Abstract
Background
Sleep apnea is a chronic, widely underdiagnosed condition characterized by disruption of sleep architecture and intermittent hypoxia due to short cessations of breathing. It is a major independent risk factor for myocardial infarction, congestive heart failure and stroke as well as one of the rare modifiable risk factors for Alzheimer’s Dementia. Reliable animal disease models are needed to understand the link between sleep apnea and the various clinically linked disorders.
New method
An automated system for inducing hypoxia was developed, in which the major improvement was the possibility to efficiently adjust the length and intensity of hypoxia in two different periods. The chamber used a small volume of gas allowing for fast exchanges of different oxygen levels. The mice were kept in their cages adapted with the system on the cage lid. As a proof of principle, they were exposed to a three week period of intermittent hypoxia for 8 hours a day, with 90 s intervals of 5, 7% and 21% oxygen to validate the model. Treated (n = 8) and control mice (no hypoxia, n = 7) were handled in the same manner and their hippocampal brain regions compared by histology.
Results
The chamber provided a fast, reliable and precise intermittent hypoxia, without inducing noticeable side effects to the animals. The validation experiment showed that apoptotic neurons in the hippocampus were more numerous in the mice exposed to intermittent hypoxia than in the control group, in all tested hippocampal regions (cornu ammonis 1 (CA1) P <0.001; cornu ammonis 3 (CA3) P <0.001; and dentate gyrus (DG) P = 0.023). In both, control and hypoxic conditions, there was a significantly higher number of apoptotic neurons in the DG compared to the CA1 and CA3 subfields (P <0.001).
Conclusion
The new design of a hypoxic chamber provides a fast, adjustable and reliable model of obstructive sleep apnea, which was validated by apoptosis of hippocampal neurons.
Introduction
Sleep apnea is characterized by periodical cessation of breathing with a reduction in nasal airflow less than 30 percent of its normal level. It is present in three forms: central, obstructive or mixed. In central sleep apnea, the feedback mechanism in the respiratory centers of the ventrolateral medulla fails, resulting in a reduced or absent drive to breathe during sleep. The more common obstructive sleep apnea (OSA) is characterized by reduction in airflow to the lungs that occurs during sleep as a result of occlusion or narrowing of the respiratory tract at the pharyngeal level [1]. In all cases, sleep apnea leads to intermittent hypoxia, hypercapnia and subsequent reoxygenation as well as a disruption of sleep architecture. OSA has been reported to affect middle-aged and older individuals, with the prevalence recently estimated to be around 22% in men and 17% in women [2].
As obesity and ageing, the most important conditions that precipitate OSA are increasing, a significant rise in the prevalence of OSA is likely in the future [3]. It has been reported that sleep apnea is a major independent risk factor for cardiovascular diseases such as systemic and pulmonary hypertension, congestive heart failure and stroke [4] as well as myocardial infarction, cerebrovascular dysfunction and idiopathic sudden death [5]. Although association of OSA with cardiovascular and metabolic morbidity is well recognized and described, its potential role in the etiology of chronic kidney disease and its association with cancer was indicated only recently [6], as well as its potential role in brain injury leading to neurodegenerative disorders [7]. OSA has an important effect on cognitive function, in particular in episodic verbal learning, memory, cognitive flexibility and mental processing speed and more. It is also one of the rare modifiable risk factors for Alzheimer’s disease. The patients with Alzheimer’s disease have a five times higher chance of presenting with OSA than cognitively non-impaired individuals of similar age [8].
On the contrary, mild sleep apnea can have a beneficial preconditioning effect on the body in the context of cerebrovascular disease [9]. There have been numerous animal studies showing both beneficial and detrimental consequences in relation to the pattern and depth of hypoxia exposure. In addition, clinical research has underlined the importance of including severity of intermittent hypoxia as a relevant factor in making sense of large quantities of sometimes contradictory data [10].
An animal model, which would recreate the conditions of sleep apnea as closely as possible, is needed to clarify both the consequences and potential therapeutic strategies. Among current systems available for exposing animals to hypoxia, most are manually controlled, expensive or bulky. Although designed to provide hypoxia, their major disadvantage in modelling the obstructive sleep apnea is that they are too slow to reach set oxygen levels in the limited time period of low oxygen exposure and are therefore inadequately precise for defining experimental protocols that allow studying subtle differences between mild, moderate and severe sleep apnea. Finally, some systems require exposing the mice to a new chamber environment, thus contributing to the stress levels for the animals.
