Abstract
Background
Advanced systolic heart failure is associated with myocardial and systemic metabolic abnormalities, including low levels of total cholesterol and low-density lipoprotein. Low cholesterol and low-density lipoprotein have been associated with greater mortality in heart failure. Implantation of a left ventricular assist device (LVAD) reverses some of the metabolic derangements of advanced heart failure.
Methods and Results
A cohort was retrospectively assembled from 2 high-volume implantation centers, totaling 295 continuous-flow LVAD recipients with ≥2 cholesterol values available. The cohort was predominantly bridge-to-transplantation (67%), with median age of 59 years and 49% ischemic heart failure cause. Total cholesterol, low-density lipoprotein, high-density lipoprotein, and triglyceride levels all significantly increased after LVAD implantation (median values from implantation to 3 months post implantation 125–150 mg/dL, 67–85 mg/dL, 32–42 mg/dL, and 97–126 mg/dL, respectively). On Cox proportional hazards modeling, patients achieving recovery of total cholesterol levels, defined as a median or greater change from pre implantation to 3 months post-LVAD implantation, had significantly better unadjusted survival (hazard ratio, 0.445; 95% confidence interval, 0.212–0.932) and adjusted survival (hazard ratio, 0.241; 95% confidence interval, 0.092–0.628) than those without cholesterol recovery after LVAD implantation. The continuous variable of total cholesterol at 3 months post implantation and the cholesterol increase from pre implantation to 3 months were also both significantly associated with survival during LVAD support.
Conclusions
Initiation of continuous-flow LVAD support was associated with significant recovery of all 4 lipid variables. Patients with a greater increase in total cholesterol by 3 months post implantation had superior survival during LVAD support.
Keywords: cholesterol, heart failure, heart-assist devices, lipids, transplantation
Advanced heart failure with reduced ejection fraction (HFrEF) is associated with myocardial and systemic metabolic abnormalities and a catabolic state. These metabolic derangements include systemic inflammation, insulin resistance, and hyperglycemia.1 Although elevated cholesterol levels are associated with increased incidence of HFrEF,2–4 paradoxically low serum cholesterol is a feature of advanced HF. Lower total cholesterol,5–7 low-density lipoprotein (LDL),8,9 high-density lipoprotein (HDL),10 and triglyceride serum levels11 have all been associated with worse outcomes in HF cohorts. The prognostic significance of low lipid levels in established HF is sufficiently strong that total cholesterol is included in the Seattle Heart Failure Model for prediction of HF survival.12 Furthermore, cholesterol reduction with statin therapy has proven ineffective in promoting survival in patients with HFrEF.13,14
See Clinical Perspective
Many patients with advanced HF have coronary artery disease and are hyperlipidemic early in their disease course, but by the time of left ventricular assist device (LVAD) implantation, they may be cachectic and frail, which confers greater mortality risk.15,16 It has been observed that patients who undergo LVAD implantation with lower cholesterol levels have higher perioperative mortality.17 Existing data suggest a positive impact of continuous-flow LVAD support on other metabolic derangements of the decompensated HF state, including improvements in glycemic control for patients with diabetes mellitus, and improved insulin and adiponectin resistance.18,19 Therefore, we sought to define the impact of LVAD implantation on lipid profiles in advanced HFrEF and to determine whether changes in cholesterol levels after LVAD implantation affect survival during LVAD support.
Methods
Patient Cohort
The retrospective study cohort was assembled from 2 high-volume LVAD implantation centers, Cleveland Clinic and Columbia University Medical Center. Each cohort included all adults (≥18 years at the time of surgery) who underwent primary continuous-flow LVAD implantation (Heartmate II or Heartware heartware ventricular assist device) within a specified time period (January 1, 2006 to December 31, 2013 for Cleveland Clinic and October 1, 2006 to October 8, 2013 for Columbia University) and who had ≥2 total cholesterol results falling within the specified 3-monthly time points (±30 days) during the period of LVAD support. Lipid values were collected within the period 9 months before LVAD implant until death on LVAD support, transplantation, LVAD explantation, transition of follow-up care to another institution (n=11 patients), or administrative censoring (March 1, 2014 Cleveland Clinic, September 1, 2014 Columbia University). If the LVAD was replaced with a new pump, the complete period of both the pump implantations was counted as the total duration of LVAD support. Patient demographics, clinical data, and additional laboratory values were also recorded, as well as the dates of death on LVAD support. Mortality outcomes were recorded until heart transplantation, LVAD explantation, transition of follow-up care to another institution, or administrative censoring. Total mortality after transplantation or explantation was also collected until censoring at each site. Institutional Review Board approval was obtained separately at each site, and the requirement for informed consent was waivered at both institutions.
