assay at the pathology department of Sichuan Cancer Hospital. Positive ER and PR status was defined as ≥1% of tumor cells presenting positive nuclear staining. The results of HER2 were scored semi-quantitatively according to the estimated percentage of positively stained tumor cell nuclei and the intensity of nuclear staining (−for no staining, +1 for weak intensity, +2 for intermediate intensity, and +3 for strong intensity). Results of “−” or “+1” were classed as HER2 negative and “+3” as positive.20 A fluorescence in situ hybridization (FISH) test was recommended to “+2” patients to determine the HER2 expression status in the breast tumor. In our study, 20% of “+2” patients opted for the FISH test. Considering that less than 20% of women with “+2” were diagnosed as HER2 positive by FISH, we treated those who did not opt for FISH as HER2 negative.21
All the patients were grouped into one of the following three categories: luminal (ER+ or PR+, HER2+ or HER2-), HER2-enriched (ER−, PR−, HER2+), or triple-negative (ER−, PR−, HER2−).
Data analysis
BMI was categorized using the World Health Organization (WHO) definition of <18.5 kg/m2 as underweight, 18.5–24.9 kg/m2 as normal, 25–29.9 kg/m2 as overweight, and ≥30 kg/m2 as obese. We combined the overweight and obese groups due to the low proportion of obesity in the subjects (2%). Age at menarche and age at first live birth were divided into two categories according to common cutoff values reported previously.22 Due to the small number of nulliparous women (<5%), we did not include parity in the regression analysis. In the subgroup analysis performed according to menopausal status, all continuous variables were categorized into two levels due to the limited sample size in tumor subgroups. One-way analysis of variance and chi-square tests were used to compare the distribution of selected factors between controls and subgroups of cases. The distribution differences between groups were measured using trend chi-square tests. Polytomous logistic regression, which is suitable for multiple outcomes data, was used to evaluate the associations between BMI, reproductive factors, and risks for molecular subtypes. The weighted least square (WLS) method was used to test the heterogeneity of associations between risk factors and breast cancer subtypes. Data were analyzed with SPSS 17.0 software (IBM Corp, Armonk, NY, USA). All p-values were subjected to a two-tailed test, with an alpha level of 0.05 for significance testing.
Meta-analysis
Literature search
We searched for studies on the relationship between common factors and risk of breast cancer molecular subtypes among East Asian women in PubMed, Ovid, the Chinese Biomedical Literature Analysis and Retrieval System, and the Chinese National Knowledge Infrastructure for the period up to August 2015 using the following keyword combinations: (‘‘risk factors’’ OR “reproductive factors” OR “body mass index”) AND (‘‘breast cancer’’ OR “breast tumor” OR “breast malignancy”) AND (“subtypes” OR “hormonal status”) AND (‘‘East Asia’’ OR ‘‘China’’ OR “Japan” OR “Korea” OR “Mongolia”). In addition, we checked the references of relevant papers for citations of similar studies. We included in our analysis studies with molecular subtypes determined by joint ER/PR status or ER/PR/HER2 status. Only studies with sufficient data to estimate an odds ratio (OR) with 95% confidence interval (CI) were included. If multiple publications reported the same or overlapping data, the publication with the largest sample size or most recent publication was selected for inclusion.
Quality assessment
The Newcastle-Ottawa Quality Assessment Scale was used to assess the validity of the selected studies. Two of the items were not applicable for evaluating the included studies: (a) ‘‘structured interview where blind to case/control status’’; and (b) “same method of ascertainment for cases and controls” (the invasive histopathological diagnostic procedure is unsuitable for healthy control subjects). Thus, a total of six criteria with seven scores were used in the quality assessment. A study that met six or seven criteria was ranked as “A”, a study that met four or five criteria was ranked “B”, and a study was ranked “C” if it met fewer than four criteria.
Data extraction
For each eligible study, the following information was extracted: the first author's name, year of publication, country of origin, study design, menopausal status of cases, year subjects were recruited, sample size, tumor subtypes, matched factors, and adjusted confounding factors. The literature search, quality assessment, and data extraction were independently performed by two researchers. If divergence existed, they discussed with the third investigator and came to a final decision.
Statistical analysis
Pooled ORs and 95% CIs for the associations between BMI, reproductive factors, and breast cancer molecular subtypes were calculated via dividing the observed frequencies of categories by the unified cutoff values of eligible studies. Heterogeneity among the included articles was estimated by a chi-square-based Q-test. If significant heterogeneity was not detected (P > 0.05), the Mantel-Haenszel method was used to estimate the pooled OR and 95% CI. Otherwise, the DerSimonian and Laird method was selected. The WLS method was used to quantify the heterogeneity of associations between risk factors and breast cancer subtypes. Egger's regression test was used to determine publication bias.23 Publication bias is considered to be present if the p-value of the intercept (a) is less than 0.05. Sensitivity analysis was performed via excluding the study with the largest weight and then evaluating the impact of the change on the pooled OR. Crude ORs with 95% CIs for each study, Q-tests, and pooled ORs with 95% CI were calculated using Review Manager 5.0 (Nordic Cochrane Center, Copenhagen, Denmark). The Egger's and WLS tests were performed with SPSS 17.0. The p-values were subjected to a two-tailed test, with an alpha level of 0.05 for significance testing.
Results
The case-control study
Of the 1256 cases, 898 tumors (71.4%) were classified as luminal, 55(4.3%) as HER2-enriched, and 303(24.1%) as triple-negative (Table 1). There was no statistical difference in average age, postmenopausal age, parity, or breastfeeding status among controls and the subtype groups (P > 0.05) (Table 1). The distribution of BMI, age at menarche, age at first live birth, and menopausal status varied among groups (P ≤ 0.05) (Table 1).
Table 1.
Characteristics of breast cancer cases and controls.a
| Risk factors | Controls n = 1416 | Luminal n = 898 | HER2-enriched n = 55 | Triple-negative n = 303 | P-valueb |
|---|---|---|---|---|---|
| Age, years | 47.96 (10.07) | 47.73 (10.93) | 48.62 (8.97) | 49.54 (10.74) | 0.06 |
| BMI, kg/m2 | 22.32 (2.66) | 23.01 (2.88) | 22.65 (2.26) | 23.20 (3.03) | <0.001 |
| Age at menarche | 14.28 (1.68) | 14.27 (1.82) | 14.87 (1.78) | 14.49 (1.84) | 0.02 |
| Age at first live birth | 25.04 (2.68) | 24.50 (2.78) | 24.51 (2.50) | 24.18 (2.69) | <0.001 |
| Age at menopause | 48.98 (2.97) | 48.97 (4.29) | 49.24 (4.12) | 48.52 (4.35) | 0.44 |
| Menopause status | |||||
| No | 831 (58.8) | 554 (61.7) | 30 (54.5) | 154 (50.8) | 0.01 |
| Yes | 585 (41.2) | 344 (38.3) | 25 (45.5) | 149 (49.2) | |
| Parity | |||||
| 0 | 39 (2.8) | 28 (3.1) | 0 (0.0) | 15 (5.0) | 0.11 |
| ≥1 | 1377 (97.2) | 870 (96.9) | 55 (100.0) | 288 (95.0) | |
| Breastfeedingc | |||||
| No | 95 (6.9) | 49 (5.6) | 5 (9.1) | 21 (7.3) | 0.51 |
| Yes | 1282 (93.1) | 821 (94.4) | 50 (90.9) | 267 (92.7) | |
| Family history of breast cancer | |||||
| No | 1391 (98.2) | 860 (95.8) | 54 (98.2) | 290 (95.7) | 0.002 |
| Yes | 25 (1.8) | 38 (4.2) | 1 (1.8) | 13 (4.3) | |
BMI, body mass index.
