Abstract
Objective
Citrullinated proteins have been found within atherosclerotic plaque. However, to date studies evaluating the association between anti-citrullinated peptide antibodies (ACPAs) and imaging measures of atherosclerosis in patients with rheumatoid arthritis (RA) have been limited to citrullinated-fibrinogen or citrullinated-vimentin seroreactivities and rendered contradictory results. Therefore, our objective was to evaluate this association using an extended panel of ACPAs in a larger sample of RA patients without clinical cardiovascular disease (CVD).
Methods
ACPAs were identified by a custom Bio-Plex bead assay in 270 patients from two independent RA cohorts without clinical CVD consisting of 195 and 75 patients, respectively. Coronary artery calcium (CAC) was assessed by computed tomography as a measure of coronary artery disease.
Results
High levels of anti-citrullinated histone 2B antibodies were strongly associated with higher CAC compared with lower antibody levels (p=0.001); this remained significant after adjusting for traditional CV and RA-specific risk factors (p=0.03). No association between levels of ACPAs and CAC progression at 3 years was seen (p=0.09), however the number of progressors was small (n=92).
Conclusion
Higher levels of ACPAs targeting cit-histone 2B were associated with higher CAC scores when compared to lower antibody levels, suggesting a potential role for histone citrullination seroreactivity in atherosclerosis.
Cardiovascular disease (CVD), including coronary heart disease (CHD), is the leading cause of death in rheumatoid arthritis (RA) (1). RA poses an increased risk for accelerated atherosclerosis that is only partially explained by traditional CV risk factors (2). Inflammatory and immune-mediated pathway interactions play an essential role in atherosclerotic plaque development and rupture (3). The recent demonstration of citrullinated proteins within atherosclerotic plaque (4), and the relative specificity of anti-citrullinated protein antibodies (ACPAs) for RA, suggest a role for ACPAs in accelerating atherosclerosis in RA. However, investigations to date in this regard have been limited to anti-citrullinated fibrinogen and anti-citrullinated vimentin antibodies and have yielded contradictory results (4–6). We investigated the association of seroreactivities to a broader panel of autoantigens with coronary artery calcification (CAC), as a surrogate measure of atherosclerosis, in a larger sample consisting of two independent RA cohorts without clinical CVD.
MATERIALS AND METHODS
Patients
The study included 195 patients from the ESCAPE-RA (Evaluation of Subclinical CArdiovascular disease and Predictors of Events in Rheumatoid Arthritis) study who underwent cardiac computed tomography for CAC score measurement and concurrent serum testing for ACPAs. Briefly, ESCAPE-RA participants were 45–84 years old, met 1987 American College of Rheumatology RA criteria, had RA for ≥6 months, and no clinical CVD (defined as coronary artery disease, myocardial infarction, heart failure, stroke) (7). A second independent cohort consisted of the first 75 participants in RHYTHM (RHeumatoid arthritis studY of THe Myocardium), an ongoing study to identify factors associated with myocardial phenotypes in RA patients without CVD. Participants had similar inclusion criteria as ESCAPE-RA (except for an age requirement of ≥18 years), underwent similar CAC score measurement and concurrent testing for an expanded ACPA panel. The studies were approved by the Johns Hopkins Medical Institutions and Columbia University Institutional Review Boards, respectively. Enrolment for ESCAPE-RA occurred from 2004–2006. Enrolment for RHYTHM started in 2011 and is ongoing.
Outcome assessment
Coronary artery calcification
All participants underwent cardiac CT scanning as previously described (7, 8). CAC, quantified by the Agatston method (9), was used as the primary outcome given its non-invasiveness, high reproducibility, sensitivity and specificity in measuring atherosclerosis; and its predictability of CV events (10).
