Abstract
Background
Bupropion is used to treat depression during pregnancy. However, its usefulness as a smoking cessation aid for pregnant women is not fully known.
Objective
To evaluate the preliminary efficacy of bupropion sustained-release for smoking cessation during pregnancy.
Study design
We conducted a randomized, prospective, double-blind, placebo-controlled, pilot trial. Pregnant women who smoked daily received individualized behavior counseling and were randomly assigned to a 12-week, twice-a-day treatment with 150 mg bupropion sustained-release or placebo. The primary study objectives were to 1) determine whether bupropion sustained-release reduces nicotine withdrawal symptoms on the quit date and during treatment period compared to placebo; and 2) whether it increases 7-day point prevalence abstinence at the end of treatment period and at the end of pregnancy.
Results
Subjects in the bupropion (n = 30) and placebo (n = 35) groups were comparable in age, smoking history, number of daily smoked cigarettes, and nicotine dependence. After controlling for maternal age and race, bupropion sustained-release reduced cigarette cravings (1.5 ± 1.1 vs 2.1 ± 1.2, P = 0.02) and total nicotine withdrawal symptoms (3.8 ± 4.3 vs 5.4 ± 5.1, P = 0.028) during the treatment period. Administration of bupropion sustained-release reduced tobacco exposure, as determined by levels of carbon monoxide in exhaled air (7.4 ± 6.4 vs 9.1 ± 5.8, P = 0.053) and concentrations of cotinine in urine (348 ± 384 ng/mL vs 831 ± 727 ng/mL, P = 0.007), and increased overall abstinence rates during treatment (19% vs 2%, P = 0.003). However, there was no significant difference in 7-day point prevalence abstinence rates between the two groups at the end of medication treatment (17% vs 3%, P = 0.087) and at the end of pregnancy (10% vs 3%, P = 0.328).
Conclusion
Individual smoking cessation counseling along with the twice-daily use of 150 mg bupropion sustained-release increased smoking cessation rates and reduced cravings and total nicotine withdrawal symptoms during the treatment period. However, there was no significant difference in abstinence rates between groups at the end of medication treatment and at end of pregnancy, likely due to the small sample size. A larger study is needed to confirm these findings and to examine the potential benefit/ risk ratio of bupropion sustained-release for smoking cessation during pregnancy.
Keywords: bupropion sustained-release, pregnancy, smoking cessation
Introduction
Despite the well-known obstetrical, fetal, and developmental complications associated with cigarette smoking, 15.9% of all pregnant women continue to smoke throughout pregnancy.1 Behavioral interventions are only modestly effective in helping pregnant women quit smoking.2,3 Smoking cessation medications increase the chance of quitting smoking in men and non-pregnant women,2 and they could also be used to reduce cigarette cravings and withdrawal and to enhance quit rates during pregnancy.
Bupropion sustained-release (SR), an antidepressant, is commonly used to promote smoking cessation in males and non-pregnant females.4 The effectiveness of bupropion (Zyban ®, Wellbutrin ®) as a smoking cessation aid for pregnant women was suggested in a prospective observational study, in which pregnant smokers receiving bupropion 150 or 300 mg daily had higher quit rates than controls.5
Based on this information, we conducted a randomized, prospective, double-blind placebo-controlled, pilot trial of the preliminary efficacy of bupropion SR in combination with behavioral counseling for smoking cessation during pregnancy. The primary study objectives were to determine 1) whether bupropion SR reduces nicotine withdrawal symptoms on the quit date and during medication treatment; and 2) whether bupropion SR increases 7-day point prevalence abstinence at the end of medication treatment and at the end of pregnancy compared to placebo. Secondary objectives included assessment of bupropion SR on overall quit rates during treatment and on adverse effects during pregnancy when compared to placebo.
Materials and Methods
Prior to implementation, the study protocol was reviewed and approved by The University of Texas Medical Branch (UTMB) Institutional Review Board and was registered on Clinicaltrials.gov (ClinicalTrials.gov Identifier NCT01390246). An independent Data Safety and Monitoring Board (DSMB) reviewed ongoing trial data, including efficacy rates and serious adverse events (SAEs) through the study.
Recruitment
Pregnant smokers were recruited through the UTMB Ob/Gyn Department clinics and the UTMB Regional Maternal Child Health Program (RMCHP) clinics. Referrals from heath care providers were also accepted. In addition, the study was advertised through printed flyers, posters, and electronic media in RMCHP clinic waiting areas. Pregnant smokers who expressed interest in the research study were then screened for eligibility using inclusion and exclusion criteria.
Inclusion and Exclusion Criteria
Inclusion criteria were: women ≥ 18 years of age between 13 and 30 weeks’ gestation, smoking ≥ 10 cigarettes per day (CPD) prior to pregnancy and 5 CPD for the preceding 7 days, English or Spanish speaking, and having the intent to carry to term.
Exclusion criteria were: current illicit drug or alcohol abuse or dependence; multiple gestation; treatment for psychiatric disorder within the last 6 months; unstable medical problems (pregnancy-induced hypertension [blood pressure (BP) > 140/90 mg Hg], preeclampsia, threatened abortion, hyperemesis gravidarum); known fetal congenital abnormality; seizure disorder; use of psychotropic medication; use of medication known to lower the seizure threshold; anorexia/bulimia; a personal history of closed head trauma with > 30 minutes of loss of consciousness or amnesia or resulting in skull fracture or subdural hematoma/ brain contusion; current use of any other smoking cessation treatment; and current use of methadone.
Consent and Randomization
Prior to participation in the study, all subjects agreed and signed the IRB-approved informed consent. To ensure comparability of subjects in the treatment groups, we employed the urn randomization method.6 The groups were balanced for 2 variables: gestational age at study entry and number of CPD. Subjects, primary investigators, and research nurses were blinded to pharmacotherapy group assignment.
