Abstract
Mice homozygous for the mutation scid (scid mice) are severely immunodeficient and generally lack detectable numbers of pre-B, B, and T cells. This condition is believed to result from a defect in the mechanism responsible for rearrangement of immunoglobulin and T-cell receptor genes in developing B and T lymphocytes. To test this hypothesis and evaluate whether scid affects only the process of gene recombination, we introduced functionally rearranged immunoglobulin genes into the scid mouse genome. As scid mice appear to contain early lymphoid cells committed to the B lineage (pro-B cells), we asked whether the introduction of an IgM heavy-chain gene alone (mu-transgenic scid mice) or both IgM heavy- and kappa light-chain genes (mu kappa-transgenic scid mice) would allow further differentiation of scid pro-B cells into pre-B and B cells. We found that normal numbers of pre-B cells appeared in the bone marrow of mu-transgenic scid mice and that both pre-B and B cells appeared in the bone marrow of mu kappa-transgenic scid mice. However, in the latter case, the number of pre-B and B cells was 2- to 3-fold less than in the controls (mu kappa-transgenic scid heterozygotes) and few, if any, B cells were detectable in the peripheral lymphoid tissues. The implications of these results for the above hypothesis are discussed.
Full text
PDF

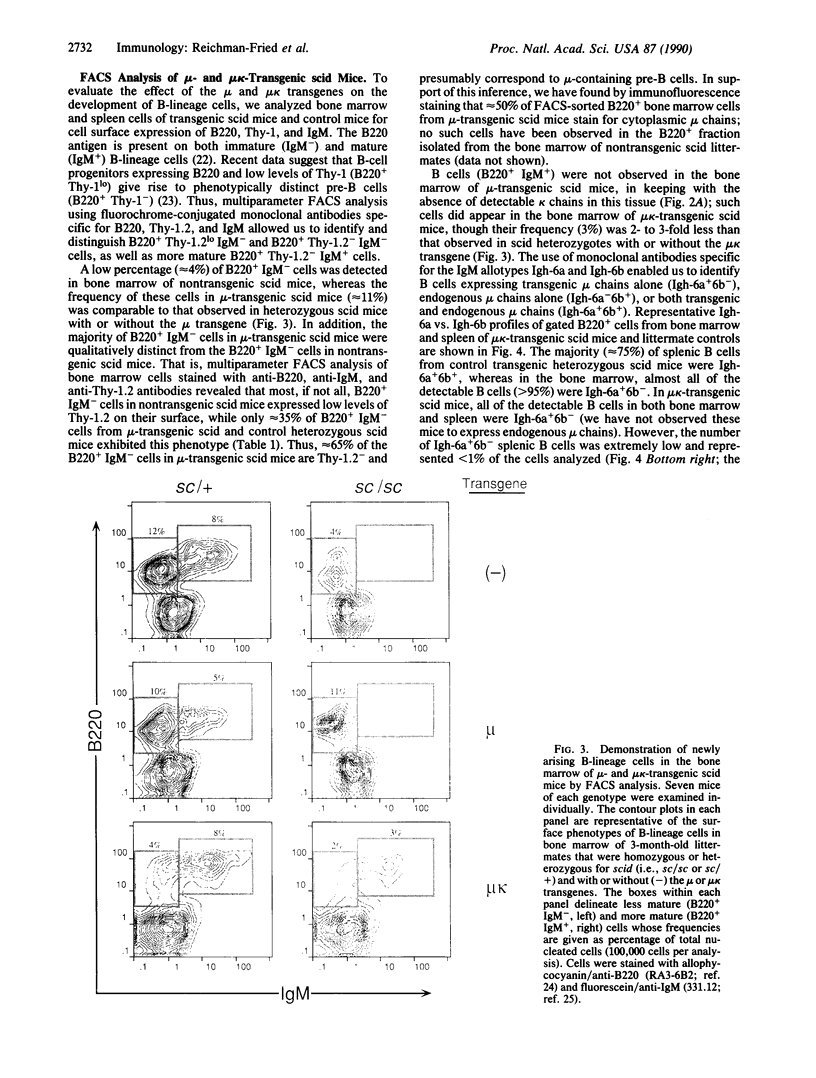

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arp B., McMullen M. D., Storb U. Sequences of immunoglobulin lambda 1 genes in a lambda 1 defective mouse strain. Nature. 1982 Jul 8;298(5870):184–187. doi: 10.1038/298184a0. [DOI] [PubMed] [Google Scholar]
- Blackwell T. K., Malynn B. A., Pollock R. R., Ferrier P., Covey L. R., Fulop G. M., Phillips R. A., Yancopoulos G. D., Alt F. W. Isolation of scid pre-B cells that rearrange kappa light chain genes: formation of normal signal and abnormal coding joins. EMBO J. 1989 Mar;8(3):735–742. doi: 10.1002/j.1460-2075.1989.tb03433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosma G. C., Custer R. P., Bosma M. J. A severe combined immunodeficiency mutation in the mouse. Nature. 1983 Feb 10;301(5900):527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- Bosma G. C., Davisson M. T., Ruetsch N. R., Sweet H. O., Shultz L. D., Bosma M. J. The mouse mutation severe combined immune deficiency (scid) is on chromosome 16. Immunogenetics. 1989;29(1):54–57. doi: 10.1007/BF02341614. [DOI] [PubMed] [Google Scholar]