Measurement of anti-citrullinated peptide antibodies (ACPAs)
Serum samples were obtained the same day as the cardiac CT scanning for both cohorts. Reactivities to citrullinated and non-citrullinated/control (native) proteins/peptides were quantified using a custom Bio-Plex bead assay as previously described (11, 12). A high autoreactivity level was defined as ≥75th percentile. The ESCAPE-RA patients underwent ACPA testing using a panel of 17 citrullinated and 3 native proteins/peptides (11). For the RHYTHM patients an expanded panel of 30 citrullinated and 5 native proteins/peptides was used (12), of which 16 citrullinated and 2 native proteins/peptides were overlapped with the panel used for the ESCAPE-RA cohort.
Assessment of covariates
Clinical Characteristics
Demographics and smoking history were self-reported and collected from standardized study questionnaires. The body mass index, blood pressure, hypertension, and diabetes were defined and measured as previously described (7, 8).
RA-specific covariates
RA disease duration was calculated from the time of physician diagnosis. RA activity was calculated using the Disease Activity Score in 28 joints (DAS28) with C-reactive protein (CRP) level (7, 8). Use of glucocorticoids, biologic and non-biologic DMARDs, aspirin and cholesterol medications was ascertained by patient interview.
Laboratory covariates
Serum and plasma were separated by centrifugation and stored at −70°C. High-sensitivity CRP, IL-6, cholesterol levels, rheumatoid factor (RF) IgM and anti–cyclic citrullinated peptide (anti-CCP) antibodies were assessed as previously described (7, 8). RF and anti-CCP antibodies were defined as positive if ≥40 units and ≥60 units, respectively.
Statistical methods
Baseline characteristics were summarized and expressed as the mean ± standard deviation (SD) or median (interquartile range) for continuous variables. Counts and percentages were calculated for categorical variables. CAC was analyzed as a linear variable: log-transformed CAC score + 1. ACPA levels were dichotomized at the 75th percentile. The association between seroreactivity to a panel of citrullinated, and native (non-citrullinated) proteins/peptides with CAC was assessed using linear regression with adjustment for confounders and variables significantly associated with CAC. The association of CAC with ACPAs that were measured in only one of the cohorts was examined in a similar manner. An alpha value of 0.05 was defined as statistically significant. Statistical calculations were performed using SAS 9.4.