Study design
The study protocol consisted of 10 visits: first—enrollment, randomization, and study medication dispensation; second—the scheduled quit date; third to sixth— medication therapy progress assessment; seventh—the end of pregnancy (36–38 weeks’ gestation); and eighth to tenth—1-, 3-, and 6-month postnatal assessments of abstinence (Figure 1). Participants received either bupropion SR or matched placebo orally once daily for three days followed by twice daily for a total medication treatment of 12 weeks.
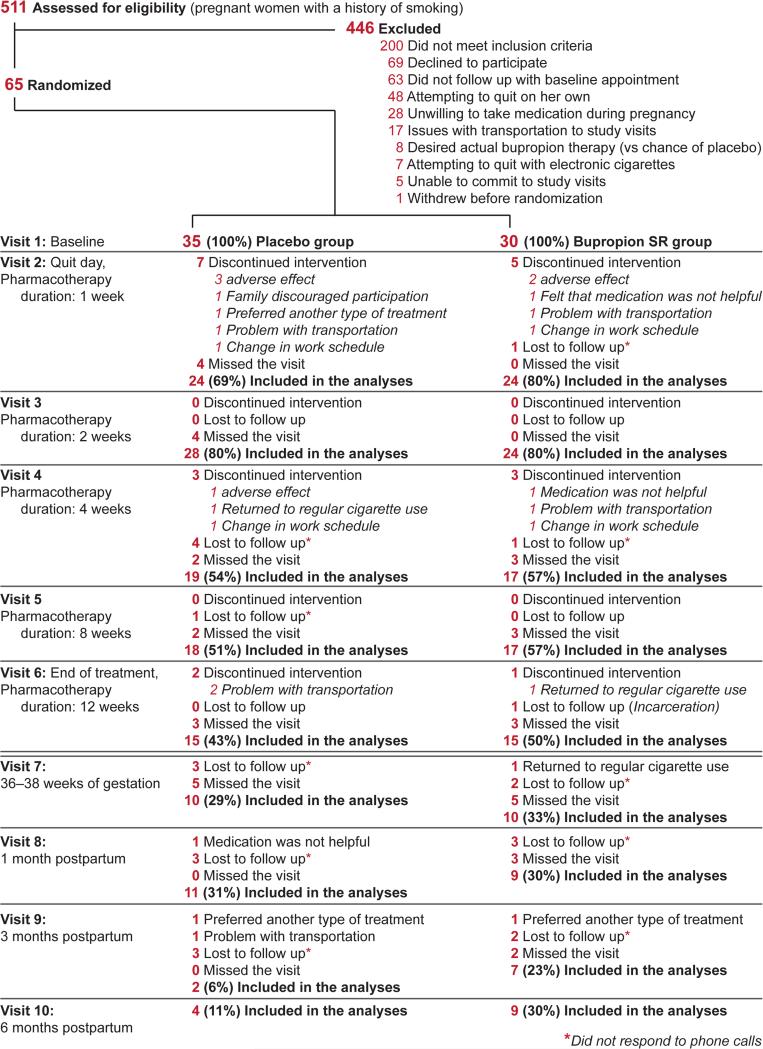
Figure 1.
Consort diagram.
The University of Iowa Pharmaceuticals was contracted for production, labeling, and bottling of the active drug and placebo tablets. To confirm patients’ proper use of medication, participants were asked to keep a daily diary of the number of pills taken. Subjects then returned the study drug bottles at the next study visit to the research nurse who performed pill counts for compliance records. Compliance to study medication was also biochemically confirmed as described below in Biochemical measures section.
Both groups received behavioral interventions, which included 35-minute counseling sessions at each of the first 2 visits (enrollment and on the quit day) and 10 minutes of smoking cessation counseling at subsequent visits. Counseling sessions were delivered by a research nurse using a motivational interviewing approach,7,8 which was previously shown to be effective in pregnant smokers.9
Measures
Prior to the smoking intervention, baseline standardized questionnaires assessed demographics, smoking history/status, as well as medical and obstetrical history. Additional questionnaires assessed confidence and motivation to quit, concerns about excessive weight gain, and confidence in bupropion as a smoking cessation aid. The questionnaires were based on the clinical trials published previously, 10,11 and each parameter was measured using a 5-point scale (1=least likely/mostly disagree, 5=highly likely/mostly agree). Nicotine dependence was assessed using the 6-item Fagerstrom Test of Nicotine Dependence (FTND).12 The Minnesota Nicotine Withdrawal Scale (MNWS)13 and the Prime Score14 questionnaires were completed at every visit, including baseline.
At every visit, a research nurse monitored the smoking status of all subjects (i.e., CPD, exhaled carbon monoxide [CO]) and adverse events (AEs). Exhaled CO was measured using a Vitalograph carbon monoxide monitor (Lenexa, KS) according to the manufacturer's recommendations. A urine sample was collected at each visit and stored at −80°C. AEs, including SAEs, were assessed on all subjects during interviews by the research nurse at each study visit. We monitored for maternal AEs that could be related to bupropion, such as seizures, BP > 140/90 mm Hg, headache, insomnia, rhinitis, dry mouth, and anxiety. We defined apriori which AEs would be considered fetal and neonatal SAEs: intrauterine fetal demise, preterm delivery (< 34 weeks), clinically suspected fetal growth restriction, congenital malformations, cardiovascular anomalies, low birth weight (< 10%), Apgar scores < 7 at 5 minutes, and neonatal length of hospital stay > 3 days. Research nurses also abstracted data on pregnancy and neonatal outcomes from electronic medical records after delivery.