Therefore, the aim of our study was to develop an automated system that could mimic the gas exchanges and create intermittent hypoxia similar to those occurring in patients during sleep. Moreover, it was desirable to allow maximal adaptability of experimental protocol by controlling different oxygen levels, their duration, and fast exchanges. As the periods of hypoxia are short but repetitive, it was important to develop a system that is fast to reach the set value of oxygen in the chamber, permitting the animal exposure to very short periods of hypoxia. To minimize animal stress, the system was made to be compatible with an existing animal housing cage to reduce the effect of changing the environment for the experimental animals. The system had to meet the needs of noise, humidity and gas flow control and assure the reliability of the experimental protocol.
Given the vulnerability of the region to a range of pathologies and considering the consistency of the presentation of neurocognitive impairments in sleep apnea [11, 12], neuronal apoptosis in the hippocampus was evaluated in this study in the context of chronic intermittent hypoxia. The hippocampus was selected for the validation of our new mouse model because clinical research points to the degeneration of the same anatomical regions in the patients suffering from obstructive sleep apnea as it does in mice [9, 13].
Materials and methods
The mouse model of intermittent hypoxia
The mice were bred and housed in standard animal cages in the animal facility of the Croatian Institute for Brain Research, University of Zagreb School of Medicine. Light-dark cycle was set to 12 h-12 h beginning at 7 h and 19 h respectively. Food and water was available ad libitum in the cages as well as during the intermittent hypoxia treatment. The experiments were approved by the Ethical Committee of the University of Zagreb School of Medicine (permit number: 380-59-10106-14-55/230).
Male mice of C57BL6 inbred strain aged 85 ± 20 days were randomly allocated into two groups, animals exposed to intermittent hypoxia (IH, n = 8) and a control group (CTRL, n = 7). CTRL mice were handled, and housed in a manner consistent with that of the IH mice, most importantly being in the same size cages and in the same room as the treated animals, but not being exposed to hypoxia. This allowed controlling for the effects of the handling-stress, noise and disturbance being same as in the intervention group.
The IH mice were exposed to a 21 day protocol for 8 hours per day of 90 s periods of 5.7% of oxygen followed by 90 s period of 21% oxygen. The flow of gases was 50 L/min with the set pressures of 2 bars of nitrogen and 1.2 bars of air. The protocol was administered using a new designed system explained in this article.
Brain histology
After the 21-day study period, mice were anaesthetized by administration of 0.5 g/Kg tribromoethanol (Avertin, Sigma-Aldrich, USA) and transcardially perfused with 10 ml phosphate buffered saline (PBS) followed by 10 ml of 4% paraformaldehyde solution, and the brains isolated. The isolated brains were kept for 24 h immersed in 4% PFA, kept in PBS with 0.05% sodium azide and transferred to 30% sucrose solution 3 days before cutting.
The brains were embedded into TissueTek fluid (Sakura, Netherlands), mounted on the platform, frozen with dry ice and cut using a microtome (HM 430 TermoFisher, USA) in 35 μm thick coronal sections. The glass slides with tissue sections were immersed in 0.05% Nissl stain solution mixed with few drops of 10% acetic acid for 30 minutes, rinsed in distilled water and dehydrated in ascending concentrations of ethyl alcohol. Samples were cleared in xylene and mounted.
For every mouse 4 representative coronal sections containing the hippocampal formation were selected using the Brain Allen Mouse Atlas as a reference (http://mouse.brain-map.org) and the regions of interest Cornu ammonis 1 (CA1), Cornu ammonis 3 (CA3) and the dentate gyrus (DG) were identified. In order to eliminate the possibility of a systematic error due to a difference in area of selected regions of interest, same sizes of examined areas were selected.
The apoptotic neurons were stereologically counted within the 280 μm x 280 μm counting frame for the regions of interest on a Nikon YS100 microscope, 40x magnification, and subsequently numbers of apoptotic neurons per mm3 were calculated. The experimenter was blinded to the mouse label and group affiliation.
All data was processed and plotted using GraphPad Prism 6 software, tested for normality of distribution using Shapiro-Wilk test and analyzed using ANOVA with Holm-Sidak’s multiple comparison test. Statistical significance was defined as lower than P<0.05.
Results
Intermittent hypoxia system design
The system was designed to address the requirements for adjustable, fast and precise experimental protocols of intermittent hypoxia allowing the improved modelling of sleep apnea (Table 1).