RESULTS
Clinical characteristics
Patient characteristics are summarized in Table 1. RHYTHM patients were more likely to be non-white and to have diabetes than the ESCAPE-RA participants. In ESCAPE-RA current smoking, ever smoking, and having a CAC >0 were more common than in RHYTHM.
Table 1.
Characteristics of patients in the ESCAPE-RA and RHYTHM cohorts.
| ESCAPE-RA (n=195) |
RHYTHM (n=75) |
Total (n=270) |
|
|---|---|---|---|
| Demographics | |||
| Age (years) | 59 ± 9 | 54 ± 13 | 58 ± 10 |
| Female | 118 (60%) | 64 (85%) | 182 (68%) |
| White | 170 (87%) | 27 (37%) | 197 (73%) |
| RA Characteristics | |||
| Disease duration (years) | 9 (4–17) | 7 (3–17) | 8 (4–17) |
| DAS28 C-RP | 3.6 ± 1.1 | 3.7 ± 1.2 | 3.7 ± 1.1 |
| RF or anti-CCP positivity | 126 (65%) | 56 (79%) | 182 (68%) |
| Any HLA-DRB1 shared epitope alleles, no (%) | 134 (68%) | 43 (61%) | 177 (67%) |
| IL-6 (pg/ml) | 3.8 (1.7–7.7) | 2.8 (1.4–8.7) | 3.6 (1.7–8.2) |
| C-Reactive Protein (mg/l) | 2.4 (1.1–7.2) | 2.7 (0.6–6.7) | 2.6 (1–6) |
| HAQ score (0 to 3) | 0.6 (0.1–1.2) | 1.0 (0.5–1.7) | 0.7 (0.2–1.3) |
| Non-biologic DMARD use | 164 (84%) | 52 (73%) | 216 (80%) |
| Biologic DMARD use (current) | 89 (46%) | 23 (32%) | 112 (42%) |
| TNF-inhibitor use (current) | 85 (44%) | 19 (27%) | 104 (39%) |
| Glucocorticoids (current) | 75 (38%) | 25 (35%) | 100 (37%) |
| Current prednisone dose (mg) | 0 (0–5) | 5 (4–10) | 0 (0–5) |
| Cardiovascular risk factors | |||
| Diabetes | 13 (7%) | 9 (13%) | 22 (8%) |
| Systolic blood pressure (mmHg) | 128 ± 19 | 118 ± 19 | 125 ± 19 |
| Diastolic blood pressure (mmHg) | 76 ± 9 | 70 ± 10 | 74 ± 10 |
| Hypertension | 79 (40%) | 31 (41%) | 110 (41%) |
| Total cholesterol (mg/dl) | 195 ± 38 | 192 ± 39 | 194 ± 39 |
| LDL cholesterol (mg/dl) | 116 ± 31 | 107 ± 35 | 113 ± 32 |
| HDL cholesterol (mg/dl) | 55 ± 19 | 61 ± 20 | 56 ± 19 |
| Triglycerides (mg/dl) | 107 (68–151) | 93 (77–140) | 100 (74–148) |
| Current smoking | 23 (12%) | 5 (7%) | 28 (11%) |
| Ever smoking history | 116 (59%) | 30 (43%) | 146 (55%) |
| Body mass index (kg/m2) | 28 ± 5 | 28 ± 6 | 28 ± 5 |
| Any CAC | 106 (54%) | 25 (33%) | 131 (49%) |