Mean (standard deviation): age, BMI, age at menarche, age at fist live birth, age at menopause. n (%): menopause status, parity, breast feeding, tumor subtype, family history of breast cancer.
One-way analysis of variance: age, BMI, age at menarche, age at first live birth, age at menopause; Chi-square test: menopause status; Fisher's exact test: parity, breast feeding, family history of breast cancer.
Among parous women.
Among all subjects, early age at menarche (≤13 years old) increased the risk of luminal tumor compared with women who had undergone menarche at 15 years of age or older (ORluminal1.28; 95%CI, 0.99–1.67; Ptrend<0.05). The risk of luminal tumor rose along with increasing BMI (18.5–24.9 vs. <18.5: ORluminal1.58; 95%CI, 1.02–2.43; ≥25 vs. <18.5: ORluminal2.58; 95%CI, 1.60–4.14; Ptrend<0.001). A BMI of 25 kg/m2 or more also increased triple-negative tumor risk (ORtriple-negative2.96; 95%CI, 1.34–6.54; Ptrend<0.001). Compared with women who had their first live birth when younger than 25 years old, those with later age at first live birth (25–29 years old) had an elevated risk of all subtypes (ORluminal1.41; 95%CI, 1.17–1.70; ORHER2-enriched2.08; 95%CI, 1.15–3.77; ORtriple-negative1.34; 95%CI, 1.02–1.76). The difference in associations between menarche age and the three subtypes of breast cancer was statistically significant (Pheterogeneity<0.05) (Table 2).
Table 2.
Associations between risk factors and breast cancer subtypes in overall cases and controls.a
| Risk factors | Control, n (%) n = 1416 | Luminal n = 898 |
HER2-enriched n = 55 |
Triple-negative n = 303 |
Phd | |||
|---|---|---|---|---|---|---|---|---|
| n (%) | OR (95% CI) | n (%) | OR (95% CI) | n (%) | OR (95% CI) | |||
| Age, years | ||||||||
| <40 | 313 (22.1) | 221 (24.6) | 1.00 | 8 (14.5) | 1.00 | 48 (15.8) | 1.00 | |
| 40–59 | 896 (63.3) | 542 (60.4) | 0.72 (0.50–1.06) | 41 (74.5) | 0.53 (0.15–1.91) | 206 (68.0) | 0.67 (0.38–1.19) | 0.01 |
| ≥60 | 207 (14.6) | 135 (15.0) | 0.85 (0.67–1.07) | 6 (11.0) | 1.41 (0.62–3.24) | 49 (16.2) | 1.27 (0.86–1.87) | 0.15 |
| Ptrend = 0.42b | Ptrend = 0.64b | Ptrend = 0.04b | ||||||
| Menopausal status | ||||||||
| Yes | 585 (41.3) | 343 (38.2) | 1.00 | 25 (45.5) | 1.00 | 149 (49.2) | 1.00 | |
| No | 831 (58.7) | 555 (61.8) | 1.32 (1.05–1.65) | 30 (54.5) | 1.03 (0.54–1.96) | 154 (50.8) | 0.96 (0.71–1.33) | 0.02 |
| BMI, kg/m2 | ||||||||
| <18.5 | 82 (5.8) | 35 (3.9) | 1.00 | 2 (3.6) | 1.00 | 8 (2.6) | 1.00 | |
| 18.5–24.9 | 1149 (81.1) | 683 (76.1) | 1.58 (1.02–2.43) | 46 (83.6) | 1.41 (0.33–5.96) | 223 (73.6) | 1.77 (0.84–3.74) | 0.70 |
| ≥25 | 185 (13.1) | 180 (20.0) | 2.58 (1.60–4.14) | 7 (12.8) | 1.27 (0.25–6.36) | 72 (23.8) | 2.96 (1.34–6.54) | 0.39 |
| Ptrend < 0.001b | Ptrend = 0.76b | Ptrend < 0.001b | ||||||
| Age at menarche, years | ||||||||
| ≥15 | 925 (65.3) | 550 (61.2) | 1.00 | 44 (80.0) | 1.00 | 206 (68.0) | 1.00 | |
| 14 | 313 (22.1) | 213 (23.7) | 1.16 (0.94–1.43) | 6 (10.9) | 0.45 (0.19–1.08) | 59 (19.5) | 1.01 (0.73–1.41) | 0.03 |
| ≤13 | 178 (12.6) | 135 (15.1) | 1.28 (0.99–1.67) | 5 (9.1) | 0.75 (0.29–1.95) | 38 (12.5) | 1.22 (0.81–1.83) | 0.18 |
| Ptrend = 0.034b | Ptrend = 0.06b | Ptrend = 0.55b | ||||||
| Age at first live birth, yearsc | ||||||||
| ≤24 | 634 (46.0) | 370 (42.5) | 1.00 | 20 (36.4) | 1.00 | 134 (46.5) | 1.00 | |
| 25–29 | 682 (49.5) | 469 (53.9) | 1.41 (1.17–1.70) | 34 (61.8) | 2.08 (1.15–3.77) | 147 (51.0) | 1.34 (1.02–1.76) | 0.34 |
| ≥30 | 61 (4.6) | 31 (3.6) | 1.19 (0.75–1.90) | 1 (1.8) | 0.86 (0.11–6.72) | 7 (2.4) | 0.93 (0.41–2.13) | 0.56 |
| Ptrend = 0.28b | Ptrend = 0.37b | Ptrend = 0.50b | ||||||
| Breastfeedingc | ||||||||
| Yes | 1282 (93.1) | 821 (94.4) | 1.00 | 50 (90.9) | 1.00 | 267 (92.7) | 1.00 | |
| No | 95 (6.9) | 49 (5.6) | 0.92 (0.64–1.32) | 5 (9.1) | 1.57 (0.60–4.10) | 21 (7.3) | 1.24 (0.75–2.05) | 0.47 |
BMI, body mass index; CI, confidence interval; OR, odds ratio.