Biochemical measures
The accuracy of self-reported smoking abstinence during study visits was confirmed by an exhaled CO level of < 4 parts per million (ppm)15,16 and by urinary cotinine level of < 50 ng/mL.17,18 Cotinine in urine was quantified using the validated liquid chromatography-mass spectrometry (LC/MS) method;19,20 sample extraction was based on the procedure described by Peterson et al.21 Patients’ compliance to study medication was confirmed by concentrations of bupropion in urine and its metabolites (hydroxybupropion [OHBUP] and threohydrobupropion [TB]) using the method reported previously.22,23
Retention
Subjects received phone call reminders prior to each study visit. If a participant missed the appointment, the research staff called to reschedule. If research personnel were unable to reach a subject after multiple attempts, the reason for withdrawal was identified as “lost to follow-up”. These subjects were classified as smokers for the purpose of analysis. Subjects were reimbursed as follows: $35 for each of the visits 1-7, $50 for each of the visits 8-9 and $75 for visit 10. The participants received the compensation at the end of study visits 3, 6, 7, 8, 9, and 10.
Data analysis
The original sample was based on detecting a difference in MNWS symptoms (i.e., cigarette craving and total withdrawal scores) between bupropion and placebo groups as a measure of potential efficacy. The study was powered for three outcomes: cigarette cravings, total MNWS withdrawal scores, and quit rates. A previous study of smoking cessation in pregnant women reported a standardized difference of d = 1.0 for cravings and d = 0.6 for total MNWS.24 In addition, a study of Hurt et al, 1997, reported a 45% quit rate among male and non-pregnant female smokers treated with bupropion SR for cessation, while 19% quit rate was observed among those receiving placebo.25 The initial targeted enrollment of 50 women per group would yield a power of 0.85 with an alpha of 0.05 to detect differences in total MNWS scores, 0.99 power to detect differences in craving scores, and 0.80 power to detect differences in quit rates. However, two years after the trial had initiated, the sample size was re-estimated because the original calculations were overpowered for some measures and the enrollment into the study was more difficult than expected. Thus, 30 subjects per group was still sufficiently powered (0.80) for the craving score; however, the total MNWS score was powered to 0.65 and quit rate to 0.60.
Baseline characteristics between groups were compared using a 2-sample t test for continuous variables and Chi-square (or exact test) for categorical variables. Likewise, the t and Chi-square tests were used to test for differences between groups on birth outcomes. For continuous outcome variables measured at each visit (MNWS, most smoking outcomes), a linear mixed model with a random effect for subject and fixed effects for group, time point, and the interaction was run. For dichotomous outcomes measured at each visit (abstinence, AE), a general estimating equation with a binomial distribution and logit link function and effects for group, time point, and the interaction was evaluated.
Results
Five hundred and eleven pregnant women were approached by research nurses to determine their study eligibility. Of these subjects, 200 (39%) did not meet inclusion criteria, 246 subjects (48%) were not enrolled for various reasons (Figure 1), and 65 pregnant women (13%) were enrolled in the study. Of the recruited 65 subjects, 30 were randomized to the bupropion group and 35 to the placebo group.
Demographic and Baseline Characteristics
Table 1 presents demographic and baseline characteristics of study participants. The average gestational age at enrollment was 18.9 ± 4.5 weeks. Participants reported average smoking of 18 ± 8 CPD prior to pregnancy and 12 ± 8 CPD at enrollment. The distribution of demographic and baseline characteristics was similar in both groups except race/ ethnicity (49% white/ non-Hispanic in the placebo group vs 77% white/ non-Hispanic in the bupropion group, P= 0.011) and age (27.5 ± 6.5 years vs 24.5 ± 5.6 years, P= 0.051).
Table 1.
Baseline characteristicsa
| Characteristics | Placebo (n = 35) | Bupropion (n = 30) | P value |
|---|---|---|---|
| Demographics | |||
| Age, in years, mean (SD) | 27.5 (6.52) | 24.5 (5.56) | 0.051 |
| Race/ethnicity, n (%) | |||
| White/ non-Hispanic | 17 (49) | 23 (77) | 0.011 |
| White/ Hispanic | 5 (14) | 3 (10) | |
| White/ none reported | 1 (3) | ||
| Black/ non-Hispanic | 13 (37) | 2 (7) | |
| Black/ Hispanic | 0 (0) | 1 (3) | |
| Marital status, n (%) | |||
| Single | 27 (77) | 23 (77) | 0.946 |
| Married | 8 (23) | 7 (23) | |
| Educational status, n (%) | |||
| ≤ High school graduate | 25 (71) | 20 (67) | 0.831 |
| Some college | 10 (29) | 10 (33) | |
| Employment status, n (%) | |||
| Unemployed | 19 (54) | 16 (53) | 0.199 |
| Employed part- or full-time | 16 (46) | 14 (47) | |
| Income estimate per year, n (%) | |||
| < $10,000 | 18 (51) | 14 (47) | 0.679 |
| $10,000–$30,000 | 11 (31) | 11 (37) | |
| $30,000–$100,000 | 5 (14) | 5 (17) | |
| Not reported | 1 (3) | ||
| Obstetrical | |||
| Gestational age at randomization (weeks.days), mean (SD) | 18.2 (1.2) | 18.5 (1.4) | 0.826 |
| Smoking history | |||
| Age when started smoking in years, mean (SD) | 15.7 (5.4) | 14.8 (2.8) | 0.414 |
| Number of cigarettes per day before pregnancy, mean (SD) | 16.1 (6.2) | 19.6 (10.1) | 0.092 |
| History of drug use, n (%) | |||
| None | 24 (68) | 21 (70) | 0.901 |
| Marijuana | 9 (26) | 7 (23) | |
| Cocaine | 1 (3) | 1 (3) | |
| Street drugs | 1 (3) | 1 (3) | |
| Living with smoker, n (%) | |||
| No | 12 (34) | 6 (20) | 0.228 |
| Yes | 23 (66) | 23 (77) | |
| Not reported | 1 (3) | ||
| Nicotine dependence | |||
| FTND score, mean (SD) | 4.6 (1.9) | 3.9 (1.7) | 0.147 |
| Psychosocial status | |||
| Total PRIME score, mean (SD) | 4.3 (4.4) | 3.6 (4.9) | 0.127 |
| Motivation and confidence to quit | |||
| Motivation to quit smoking at this time, mean (SD) | 4.3 (0.9) | 4.0 (0.7) | 0.182 |
| Confidence to quit smoking at this time, mean (SD) | 3.5 (1.1) | 3.6 (0.8) | 0.989 |
Values are numbers of participants unless stated otherwise
SD = standard deviation
Fifty-seven percent of women enrolled had previously tried to quit smoking during their current pregnancy. The average total FTND and PRIME scores as well as the average scores for motivation and confidence to quit did not differ among study groups (Table 1). The average level of worries about excessive weight gain due to intervention was not very high (2.4 ± 1.6), and many participants believed that bupropion would be helpful (mean score 3.9 ± 0.8) to them for smoking cessation.