Analysis of Baseline Characteristics and Univariate Outcomes
Preimplantation demographics, clinical and laboratory characteristics were tabulated both by site and as a combination of patients from both sites. The duration of LVAD support, time from LVAD implantation to mortality/censoring, and frequency of transplantation were also tabulated for the combined group. Comparisons by site were made by Wilcoxon–Mann–Whitney tests (with normal approximation) for continuous data, due to non-normal distributions of some variables, and Fisher exact tests for categorical data. Analyses were performed using SAS release 9.4 (SAS Institute, Cary, NC).
Analysis of Change in Lipid Variables
Total cholesterol, LDL, HDL, and triglyceride results were grouped by their proximity to 3-monthly time points before and after implantation (LVAD implantation designated time 0). Each time point incorporated values sampled±30 days from specified reference time. The total time interval examined extended from 300 days pre implantation to 300 days post implantation. The median and interquartile ranges of these cholesterol measurements over time were displayed graphically and in tabular format. Total cholesterol, LDL, HDL, and triglyceride levels were each compared between time intervals relative to LVAD implantation. Lipid values were evaluated for normality, and accordingly, comparisons were performed with the Wilcoxon matched-pairs signed-rank test between the time 0 point and each other time points. These analyses were performed using GraphPad Prism version 6.0b for Mac (GraphPad Software, La Jolla, CA).
Impact of Lipid Variables on Survival
To examine the relationship between mortality and cholesterol level, we compared the median lipid values at 3 months post implantation for patients who survived LVAD support to transplantation, explantation, or administrative censoring versus those patients who expired on LVAD support. The 3-month time point was chosen because the comparisons described above showed the greatest increase in cholesterol level occurred between time point 0 and +3 months. We then constructed a Kaplan–Meier plot with log-rank testing for significance, dividing the cohort into 2 halves based on the binary definition of lipid recovery (curve constructed with GraphPad Prism version 6.0b for Mac). Lipid recovery was defined as a median or greater change in lipid level from time 0 (implantation) to 3 months post implantation. The same analysis was also repeated for the LDL, HDL, and triglyceride variables. We also assessed the impact of lipid levels on total mortality after LVAD implantation by including the total survival time right until administrative censoring for all patients. Baseline characteristics were compared for patients with/without cholesterol recovery at 3 months post implantation, using Wilcoxon–Mann–Whitney tests and Fisher exact tests. Only patients who survived to 90 days post-LVAD implantation were included in these analyses.
To determine the independent relationship between survival and recovery from low pre-LVAD cholesterol, we constructed a series of Cox proportional hazards models (PROC PHREG SAS release 9.4, SAS Institute, Cary, NC, using Efron method for examination of ties). The time period analyzed by the Cox models was from 90 days post-LVAD implantation, because subjects needed to have survived to 3 months post implantation to have a qualifying 3-month cholesterol level and hence were not at risk of mortality before 90 days post implantation. The covariate of primary interest was cholesterol recovery (presence/absence of a change in cholesterol from baseline to 3 months post implantation ≥median). We also, in turn, substituted the baseline cholesterol, the change in cholesterol from baseline to 3 months, and the absolute value of cholesterol at 3 months post implantation, as the primary covariate. These 4 models were each replicated for the LDL, HDL, and triglyceride data. Triglyceride values were positively skewed and so this variable was log transformed when used as a continuous variable.
Adjusted Cox models were constructed with the addition of key clinical variables likely to have a significant impact on survival after LVAD implantation. Model covariates were selected from the variables that were most unequally distributed with/without cholesterol recovery including baseline cholesterol to adjust for the pre-LVAD lipid status. Preoperative statin use (or other potent lipid-lowering therapy) was added as an extra covariate to determine whether statins changed the model fit for the relationship between cholesterol recovery and mortality. This was judged by comparing the respective Akaike information criteria values (rather than −2 logL, to account for the difference in the number of model covariates) for the models with and without statins.