Table 1. Requirements and technical solution applied in the new intermittent hypoxia system.
| Requirements | Technical solutions |
|---|---|
| Maximum adjustability in the design of the experimental protocols in terms of hypoxia levels and exposure times | Controller enabling setting two different oxygen levels and two time periods |
| Fast transition between different oxygen levels to allow short hypoxia periods | Dual sensor input to the controller board |
| Mixing antechamber with oxygen sensor | |
| Small volume | |
| High gas flow | |
| Precise oxygen control in the chamber | Mixing antechamber with oxygen sensor |
| Dual-sensor input to the controller board | |
| Use of Techniplast GM500 cage with patented sealing design | |
| Evenly distributed gas flow throughout the chamber | Modification of Techniplast GM500 cage with patented ergonomic design |
| Minimizing animal suffering | System compatible with the housing cage |
| Humidifier | |
| Muffler system | |
| Standard housing space for animals |
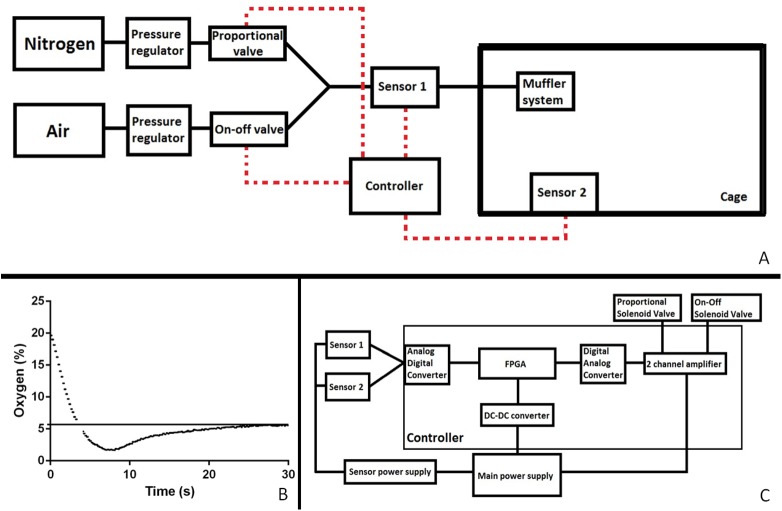
A block diagram of the entire setup is represented by Fig 1. Air supplied through a compressor (SAI2088, SMC, USA) and bottled nitrogen (99,996%, Messer, Croatia) entered the system via separate inlets. Rough pressures were manually defined on the manometers of the compressor and the bottle respectively. Upon entering the system, both tubes were connected to more precise manometers and finer pressure regulators, where the pressure could be manually fine-tuned. The air tube was then connected to two valves, an on- off (D885, M&M International, USA) and a small proportional valve (PVQ13, SMC, USA). The nitrogen tube was connected to a bigger proportional valve (PVQ31, SMC, USA). The tubes converged and another manometer was placed to check the pressure of the mix. Subsequently, the tubes diverged, one arm led to a mixing antechamber (10mm x 10mm x 500mm) mounted with its own oxygen sensor (O2-A2, Alphasense, UK), and the other arm passed through a water humidifier. The two arms converged to enter the hypoxia chamber, through the inlet originally designed to fit with the individually ventilated cage (IVC) system for the cage. A muffler system was mounted on the inside of the lid, augmenting the gas entrance area by 4 times. A second oxygen sensor (O2-A2, Alphasense, UK) was mounted from the inside of the cage lid and monitored the actual gas mix in the cage. The outlet from the cage, designed for the IVC system, remained open throughout the protocol to prevent any pressure from rising in the chamber.
Fig 1.
A block diagram of the complete setup (A), the speed and pattern of lowering the oxygen to a set concentration (B), a block diagram detailing the controller and the dual sensor interface (C). The solid line denotes airflow and the dashed line the electrical connections. FPGA, field programmable gate array; DC-DC digital to digital converter.
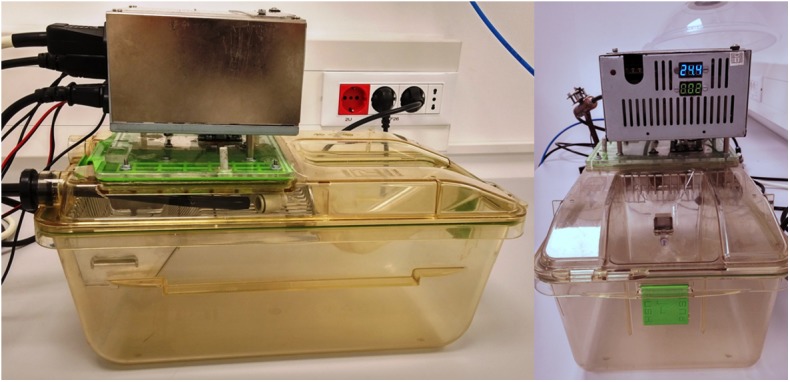
To address the demand of small volume and minimizing the stress of changed environment to the animals the system was developed to be compatible with a sealed mouse cage 391 x 199 x 160mm (GM500 Tecniplast, Italy, Patent No. ZL200610076641.0; 8.037.847; 1719406), making it possible to apply experimental hypoxia without the need to move the mice from their original housing. A lid of the mouse cage was modified to fit the regulating apparatus of the system (Fig 2). The sealing of the cage and the ergonomically-designed flow of gas was primarily intended for individually ventilated animal housing and patented by Tecniplast, but through our adaptation solved the necessity for a sealed volume and evenly distributed gas flow in the hypoxia chamber.