Characteristics are expressed as n (%), as the mean ± standard deviation, or as the median (interquartile range).
DAS, disease activity score; RF, rheumatoid factor; anti-CCP, anti-citrullinated cyclic peptide; HAQ, health assessment questionnaire; DMARD, disease modifying anti-rheumatic drug; TNF, tumoral necrosis factor; LDL, low-density lipoprotein; HDL, high-density lipoprotein; CAC, coronary artery calcium.
Association of ACPAs and CAC
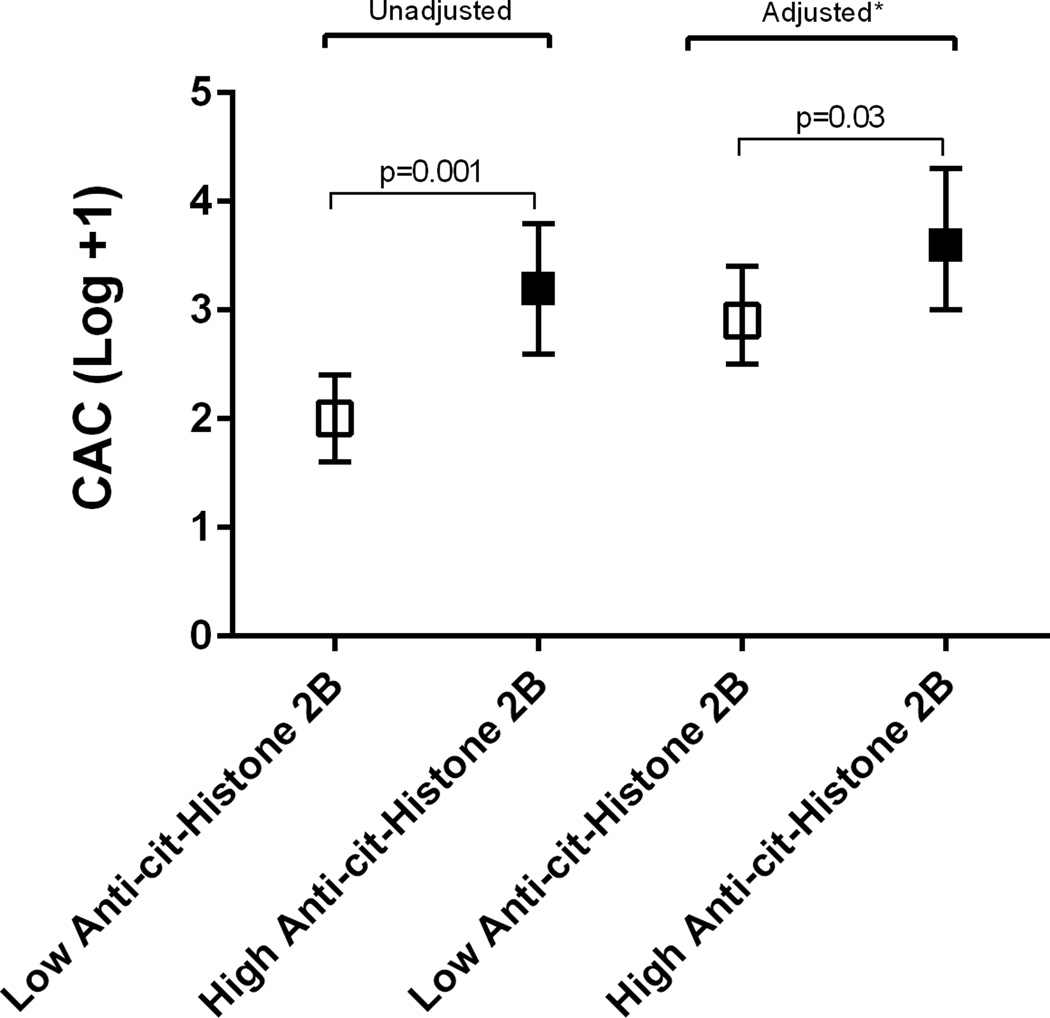
No statistically significant association between ACPAs and CAC score was identified in the ESCAPE-RA cohort although a trend was noted with high levels of anti-cit-H2B (Table 2). In the RHYTHM cohort, high levels of seroreactivities targeting cit-vimentin, cit-H2A and cit-H2B were associated with CAC score in univariable analysis; however, statistical significance was lost in the adjusted analysis (Table 2). Analyzing the combined cohorts, high levels of anti-cit-H2B antibodies were significantly associated with higher CAC scores in unadjusted (p=0.001), and adjusted analyses (p=0.03) (Figure 1). No association was seen between CAC and levels of the non-citrullinated control proteins in either cohort or in the pooled analysis (Table 2).
Table 2.
Association of CAC and ACPA reactivities.
| ESCAPE-RA (N=195) | RHYTHM (n=75) | Combined cohorts Total (n=270) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Univariate Model | Univariate Model | Multivariate Model | Univariate Model | Multivariate Model | ||||||
| AutoAntigens | Coefficient | p value | Coefficient | p value | Coefficient* | p value | Coefficient | p value | Coefficientδ | p value |
| Anti–fibrinogen cit | 0.30 | 0.50 | 0.43 | 0.52 | - | - | 0.30 | 0.42 | - | - |
| Fibrinogen A 41–60 cit | 0.68 | 0.13 | 1.17 | 0.08 | - | - | 0.79 | 0.03 | 0.22 | 0.54 |
| Fibrinogen A 556–575 cit | 0.32 | 0.48 | 0.63 | 0.35 | - | - | 0.36 | 0.33 | - | - |
| Fibrinogen A 211–230 cit | 0.44 | 0.33 | 1.01 | 0.13 | - | - | 0.50 | 0.19 | - | - |
| Fibrinogen A 616–635 cit | 0.44 | 0.33 | 0.49 | 0.46 | - | - | 0.53 | 0.16 | - | - |
| Fibrinogen A 582–599 cit | - | - | 0.54 | 0.42 | - | - | - | - | - | - |
| Fibrinogen A 27–43 cit | - | - | 0.54 | 0.42 | - | - | - | - | - | - |
| Fibrinogen B 36–52 cit | - | - | 0.88 | 0.18 | - | - | - | - | - | - |
| Fibrinogen B 54–72 cit | - | - | 0.15 | 0.82 | - | - | - | - | - | - |