Smoking abstinence
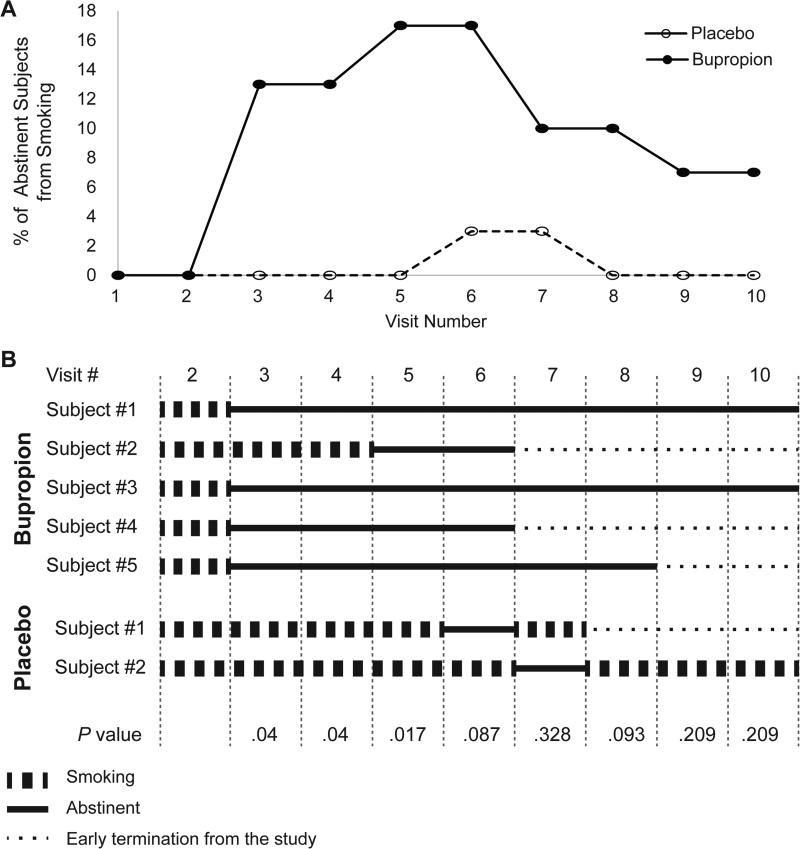
Seven-day point prevalence abstinence was defined at each visit as no cigarettes (not even a puff) in the last 7 days, levels of CO in exhaled air < 4 ppm,15,16 and concentrations of cotinine in urine < 50 ng/mL.17,18 The point prevalence abstinence rates during the treatment assessment period (visits 2–6) between study groups were significant (2% in the placebo group vs 19% in the bupropion group, P= 0.003, Figure 2). There was no significant difference in abstinence rates between bupropion and placebo groups at the end of medication treatment, visit 6, (17% vs 3%, P= 0.087) and at the end of pregnancy, visit 7 (10% vs 3%, P= 0.328), and during the postpartum period (visits 8–10).
Figure 2.
Rates of confirmed abstinence from smoking (A) and assessment of smoking status in confirmed quitters (B) during treatment period (visits 2–6), at the end of pregnancy (visit 7), and in the postpartum period (visits 8–10).
Abstinence was defined as no cigarettes (even a puff) in the last 7 days, levels of carbon monoxide (CO) in exhaled air < 4 ppm, and concentration of cotinine in urine less than 50 ng/mL. Five subjects in the bupropion group achieved abstinence during the treatment period. Four out of 5 subjects achieved 7-day point prevalence abstinence one week after the quit date (visit 3) and one subject 7 weeks after the quit date (visit 5). All 5 subjects in the bupropion group remained abstinent up to the last visit during the treatment period (visit 6). In the placebo group, one subject achieved 7-day point prevalence abstinence at visit 6, and another subject was abstinent at visit 7. Only 3/5 subjects who achieved abstinence during treatment in bupropion group, continued the study after visit 6 (end of treatment period) and remained abstinent at the end of pregnancy (visit 7).
If a subject missed a visit, she was counted as abstinent if she met the above criteria before and after this visit. All subjects are included at all time points. Missing data are recorded as smoking.
Compared to baseline levels of self-reported cigarette consumption, there was at least a 50% reduction in cigarettes smoked per day during treatment, at the end of pregnancy, and postpartum in both groups (Table 2). Although, this reduction in cigarette consumption was not statistically significant between the groups, exhaled CO and urinary cotinine concentrations during treatment (visits 2–6) were higher in the placebo group than the bupropion group (P= 0.011 and P< 0.001, respectively, Table 2).
Table 2.