The proportional hazards assumption of the Cox model for the binary variable of cholesterol recovery (presence/absence of a change in cholesterol from baseline to 3 months post implantation ≥ median) was assessed using the Epanechnikov Kernel-smoothed hazard function. The proportional hazards assumption for other variables was tested using Schoenfeld residuals and assessing the Pearson correlation coefficients (and their corresponding P values) between the residuals and the ranks of the observed failure times. Nonlinearity of the continuous covariates was also explored. The significance level of all tests was set at 0.05.
A threshold total cholesterol level at 3 months that best predicted future survival during LVAD support was sought. This was achieved using the effect statement in proc phreg with unadjusted and adjusted Cox proportional hazards models to visually determine the mortality hazard ratio (HR) across a spectrum of values for the cholesterol level at 3 months post-LVAD implantation. Finally, to determine possible mechanisms of the observed changes in lipids after LVAD implantation, we calculated the Spearman correlation coefficients (because of non-normal distribution) between baseline and 3-month percent change in cholesterol, with percent change in albumin, bilirubin, and creatinine, respectively.
Results
There were 332 LVAD implants at Cleveland Clinic and 273 at Columbia University Medical Center, during the specified date ranges. A total of 295 HeartMate II and Heartware heartware ventricular assist device recipients met the inclusion criteria of 2+ total cholesterol results falling within the specified time ranges around 3-monthly time points (±30 days) during LVAD support (n=113 Cleveland Clinic and n=182 Columbia University Medical Center). The median age at implantation in the combined cohort was 59 years and 18% were female (Table 1). Diabetes mellitus was diagnosed preoperatively in 39%, 54% were taking a statin before LVAD implantation and 49% had an ischemic HF cause. Sixty-seven percent of LVADs were implanted with a bridge-to-transplantation strategy.
Table 1.
Baseline Characteristics for the Combined Cohort
| Variable | Overall Cohort (n=295) | Cleveland Clinic (n=113) | Columbia University (n=182) | P Value for Comparison Between Sites |
|---|---|---|---|---|
| Female sex | 53 (18%) | 20 (18%) | 33 (18%) | 1.0 |
| Age, y | 59 (48, 66) | 58 (51, 65) | 61 (47, 67) | 0.38 |
| HMII device | 272 (92%) | 106 (94%) | 166 (91%) | 0.51 |
| Implanted with BTT strategy | 197 (67%) | 55 (49%) | 142 (78%) | <0.001 |
| BMI, kg/m2 | 26.7 (22.9, 31.0) | 27.8 (22.9, 32.6) | 26.4 (22.8, 30.0) | 0.09 |
| Baseline statin | 159 (54%) | 61 (54%) | 98 (54%) | 1.0 |
| Ischemic HF cause | 144 (49%) | 55 (49%) | 89 (49%) | 1.0 |
| Smoking history | 168 (57%) | 71 (63%) | 97 (53%) | 0.12 |
| Hypertension history | 155 (53%) | 73 (65%) | 82 (45%) | 0.001 |
| Diabetes mellitus history | 114 (39%) | 49 (41%) | 68 (37%) | 0.62 |
| Creatinine, mg/dL | 1.3 (1.0, 1.7) | 1.3 (1.0, 1.7) | 1.3 (1.1, 1.7) | 0.09 |
| Hemoglobin, g/dL | 9.9 (8.8, 11.8) | 9.0 (8,2, 9.8) | 11.1 (9.7, 12.4) | <0.001 |
| Platelets, 109/L | 173 (128, 223) | 134 (103, 173) | 198 (148, 251) | <0.001 |
| Sodium, mmol/L | 135 (132, 137) | 135 (132, 138) | 135 (132, 137) | 0.96 |
| Albumin, g/dL | 3.4 (2.9, 3.8) | 3.0 (2.5, 3.4) | 3.6 (3.2, 3.9) | <0.001 |
| Total bilirubin, mg/dL | 1.3 (0.8, 2.2) | 1.6 (1.1, 2.5) | 1.1 (0.7, 2.0) | <0.001 |
| ALT, U/L | 23 (16, 41) | 29 (21, 45) | 21 (15, 33) | <0.001 |
| AST, U/L | 37 (22, 61) | 62 (47, 89) | 25 (19, 39) | <0.001 |
| Total cholesterol, mg/dL | 125 (101, 151) | 116 (96, 149) | 129 (107, 156) | 0.04 |
| LDL, mg/dL | 67 (50, 92) | 63 (47, 85) | 69 (52, 103) | 0.02 |
| HDL, mg/dL | 32 (25, 39) | 33 (26, 39) | 31 (24, 39) | 0.31 |
| Triglycerides, mg/dL | 97 (71, 134) | 90 (70, 117) | 100 (72, 140) | 0.07 |
| BNP, pg/mL | 993 (446, 1776) | 749 (281, 1359) | 1190 (616, 2034) | <0.001 |
| HbA1c, % | 6.3 (5.8, 6.9) | 6.2 (5.8, 6.5) | 6.3 (5.9, 7.1) | 0.05 |
| LVEF, % | 15.0 (12.5, 20.0) | 15.0 (10.0, 20.0) | 17.5 (12.5, 20.0) | 0.20 |
Values expressed as median (25th, 75th percentiles) or value (percentage of group). Wilcoxon–Mann–Whitney tests for continuations variables and Fisher exact tests for categorical variables. ALT indicates alanine transaminase; AST, aspartate transaminase; BMI, body mass index; BNP, brain natriuretic peptide; BTT, bridge to transplantation; CAD, coronary artery disease; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; HMII, Heartmate II; LDL, low-density lipoprotein; and LVEF, left ventricular ejection fraction.