| Fibrinogen B 246–267 cit | - | - | 0.15 | 0.82 | - | - | - | - | - | - |
| Vimentin cit | −0.05 | 0.92 | 1.37 | 0.04 | 0.96 | 0.08 | 0.56 | 0.14 | - | - |
| Vimentin 58–77 cit | 0.12 | 0.79 | 1.31 | 0.05 | 0.94 | 0.11 | 0.07 | 0.86 | - | - |
| Vimentin 1–16 cit | - | - | 1.13 | 0.09 | - | - | - | - | - | - |
| Apolipoprotein A1 cit | −0.43 | 0.34 | 0.79 | 0.24 | - | - | 0.26 | 0.50 | - | - |
| Apolipoprotein A1 231–248 cit | - | - | −0.74 | 0.26 | - | - | - | - | - | - |
| Apolipoprotein E cit | 0.01 | 0.97 | 0.04 | 0.95 | - | - | 0.41 | 0.33 | - | - |
| Apolipoprotein E 277–296 cit | 0.61 | 0.18 | 0.61 | 0.36 | - | - | 0.38 | 0.31 | - | - |
| Filaggrin 48–65 cit | 0.62 | 0.17 | 0.85 | 0.20 | - | - | 0.63 | 0.09 | - | - |
| Filaggrin 48–65 cyclic cit | - | - | −0.34 | 0.59 | - | - | - | - | - | - |
| Biglycan 247–266 cit | 0.58 | 0.20 | 0.66 | 0.32 | - | - | 0.47 | 0.21 | - | - |
| Histone 2A cit | - | - | 1.28 | 0.05 | 0.90 | 0.14 | - | - | - | - |
| Histone 2A 1–20 cit | 0.38 | 0.40 | 0.94 | 0.16 | - | - | 0.77 | 0.04 | 0.33 | 0.32 |
| Histone 2A 1–20 cit sm | - | - | 0.46 | 0.50 | - | - | - | - | - | - |
| Histone 2B cit | 0.80 | 0.08 | 1.34** | 0.05 | 0.84 | 0.15 | 1.21 | 0.001 | 0.74 | 0.03 |
| Histone 2B62–81 cit | 0.07 | 0.88 | 1.45 | 0.03 | 0.52 | 0.38 | 0.60 | 0.11 | - | - |
| Clusterin 231–250 cit | 0.09 | 0.83 | 1.26 | 0.06 | - | - | 0.44 | 0.24 | - | - |
| Clusterin 221–240 cit | - | - | 0.99 | 0.14 | - | - | - | - | - | - |
| Enolase cit | 0.16 | 0.72 | - | - | - | - | - | - | - | - |
| Enolase A 5–21 cit | - | - | 0.76 | 0.26 | - | - | - | - | - | - |
| Fibronectin cit | - | - | −0.17 | 0.80 | - | - | - | - | - | - |
| Fibronectin 1029–1042 cit | - | - | 0.03 | 0.97 | - | - | - | - | - | - |
| Non-citrullinated Controls | ||||||||||
| Fibrinogen | 0.37 | 0.41 | −0.16 | 0.80 | - | - | 0.38 | 0.31 | - | - |
| Apo A1 | 0.29 | 0.47 | −0.64 | 0.33 | - | - | 0.59 | 0.11 | - | - |
| Apo E | 0.06 | 0.89 | - | - | - | - | - | - | - | - |
| Vimentin | - | - | −0.36 | 0.59 | - | - | - | - | - | - |
| Histone 2A | - | - | −0.22 | 0.72 | - | - | - | - | - | - |
| Histone 2B | - | - | −0.17 | 0.80 | - | - | - | - | - | - |
Linear regression parameter estimates of the log transformed coronary artery calcium score (CAC)+1 per category of ACPA reactivity (dichotomized at the 75th percentile) in ESCAPE-RA, RHYTHM, and the combination of the cohorts.
