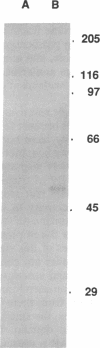
Abstract
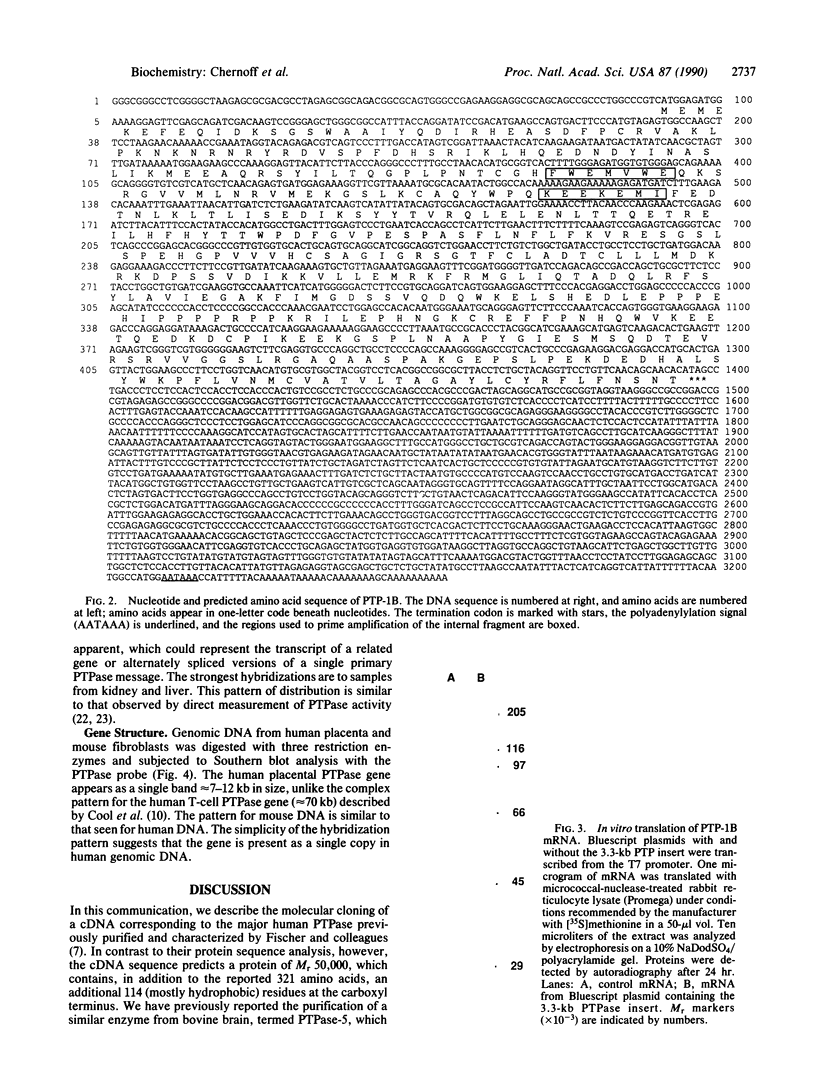
We have isolated a cDNA clone encoding the major protein-tyrosine-phosphatase (protein-tyrosine-phosphate phosphohydrolase, EC 3.1.3.48) of human placenta. Degenerate oligonucleotides, based on the amino acid sequence of the protein, were used to amplify an internal fragment of the gene from human placental cDNA by the polymerase chain reaction. This fragment was then used to probe a human placental cDNA library. A 3.3-kilobase (kb) insert was isolated and sequenced. The insert has a single extended open reading frame that predicts a 435 amino acid protein of Mr approximately 50,000. From the amino terminus to residue 321, the deduced amino acid sequence is identical to that previously determined by peptide sequencing [Charbonneau, H., Tonks, N. K., Kumar, S., Diltz, C. D., Harrylock, M., Cool, D. E., Krebs, E. G., Fischer, E. H. & Walsh, K. A. (1989) Proc. Natl. Acad. Sci. USA 86, 5252-5256]; however, the sequence predicts that the protein contains an additional 114 amino acids not present in the reported peptide sequence. In vitro translation of the 3.3-kb insert produces a protein of Mr 56,000, in general agreement with the predicted size. The phosphatase gene appears to be present as a single copy in human genomic DNA and is transcribed into a 3.5-kb message in a variety of tissues.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baer R., Bankier A. T., Biggin M. D., Deininger P. L., Farrell P. J., Gibson T. J., Hatfull G., Hudson G. S., Satchwell S. C., Séguin C. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984 Jul 19;310(5974):207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- Charbonneau H., Tonks N. K., Kumar S., Diltz C. D., Harrylock M., Cool D. E., Krebs E. G., Fischer E. H., Walsh K. A. Human placenta protein-tyrosine-phosphatase: amino acid sequence and relationship to a family of receptor-like proteins. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5252–5256. doi: 10.1073/pnas.86.14.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernoff J., Li H. C. A major phosphotyrosyl-protein phosphatase from bovine heart is associated with a low-molecular-weight acid phosphatase. Arch Biochem Biophys. 1985 Jul;240(1):135–145. doi: 10.1016/0003-9861(85)90016-5. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cool D. E., Tonks N. K., Charbonneau H., Walsh K. A., Fischer E. H., Krebs E. G. cDNA isolated from a human T-cell library encodes a member of the protein-tyrosine-phosphatase family. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5257–5261. doi: 10.1073/pnas.86.14.5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunphy W. G., Newport J. W. Fission yeast p13 blocks mitotic activation and tyrosine dephosphorylation of the Xenopus cdc2 protein kinase. Cell. 1989 Jul 14;58(1):181–191. doi: 10.1016/0092-8674(89)90414-5. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gattoni S., Kirschmeier P., Weinstein I. B., Escobedo J., Dina D. Cellular Moloney murine sarcoma (c-mos) sequences are hypermethylated and transcriptionally silent in normal and transformed rodent cells. Mol Cell Biol. 1982 Jan;2(1):42–51. doi: 10.1128/mcb.2.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould K. L., Nurse P. Tyrosine phosphorylation of the fission yeast cdc2+ protein kinase regulates entry into mitosis. Nature. 1989 Nov 2;342(6245):39–45. doi: 10.1038/342039a0. [DOI] [PubMed] [Google Scholar]