Cigarette consumption, carbon monoxide and cotinine levels, tobacco cravings, and total score of withdrawal symptoms
| P value | ||||
|---|---|---|---|---|
| Placebo, mean (SD) | Bupropion, mean (SD) | controlling for baseline | controlling for baseline, age & race | |
| Cigarettes per day | ||||
| Baseline, Visit 1 | 10.7 (6.9) | 13.4 (9.3) | 0.202 | |
| Quit date, Visit 2 | 5.8 (4.3) | 7.2 (7.6) | 0.959 | 0.93 |
| Treatment assessment period, Visits 2–6 | 5.0 (4.8) | 4.7 (6.3) | 0.068 | 0.216 |
| Weeks 36–38, Visit 7 | 1.6 (2.5) | 4.6 (5.9) | 0.665 | 0.441 |
| Postpartum, Visits 8–10 | 4.3 (3.9) | 4.1 (3.5) | 0.55 | 0.749 |
| Exhaled carbon monoxide (ppm) | ||||
| Baseline, Visit 1 | 10.2 (6.5) | 13.4 (8.5) | 0.117 | |
| Quit date, Visit 2 | 8.7 (5.4) | 9.2 (7.3) | 0.509 | 0.988 |
| Treatment assessment period, Visits 2–6 | 9.1 (5.8) | 7.4 (6.4) | 0.011 | 0.053 |
| Weeks 36–38, Visit 7 | 5.5 (5.1) | 7.2 (4.7) | 0.514 | 0.662 |
| Postpartum, Visits 8–10 | 8.6 (5.7) | 10.5 (8.6) | 0.613 | 0.745 |
| Cotinine (ng/mL) | ||||
| Baseline, Visit 1 | 822.7 (685.9) | 657.6 (479.4) | 0.295 | |
| Quit date, Visit 2 | 843.5 (692.4) | 404.7 (402.9) | 0.066 | 0.24 |
| Treatment assessment period, Visits 2–6 | 830.5 (727.3) | 347.8 (383.9) | <0.001 | 0.007 |
| Weeks 36–38, Visit 7 | 542.6 (671.2) | 484.1 (462.1) | 0.96 | 0.666 |
| Postpartum, Visits 8–10 | 997.1 (609.8) | 755.5 (729.0) | 0.365 | 0.749 |
| Total score of withdrawal symptoms (no craving) | ||||
| Baseline, Visit 1 | 4.43 (5.77) | 5.22 (6.05) | 0.62 | |
| Quit date, Visit 2 | 4.88 (4.61) | 4.75 (4.87) | 0.978 | 0.713 |
| Treatment assessment period, Visits 2–6 | 5.35 (5.14) | 3.77 (4.27) | 0.068 | 0.028 |
| Craving for tobacco | ||||
| Baseline, Visit 1 | 1.79 (1.64) | 1.63 (1.55) | 0.742 | |
| Quit date, Visit 2 | 2.33 (1.31) | 2.04 (1.08) | 0.786 | 0.622 |
| Treatment assessment period, Visits 2–6 | 2.07 (1.23) | 1.50 (1.11) | 0.08 | 0.02 |
SD =standard deviation
There was no significant difference between the groups in total nicotine withdrawal symptoms (P= 0.068) and tobacco cravings (P = 0.08) in the unadjusted analysis; however, after controlling for maternal age and race, these measures were statistically significant between groups with the placebo groups having greater withdrawal symptoms and craving during treatment (P= 0.028 and P= 0.02, respectively, Table 2).
Birth and delivery outcomes
There were no significant differences between treatment groups in birth weight, infant length, head circumferences, Apgar score at 5 minutes, and pH values in arterial and venous cord blood (Table 3). The gestational age (P = 0.058) and Apgar score at 1 minute (P = 0.064), although not statistically significant, trended higher in the bupropion group.
Table 3.
Birth and delivery outcomes.
| Outcome measures | Placebo/BUP, N° of subjects | Placebo, mean (SD) | Bupropion, mean (SD) | Placebo/BUP | P value | |
|---|---|---|---|---|---|---|
| Minimum | Maximum | |||||
| Birth weight, gr | 30 / 27 | 3111 (543) | 3223 (501) | 1860 / 2087 | 4536 / 4055 | 0.299 |
| Infant length at birth, cm | 28 / 27 | 49 (2.5) | 50 (2.3) | 41 / 45 | 53 / 55 | 0.25 |
| Head circumference, cm | 26 / 26 | 33.5 (1.8) | 34.1 (1.22) | 30 / 32 | 37 / 37 | 0.265 |
| Apgar score at 1 minute | 29 / 27 | 7.8 (1.6) | 8.3 (1.0) | 2 / 5 | 9 / 9 | 0.064 |
| Apgar score at 5 minute | 29 / 27 | 8.8 (0.6) | 9.0 (0.3) | 7 / 8 | 10 / 10 | 0.201 |
| Cord blood arterial pH | 8 / 9 | 7.3 (0.04) | 7.3 (0.06) | 7.2 / 7.2 | 7.32 / 7.37 | 0.541 |
| Cord blood venous pH | 11 / 11 | 7.3 (0.05) | 7.3 (0.12) | 7.26 / 7.02 | 7.42 / 7.42 | 0.898 |
| Infant length of hospital stay, days | 28 / 27 | 2.8 (3.1) | 2.4 (2.8) | 1 / 1 | 14 / 16 | 0.612 |
| Neonatal intensive care unit admission, number (%) | 28 / 27 | 3 (11%) | 1 (4%) | N/A | N/A | 0.611 |
| Gestational age, weeks.days | 30 / 26 | 38.2 (1.4) | 38.7 (1.6) | 33.5 / 32.3 | 40 /40.3 | 0.058 |
| Preterm birth < 34 weeks, number (%) | 30 / 26 | 1 (3%) | 1 (4%) | N/A | N/A | 1.0 |
BUP =-bupropion; SD = standard deviation, N/A = non-applicable
SAEs occurred in five subjects in the placebo group and two in the bupropion group. In the placebo group, four involved an infant stay greater than three days (two cases of premature delivery, one for respiratory distress, and one for hyperbilirubinemia). The other SAE in the placebo group was due to a maternal hospitalization for diabetes management. The two SAEs in the bupropion group involved a subject who developed superimposed preeclampsia requiring delivery at 32 weeks 2 days and an infant with a cord blood pH value of 7.01. Following clinical review, both events were considered unlikely related to the study medication.