Fig 2. A photograph of how the mouse cage lid was modified to fit the regulating apparatus of the system.
The controller consisted of a field programmable gate array (FPGA) (Basys2, Digilent, USA) card with a LED display and a user interface mounted on the cage lid. The interface used 4 buttons where the percentage of oxygen and the time of maintaining the set oxygen level could be specified for two distinct time periods and two distinct oxygen levels. The buttons allowed changing the parameter displayed on the LED display, setting the parameter value, saving the parameter setting, and starting the protocol.
A computer program in VHSIC Hardware Description Language (VHDL) was written for the FPGA within the regulating device to use the input from both sensors and the set parameters to calculate the current needed to control the diameter of the opening of 2 proportional valves and a third on-off valve (S1 Computer Code). The program simulated a proportional- integral-derivative controller. The FPGA was fitted with an analogue- digital and digital- analogue converters to be able to process the signals from the sensors and to the valves (Fig 1C). The program used the antechamber sensor input to modify the gas mix rapidly, while the cage sensor input signaled to turn off the gas flow when the set oxygen level was reached. This also allowed minimizing the use of nitrogen and air.
The compressor supplying air was connected to two separately controlled valves to lower the risk of the system undershooting. Namely, we have noticed that in experimental protocols using low levels of oxygen, before some systems reach the set level there is a transitory drop in oxygen below the set level. This risk of transitory undershoot can have a catastrophic impact on the experimental animals, sometimes suffocating or unacceptably injuring them, but as well significantly influence the experimental results. For this reason, we decided to add a small proportional valve that would continuously stay open, minimizing the length and the effect of the undershoot (Fig 1B). A bigger on-off valve would nonetheless permit the fast and precise elevation of oxygen level when needed.
The muffler system was designed to slow down the flow of gas to minimize the stress of drought, noise and drying to the animals by increasing the entrance area of gas by 4 times of the original inlet.
The hypoxia chamber developed in our laboratory has been submitted for a patent, which is currently pending (GB 16602071.1).
Intermittent hypoxia was achieved by the automated system
The intermittent hypoxia system allowed various complex hypoxia exposure patterns. The system enabled defining two different oxygen levels and two time period lengths.
The system was fast to reach the set parameters, with a mild transitory undershoot. In our experiment, when changing from atmospheric (21%) to hypoxic (5.7%) levels the system reached hypoxia in less than 5 seconds and stabilized the oxygen level to 5.7% in 27 seconds. The undershooting was also short, lasting approximately 12 seconds with a nadir of 2% of oxygen (Fig 1B).
The system was also minimally stressful for the animals, as they were able to stay in their original housing cages, with humidity changes, airflow and noise kept at minimum levels. Although the animals were more immobile and quiet then usual while the hypoxia was ongoing, they showed no signs of uneasiness or obvious effects during or after hypoxia.
Intermittent hypoxia induces neuronal apoptosis in the hippocampus
To validate the intermittent hypoxia system, the animals were subjected to a protocol lasting 21 days and assessed for the effect of the hypoxic microenvironment on hippocampal neurons to verify whether the expected biological effects will be achieved. Neuronal apoptosis was taken as a hallmark consequence of intermittent hypoxia. A similar protocol of intermittent hypoxia using Oxycycler model A44XO (Reming Bioinstruments, USA) showed a sevenfold increase of TUNEL stained neuronal apoptosis in the CA1 region of the rat hippocampus after acute hypoxia exposure and a smaller, but statistically significant difference after 14 days of chronic intermittent hypoxia [13].