- Gould S. J., Subramani S., Scheffler I. E. Use of the DNA polymerase chain reaction for homology probing: isolation of partial cDNA or genomic clones encoding the iron-sulfur protein of succinate dehydrogenase from several species. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1934–1938. doi: 10.1073/pnas.86.6.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Horn T., Urdea M. S. Solid supported hydrolysis of apurinic sites in synthetic oligonucleotides for rapid and efficient purification on reverse-phase cartridges. Nucleic Acids Res. 1988 Dec 23;16(24):11559–11571. doi: 10.1093/nar/16.24.11559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Protein-tyrosine kinases. Annu Rev Biochem. 1985;54:897–930. doi: 10.1146/annurev.bi.54.070185.004341. [DOI] [PubMed] [Google Scholar]
- Jones S. W., Erikson R. L., Ingebritsen V. M., Ingebritsen T. S. Phosphotyrosyl-protein phosphatases. I. Separation of multiple forms from bovine brain and purification of the major form to near homogeneity. J Biol Chem. 1989 May 5;264(13):7747–7753. [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Lau K. H., Farley J. R., Baylink D. J. Phosphotyrosyl protein phosphatases. Biochem J. 1989 Jan 1;257(1):23–36. doi: 10.1042/bj2570023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morla A. O., Draetta G., Beach D., Wang J. Y. Reversible tyrosine phosphorylation of cdc2: dephosphorylation accompanies activation during entry into mitosis. Cell. 1989 Jul 14;58(1):193–203. doi: 10.1016/0092-8674(89)90415-7. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M. D., Fink G. R. KAR1, a gene required for function of both intranuclear and extranuclear microtubules in yeast. Cell. 1987 Mar 27;48(6):1047–1060. doi: 10.1016/0092-8674(87)90712-4. [DOI] [PubMed] [Google Scholar]
- Rotenberg S. A., Brautigan D. L. Membrane protein phosphotyrosine phosphatase in rabbit kidney. Proteolysis activates the enzyme and generates soluble catalytic fragments. Biochem J. 1987 May 1;243(3):747–754. doi: 10.1042/bj2430747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shriner C. L., Brautigan D. L. Cytosolic protein phosphotyrosine phosphatases from rabbit kidney. Purification of two distinct enzymes that bind to Zn2+-iminodiacetate agarose. J Biol Chem. 1984 Sep 25;259(18):11383–11390. [PubMed] [Google Scholar]
- Streuli M., Krueger N. X., Hall L. R., Schlossman S. F., Saito H. A new member of the immunoglobulin superfamily that has a cytoplasmic region homologous to the leukocyte common antigen. J Exp Med. 1988 Nov 1;168(5):1523–1530. doi: 10.1084/jem.168.5.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup G., Subrahmanyam G. Purification and characterization of a protein-phosphotyrosine phosphatase from rat spleen which dephosphorylates and inactivates a tyrosine-specific protein kinase. J Biol Chem. 1989 May 15;264(14):7801–7808. [PubMed] [Google Scholar]
- Tonks N. K., Charbonneau H., Diltz C. D., Fischer E. H., Walsh K. A. Demonstration that the leukocyte common antigen CD45 is a protein tyrosine phosphatase. Biochemistry. 1988 Nov 29;27(24):8695–8701. doi: 10.1021/bi00424a001. [DOI] [PubMed] [Google Scholar]
- Tonks N. K., Diltz C. D., Fischer E. H. Purification of the major protein-tyrosine-phosphatases of human placenta. J Biol Chem. 1988 May 15;263(14):6722–6730. [PubMed] [Google Scholar]