Maternal outcomes
There were no differences between bupropion and placebo groups in body mass index (32.9 ± 9.4 vs 39.8 ± 9.6, P = 0.52) at the end of pregnancy, systolic BP (116 ± 8 mmHg vs 122 ± 14 mmHg, P = 0.464) and diastolic BP (70 ± 9 mmHg vs 75 ± 11 mmHg, P = 0.396), and pulse rate, (81 ± 12 beat per minute vs 82 ± 12 beat per minute, P = 0.721).
Subjects in both groups reported moderate or severe adverse effects that are known side effects of bupropion, including headache (29% vs 11%, P= 0.157), difficulty sleeping (25% vs 7%, P= 0.123), runny nose (17% vs 7%, P= 0.397), dry mouth (37.5% vs 14%, P= 0.308), and anxiety (33% vs 18%, P= 0.22). The percentage of subjects who experienced moderate or severe adverse effects was not statistically different between groups.
Compliance with study medication and retention
The proportion of adherence to the study medication in the bupropion group was 87.4%, compared to 82% in the placebo group (P= 0.31). While the majority of subjects in both groups were able to correctly guess which treatment they received (P= 0.049), 33% of subjects in the placebo group believed they received bupropion SR, and 29% of subjects in the bupropion group believed they received placebo.
There was a high rate of early withdrawal from the study; only 20% of all subjects completed the clinical trial (30% of subjects in the bupropion group vs 11% in the placebo group, P= 0.12). There were no differences in education level, number of adults and children in household, annual income, smoking and psychosocial variables in the subjects who withdrew from the study vs those who stayed in the study. Higher completion rates were observed among subjects who lived with a partner vs those who did not (P = 0.013). In addition, the slightly higher completion rate in the cohort was associated with Hispanic vs non-Hispanic ethnicity (P = 0.07) and part-time (P= 0.080) vs non- or full-time workers (whose completion rates were similar).
Comments
In this randomized placebo controlled pilot study of bupropion SR for smoking cessation during pregnancy, bupropion SR reduced nicotine cravings and withdrawal symptoms compared to placebo and increased overall cessation rates during medication treatment. Abstinence rates were not statistically significant between groups at the end of pregnancy and postpartum, likely due to the small sample size. The medication was relatively well tolerated, and the serious adverse event rate was similar between groups (although numerically lower in the bupropion SR compared to the placebo group). These findings suggest that bupropion SR may be a promising adjunctive medication for smoking cessation during pregnancy.
It is noteworthy that we used a conservative estimate for cigarette abstinence, counting those who were lost to follow-up as smokers. It is possible that some of the individuals who quit smoking with bupropion or placebo and were lost to follow-up may have been tobacco free at the end of treatment and after pregnancy. During the treatment period, bupropion SR significantly reduced smoking as determined by levels of CO in exhaled air and concentrations of cotinine in the urine of pregnant women. The results of our study are in agreement with a previous observational study of bupropion SR effectiveness in pregnancy5 as well as with the existing literature on non-pregnant smokers showing that compared to placebo, bupropion can approximately double the chance of smoking cessation success.25
A significant impact of bupropion SR compared to placebo on measures of cravings and nicotine withdrawal symptoms during treatment was observed. This finding is consistent with other studies showing that bupropion SR reduces cravings for tobacco and signs and symptoms of nicotine withdrawal.25 It is noteworthy that many of the signs of nicotine withdrawal can also commonly occur during pregnancy (irritability, anxiety, difficulty concentrating, insomnia, restlessness, increased appetite, depressed mood, and drowsiness),26 and use of medications that reduce nicotine withdrawal could be especially beneficial during pregnancy to improve smoking cessation rates. Since the bupropion SR compared to the placebo group had lower overall nicotine withdrawal symptoms (which include insomnia and anxiety), but higher reports of at least one episode of insomnia and anxiety during treatment, it seems likely that these two symptoms may have been in part due to the medication in the bupropion SR group.
One of the major goals of prenatal management in women who smoke during pregnancy is to decrease the preterm delivery and low birth weight in neonates. Although our study was not powered to detect differences in neonatal outcomes, the difference in gestational age and Apgar score at 1 minute was in favor of the bupropion group.
Previous studies have shown that bupropion results in modest weight loss in patients with depression27 and hypertension.28 Our study did not reveal any differences between the bupropion and placebo groups in body weight, systolic and diastolic BP, and pulse rate. SAEs were not significantly different between groups. One subject in the bupropion group at 32.0 weeks’ gestation was diagnosed with superimposed preeclampsia. The association between preeclampsia and antidepressant use during pregnancy has been previously suggested.29,30 However, a recent study of an exposure-outcome relation within a nationwide Medicaid cohort did not find an association between bupropion use during pregnancy and preeclampsia (RR: 1.06; 95% CI:0.91–1.25).31
There are some limitations to the present study. One of the factors that affected enrollment of eligible subjects to the pilot study is the social stigma associated with both prenatal smoking and prenatal use of medications. It appears that there is a general misperception of risks associated with prenatal smoking and the potential benefit-to-risk ratios of smoking cessation with bupropion SR. An integrated effort of medical providers who can directly address these issues along with education of eligible women and family members provided via clinic media and printed material should overcome the challenges associated with recruitment in future studies. A high rate of early withdrawal from the clinical trial was another challenge encountered in this study. Implementation of home visits in future studies could help retain the recruited number of subjects through 10 visits, beginning in pregnancy and following through the postpartum period (up to 6 months after delivery).
The present study provides preliminary evidence that bupropion SR could be beneficial for smoking cessation during pregnancy. This result is encouraging because placebo controlled trials of nicotine replacement therapy for smoking cessation during pregnancy have not shown increased smoking cessation rates.32 A large scale study is warranted to fully examine the efficacy and safety of bupropion SR for smoking cessation during pregnancy.