Some of the laboratory characteristics suggested a higher preoperative risk profile in the Cleveland Clinic patients (lower hemoglobin, platelets, and albumin; higher bilirubin and transaminases; Table 1). Cleveland Clinic patients also had slightly lower median total cholesterol (116 versus 129 mg/dL, P=0.04) and LDL (63 versus 69 mg/dL, P=0.02) at baseline. Median duration of LVAD support was 427 days (14 months) and overall 48% of patients were transplanted (median LVAD support duration was lower and the proportion transplanted higher at Columbia University Medical Center, Table 2). Twenty-one percent of patients died on LVAD support, of which 9 patients died before 90 days post implantation (range 3 days to 51 days post implantation).
Table 2.
Clinical Outcomes During and After LVAD Support
| Variable | Overall Cohort (n=295) |
|---|---|
| Length of LVAD support, d | 427 (217, 480) |
| Cardiac transplantation | 142 (48%) |
| Mortality ≤6 mo of LVAD support | 14 (5%) |
| Mortality ≤12 mo of LVAD support | 24 (8%) |
| Mortality during total LVAD support | 62 (21%) |
| Mortality during or after LVAD support | 82 (28%) |
Values expressed as median (25th, 75th percentiles) or value (percentage of group). Wilcoxon–Mann–Whitney tests for continuations variables and Fisher exact tests for categorical variables. LVAD indicates left ventricular assist device.
Analysis of Change in Lipid Variables
There was a significant increase in all 4 lipid variables from baseline (time of implantation±30 days) to 3 months±30 days post-LVAD implantation (Table 3). The median cholesterol, LDL, HDL, and triglyceride values were significantly higher at 3, 6, 9, and 12 months post implantation, compared with peri-implantation, with the biggest increase seen between implantation and 3 months (Figure 1). Therefore, further analyses focused on the 3-month post implantation time point as the determinant of lipid recovery. Patients with and without a cholesterol level at 3 months had similar baseline characteristics and outcomes (Table I in the Data Supplement).
Table 3.
Change in Lipid and Nutritional Variables With LVAD Implantation
| Variable | At Baseline (±30 d) of LVAD Implantation | At 3 mo (±30 d) Post-LVAD Implantation | No. of Paired Comparisons | Median Change From Baseline to 3 mo | P Value for Baseline to 3-mo Comparison |
|---|---|---|---|---|---|
| Total cholesterol, mg/dL | 125 (101, 151) | 150 (125, 179) | 129 | 22 (−3, 64) | <0.001 |
| LDL, mg/dL | 67 (50, 92) | 85 (51,109) | 63 | 12 (−7, 37) | 0.01 |
| HDL, mg/dL | 32 (25, 39) | 42 (35, 50) | 67 | 9 (1, 22) | <0.001 |
| Triglycerides, mg/dL | 97 (71, 134) | 126 (89, 170) | 75 | 28 (−13, 62) | <0.001 |
| Albumin, g/dL | 3.4 (2.9, 3.8) | 4.0 (3.5, 4.3) | 286 | 0.5 (0, 1.0) | <0.001 |
Values expressed as medians (25th, 75th percentile). Baseline-3 mo comparisons made with Wilcoxon matched-pairs signed-rank tests. HDL indicates high-density lipoprotein; LVAD, left ventricular assist device; and LDL, low-density lipoprotein.