- Coffman R. L., Weissman I. L. B220: a B cell-specific member of th T200 glycoprotein family. Nature. 1981 Feb 19;289(5799):681–683. doi: 10.1038/289681a0. [DOI] [PubMed] [Google Scholar]
- Coffman R. L., Weissman I. L. Immunoglobulin gene rearrangement during pre-B cell differentiation. J Mol Cell Immunol. 1983;1(1):31–41. [PubMed] [Google Scholar]
- Dorshkind K., Keller G. M., Phillips R. A., Miller R. G., Bosma G. C., O'Toole M., Bosma M. J. Functional status of cells from lymphoid and myeloid tissues in mice with severe combined immunodeficiency disease. J Immunol. 1984 Apr;132(4):1804–1808. [PubMed] [Google Scholar]
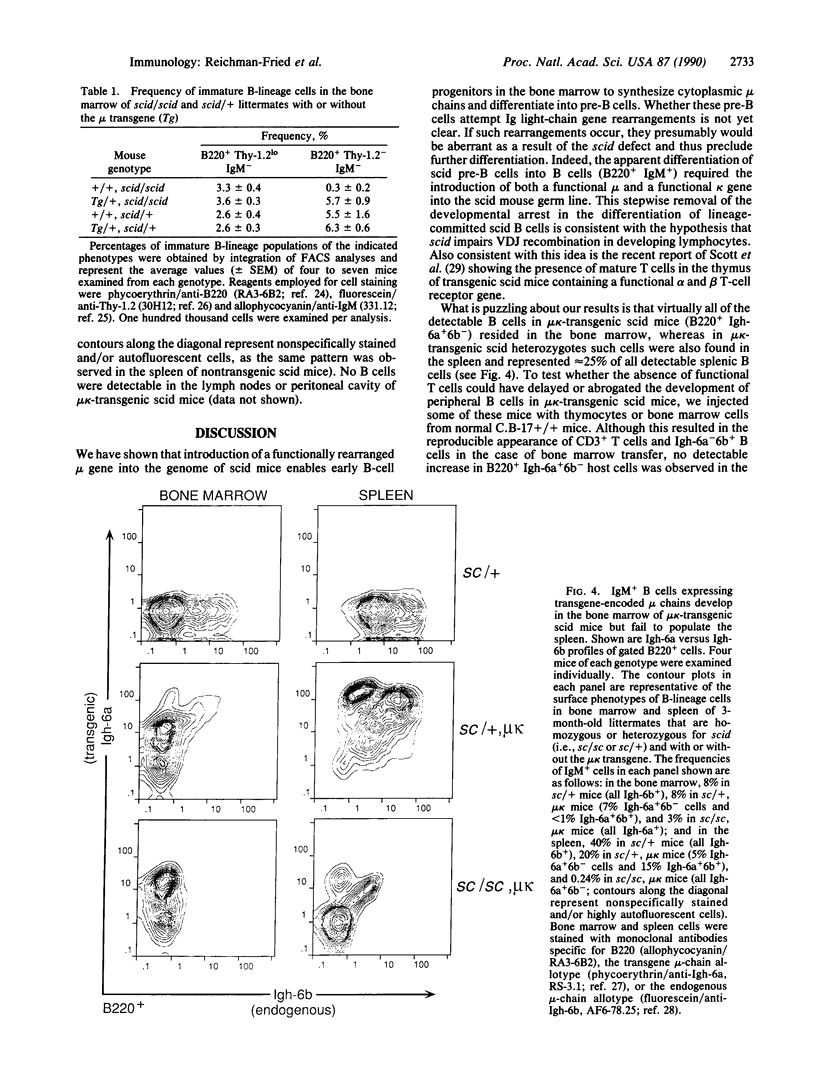
- Epstein R., Davisson M., Lehmann K., Akeson E. C., Cohn M. Position of Igl-1, md, and Bst loci on chromosome 16 of the mouse. Immunogenetics. 1986;23(2):78–83. doi: 10.1007/BF00377965. [DOI] [PubMed] [Google Scholar]
- Grosschedl R., Weaver D., Baltimore D., Costantini F. Introduction of a mu immunoglobulin gene into the mouse germ line: specific expression in lymphoid cells and synthesis of functional antibody. Cell. 1984 Oct;38(3):647–658. doi: 10.1016/0092-8674(84)90259-9. [DOI] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Herzenberg L. A., Herzenberg L. A. Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. J Exp Med. 1985 Jun 1;161(6):1554–1568. doi: 10.1084/jem.161.6.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson E. A., Schatz D. G., Weaver D. T. The scid gene encodes a trans-acting factor that mediates the rejoining event of Ig gene rearrangement. Genes Dev. 1988 Jul;2(7):817–829. doi: 10.1101/gad.2.7.817. [DOI] [PubMed] [Google Scholar]
- Kim M. G., Schuler W., Bosma M. J., Marcu K. B. Abnormal recombination of Igh D and J gene segments in transformed pre-B cells of scid mice. J Immunol. 1988 Aug 15;141(4):1341–1347. [PubMed] [Google Scholar]