Odds ratios adjusted for the other factors in this table and family history of breast cancer.
P-value for trends calculated by trend chi-square.
Among parous women.
P-value of the heterogeneity of exposure-disease ORs between luminal, HER2-enriched, and triple-negative subtypes.
In premenopausal women, higher BMI (≥25 kg/m2) was associated with elevated risk of luminal and triple-negative subtypes (ORluminal1.88; 95%CI, 1.31–2.69; ORtriple-negative2.51; 95%CI, 1.53–4.12), and late age at first live birth (≥25 years old) was linked with increased risk of luminal tumor (ORluminal1.39; 95%CI, 1.10–1.76) (Table 3). In postmenopausal women, overweight and obesity (BMI ≥ 25 kg/m2) was associated with an increased risk of the luminal subtype only (ORluminal1.48; 95%CI, 1.08–2.04). Increased age at first live birth (≥25 years) was associated with elevated risk of luminal tumor (ORluminal1.41; 95%CI, 1.04–1.90), and women with older menopausal age (≥55 years old) had higher risk of all subtypes (ORluminal3.11; 95%CI, 1.61–6.02; ORHER2-enriched5.56; 95%CI, 1.41–21.93; ORtriple-negative2.65; 95%CI, 1.12–6.33). In postmenopausal women, the associations between age at menarche and tumor subtypes still showed significant heterogeneity (P = 0.05) (Table 3).
Table 3.
Associations between risk factors and breast cancer subtypes by menopausal status.a
| Risk factorsb | Premenopausal |
Phd | Postmenopausal |
Phd | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control |
Luminal |
HER2-enriched |
Triple-negative |
Control |
Luminal |
HER2-enriched |
Triple-negative |
|||||||||
| n = 831 |
n = 555 |
n = 30 |
n = 154 |
n = 585 |
n = 343 |
n = 25 |
n = 149 |
|||||||||
| n (%) | n (%) | OR (95% CI) | n (%) | OR (95% CI) | n (%) | OR (95% CI) | n (%) | n (%) | OR (95% CI) | n (%) | OR (95% CI) | n (%) | OR (95% CI) | |||
| Age, years | ||||||||||||||||
| <40 | 313 (37.7) | 221 (39.8) | 1.00 | 8 (26.7) | 1.00 | 48 (31.2) | 1.00 | – | ||||||||
| 40–59 | 518 (62.3) | 334 (60.2) | 0.85 (0.67–1.07) | 22 (73.3) | 1.43 (0.62–3.29) | 106 (68.8) | 1.25 (0.84–1.84) | 0.11 | 378 (64.6) | 208 (60.6) | 1.00 | 19 (76.0) | 1.00 | 100 (67.1) | 1.00 | |
| ≥60 | – | 207 (35.4) | 135 (39.4) | 0.80 (0.58–1.09) | 6 (24.0) | 0.31 (0.11–0.83) | 49 (32.9) | 0.52 (0.33–0.81) | 0.22 | |||||||
| BMI, kg/m2 | ||||||||||||||||
| <25 | 765 (92.1) | 478 (86.1) | 1.00 | 25 (83.3) | 1.00 | 126 (81.8) | 1.00 | 467 (79.8) | 240 (70.0) | 1.00 | 23 (92.0) | 1.00 | 106 (71.1) | 1.00 | ||
| ≥25 | 66 (7.9) | 77 (13.9) | 1.88 (1.31–2.69) | 5 (16.7) | 2.25 (0.82–6.17) | 28 (18.2) | 2.51 (1.53–4.12) | 0.55 | 118 (20.2) | 103 (30.0) | 1.48 (1.08–2.04) | 2 (8.0) | 0.31 (0.07–1.35) | 43 (28.9) | 1.24 (0.80–1.92) | 0.11 |
| Menarche age, years | ||||||||||||||||
| ≥14 | 507 (61.0) | 318 (57.3) | 1.00 | 20 (66.7) | 1.00 | 99 (64.3) | 1.00 | 418 (71.5) | 232 (67.6) | 1.00 | 24 (96.0) | 1.00 | 107 (71.8) | 1.00 | ||
| <14 | 324 (39.0) | 237 (42.7) | 1.17 (0.93–1.47) | 10 (33.3) | 0.83 (0.38–1.83) | 55 (35.7) | 0.98 (0.67–1.43) | 0.29 | 167 (28.5) | 111 (32.4) | 1.25 (0.92–1.69) | 1 (4.0) | 0.14 (0.02–1.03) | 42 (28.2) | 1.20 (0.79–1.83) | 0.05 |
| Age at first live birth, yearsc | ||||||||||||||||
| <25 | 391 (49.1) | 238 (44.4) | 1.00 | 12 (40.0) | 1.00 | 70 (47.3) | 1.00 | 243 (41.9) | 132 (39.5) | 1.00 | 8 (32.0) | 1.00 | 64 (45.7) | 1.00 | ||
| ≥25 | 406 (50.9) | 298 (55.6) | 1.39 (1.10–1.76) | 18 (60.0) | 1.72 (0.79–3.74) | 78 (52.7) | 1.43 (0.98–2.09) | 0.74 | 337 (58.1) | 202 (60.5) | 1.41 (1.04–1.90) | 17 (68.0) | 2.45 (0.97–6.18) | 76 (54.3) | 1.21 (0.81–1.81) | 0.40 |
| Breastfeedingc | ||||||||||||||||
| Yes | 748 (93.9) | 507 (94.6) | 1.00 | 27 (90.0) | 1.00 | 140 (94.6) | 1.00 | 534 (92.1) | 314 (94.0) | 1.00 | 23 (92.0) | 1.00 | 127 (90.7) | 1.00 | ||
| No | 49 (6.1) | 29 (5.4) | 0.98 (0.60–1.59) | 3 (10.0) | 1.87 (0.54–6.47) | 8 (5.4) | 1.00 (0.46–2.20) | 0.60 | 46 (7.9) | 20 (6.0) | 0.83 (0.48–1.46) | 2 (8.0) | 1.40 (0.31–6.40) | 13 (9.3) | 1.44 (0.74–2.80) | 0.54 |
| Age at post-menopause, years | ||||||||||||||||
| <55 | – | 570 (97.4) | 316 (92.1) | 1.00 | 22 (88.0) | 1.00 | 140 (94.0) | 1.00 | ||||||||
| ≥55 | – | 15 (2.6) | 27 (7.9) | 3.11 (1.61–6.02) | 3 (12.0) | 5.56 (1.41–21.93) | 9 (6.0) | 2.65 (1.12–6.33) | 0.63 | |||||||
BMI, body mass index; CI, confidence interval; OR, odds ratio.
Odds ratios adjusted for the other factors in this table and family history of breast cancer.
Considering the limited sample sizes in tumor subgroups, all the continuous variables were categorized into two levels, including BMI, age at menarche, and age at first live birth.
Among parous women.