Figure 1.

A to D, Trend in median lipid values over time with reference to the time of left ventricular assist device (LVAD) implantation. Total cholesterol, low-density lipoprotein (LDL), and triglyceride levels fell in the 9 mo preceding LVAD implantation. After a nadir around the time of LVAD implantation, all 4 lipid variables significantly increased in the following 9 mo of LVAD support, with the steepest gradients between the time of implantation and 3 mo post-LVAD implantation. Lipid values were assigned to time points as follows: −9 mo, −270±30 d (−300 to −240); −6 mo, −180±30 d (−210 to −150); −3 mo: −90±30 d (−120 to −60); implant date: 0±30 d (−30 to +30); 3 mo: 90±30 d (60–120); 6 mo: 180±30 d (150–210); and 9 mo: 270±30 d (240–300). *P≤0.05; **P≤0.01; ***P≤0.001.
Impact of Lipid Variables on Survival
Patients who survived on LVAD support to reach cardiac transplantation, LVAD explantation, or administrative censoring had a significantly higher total cholesterol at 3 months post implantation than patients who expired on LVAD support (Figure 2). Patients who achieved a median or greater change in cholesterol level from baseline to 3 months (±30 days) post-LVAD implantation were younger, more likely to have a nonischemic HF cause and a bridge-to-transplantation strategy and had a lower LVEF than those who did not achieve cholesterol recovery (Table 4). The recovery group also had significantly lower baseline lipid levels, hence the inclusion of baseline cholesterol in the multivariable models below.
Figure 2.
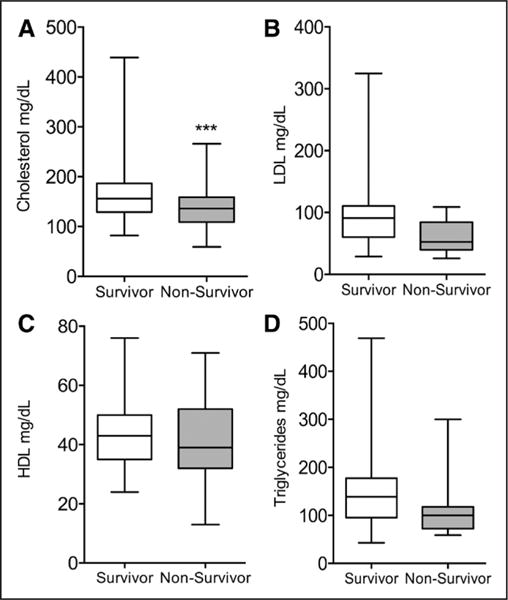
A to D, Median lipid values at 3 mo post implantation in survivors vs nonsurvivors of left ventricular assist device (LVAD) support. The values displayed are the median levels for each of the 4 lipid variables at 3 mo (±30 d) post-LVAD implantation. Individuals who survived LVAD support to the time of cardiac transplantation, LVAD explantation, or administrative censoring, had a significantly higher median total cholesterol value than individuals who died on LVAD support. There was no significant association between survival status and median low-density lipoprotein (LDL), high-density lipoprotein (HDL), or triglyceride levels. *P≤0.05; **P≤0.01; ***P≤0.001.
Table 4.