- Kincade P. W., Lee G., Sun L., Watanabe T. Monoclonal rat antibodies to murine IgM determinants. J Immunol Methods. 1981;42(1):17–26. doi: 10.1016/0022-1759(81)90220-9. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Lieber M. R., Hesse J. E., Lewis S., Bosma G. C., Rosenberg N., Mizuuchi K., Bosma M. J., Gellert M. The defect in murine severe combined immune deficiency: joining of signal sequences but not coding segments in V(D)J recombination. Cell. 1988 Oct 7;55(1):7–16. doi: 10.1016/0092-8674(88)90004-9. [DOI] [PubMed] [Google Scholar]
- Malynn B. A., Blackwell T. K., Fulop G. M., Rathbun G. A., Furley A. J., Ferrier P., Heinke L. B., Phillips R. A., Yancopoulos G. D., Alt F. W. The scid defect affects the final step of the immunoglobulin VDJ recombinase mechanism. Cell. 1988 Aug 12;54(4):453–460. doi: 10.1016/0092-8674(88)90066-9. [DOI] [PubMed] [Google Scholar]
- Manz J., Denis K., Witte O., Brinster R., Storb U. Feedback inhibition of immunoglobulin gene rearrangement by membrane mu, but not by secreted mu heavy chains. J Exp Med. 1988 Oct 1;168(4):1363–1381. doi: 10.1084/jem.168.4.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks R., Bosma M. J. Truncated mu (mu') chains in murine IgM. Evidence that mu' chains lack variable regions. J Exp Med. 1985 Dec 1;162(6):1862–1877. doi: 10.1084/jem.162.6.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki K., Nishikawa S., Sakano H. Aberrant immunoglobulin gene rearrangement in scid mouse bone marrow cells. J Immunol. 1988 Aug 15;141(4):1348–1352. [PubMed] [Google Scholar]
- Salacinski P. R., McLean C., Sykes J. E., Clement-Jones V. V., Lowry P. J. Iodination of proteins, glycoproteins, and peptides using a solid-phase oxidizing agent, 1,3,4,6-tetrachloro-3 alpha,6 alpha-diphenyl glycoluril (Iodogen). Anal Biochem. 1981 Oct;117(1):136–146. doi: 10.1016/0003-2697(81)90703-x. [DOI] [PubMed] [Google Scholar]
- Schuler W., Schuler A., Lennon G. G., Bosma G. C., Bosma M. J. Transcription of unrearranged antigen receptor genes in scid mice. EMBO J. 1988 Jul;7(7):2019–2024. doi: 10.1002/j.1460-2075.1988.tb03041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler W., Weiler I. J., Schuler A., Phillips R. A., Rosenberg N., Mak T. W., Kearney J. F., Perry R. P., Bosma M. J. Rearrangement of antigen receptor genes is defective in mice with severe combined immune deficiency. Cell. 1986 Sep 26;46(7):963–972. doi: 10.1016/0092-8674(86)90695-1. [DOI] [PubMed] [Google Scholar]