Adjusted for anti-histone 2B.
Adjusted for age, RA disease duration, hypertension, diabetes and TNF-inhibitor use.
Adjusted for age, gender, RA disease duration, ever smoking, hypertension, body mass index, high-density lipoprotein cholesterol, triglycerides, lipid medication, and aspirin use.
Figure 1.
Coronary artery calcification association with levels of anti-citrullinated histone 2B antibody (75th percentile vs. lower percentiles).
Linear regression of the association of the log transformation of coronary artery calcium score (CAC)+1 and anti-citrullinated histone 2B antibody level (75th percentile vs. lower percentiles). *Adjusted for age, gender, RA disease duration, ever smoking, hypertension, body mass index, high-density lipoprotein (HDL) cholesterol, triglycerides, lipid medication, and aspirin use.
The association of ACPAs with CAC progression over 3 years was also investigated. Although no association was found (p= 0.09), only the ESCAPE cohort had repeat CAC measurements and, of those, the number of progressors was small (n=92).
The total number of high-level ACPAs present per patient was also not associated with either baseline CAC or CAC progression (data not shown).
Association of ACPAs and CAC per strata of patient characteristics
The association between CAC and high levels of ACPAs targeting cit-apoE277–296, cit-biglycan247–266, and cit-filaggrin48–65, varied per gender (interaction term p value <0.05 for each). However, although these 3 ACPAs were associated with CAC in univariable analysis, statistical significance was lost in multivariable models (data not shown). In addition, there was an interaction between ever-smoking history and CAC, with higher CAC scores seen in ever-smokers with higher ACPA levels, but significance was lost in adjusted analyses (data not shown). No difference in the association of high-levels of ACPAs with CAC was noted by race, shared epitope status and/or rheumatoid factor or anti-CCP positivity (data not shown).
DISCUSSION
This study shows that in RA patients without clinical CVD, high levels of ACPAs targeting cit-H2B proteins, but not ACPAs targeting other citrullinated and non-citrullinated antigens, were significantly associated with higher CAC scores when compared with lower levels of these antibodies.
RA patients have an increased risk of atherosclerosis and coronary heart disease only partially explained by traditional cardiovascular risk factors (1–3). RA itself is an independent risk factor for this excess risk (2,3) and although autoimmunity has been proposed as the possible link, the exact pathogenesis remains poorly understood.
Citrullination is a post-translational protein modification that occurs as part of inflammation within the atherosclerotic plaque as it does in the synovium (4). Recent data suggest a role for ACPAs in atherosclerosis: anti-cit-vimentin antibodies correlated with subclinical atherosclerosis in early RA (12); cit-fibrinogen and colocalizing peptidylarginine deiminase type 4 enzyme were identified within the atherosclerotic plaque of autopsied non-RA patients, and ACPAs in RA subjects targeting cit-fibrinogen and cit-vimentin were associated with an increase in aortic plaque burden (4). However, a study by Montes et al found no association between three different measures of atherosclerosis, including CAC, and ACPAs targeting cit-fibrinogen and its B36–52 peptide (5). Our study examined a much broader array of autoantigens than the prior studies and in a larger combined cohort. We failed to find an association between anti-cit-fibrinogen or anti-cit-vimentin antibodies with CAC, but identified a strong association with ACPAs targeting cit-H2B. Several reasons could account for these differences. Sokolove studied aortic calcification and exclusively in RA females, while El-Barbary evaluated the association of anti-cit-vimentin antibodies with carotid intima-media thickness, in contrast to our study which utilized a direct measure of coronary artery disease (CAC) (6). Montes also investigated CAC in addition to carotid artery measures but limited the ACPA investigations to anti-cit-fibrinogen and its B36–52 peptide (5) while our study addressed a much broader array of citrullinated autoantigens.