Baseline Characteristics of Patients by Total Cholesterol Recovery Status at 3 Months Post-LVAD Implantation
| Variable | No Cholesterol Recovery* at 3 mo (n=64) | Cholesterol Recovery* at 3 mo (n=65) | P Value |
|---|---|---|---|
| Female sex | 7 (11%) | 13 (20%) | 0.22 |
| Age, y | 62 (51, 69) | 53 (46, 61) | 0.001 |
| HMII device | 57 (89%) | 61 (94%) | 0.36 |
| Implanted with BTT strategy | 38 (59%) | 50 (77%) | 0.04 |
| BMI, kg/m2 | 26.2 (22.8, 31.0) | 25.3 (22.1, 29.9) | 0.67 |
| Baseline statin | 36 (56%) | 35 (54%) | 0.86 |
| Ischemic HF cause | 40 (63%) | 24 (37%) | 0.005 |
| Smoking history | 40 (63%) | 33 (51%) | 0.21 |
| Hypertension history | 36 (56%) | 37 (57%) | 1.0 |
| Diabetes mellitus history | 29 (45%) | 25 (38%) | 0.48 |
| Creatinine, mg/dL | 1.4 (1.1, 1.8) | 1.3 (1.0, 1.7) | 0.41 |
| Hemoglobin, g/dL | 10.0 (9.1, 12.2) | 9.9 (8.6, 11.9) | 0.58 |
| Platelets, 109/L | 183 (132, 243) | 178 (127, 240) | 0.76 |
| Sodium, mmol/L | 135 (133, 137) | 134 (131, 137) | 0.19 |
| Albumin, g/dL | 3.3 (2.9, 3.8) | 3.3 (2.8, 3.7) | 0.64 |
| Total bilirubin, mg/dL | 1.3 (0.7, 2.1) | 1.4 (0.9, 2.7) | 0.22 |
| ALT, U/L | 23 (17, 34) | 24 (17, 46) | 0.40 |
| AST, U/L | 34 (21, 58) | 38 (25, 65) | 0.29 |
| Total cholesterol, mg/dL | 137 (113, 167) | 109 (94, 127) | <0.001 |
| LDL, mg/dL | 68 (49, 119) | 62 (45, 72) | 0.03 |
| HDL, mg/dL | 35 (29, 47) | 27 (21, 37) | 0.01 |
| Triglycerides, mg/dL | 103 (77, 175) | 89 (72, 113) | 0.04 |
| BNP, pg/mL | 1080 (377, 2043) | 1296 (787, 2277) | 0.38 |
| HbA1c, % | 6.3 (5.8, 7.6) | 6.3 (5.9, 6.8) | 0.71 |
| LVEF, % | 17.5 (13, 20) | 15 (12, 20) | 0.04 |
| Duration of LVAD support, d | 419 (216, 882) | 427 (253, 840) | 0.84 |
| Cardiac Transplanation | 28 (44%) | 34 (52%) | 0.38 |
| Mortality during LVAD support | 24 (38%) | 10 (15%) | 0.005 |
| Mortality during or after LVAD support | 30 (47%) | 12 (18%) | <0.001 |
Values expressed as median (25th, 75th percentiles) or value (percentage of group). Wilcoxon–Mann–Whitney tests for continuations variables and Fisher exact tests for categorical variables. ALT indicates alanine transaminase; AST, aspartate transaminase; BMI, body mass index; BNP, brain natriuretic peptide; BTT, bridge to transplantation; CAD, coronary artery disease; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; HMII, Heartmate II; LDL, low-density lipoprotein; LVAD, left ventricular assist device; and LVEF, left ventricular ejection fraction.
Recovery defined as change from baseline to 3 mo post implantation ≥median change.
On dividing the cohort into halves at median change values, patients with greater recovery of total cholesterol or triglycerides at 3 months post implantation had significantly lower unadjusted mortality (log-rank P=0.03 and P=0.02, respectively; Figure 3). This mortality association was not seen with the LDL and HDL variables (P=0.29 and P=0.77, respectively). For total mortality after LVAD implantation (mortalities on LVAD support and after transplantation or LVAD removal), recovery of cholesterol or triglycerides were similarly associated with lower unadjusted mortality (P=0.006 and P=0.04, respectively) but not recovery of LDL or HDL (P=0.06 and P=0.55, respectively).
Figure 3.

A to D, Kaplan–Meier plots for unadjusted survival by degree of change in lipids from left ventricular assist device (LVAD) implantation to 3 mo post implantation (above/below median change). Incorporation of time-to-event information shows superior survival on LVAD support for individuals whose change in total cholesterol or triglyceride levels were above the median at 3 mo (±30 d) post-LVAD implantation. There was no significant association between the low-density lipoprotein (LDL) or high-density lipoprotein (HDL) level at 3 months post-LVAD implantation and survival on LVAD support.
Unadjusted Cox models showed no association between baseline (time of implantation±30 days) cholesterol, LDL, HDL, or triglyceride levels and mortality during LVAD support (mortality from 3 months onwards for fair comparison; Table 5). These results were unchanged when the analysis was broadened out to include all patients with baseline lipid results, not just those with 3-month data (data not shown). However, the binary variable of total cholesterol recovery—defined as change from time 0 to 3 months post-LVAD implantation that was greater or equal to the median change—strongly predicted survival during LVAD support (unadjusted HR, 0.445; 95% confidence interval, 0.212–0.932; P=0.03). In addition, both change in cholesterol (from time 0–3 months post implantation) and absolute cholesterol levels at 3 months (±30 days) post-LVAD implantation were significantly associated with lower mortality during LVAD support. Triglyceride recovery was also associated with lower mortality during LVAD support (Table 5).