P-value of heterogeneity of exposure-disease odds ratios among luminal, HER2-enriched, and triple-negative subtypes.
Meta-analysis
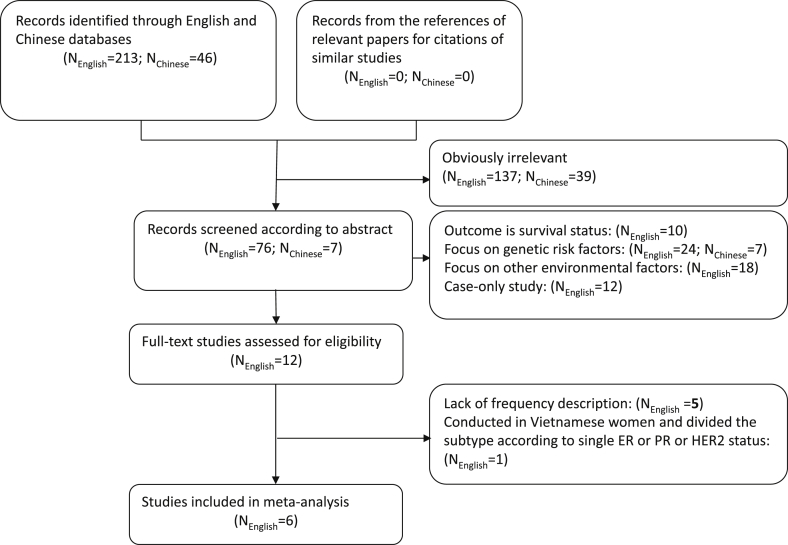
Among the 259 retrieved studies, 176 studies were irrelevant, 12 were case series studies, 49 focused on genetic and other environmental factors, and the outcome of 10 studies was survival status. Of the remaining 12 studies, the risk factors in Yoo's,24 Suzuki's,25 and Tamaki's26 studies from Japan, Chung's study from Korea,27 and Adam's study from China28 lacked frequencies, and one study including subjects from Vietnam and China reported the molecular subtypes according to single ER status, PR status, or HER2 status.29 Among the remaining six studies,18, 19, 30, 31, 32, 33 two studies from Japan were performed on an overlapping population. In considering whether it was feasible to pool the data, we used the frequencies of BMI from Sueta et al33 and that of reproductive factors reported by Islam30 to calculate pooled effects separately. The flow diagram of the study selection is shown in Fig. 1.
Fig. 1.
The flow diagram of the study selection.
Among the six included studies, two were ranked as “A” and four were ranked as “B” quality. Two studies were from China and four were from Japan (Table 4). Few studies were eligible to be included in the pooled analysis according to menopausal status, so we only conducted the meta-analysis in controls. Thus, a total of 8,637cases and 13,001 controls (including those from our own case-control study) were included in the final analysis. The categories of menopausal age and age at menarche in our own case-control study were re-divided to be consistent with the majority of the selected studies. The breast tumor molecular subtypes were classified as luminal (ER+/PR+) and ER−PR−. When possible, the latter was further divided into two groups: HER2-enriched and triple-negative.
Table 4.
Characteristics of included studies in meta-analysis.
| First author (published year) | Region | Menopausal status | Study design | Year subjects were recruited | Sample size, case/control | Type of tumor | Risk factorsa | Matched factor | Adjusted factorsb | Rank (Number of qualified items)c |
|---|---|---|---|---|---|---|---|---|---|---|
| Bao et al. 2011.18 | China | total/pre/post | PCC | 1996–1998/2002–2005 | 2676/3474 | ER+/PR+, ER−/PR−, ER+/PR−, ER−/PR+ | B,C,D,E,F | age | 1, 2, 3, 4, 5, 6, 7, 8, 9, 10. | A (6) |
| Islam et al. 2012.25 | Japan | total | HCC | 2003–2005 | 706/1412 | Luminal, HER2-enriched, TN | B,C,D,E,F | age, menopausal status | 1, 4, 5, 6, 13, 17, 18 | B (5) |
| Sueta et al. 2012.28 | Japan | total/pre/post | HCC | 2001–2005 | 715/1430 | Luminal, HER2-enriched, TN | A | age, menopausal status | 1, 4, 5, 9, 12, 13, 14,15,16. | B (5) |
| Kawai et al. 2012.26 | Japan | total | HCC | 1997–2009 | 1092/3160 | ER+/PR+,ER−/PR−, ER+/PR−,ER−/PR+ | A,B,C,D,E | – | 1, 5, 6,17,18, 19,20, 21. | A (6) |
| Miyagawa et al. 2013.27 | Japan | total/pre/post | PCC | 2005–2012 | 615/682 | Luminal A, Luminal B, HER2-enriched, TN | C | – | 1,4,9,12,22 | B (5) |
| Xing et al. 2009.19 | China | total | PCC | 2001–2009 | 1417/1587 | Luminal A, Luminal B, HER2-enriched, TN | B,C,D,E | age | 1,4,9,12,13,23,24,25 | B (4) |
| Present study | China | total/pre/post | PCC | 2002–2012 | 1416/1256 | Luminal, HER2-enriched, TN | A,B,C,D,E,F | age | 1,6,9,12,13,14,22, 26 | – |
ER, estrogen receptor; HCC, hospital-based case-control study; PCC, population-based case-control study; PR. progesterone receptor; TN, triple-negative.
Risk factors included in this meta-analysis: A. BMI B. age at menarche C. parity D. age at first live birth E. breastfeeding F. menopausal age.
Risk factors adjusted for in the studies: 1. age, 2. education, 3. history of breast fibroadenoma, 4. family history of breast cancer, 5. physical exercise, 6. BMI, 7. waist-to-hip ratio, 8. history of live birth, 9. parity, 10. study phase, 11. years of menstruation, 12. age at menarche, 13. menopausal status, 14. age at first live birth, 15. hormone use, 16. referral pattern, 17. smoking, 18. alcohol use, 19. occupation, 20. year of recruitment, 21. area of residence, 22. age at menopause, 23. induced abortion, 24. spontaneous abortion, 25. history of hysteromyoma, and 26. breastfeeding.
Results of quality assessment and number of qualified items according to the Newcastle-Ottawa Quality Assessment Scale (NOS) for case-control studies.