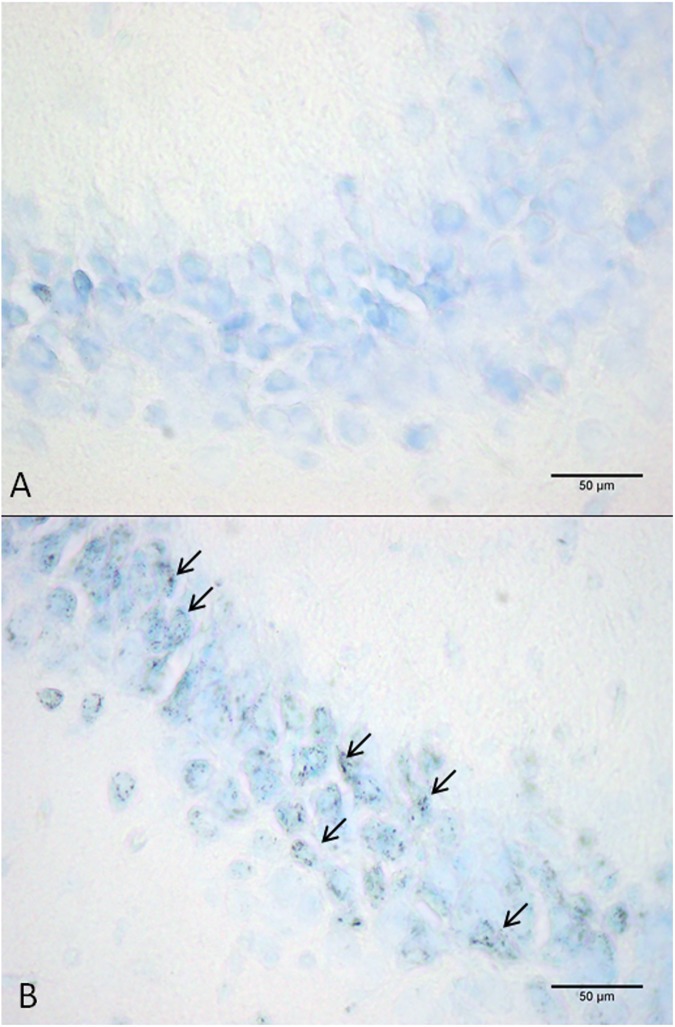
The apoptotic neurons were determined by hyperchromia, condensation and fragmentation of chromatin, shrinking of neuron’s soma (Fig 3). By quantifying the number of apoptotic neurons in the hippocampal subfields of the exposed (n = 8) and the control groups (n = 7), we have shown statistically significant apoptosis in all three examined subfields of the hippocampus, the CA1, CA3 and DG (Fig 4A, 4B and 4C). When the means of treated and control mice were compared the increase of apoptosis in hippocampus was around 30%, which is in compliance with the existing literature for chronic IH exposure at a similar time point [13, 14], and confirms the validity of the intermittent hypoxia system applied.
Fig 3.
Representative images of neuronal apoptosis in the hippocampal region cornu ammonis 3 (CA3) of mouse from the control group (A) and intermittent hypoxia group (B). The arrows show apoptotic neurons.
Fig 4.
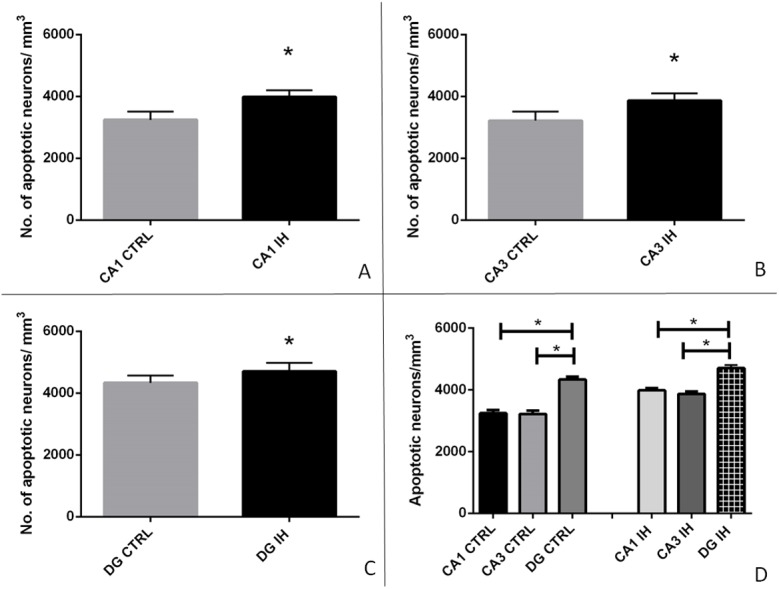
Apoptotic neurons per mm3 of tissue in the control group (CTRL) compared to the intermittent hypoxia group (IH) in cornu ammonis 1(CA1) subfield (P< 0.001) (A), cornu ammonis 3 (CA3) subfield (P = 0.001) (B), and the dentate gyrus (DG) (P = 0.023) (C) shown as columns representing means and standard deviations. The number of apoptotic neurons differed significantly in the DG IH group opposed to CA1 IH (P<0,001), and CA3 IH (P<0,001), as well as in the DG CTRL group opposed to CA1 CTRL (P<0,001), and CA3 CTRL (P<0,001) (D). Asterisks denote statistically significant differences.