Table 5.
Unadjusted Hazard Ratio Estimates for All-Cause Mortality for Each Lipid Variable
| Baseline (Time 0), per 1 mg/dL/per per Log Unit* for Triglyceride | 3 Mo Post Implantation, per 1 mg/dL/per Log Unit* for Triglyceride | Change From Baseline to 3 Mo Post Implantation, per 1 mg/dL/per Log Unit* for Triglyceride | Recovery† (Binary Variable) at 3 Mo Post Implantation | |
|---|---|---|---|---|
| Cholesterol unadjusted mortality hazard ratios (95% confidence interval) | 0.998 (0.991–1.004) | 0.983‡ (0.973–0.993) | 0.993§ (0.986–0.999) | 0.445§ (0.212–0.932) |
| No. of subjects, No. of events | n=255, events=51 | n=134, events=34 | n=129, events=34 | n=129, events=34 |
| LDL unadjusted mortality hazard ratios (95% confidence interval) | 0.995 (0.986–1.004) | 0.971‡ (0.954–0.989) | 0.983 (0.966–1.001) | 0.549 (0.179–1.684) |
| No. of subjects, No. of events | n=208, events=39 | n=80, events=16 | n=63, events=13 | n=63, events=13 |
| HDL unadjusted mortality hazard ratios (95% confidence interval) | 1.013 (0.990–1.036) | 0.969 (0.931–1.009) | 0.980 (0.952–1.009) | 0.853 (0.299–2.437) |
| No. of subjects, No. of events | n=210, events=39 | n=84, events=17 | n=67, events=14 | n=67, events=14 |
| Triglyceride unadjusted mortality hazard ratios (95% confidence interval) | 0.751 (0.398–1.417) | 0.672 (0.264–1.711) | 0.532 (0.186–1.525) | 0.282§ (0.089–0.890) |
| No. of subjects, No. of events | n=227, events=41 | n=86, events=17 | n=75, events=15 | n=75, events=15 |
Time 0=date of left ventricular assist device implantation. LDL indicates low-density lipoprotein; and HDL, high-density lipoprotein.
Triglyceride log transformed because of positive skew.
Recovery’ defined as change from baseline to 3 mo post implantation ≥median change.
P≤0.01;
P≤0.05.
When adjusted for baseline age, ischemic HF cause, bridge-to-transplantation status, LVEF, and cholesterol at baseline, the change in total cholesterol from baseline to 3 months post-LVAD remained an independent predictor of survival on LVAD support (Table 6). Patients with cholesterol recovery had a 76% lower adjusted mortality hazard during LVAD support compared with patients without recovery (Table 7). The continuous variable of total cholesterol at 3 months post implantation was also significantly associated with survival during LVAD support (adjusted HR, 0.978 per 1 mg/dL; 95% confidence interval, 0.967–0.990; P<0.001). Adjusted models were not constructed for the other lipid variables because of the small numbers available for these analyses.
Table 6.
Cox Proportional Hazards Model Parameter Estimates for Change in Total Cholesterol From Baseline to 3 Months Post-LVAD Implantation as a Continuous Variable
| Variable | Hazard Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|
| Change in cholesterol, per 1 mg/dL change | 0.978 | 0.967–0.990 | <0.001 |
| Age at implant, per 1 y | 0.975 | 0.944–1.008 | 0.14 |
| Ischemic HF cause | 0.694 | 0.294–1.637 | 0.40 |
| Bridge to transplantation strategy | 0.619 | 0.272–1.410 | 0.25 |
| Left ventricular ejection fraction at baseline, per 1% | 1.054 | 0.983–1.130 | 0.14 |
| Baseline cholesterol, per 1 mg/dL | 0.979 | 0.967–0.991 | <0.001 |
Wald χ2=16.7, 6 degrees of freedom, P for model fit=0.0106, n=129 (34 events). HF indicates heart failure; and LVAD, left ventricular assist device.
Table 7.