Compared with women with a BMI of less than 18.5 kg/m2, those with a BMI between 18.5 kg/m2 and 24.9 kg/m2 had marginally increased risk of both luminal (ORluminal1.20; 95%CI, 0.96–1.49) and ER−PR− tumors (ORER−PR−1.43; 95%CI, 1.00–2.05). A BMI of more than 25 kg/m2 conferred higher risks of both subtypes (ORluminal1.75; 95%CI, 1.30–2.35 and ORER−PR−1.95; 95%CI, 1.04–3.65). Women who experienced menopause after 50 years of age also had elevated risk of luminal and ER−PR− tumors (ORluminal1.15; 95%CI, 1.00–1.32 and ORER−PR−1.19; 95%CI, 1.00–1.43). Younger age at menarche (≤12 years old) (ORluminal1.39; 95%CI, 1.23–1.57) and nulliparity (ORluminal1.26; 95%CI, 1.11–1.44) only increased the risk of luminal tumor. Lack of breastfeeding history increased the risk of all the molecular subtypes (ORluminal1.35; 95%CI, 1.05–1.74; ORHER2-enriched1.97; 95%CI, 1.39–2.80; and ORtriple-negative1.85; 95%CI, 1.06–3.21). The differences in associations between age at menarche, parity, age at first live birth, breastfeeding, and breast tumor subtypes were significant (all Pheterogeneity<0.05) (Table 5). After removing the study with the largest weight, the significant associations between menopausal age and luminal tumor risk and between breastfeeding and triple-negative tumor disappeared. The other pooled ORs and 95% CIs were stable (eTable 1). Publication bias was not detected for the overall analysis (all P > 0.05) (eTable 2).
Table 5.
Pooled odds ratios and 95% confidence intervals for breast cancer molecular subtype associated with common risk factors.
| Risk factors | Nstudy | Ncontrol | Luminal |
ER-PR- |
PH1b | Nstudy | Ncontrol | HER2-enriched |
Triple-negative |
PH2c | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ncase | ORpooled | Pha | Ncase | ORpooled | Pha | Ncase | ORpooled | Pha | Ncase | ORpooled | Pha | |||||||
| BMI, kg/m2 | ||||||||||||||||||
| <18.5 | 3 | 415 | 110 | 1.00 | 35 | 1.00 | – | – | – | – | – | – | – | – | ||||
| 18.5–24.9 | 3 | 4210 | 1432 | 1.20 (0.96–1.49) | 0.68 | 553 | 1.43 (1.00–2.05) | 0.57 | 0.66 | – | – | – | – | – | – | – | – | |
| ≥25 | 3 | 1349 | 534 | 1.75 (1.30–2.35) | 0.22 | 191 | 1.95 (1.04–3.65) | 0.07 | 0.87 | – | – | – | – | – | – | – | – | |
| Ptrend<0.001d | Ptrend = 0.03d | |||||||||||||||||
| Menopausal age, years | ||||||||||||||||||
| <50 | 3 | 1261 | 547 | 1.00 | 260 | 1.00 | – | – | – | – | – | – | – | – | ||||
| ≥50 | 3 | 1363 | 682 | 1.15 (1.00–1.32) | 0.26 | 369 | 1.19 (1.00–1.43) | 0.06 | 0.32 | – | – | – | – | – | – | – | – | |
| Age at menarche, years | ||||||||||||||||||
| >12 | 4 | 5968 | 2146 | 1.00 | 948 | 1.00 | 3 | 3773 | 265 | 1.00 | 505 | 1.00 | ||||||
| ≤12 | 4 | 1307 | 525 | 1.39 (1.23–1.57) | 0.28 | 168 | 1.20 (0.91–1.58) | 0.11 | 0.01 | 3 | 616 | 28 | 0.76 (0.50–1.16) | 0.15 | 59 | 1.28 (0.94–1.73) | 0.76 | 0.001 |
| Parity | ||||||||||||||||||
| ≥1 | 6 | 10,604 | 5032 | 1.00 | 2012 | 1.00 | 4 | 4684 | 364 | 1.00 | 730 | 1.00 | ||||||
| 0 | 6 | 771 | 408 | 1.26 (1.11–1.44) | 0.14 | 128 | 1.03 (0.84–1.26) | 0.28 | 0.04 | 4 | 406 | 38 | 1.02 (0.70–1.47) | 0.19 | 50 | 1.34 (0.96–1.85) | 0.66 | 0.10 |
| Age at first live birth, years | ||||||||||||||||||
| <25 | 5 | 3829 | 1592 | 1.00 | 821 | 1.00 | 3 | 1628 | 119 | 1.00 | 306 | 1.00 | ||||||
| ≥25 | 5 | 6087 | 3093 | 1.14 (0.86–1.52)e | <0.001 | 1149 | 0.83 (0.62–1.10)e | <0.001 | <0.001 | 3 | 2443 | 160 | 0.93 (0.56–1.56)e | <0.001 | 361 | 0.80 (0.56–1.14)e | <0.001 | <0.001 |
| Breastfeeding | ||||||||||||||||||
| Yes | 5 | 8430 | 3806 | 1.00 | 1529 | 1.00 | 3 | 3626 | 222 | 1.00 | 585 | 1.00 | ||||||
| No | 5 | 1579 | 851 | 1.35 (1.05–1.74)e | <0.001 | 186 | 1.74 (1.41–2.15) | 0.23 | <0.001 | 3 | 484 | 48 | 1.97 (1.39–2.80) | 0.23 | 78 | 1.85 (1.06–3.21)e | 0.02 | 0.07 |
P-values (two-sided) were based on a Q-test of heterogeneity between included studies.
PH1: P-value of heterogeneity of exposure-disease ORs among luminal and ER−/PR− subtypes.
PH2: P-value of heterogeneity of exposure-disease ORs among luminal, HER2-enriched and triple-negative subtypes.
P-value for trends calculated by trend chi-square.
The pooled odds ratio was calculated by a random-effects model.
Discussion
In this population-based case-control study in southwestern China, we found that overweight and obesity and late menopause increased the risk of both luminal and triple-negative tumors. These findings were consistent with those of our meta-analysis of risk factors for East Asian women. Overweight and obesity may affect breast cancer by various mechanisms, including increasing estrogen synthesis, causing insulin resistance, inhibiting the synthesis of sex hormone-binding globulin (SHBG), and promoting systemic inflammation.34
In our case-control study, we observed that, in postmenopausal women, higher BMI only elevated risk of the luminal tumor subtype. Previous studies observed similar results, including the California Teacher Study35 and the Breast Cancer Surveillance Consortium (BCSC) cohort study36 from the United States, the European Prospective Investigation into Cancer and Nutrition (EPIC) study, and case-control studies from Japan and China32, 33, 37 (RRs and ORs ranged from 1.17 to 2.14, with 95%CIs excluding 1). In general, the magnitude of the associations observed in these studies was similar to that observed in the present study. We also found that overweight and obesity were associated with increased premenopausal risk of both luminal and triple-negative tumors. For triple-negative tumors, a meta-analysis of case-case studies found that obesity was associated with an increased triple-negative tumor risk among premenopausal women (OR1.43; 95% CI, 1.23–1.65),38 which was similar to our results. For luminal subtypes, Yang's meta-analysis,39 which included 12 population-based American studies, found a 1.36-times higher risk of premenopausal luminal tumors for those with higher BMI. However, the EPIC study37 and the case-control study from Japan32 reported a small inverse association between BMI and premenopausal luminal tumors (HRs and ORs ranged from 0.79 to 0.93, with 95%CIs excluding 1), while the Shanghai study18 found no significant association. Differing prevalence of obesity and subtypes in different populations may contribute to the disparity of these results.