The number of apoptotic neurons differed significantly in the DG of IH group opposed to CA1 IH (P<0,001) and CA3 IH (P<0,001). In addition, the number of apoptotic neurons in the DG CTRL group differed significantly opposed to CA1 CTRL (P<0, 001) and CA3 CTRL (P<0,001) implicating the DG as the most dynamic region of hippocampus. No differences were found between the number of apoptotic neurons in CA1 and CA3 regions of IH mice or CTRL mice (Fig 4D).
Discussion
Approaches to modeling intermittent hypoxia
The intermittent hypoxia system for laboratory mice, intended to model human sleep apnea was designed, applied and verified in this study. The major improvements in relation to previous solutions was that (A) the system was fast to reach set oxygen levels with the dual sensor input to the controller improving precision of control and minimizing the time lag between set and actual parameters in the chamber, and (B) it was compact and compatible with an individually ventilated mouse cage allowing controlled gas flow and appropriate sealing.
Most non- commercial systems for inducing hypoxia incorporate a Plexiglas chamber with limited control of gas mixing and sealing, a time-regulated opening and closing of solenoid valves and a sensor for monitoring the gas in the chamber, but with no backward loop to the valves [15–18]. Most of them are voluminous cabinets, which could host many animals, but the ability of fast exchange of gasses in such volume (the requirement of intermittent hypoxia protocol is 90 seconds) remains questionable. On the other hand, some of them are very small, being able to contain only a single animal per box, prolonging and complicating the experiment [19].
The novelty of our system is the control of the solenoid valves using input from two sensors. An antechamber with an oxygen sensor is used to pre-mix the gases to a set value while another sensor monitors the actual oxygen percentage in the hypoxia chamber. Most developed systems monitor the percentage of oxygen in the chamber and thus are subjected to a time lag between set parameters and the actual chamber conditions, which in our experience can be quite significant, especially in very short hypoxia exposures.
Another approach to modelling intermittent hypoxia is using a commercial oxygen controller that is made to be compatible with an incubator where a cage or several cages can be placed within [14]. These systems are very practical, being almost completely automated throughout the several week periods. However, systems with big volumes can be slow to reach set parameters in short periods of time. For illustration, Xu et al. used a chamber with dimensions of 30x20x20 inches (V = 12000 in3; 196.6L) while our chamber measures 15.40x7.83x6.30 inches (V = 759, 66 inches3; 12.4 L) [20]. The gas flow to the chamber used was roughly the same, measuring 60 l/min in the experiment by Xu et al. and 50L/min in our experiment.
There have been several other comparable models of intermittent hypoxia, such as a system described by Li et al. where the animal chamber is inserted into a larger hypoxia box and moved out into ambient air in relation to the period of exposure [21]. In an original solution by Tagaito et al. a bell-shaped chamber floating on water was designed that enabled to monitor EEG and EMG from the exposed animal during the hypoxic exposure [22]. In our opinion, although inventive and important, these designs are limited in providing a high throughput, reproducible and easily available animal model needed for large scale experiments.
Intermittent hypoxia induces neuronal apoptosis in the hippocampus
The biological validation of the system showed a significant increase of apoptosis in the hippocampus. This finding is in partial contradiction to previous findings [14, 23], where the authors established an increase in apoptosis in the CA1 subfield of the rat hippocampus, but found no significant increase in apoptosis in the CA3 area. In previous studies the dentate gyrus was implicated as the location of hippocampal neurogenesis following intermittent hypoxia, showing cells co-positive for neurofilament and BrDU [24]. Recently, lower numbers of neurons after intermittent hypoxia have been shown in the mouse [25] and rat DG as well [26].
The reasons for the diverging of the finding of CA3 neuronal apoptosis in our results might have twofold explanation. The intermittent hypoxia studies dealing with differences of the sensitivity of hippocampal subfields have been done on rats [14, 23], while our model vas validated studying mice. Secondly, the CA1 subfield has been shown to be more susceptible to hypoxic damage then CA3 subfield in the models using large- volume chambers. We propose that our system was faster to reach the set hypoxic parameters and more precise in keeping the parameters stable and thus resulted in a more severe hypoxia, possibly thus affecting the less sensitive CA3 subfield.