Histones, DNA-packaging nuclear proteins released during inflammation, are thought to be pro-atherogenic at least in part by promoting aggregation and uptake of LDL into foam cells (13). Although this relates to non-citrullinated histones, it is tempting to speculate a role for the citrullinated forms of these proteins in atherosclerosis. In support of this, cit-histones are considered to be a biomarker for netosis. Netosis is a neutrophil extracellular aggregate (trap) consisting of DNA segments wound in a mixture of nuclear and cytoplasmic proteins including histones, myeloperoxidase, and neutrophil elastase, known to have potent pro-inflammatory, cytotoxic, and pro-thrombotic effects that are independently associated with coronary atherosclerosis and ischemic cardiac events (14). Moreover, immune complexes containing citrullinated H2B have the capacity to initiate inflammation including inducing neutrophil activation (15). Our data extend these findings by suggesting a potential role for anti-cit-histone antibodies in the evolution of atherosclerosis in RA. Interestingly, no additive effect was seen in the number of high-level ACPAs and their association with CAC, supporting specificity in the association with anti-cit-H2B antibodies. Although anti-cit-histone reactivity was not associated with progression of CAC, the number of progressors in the ESCAPE-RA cohort was small.
The strengths of our study include its sample size derived from two independent cohorts of RA patients without clinical CVD who were well phenotyped for CV risk. Furthermore, our expanded panel of ACPAs allowed testing of additional seroreactivities of potential interest in atherosclerosis.
Limitations include using an ACPA panel that consisted of candidate autoantigens identified through synovial analysis; thus, potential additional autoantigens more relevant in atherosclerosis were conceivably missed.
In conclusion, high-levels of anti-cit-H2B antibodies were associated with a higher burden of atherosclerosis, measured by coronary artery calcification, in RA patients without CVD. Future studies to explain their role in the pathogenesis of atherosclerosis in RA are warranted.
SIGNIFICANCE AND INNOVATION.
This study is the first to test the association of anti-citrullinated peptide antibodies (ACPAs) and coronary artery calcification (CAC) using a broad panel of ACPAs in a large sample of rheumatoid arthritis (RA) patients without clinical cardiovascular disease (CVD) and comprehensively phenotyped for CV risk factors.
The main finding in our study was that high levels of anti-cit-H2B antibodies were associated with a higher burden of atherosclerosis, measured by coronary artery calcification, in RA patients without CVD. This is innovative as it adds to mechanistic understanding of the increased prevalence of atherosclerosis and CVD in RA by suggesting a potential role for anti-cit-H2B in this process.