An experimental study found that obesity was positively correlated with levels of leptin, the addition of which increased cell proliferation in ERα-positive breast cancer cell lines.40 And an experiment using the C3(1)-TAg murine model demonstrated that weight loss could prevent basal-like breast cancer by blocking the obesity-responsive pro-tumorigenic hepatocyte growth factor/c-Met pathway.41 Thus, theoretically, obesity may increase both luminal and triple-negative tumor risk in premenopausal and postmenopausal women. Accordingly, the heterogeneous results for the effects of BMI on breast tumor subtypes were not significant in our case-control study and meta-analysis. The mechanism of obesity's impact on tumor risk in women of different menopausal statuses merits further study. Based on our results, it could be deduced that, although the rate of obesity is relatively low for Chinese women (5.6%),42 premenopausal women may be relatively sensitive to obesity as a risk factor for breast cancer. Our results highlight the importance of weight control in premenopausal women.
Early age at menarche and delayed menopause may prolong the exposure period of estrogen and enhance breast cancer risk. Our case-control study found that, of these two factors, only early menarche was associated with luminal tumor risk, a relationship also found in our subsequent meta-analysis in East Asians. This finding also accords with Yang's meta-analysis of 12 population-based studies, which observed 1.16-times higher ER+ tumor risk for women who experienced menarche younger than 12 years old.39 Although our subgroup analysis on menopausal status did not yield significant relationships, we noticed that the proportion of early menarche was higher in luminal cases than in HER2-enriched or triple-negative patients for both pre- and postmenopausal women. Both our case-control study and meta-analysis found significant heterogeneity of the associations between menarche age and breast tumor subtypes. This indicated that menarche age may mainly contribute to the risk of luminal tumor. The negative results of the stratified analysis may be explained by the limited sample size in each layer. Both this case-control study and meta-analysis in East Asians found that women who had older menopausal age had higher risks of luminal and ER−PR− tumors. This was consistent with the results of the Nurses' Health Study of 121,700 participants from United Study (for the three subtypes, HRs ranged from 1.01 to 1.07, with 95% CIs excluding1).43 The mechanism for the role of menopausal age in development of HER2-enriched and triple-negative tumors was unclear. Our results need to be verified in a larger sample size in East Asia.
Pregnancy initiates cellular differentiation in mammary glands and lowers susceptibility to carcinogenesis.44 Previous studies have observed a negative relationship between parity and breast cancer.18, 31, 45, 46, 47 In our case-control study, the number of nulliparous women was small, especially in the sets of molecular subtypes. Thus, it was unlikely that we would observe an effect of nulliparity on tumor subtype. In the meta-analysis, which included our data, we found that nulliparity increased risk only of the luminal subtype. This finding is supported by Yang's pooled analysis,39 Ma's meta-analysis of seven epidemiological studies,48 and Anderson's recent systematic review, which qualitatively summarized the results of associations between reproductive factors and molecular subtypes.11 Combined with the known effects of pregnancy, these results indicate that nulliparity may increase the risk of breast cancer via a hormonal pathway.
In this case-control study, late age at first live birth increased the risk of all three subtypes. However, in the meta-analysis, we did not find an association between age at first live birth and any breast cancer subtype. When we excluded one study from the analysis, late age at first live birth showed a positive association with risk of luminal tumor (OR1.33; 95%CI, 1.22–1.44). The associations between age at first live birth and other subtypes in the meta-analysis were stable, and the heterogeneity of the included studies disappeared (P > 0.05). Then, we re-calculated the heterogeneity of associations between age at first delivery and tumor subtypes and found that it was significant (P < 0.001) (data not shown). In our case-control study, we observed that increased age at first live birth was associated with risk of both pre- and postmenopausal luminal tumor. This was accordant with Ma's meta-analysis of nine population-based studies.48
Breastfeeding may increase the protective effect of pregnancy by inducing terminal differentiation, removal of initiated breast epithelial cells, excretion of carcinogenic agents, and delay in ovulation.49 In the present case-control study, we found no association between breastfeeding and any breast cancer molecular subtype in total, pre-, or postmenopausal women. However, the meta-analysis demonstrated that lack of breastfeeding increased the risk of all breast cancer subtypes, and the effect of non-breastfeeding on ER−PR− tumor risk was significantly stronger than that on risk of the luminal subtype. This accords with Ma's meta-analysis48 and Anderson's recent systematic review.11 Our results indicate that age at first delivery and breastfeeding may affect breast cancer risk via various mechanisms.
With increasing economic prosperity, more East Asian women exercise reproductive control. In China, for example, between 1982 and 2001, the proportion of women whose first live birth occurred at 30 years of age or older increased from 1.35% to 4.86%,50 and the prevalence of non-lactation in premenopausal women increased from 9.5% to 22.7% from births in 1950 to 1970.13 This information, combined with our results, suggests the role of health education in childbearing choices and the need to encourage breastfeeding for young Chinese mothers.
This case-control study has several limitations. First, some of the subsets of interest were limited in sample size, and other population-based studies are needed to replicate the results. Second, only 20% of “+2” patients were FISH-tested, so a non-differential bias may exist. Considering that fewer than 20% of women with “+2” were diagnosed as HER2-positive using FISH, the non-differential bias may be small. Third, in this study, we included common risk factors that were previously hypothesized to affect peripheral estrogen levels. Although we adjusted for the most important known risk factors (age and family history of breast cancer) for breast cancer, several other well-known risk factors for breast cancer were not included. Further study including other well-known risk factors is needed to verify our results. In addition, we measured BMI when patients were admitted to the hospital for the first time. Since their body weight might be changed by presence of breast cancer, the observed associations might be due to reverse causality, and our study design did not allow us to make definitive causal inferences.
Sichuan Cancer Hospital is the leading tertiary hospital providing diagnosis, treatment, and routine follow-up care for patients with breast cancer in Sichuan Province, and Chengdu Children's and Women's Hospital is the leading tertiary institution providing health care for women and children in Sichuan Province. In Sichuan Cancer Hospital, more than 70% of patients came from Chengdu and areas within a radius of <150 km, and the controls were community women who attended routine physical examinations in Chengdu or suburban areas nearby. Thus, we believe that most women in the control group would go to Sichuan Cancer Hospital if they had breast cancer, so the controls represent the source population of cases to a certain degree. Our results in this case-controls study may be specific to Chengdu. In order to better observe the association between BMI, reproductive factors, and breast cancer subtypes, we conducted a meta-analysis in East Asia women. The results of studies included in the meta-analysis were homogenous, and the effects for most of the risk factors were stable. Thus, we believe the results of meta-analysis were acceptable.