The presented intermittent hypoxia system is intended to model human sleep apnea. Still the relevance of the presented animal study has limitations. The young healthy mice were used in the current study, while the patients with obstructive sleep apnea are usually elderly people with several associated diseases. Moreover, although the 8 hour protocol was administered during the day when mice predominantly sleep with intention to mimic the sleep fragmentation, mice sleep in phases with arousals every 15 to 20 minutes, which is uncommon in humans.
Conclusion
The design of a new hypoxic chamber provided a fast, adjustable and compact model of obstructive sleep apnea, which was validated by showing apoptosis of neurons in the hippocampus.
Supporting information
(ZIP)
Acknowledgments
This work was funded by the FP7 project GlowBrain funded by the European Commission (FP7-REGPOT-2012-CT2012-316120) as well as Young Brain (HR.3.2.01; 2015–2016) funded by European Social Fund. The authors would like to thank dr. Camilla Simmons and dr. Diana Cash of the Neuroimaging department, IoPPN, KCL for their valuable advice in developing the quantification method used in the biological part of the study. The authors would also like to thank Károly Péter Balázs, Márton Németh and Gyula Németh for the technical support in developing the intermittent hypoxia system.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was funded by the FP7 project GlowBrain funded by the European Commission (FP7-REGPOT-2012-CT2012-316120, https://ec.europa.eu/research/fp7/index_en.cfm) awarded to SG, as well as Young Brain (HR.3.2.01; 2015-2016) funded by European Social Fund, http://ec.europa.eu/esf/home.jsp and provided support to DP and SG. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Lévy P, Kohler M, McNicholas WT, Barbé F, McEvoy RD, Somers VK, et al. Obstructive sleep apnoea syndrome. Nature Reviews. Disease Primers. 2014. December;1:15015. [DOI] [PubMed] [Google Scholar]
- 2.Franklin KA, Lindberg E. Obstructive sleep apnea is a common disorder in the population—a review on the epidemiology of sleep apnea. Journal of Thoracic Disease. 2015. August;7(8):1311 10.3978/j.issn.2072-1439.2015.06.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kent BD, McNicholas WT, Ryan S. Insulin resistance, glucose intolerance and diabetes mellitus in obstructive sleep apnoea. Journal of Thoracic Disease. 2015. August;7(8):1343 10.3978/j.issn.2072-1439.2015.08.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kent BD, Ryan S, McNicholas WT. Obstructive sleep apnea and inflammation: relationship to cardiovascular co-morbidity. Respiratory Physiology & Neurobiology. 2011. September 30;178(3):475–81. [DOI] [PubMed] [Google Scholar]
- 5.Bradley TD, Floras JS. Obstructive sleep apnoea and its cardiovascular consequences. The Lancet. 2009. January 9;373(9657):82–93. [DOI] [PubMed] [Google Scholar]
- 6.Gildeh N, Drakatos P, Higgins S, Rosenzweig I, Kent BD. Emerging co-morbidities of obstructive sleep apnea: cognition, kidney disease, and cancer. Journal of Thoracic Disease. 2016. September;8(9):E901 10.21037/jtd.2016.09.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenzweig I, Glasser M, Polsek D, Leschziner GD, Williams S, Morrell MJ. Sleep apnoea and the brain: a complex relationship. Lancet Respiratory Medicine. 2015;3:404–14. 10.1016/S2213-2600(15)00090-9 [DOI] [PubMed] [Google Scholar]
- 8.Emamian F, Khazaie H, Tahmasian M, Leschziner GD, Morrell MJ, Hsiung GY, et al. The association between obstructive sleep apnea and Alzheimer’s disease: a meta-analysis perspective. Frontiers in Aging Neuroscience. 2016;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenzweig I, Williams SC, Morrell MJ. The impact of sleep and hypoxia on the brain: potential mechanisms for the effects of obstructive sleep apnea. Current Opinions in Pulmonary Medicine. 2014. November 1;20(6):565–71. [DOI] [PubMed] [Google Scholar]