Acknowledgements
The authors appreciate the support of National Institute on Drug Abuse RO1 DA030998; the physicians and nurses of the Labor & Delivery Ward of UTMB, Galveston, TX, and affiliated Regional Maternal Child Health Program clinics; and the assistance of the Publication, Grant, & Media Support Office of the Department of Obstetrics & Gynecology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The study was conducted at: Department of Obstetrics & Gynecology, The University of Texas Medical Branch (UTMB) at Galveston, TX; UTMB Regional Maternal Child Health program (RMCHP) clinics at Pearland, TX, and Pasadena, TX.
Conflict of interest: The authors report no conflict of interest
Clinical trial registration: ClinicalTrials.gov, www.clinicltrials.gov, NCT01390246).
Condensation: Adjunctive use of bupropion sustained-release compared to placebo for smoking cessation during pregnancy reduced cravings and nicotine withdrawal symptoms and increased quit rates during treatment.
References
- 1.Patnode CP, Henderson JT, Thompson JH, Senger CA, Fortmann SP, Whitlock EP. Behavioral Counseling and Pharmacotherapy Interventions for Tobacco Cessation in Adults, Including Pregnant Women: A Review of Reviews for the U.S. Preventive Services Task Force. Agency for Healthcare Research and Quality; Rockville, MD: Sep, 2015. Evidence Synthesis No. 134. AHRQ Publication No. 14-05200-EF-1. (Evidence Syntheses, No. 134. [PubMed] [Google Scholar]
- 2.Fiore MC, Jaén CR, Baker TB, et al. Treating tobacco use and dependence: 2008 update U.S. Public Health Service Clinical Practice Guideline executive summary. Respir Care. 2008;53(9):1217–22. PubMed PMID: 18807274. [PubMed] [Google Scholar]
- 3.Lumley J, Oliver SS, Chamberlain C, Oakley L. Interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev. 2004;(4):CD001055. doi: 10.1002/14651858.CD001055.pub2. DOI: 10.1002/14651858.CD001055.pub2. PubMed PMID: 15495004. [DOI] [PubMed] [Google Scholar]
- 4.Raupach T, van Schayck CP. Pharmacotherapy for smoking cessation: current advances and research topics. CNS Drugs. 2011;25(5):371–82. doi: 10.2165/11590620-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 5.Chan B, Einarson A, Koren G. Effectiveness of bupropion for smoking cessation during pregnancy. J Addict Dis. 2005;24(2):19–23. doi: 10.1300/J069v24n02_02. DOI: 10.1300/J069v24n02_02. PubMed PMID: 15784520. [DOI] [PubMed] [Google Scholar]
- 6.Stout RL, Wirtz PW, Carbonari JP, Del Boca FK. Ensuring balanced distribution of prognostic factors in treatment outcome research. J Stud Alcohol Suppl. 1994;12:70–5. doi: 10.15288/jsas.1994.s12.70. PubMed PMID: 7723001. [DOI] [PubMed] [Google Scholar]
- 7.Rollnick S, Butler CC, Stott N. Helping smokers make decisions: the enhancement of brief intervention for general medical practice. Patient Educ Couns. 1997;31(3):191–203. doi: 10.1016/s0738-3991(97)01004-5. PubMed PMID: 9277242. [DOI] [PubMed] [Google Scholar]
- 8.Rollnick S, Heather N, Gold R, Hall W. Development of a short 'readiness to change' questionnaire for use in brief, opportunistic interventions among excessive drinkers. Br J Addict. 1992;87(5):743–54. doi: 10.1111/j.1360-0443.1992.tb02720.x. PubMed PMID: 1591525. [DOI] [PubMed] [Google Scholar]
- 9.Dornelas EA, Magnavita J, Beazoglou T, et al. Efficacy and cost-effectiveness of a clinic-based counseling intervention tested in an ethnically diverse sample of pregnant smokers. Patient Educ Couns. 2006;64(1-3):342–9. doi: 10.1016/j.pec.2006.03.015. Epub 2006 Jul 21. DOI: 10.1016/j.pec.2006.03.015. PubMed PMID: 16859864. [DOI] [PubMed] [Google Scholar]
- 10.Berg CJ, Park ER, Chang Y, Rigotti NA. Is concern about post-cessation weight gain a barrier to smoking cessation among pregnant women? Nicotine Tob Res. 2008 Jul;10(7):1159–63. doi: 10.1080/14622200802163068. doi: 10.1080/14622200802163068.PubMed PMID: 18629725. [DOI] [PubMed] [Google Scholar]
- 11.Fucito LM, Toll BA, Salovey P, O'Malley SS. Beliefs and attitudes about bupropion: implications for medication adherence and smoking cessation treatment. Psychol Addict Behav. 2009 Jun;23(2):373–9. doi: 10.1037/a0015695. doi: 10.1037/a0015695. PubMed PMID: 19586156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. PubMed PMID: 1932883. [DOI] [PubMed] [Google Scholar]
- 13.Hughes J, Hatsukami DK. Errors in using tobacco withdrawal scale. Tob Control. 1998;7(1):92–3. doi: 10.1136/tc.7.1.92a. PubMed PMID: 9706762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282(18):1737–44. doi: 10.1001/jama.282.18.1737. PubMed PMID: 10568646. [DOI] [PubMed] [Google Scholar]
- 15.Bailey BA. Using expired air carbon monoxide to determine smoking status during pregnancy: preliminary identification of an appropriately sensitive and specific cut-point. Addict Behav. 2013;38(10):2547–50. doi: 10.1016/j.addbeh.2013.05.011. DOI: 10.1016/j.addbeh.2013.05.011. PubMed PMID: 23793041. [DOI] [PubMed] [Google Scholar]
- 16.Higgins ST, Heil SH, Badger GJ, et al. Biochemical verification of smoking status in pregnant and recently postpartum women. Exp Clin Psychopharmacol. 2007;15(1):58–66. doi: 10.1037/1064-1297.15.1.58. DOI: 10.1037/1064-1297.15.1.58. PubMed PMID: 17295585. [DOI] [PubMed] [Google Scholar]
- 17.Aurrekoetxea JJ, Murcia M, Rebagliato M, et al. Determinants of self-reported smoking and misclassification during pregnancy, and analysis of optimal cut-off points for urinary cotinine: a cross-sectional study. BMJ Open. 2013;3(1) doi: 10.1136/bmjopen-2012-002034. DOI: 10.1136/bmjopen-2012-002034. PubMed PMID: 23355667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stragierowicz J, Mikołajewska K, Zawadzka-Stolarz M, Polańska K, Ligocka D. Estimation of cutoff values of cotinine in urine and saliva for pregnant women in Poland. Biomed Res Int. 2013;2013:386784. doi: 10.1155/2013/386784. DOI: 10.1155/2013/386784. PubMed PMID: 24228246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.FDA, Guidance for Industry, Bio-analytical Method Validation, Food and Drug Administration, Centre for Drug Evaluation and Research 2011 May; http://www.fda.gov/AnimalVeterinary/GuidanceComplianceEnforcement/ GuidanceforIndustry/ucm123635.htm.