In summary, for East Asian women, overweight, late menopause, and lack of breastfeeding appear to increase risks of both luminal and ER−PR− tumors. Early menarche and nulliparity may contribute chiefly to the risk of luminal tumor. In our study, these associations were not impacted by menopausal status. Most of these findings were similar to those in studies conducted in Western countries. In addition, late age at first live birth may increase the risk of luminal tumor, which needs to be evaluated further in a larger East Asian population.
Conflicts of interest
None declared.
Acknowledgements
This work was supported by NO. 81302500 from the National Natural Science Foundation of China. We thank Shawna Williams for editing this article.
Footnotes
Peer review under responsibility of the Japan Epidemiological Association.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.je.2016.05.002.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Ferlay J.S.I., Ervik M., Dikshit R. International Agency for Research on Cancer; Lyon, France: 2013. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. [Internet] Available from: http://globocaniarcfr, accessed on day/month/year. 2012;Available from: http://globocan.iarc.fr, Accessed on day/month/year. [Google Scholar]
- 2.Nielsen T.O., Hsu F.D., Jensen K. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004;10:5367–5374. doi: 10.1158/1078-0432.CCR-04-0220. [DOI] [PubMed] [Google Scholar]
- 3.Parise C.A., Bauer K.R., Brown M.M., Caggiano V. Breast cancer subtypes as defined by the estrogen receptor (ER), progesterone receptor (PR), and the human epidermal growth factor receptor 2 (HER2) among women with invasive breast cancer in California, 1999-2004. Breast J. 2009;15:593–602. doi: 10.1111/j.1524-4741.2009.00822.x. [DOI] [PubMed] [Google Scholar]
- 4.Anderson W.F., Rosenberg P.S., Prat A., Perou C.M., Sherman M.E. How many etiological subtypes of breast cancer: two, three, four, or more? J Natl Cancer Inst. 2014:106. doi: 10.1093/jnci/dju165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim M.J., Ro J.Y., Ahn S.H., Kim H.H., Kim S.B., Gong G. Clinicopathologic significance of the basal-like subtype of breast cancer: a comparison with hormone receptor and Her2/neu-overexpressing phenotypes. Hum Pathol. 2006;37:1217–1226. doi: 10.1016/j.humpath.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 6.Rouzier R., Perou C.M., Symmans W.F. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res. 2005;11:5678–5685. doi: 10.1158/1078-0432.CCR-04-2421. [DOI] [PubMed] [Google Scholar]
- 7.Haque R., Ahmed S.A., Inzhakova G. Impact of breast cancer subtypes and treatment on survival: an analysis spanning two decades. Cancer Epidemiol Biomarkers Prev. 2012;21:1848–1855. doi: 10.1158/1055-9965.EPI-12-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shim H.J., Kim S.H., Kang B.J. Breast cancer recurrence according to molecular subtype. Asian Pac J Cancer Prev. 2014;15:5539–5544. doi: 10.7314/apjcp.2014.15.14.5539. [DOI] [PubMed] [Google Scholar]
- 9.Kongsiang A., Tangvoraphonkchai V., Jirapornkul C., Promthet S., Kamsa-Ard S., Suwanrungruang K. Survival time and molecular subtypes of breast cancer after radiotherapy in Thailand. Asian Pac J Cancer Prev. 2014;15:10505–10508. doi: 10.7314/apjcp.2014.15.23.10505. [DOI] [PubMed] [Google Scholar]
- 10.Amadou A., Hainaut P., Romieu I. Role of obesity in the risk of breast cancer: lessons from anthropometry. J Oncol. 2013;2013:906495. doi: 10.1155/2013/906495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson K.N., Schwab R.B., Martinez M.E. Reproductive risk factors and breast cancer subtypes: a review of the literature. Breast Cancer Res Treat. 2014;144:1–10. doi: 10.1007/s10549-014-2852-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parsa P., Parsa B. Effects of reproductive factors on risk of breast cancer: a literature review. Asian Pac J Cancer Prev. 2009;10:545–550. [PubMed] [Google Scholar]
- 13.Lee H., Li J.Y., Fan J.H. Risk factors for breast cancer among Chinese women: a 10-year nationwide multicenter cross-sectional study. J Epidemiol. 2014;24:67–76. doi: 10.2188/jea.JE20120217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clarke C.A., Keegan T.H., Yang J. Age-specific incidence of breast cancer subtypes: understanding the black-white crossover. J Natl Cancer Inst. 2012;104:1094–1101. doi: 10.1093/jnci/djs264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song Q., Huang R., Li J. The diverse distribution of risk factors between breast cancer subtypes of ER, PR and HER2: a 10-year retrospective multi-center study in China. PLoS One. 2013;8:e72175. doi: 10.1371/journal.pone.0072175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.ZhaoP C.W. Military Medical Science Press; Beijing: 2008. Cancer incidence and Mortality in Chinese cancer Registration Areas in 2005. [Google Scholar]
- 17.Hao J CW. Beijing: Military Medical Science Press; 2012.