- Schüppel R., Wilke J., Weiler E. Monoclonal anti-allotype antibody towards BALB/c IgM. Analysis of specificity and site of a V-C crossover in recombinant strain BALB-Igh-Va/Igh-Cb. Eur J Immunol. 1987 May;17(5):739–741. doi: 10.1002/eji.1830170527. [DOI] [PubMed] [Google Scholar]
- Scott B., Blüthmann H., Teh H. S., von Boehmer H. The generation of mature T cells requires interaction of the alpha beta T-cell receptor with major histocompatibility antigens. Nature. 1989 Apr 13;338(6216):591–593. doi: 10.1038/338591a0. [DOI] [PubMed] [Google Scholar]
- Stall A. M., Loken M. R. Allotypic specificities of murine IgD and IgM recognized by monoclonal antibodies. J Immunol. 1984 Feb;132(2):787–795. [PubMed] [Google Scholar]
- Storb U., Pinkert C., Arp B., Engler P., Gollahon K., Manz J., Brady W., Brinster R. L. Transgenic mice with mu and kappa genes encoding antiphosphorylcholine antibodies. J Exp Med. 1986 Aug 1;164(2):627–641. doi: 10.1084/jem.164.2.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidmarsh G. F., Heimfeld S., Whitlock C. A., Weissman I. L., Müller-Sieburg C. E. Identification of a novel bone marrow-derived B-cell progenitor population that coexpresses B220 and Thy-1 and is highly enriched for Abelson leukemia virus targets. Mol Cell Biol. 1989 Jun;9(6):2665–2671. doi: 10.1128/mcb.9.6.2665. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Weaver D., Costantini F., Imanishi-Kari T., Baltimore D. A transgenic immunoglobulin mu gene prevents rearrangement of endogenous genes. Cell. 1985 Aug;42(1):117–127. doi: 10.1016/s0092-8674(85)80107-0. [DOI] [PubMed] [Google Scholar]
- Witte P. L., Burrows P. D., Kincade P. W., Cooper M. D. Characterization of B lymphocyte lineage progenitor cells from mice with severe combined immune deficiency disease (SCID) made possible by long term culture. J Immunol. 1987 Apr 15;138(8):2698–2705. [PubMed] [Google Scholar]