- 20.Fokina VM, Wang X, Ahmed M, Oncken C, Hankins GDV, Nanovskaya TN. Determination of cotinine in the urine of pregnant patients enrolled in a clinical trial for the use of bupropion sustained release as an aid for smoking cessation. Drug and Alcohol Dependence. 2015;146:e280. DOI: http://dx.doi.org/10.1016/j.drugalcdep.2014.09.227. [Google Scholar]
- 21.Petersen GO, Leite CE, Chatkin JM, Thiesen FV. Cotinine as a biomarker of tobacco exposure: development of a HPLC method and comparison of matrices. J Sep Sci. 2010;33(4-5):516–21. doi: 10.1002/jssc.200900575. DOI: 10.1002/jssc.200900575. PubMed PMID: 20155742. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Abdelrahman DR, Fokina VM, Hankins GD, Ahmed MS, Nanovskaya TN. Metabolism of bupropion by baboon hepatic and placental microsomes. Biochem Pharmacol. 2011;82(3):295–303. doi: 10.1016/j.bcp.2011.04.014. DOI: 10.1016/j.bcp.2011.04.014. PubMed PMID: 21570381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fokina VM, Xu M, Rytting E, et al. Pharmacokinetics of bupropion and its pharmacologically active metabolites in pregnancy. Drug Metab Dispos. 2016 doi: 10.1124/dmd.116.071530. pii: dmd.116.071530. DOI: 10.1124/dmd.116.071530 [Epub ahead of print]. PubMed PMID: 27528039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oncken C, Campbell W, Chan G, Hatsukami D, Kranzler HR. Effects of nicotine patch or nasal spray on nicotine and cotinine concentrations in pregnant smokers. J Matern Fetal Neonatal Med. 2009;22(9):751–8. doi: 10.3109/14767050902994515. DOI: 10.3109/14767050902994515. PubMed PMID: 19526424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hurt RD, Sachs DP, Glover ED, et al. A comparison of sustained-release bupropion and placebo for smoking cessation. N Engl J Med. 1997;337(17):1195–202. doi: 10.1056/NEJM199710233371703. DOI: 10.1056/NEJM199710233371703. PubMed PMID: 9337378. [DOI] [PubMed] [Google Scholar]
- 26.Oncken CA, Kranzler HR. Pharmacotherapies to enhance smoking cessation during pregnancy. Drug Alcohol Rev. 2003;22(2):191–202. doi: 10.1080/09595230100100633. DOI: 10.1080/09595230100100633. PubMed PMID: 12850906. [DOI] [PubMed] [Google Scholar]
- 27.Croft H, Houser TL, Jamerson BD, et al. Effect on body weight of bupropion sustained-release in patients with major depression treated for 52 weeks. Clin Ther. 2002;24(4):662–672. doi: 10.1016/s0149-2918(02)85141-4. PubMed PMID: 12017410. [DOI] [PubMed] [Google Scholar]
- 28.Hays JT, Ebbert JO. Bupropion for the treatment of tobacco dependence: guidelines for balancing risks and benefits. CNS Drugs. 2003;17(2):71–83. doi: 10.2165/00023210-200317020-00001. PubMed PMID: 12521356. [DOI] [PubMed] [Google Scholar]
- 29.Palmsten K, Setoguchi S, Margulis AV, Patrick AR, Hernández-Díaz S. Elevated risk of preeclampsia in pregnant women with depression: depression or antidepressants? Am J Epidemiol. 2012;175(10):988–97. doi: 10.1093/aje/kwr394. DOI: 10.1093/aje/kwr394. PubMed PMID: 22442287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Vera MA, Bérard A. Antidepressant use during pregnancy and the risk of pregnancy-induced hypertension. Br J Clin Pharmacol. 2012;74(2):362–9. doi: 10.1111/j.1365-2125.2012.04196.x. DOI: 10.1111/j.1365-2125.2012.04196.x. PubMed PMID: 22435711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palmsten K, Huybrechts KF, Michels KB, et al. Antidepressant use and risk for preeclampsia. Epidemiology. 2013;24(5):682–91. doi: 10.1097/EDE.0b013e31829e0aaa. DOI: 10.1097/EDE.0b013e31829e0aaa. PubMed PMID: 23873072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coleman T, Chamberlain C, Davey MA, Cooper SE, Leonardi-Bee J. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev. 2015;(12):CD010078. doi: 10.1002/14651858.CD010078.pub2. DOI: 10.1002/14651858.CD010078.pub2. PubMed PMID: 26690977. [DOI] [PubMed] [Google Scholar]