Acknowledgments
Financial Support
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases under Award Number AR-050026 and AR050026-08S1 (JMB), and by the Clinical and Translational Science Award (CTSA) grant UL1 TR000040 of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Solomon DH, Karlson EW, Rimm EB, Cannuscio CC, Mandl LA, Manson JE, et al. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation. 2003;107:1303–1307. doi: 10.1161/01.cir.0000054612.26458.b2. [DOI] [PubMed] [Google Scholar]
- 2.Szekanez Z, Kerekes G, Der H, Sandor Z, Shoenfeld Y, Soltesz P, et al. Accelerated Atherosclerosis in Rheumatoid Arthritis. Ann. N.Y. Acad. Sci. 2007;1108:349–358. doi: 10.1196/annals.1422.036. [DOI] [PubMed] [Google Scholar]
- 3.Bartoloni E, Alunno A, Bistoni O, Gerli R. Cardiovascular risk in rheumatoid arthritis and systemic autoimmune rheumatic disorders: a suggested model of preventive surgery. Clinic Rev Allerg Inmmunol [Epub] 2011 Jan 16; doi: 10.1007/s12016-010-8251-x. [DOI] [PubMed] [Google Scholar]
- 4.Sokolove J, Brennan MJ, Sharpe O, Lahey LJ, Kao AH, Robinson WH, et al. Brief report: citrullination within the atherosclerotic plaque: a potential target for the anti-citrullinated protein antibody response in rheumatoid arthritis. Arthritis Rheum. 2013 Jul;65(7):1719–1724. doi: 10.1002/art.37961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montes A, Corrales A, Calaza M, Lopez-Mejias R, Parra JA, Gonzalez A, et al. Lack of Replication of an Association Between Anti–Citrullinated Fibrinogen and Subclinical Atherosclerosis in Patients With Rheumatoid Arthritis. Arthritis Rheum. 2015 Nov;67(11):2861–2865. doi: 10.1002/art.39302. [DOI] [PubMed] [Google Scholar]
- 6.El-Barbary AM, Kassem EM, El-Sergany MA, Essa SA, Eltomey MA. Association of anti-modified citrullinated vimentin with subclinical atherosclerosis in early rheumatoid arthritis compared with anti-cyclic citrullinated peptide. J Rheumatol. 2011 May;38(5):828–834. doi: 10.3899/jrheum.101143. [DOI] [PubMed] [Google Scholar]
- 7.Chung CP, Giles JT, Kronmal RA, Post WS, Gelber AC, Bathon JM, et al. Progression of coronary artery atherosclerosis in rheumatoid arthritis: comparison with participants from the Multi-Ethnic Study of Atherosclerosis. Arthritis Res Ther. 2013 Sep 25;15(5):R134. doi: 10.1186/ar4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winchester R, Giles JT, Nativ S, Downer K, Zhang HZ, Bathon JM, et al. Association of Elevations of Specific T Cell and Monocyte Subpopulations in Rheumatoid Arthritis With Subclinical Coronary Artery Atherosclerosis. Arthritis Rheumatol. 2016 Jan;68(1):92–102. doi: 10.1002/art.39419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Vimonte M, Detrano R. Quantification of coronary artery calcium from electron beam tomograms. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 10.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Kronmal RA, et al. Coronary artery calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 11.Solow EB, Yu F, Thiele GM, Sokolove J, Robinson WH, Mikuls TR, et al. Vascular calcifications on hand radiographs in rheumatoid arthritis and associations with autoantibodies, cardiovascular risk factors and mortality. Rheumatology (Oxford) 2015 Sep;54(9):1587–1595. doi: 10.1093/rheumatology/kev027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sokolove J, Johnson DS, Lahey LJ, Wagner CA, Cheng D, Robinson WH, et al. Rheumatoid factor as a potentiator of anti-citrullinated protein antibody-mediated inflammation in rheumatoid arthritis. Arthritis Rheumatol. 2014 Apr;66(4):813–821. doi: 10.1002/art.38307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pemberton AD, Brown JK. In Vitro Interactions of Extracellular Histones with LDL Suggest a Potential Pro-Atherogenic Role. PLoS One. 2010;5(3):e9884. doi: 10.1371/journal.pone.0009884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borissoff JI, Joosen IA, Versteylen MO, Brill A, Fuchs TA, Kietselaer BL, et al. Elevated levels of circulating DNA and chromatin are independently associated with severe coronary atherosclerosis and a prothrombotic state. Arterioscler Thromb Vasc Biol. 2013 Aug;33(8):2032–2040. doi: 10.1161/ATVBAHA.113.301627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sohn DH, Rhodes C, Onuma K, Zhao X, Sharpe O, Sokolove J, et al. Local Joint inflammation and histone citrullination in a murine model of the transition from preclinical autoimmunity to inflammatory arthritis. Arthritis Rheumatol. 2015 Nov;67(11):2877–2887. doi: 10.1002/art.39283. [DOI] [PMC free article] [PubMed] [Google Scholar]