- 18.Bao P.P., Shu X.O., Gao Y.T. Association of hormone-related characteristics and breast cancer risk by estrogen receptor/progesterone receptor status in the shanghai breast cancer study. Am J Epidemiol. 2011;174:661–671. doi: 10.1093/aje/kwr145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xing P., Li J., Jin F. A case-control study of reproductive factors associated with subtypes of breast cancer in Northeast China. Med Oncol. 2010;27:926–931. doi: 10.1007/s12032-009-9308-7. [DOI] [PubMed] [Google Scholar]
- 20.Zheng S., Bai J.Q., Li J. The pathologic characteristics of breast cancer in China and its shift during 1999-2008: a national-wide multicenter cross-sectional image over 10 years. Int J Cancer. 2012;131:2622–2631. doi: 10.1002/ijc.27513. [DOI] [PubMed] [Google Scholar]
- 21.Phipps A.I., Malone K.E., Porter P.L., Daling J.R., Li C.I. Body size and risk of luminal, HER2-overexpressing, and triple-negative breast cancer in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2008;17:2078–2086. doi: 10.1158/1055-9965.EPI-08-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perry C.S.O.J., Palmer J.L., Gross A.S. Risk factors for breast cancer in East Asian women relative to women in the West. Asia Pac J Clin Oncol. 2009;5:219–231. [Google Scholar]
- 23.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoo K.Y., Tajima K., Miura S. Breast cancer risk factors according to combined estrogen and progesterone receptor status: a case-control analysis. Am J Epidemiol. 1997;146:307–314. doi: 10.1093/oxfordjournals.aje.a009271. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki R., Iwasaki M., Inoue M. Body weight at age 20 years, subsequent weight change and breast cancer risk defined by estrogen and progesterone receptor status–the Japan public health center-based prospective study. Int J Cancer. 2011;129:1214–1224. doi: 10.1002/ijc.25744. [DOI] [PubMed] [Google Scholar]
- 26.Tamaki K., Tamaki N., Terukina S. The correlation between body mass index and breast cancer risk or estrogen receptor status in Okinawan women. Tohoku J Exp Med. 2014;234:169–174. doi: 10.1620/tjem.234.169. [DOI] [PubMed] [Google Scholar]
- 27.Chung S., Park S.K., Sung H. Association between chronological change of reproductive factors and breast cancer risk defined by hormone receptor status: results from the Seoul Breast Cancer Study. Breast Cancer Res Treat. 2013;140:557–565. doi: 10.1007/s10549-013-2645-4. [DOI] [PubMed] [Google Scholar]
- 28.Adams S.A., Matthews C.E., Hebert J.R. Association of physical activity with hormone receptor status: the Shanghai breast cancer study. Cancer Epidemiol Biomarkers Prev. 2006;15:1170–1178. doi: 10.1158/1055-9965.EPI-05-0993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nichols H.B., Trentham-Dietz A., Love R.R. Differences in breast cancer risk factors by tumor marker subtypes among premenopausal Vietnamese and Chinese women. Cancer Epidemiol Biomarkers Prev. 2005;14:41–47. [PubMed] [Google Scholar]
- 30.Islam T., Matsuo K., Ito H. Reproductive and hormonal risk factors for luminal, HER2-overexpressing, and triple-negative breast cancer in Japanese women. Ann Oncol. 2012;23:2435–2441. doi: 10.1093/annonc/mdr613. [DOI] [PubMed] [Google Scholar]
- 31.Kawai M., Kakugawa Y., Nishino Y., Hamanaka Y., Ohuchi N., Minami Y. Reproductive factors and breast cancer risk in relation to hormone receptor and menopausal status in Japanese women. Cancer Sci. 2012;103:1861–1870. doi: 10.1111/j.1349-7006.2012.02379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miyagawa Y., Miyake T., Yanai A. Association of body mass index with risk of luminal A but not luminal B estrogen receptor-positive and HER2-negative breast cancer for postmenopausal Japanese women. Breast Cancer. 2015;22:399–405. doi: 10.1007/s12282-013-0493-z. [DOI] [PubMed] [Google Scholar]
- 33.Sueta A., Ito H., Islam T. Differential impact of body mass index and its change on the risk of breast cancer by molecular subtype: a case-control study in Japanese women. Springerplus. 2012;1:39. doi: 10.1186/2193-1801-1-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arcidiacono B., Iiritano S., Nocera A. Insulin resistance and cancer risk: an overview of the pathogenetic mechanisms. Exp Diabetes Res. 2012;2012:789174. doi: 10.1155/2012/789174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Canchola A.J., Anton-Culver H., Bernstein L. Body size and the risk of postmenopausal breast cancer subtypes in the California Teachers Study cohort. Cancer Causes Control. 2012 doi: 10.1007/s10552-012-9897-x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phipps A.I., Buist D.S., Malone K.E. Breast density, body mass index, and risk of tumor marker-defined subtypes of breast cancer. Ann Epidemiol. 2012;22:340–348. doi: 10.1016/j.annepidem.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ritte R., Lukanova A., Berrino F. Adiposity, hormone replacement therapy use and breast cancer risk by age and hormone receptor status: a large prospective cohort study. Breast Cancer Res. 2012;14:R76. doi: 10.1186/bcr3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pierobon M., Frankenfeld C.L. Obesity as a risk factor for triple-negative breast cancers: a systematic review and meta-analysis. Breast Cancer Res Treat. 2013;137:307–314. doi: 10.1007/s10549-012-2339-3. [DOI] [PubMed] [Google Scholar]
- 39.Yang X.R., Chang-Claude J., Goode E.L. Associations of breast cancer risk factors with tumor subtypes: a pooled analysis from the Breast Cancer Association Consortium studies. J Natl Cancer Inst. 2011;103:250–263. doi: 10.1093/jnci/djq526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rose D.P., Vona-Davis L. Biochemical and molecular mechanisms for the association between obesity, chronic inflammation, and breast cancer. Biofactors. 2014;40:1–12. doi: 10.1002/biof.1109. [DOI] [PubMed] [Google Scholar]
- 41.Sundaram S., Le T.L., Essaid L. Weight loss reversed obesity-induced HGF/c-met pathway and basal-like breast cancer progression. Front Oncol. 2014;4:175. doi: 10.3389/fonc.2014.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li L.M., Rao K.Q., Kong L.Z. A description on the Chinese national nutrition and health survey in 2002. Zhonghua Liu Xing Bing Xue Za Zhi. 2005;26:478–484. [PubMed] [Google Scholar]
- 43.Tamimi R.M., Colditz G.A., Hazra A. Traditional breast cancer risk factors in relation to molecular subtypes of breast cancer. Breast Cancer Res Treat. 2012;131:159–167. doi: 10.1007/s10549-011-1702-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Althuis M.D., Fergenbaum J.H., Garcia-Closas M., Brinton L.A., Madigan M.P., Sherman M.E. Etiology of hormone receptor-defined breast cancer: a systematic review of the literature. Cancer Epidemiol Biomarkers Prev. 2004;13:1558–1568. [PubMed] [Google Scholar]
- 45.Horn J., Opdahl S., Engstrom M.J. Reproductive history and the risk of molecular breast cancer subtypes in a prospective study of Norwegian women. Cancer Causes Control. 2014;25:881–889. doi: 10.1007/s10552-014-0388-0. [DOI] [PubMed] [Google Scholar]
- 46.Li C.I., Malone K.E., Daling J.R. Timing of menarche and first full-term birth in relation to breast cancer risk. Am J Epidemiol. 2008;167:230–239. doi: 10.1093/aje/kwm271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ma H., Wang Y., Sullivan-Halley J. Use of four biomarkers to evaluate the risk of breast cancer subtypes in the women's contraceptive and reproductive experiences study. Cancer Res. 2010;70:575–587. doi: 10.1158/0008-5472.CAN-09-3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma H., Bernstein L., Pike M.C., Ursin G. Reproductive factors and breast cancer risk according to joint estrogen and progesterone receptor status: a meta-analysis of epidemiological studies. Breast Cancer Res. 2006;8:R43. doi: 10.1186/bcr1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Russo J., Balogh G.A., Heulings R. Molecular basis of pregnancy-induced breast cancer protection. Eur J Cancer Prev. 2006;15:306–342. doi: 10.1097/00008469-200608000-00006. [DOI] [PubMed] [Google Scholar]
- 50.Li L., Ji J., Wang J.B., Niyazi M., Qiao Y.L., Boffetta P. Attributable causes of breast cancer and ovarian cancer in China: reproductive factors, oral contraceptives and hormone replacement therapy. Chin J Cancer Res. 2012;24:9–17. doi: 10.1007/s11670-012-0009-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.