- 10.Jackman KA, Zhou P, Faraco G, Peixoto PM, Coleman C, Voss HU, et al. Dichotomous effects of chronic intermittent hypoxia on focal cerebral ischemic injury. Stroke. 2014. May 1;45(5):1460–7. 10.1161/STROKEAHA.114.004816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tononi G, Cirelli C. Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration. Neuron. 2014. January 8;81(1):12–34. 10.1016/j.neuron.2013.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim J, Dinges DF. A meta-analysis of the impact of short-term sleep deprivation on cognitive variables. Psychological Bulletin. 2010. May;136(3):375 10.1037/a0018883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gozal D, Daniel JM, Dohanich GP. Behavioral and anatomical correlates of chronic episodic hypoxia during sleep in the rat. The Journal of Neuroscience. 2001. April 1;21(7):2442–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gozal E, Row BW, Schurr A, Gozal D. Developmental differences in cortical and hippocampal vulnerability to intermittent hypoxia in the rat. Neuroscience Letters. 2001. June 15;305(3):197–201. [DOI] [PubMed] [Google Scholar]
- 15.Goldbart A, Row BW, Kheirandish L, Schurr A, Gozal E, Guo SZ, et al. Intermittent hypoxic exposure during light phase induces changes in cAMP response element binding protein activity in the rat CA1 hippocampal region: water maze performance correlates. Neuroscience. 2003. December 31;122(3):585–90. [DOI] [PubMed] [Google Scholar]
- 16.Phillips SA, Olson EB, Lombard JH, Morgan BJ. Chronic intermittent hypoxia alters NE reactivity and mechanics of skeletal muscle resistance arteries. Journal of Applied Physiology. 2006. April 1;100(4):1117–23. 10.1152/japplphysiol.00994.2005 [DOI] [PubMed] [Google Scholar]
- 17.Greenberg HE, Sica A, Batson D, Scharf SM. Chronic intermittent hypoxia increases sympathetic responsiveness to hypoxia and hypercapnia. Journal of Applied Physiology. 1999. January 1;86(1):298–305. [DOI] [PubMed] [Google Scholar]
- 18.Fletcher EC, Lesske J, Qian W, Miller CC, Unger T. Repetitive, episodic hypoxia causes diurnal elevation of blood pressure in rats. Hypertension. 1992. June 1;19(6 Pt 1):555–61. [DOI] [PubMed] [Google Scholar]
- 19.Lefebvre B, Godin-Ribuot D, Joyeux-Faure M, Caron F, Bessard G, Lévy P, et al. Functional assessment of vascular reactivity after chronic intermittent hypoxia in the rat. Respiratory Physiology & Neurobiology. 2006. February 28;150(2):278–86. [DOI] [PubMed] [Google Scholar]
- 20.Xu W, Chi L, Row BW, Xu R, Ke Y, Xu B, et al. Increased oxidative stress is associated with chronic intermittent hypoxia-mediated brain cortical neuronal cell apoptosis in a mouse model of sleep apnea. Neuroscience. 2004. December 31;126(2):313–23. 10.1016/j.neuroscience.2004.03.055 [DOI] [PubMed] [Google Scholar]
- 21.Li C, Lu J, Zhang B. Development of a novel chronic intermittent hypoxia chamber. Sleep and Breathing. 2012. March 1;16(1):177–9. 10.1007/s11325-010-0470-0 [DOI] [PubMed] [Google Scholar]
- 22.Tagaito Y, Polotsky VY, Campen MJ, Wilson JA, Balbir A, Smith PL, et al. A model of sleep-disordered breathing in the C57BL/6J mouse. Journal of Applied Physiology. 2001. December 1;91(6):2758–66. [DOI] [PubMed] [Google Scholar]
- 23.Klein JB, Gozal D, Pierce WM, Thongboonkerd V, Scherzer JA, Sachleben LR, et al. Proteomic identification of a novel protein regulated in CA1 and CA3 hippocampal regions during intermittent hypoxia. Respiratory Physiology & Neurobiology. 2003. July 16;136(2):91–103. [DOI] [PubMed] [Google Scholar]
- 24.Gozal D, Row BW, Gozal E, Kheirandish L, Neville JJ, Brittian KR, et al. Temporal aspects of spatial task performance during intermittent hypoxia in the rat: evidence for neurogenesis. European Journal of Neuroscience. 2003. October 1;18(8):2335–42. [DOI] [PubMed] [Google Scholar]
- 25.Pan YY, Deng Y, Xie S, Wang ZH, Wang Y, Ren J, et al. Altered Wnt Signaling Pathway in Cognitive Impairment Caused by Chronic Intermittent Hypoxia: Focus on Glycogen Synthase Kinase-3β and β-catenin. Chinese Medical Journal. 2016. April 5;129(7):838 10.4103/0366-6999.178969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pedroso D, Nunes AR, Diogo LN, Oudot C, Monteiro EC, Brenner C, et al. Hippocampal neurogenesis response: what can we expect from two different models of hypertension? Brain Research. 2016. May 26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(ZIP)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.