Cox Proportional Hazards Model Parameter Estimates for Recovery of Total Cholesterol at 3 Months Post-LVAD Implantation as a Binary Variable
| Variable | Hazard Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|
| Cholesterol recovery at 3 mo (change ≥median) | 0.241 | 0.092–0.628 | 0.004 |
| Age at implant, per 1 y | 0.983 | 0.954–1.014 | 0.28 |
| Ischemic HF cause | 0.560 | 0.242–1.299 | 0.18 |
| Bridge to transplantation strategy | 0.661 | 0.291–1.502 | 0.32 |
| Left ventricular ejection fraction at baseline, per 1% | 1.040 | 0.975–1.110 | 0.23 |
| Baseline cholesterol, per 1 mg/dL | 0.986 | 0.975–0.997 | 0.01 |
Wald χ2=14.1, 6 degrees of freedom, P for model fit=0.0285, n=129 (34 events). HF indicates heart failure; and LVAD, left ventricular assist device.
The 3-month total cholesterol threshold for transitioning into a more favorable mortality hazard during LVAD support was ≈130 mg/dL, with the lowest adjusted mortality hazard occurring ≈170 mg/dL (Figure 4). Despite this nonlinear relationship, quadratic and spline functions did not improve the fit for the 3-month cholesterol Cox model, as judged by the Akaike information criteria. The association between higher cholesterol after LVAD implantation and favorable survival was unaffected by preoperative statin therapy, and there was no significant difference in the 3-month post-LVAD cholesterol in those with or without statin therapy—median 153 (123, 184) mg/dL versus 147.5 (126, 177) mg/dL, respectively, P=1.0. To further explore the impact of statins on mortality, we also expanded the adjusted model for the outcome of cholesterol recovery with the addition of preoperative statin use as a covariate. The addition of statin use to the model did not improve the model fit (Akaike information criteria, 253.68 in the original model and 253.09 with statin covariate) and did not change the HR for 3-month cholesterol. There was no significant effect of preoperative statin use on mortality (HR, 0.528; 95% confidence interval, 0.243–1.148; P=0.10).
Figure 4.

Spline function curve for the impact of cholesterol level at 3 mo after left ventricular assist device (LVAD) implantation on mortality during LVAD support. The hazard of mortality reaches a lower threshold around a total cholesterol level of 130 mg/dL at 3 mo post-LVAD implantation. The nadir for the hazard of mortality is around 170 mg/dL. The spline curve adjusted for age, ischemic heart failure cause, ejection fraction, bridge-to-transplantation strategy, and baseline cholesterol is displayed.
To explore possible mechanisms for the observed survival advantage associated with cholesterol recovery, we examined the Spearman correlation coefficients for the percent change in cholesterol from baseline to 3 months post-LVAD with the percent change in creatinine, albumin, and bilirubin. These variables were chosen because they are markers for potential mechanisms by which the survival effect of cholesterol recovery could be mediated. There was a weak but significant correlation with change in albumin (r=0.196, P=0.03). When percent changes of these 3 variables were placed into a Cox proportional hazards model along with percent change in cholesterol from baseline to 3 months, the percent change in cholesterol retained its significance as a mortality predictor (P=0.04) and was the only significant predictor.
Discussion
Our observation of recovery in the lipid profile during LVAD support builds on previously described improvements in insulin resistance and glycemic control for patients with diabetes mellitus.18 Reduced myocardial levels of toxic lipid intermediates post-LVAD were also demonstrated in a small subgroup of patients within this study.20 Despite the deranged and insulin-resistant metabolic state of advanced HF,19,21 no existing HF therapies specifically target the progressive deterioration in lipid and glucose homeostasis. There is growing interest in the possibility that normalization of the systemic metabolic milieu and myocardial substrate delivery may improve HF survival (Figure 5). This cholesterol recovery data support lipid and glucose hemostasis as a potential target for the development of novel metabolic HF therapies.22 Therapies such as trimetazidine, which inhibits myocardial fatty acid oxidation, improve whole-body insulin sensitivity and raise HDL levels and has been associated with improvements in HF symptoms and myocardial function.23,24 Nevertheless, our retrospective analysis does not exclude the possibility that cholesterol recovery is simply a bystander of improved overall metabolic state after hemodynamic improvement.
Figure 5.

A hypothesis for metabolic recovery after left ventricular assist device (LVAD) implantation in advanced heart failure. LDL indicates low-density lipoprotein.
Second, the impact of low cholesterol levels during LVAD support is clinically relevant in refining LVAD candidate selection and optimization. Cachectic and malnourished patients are at higher risk of mortality during LVAD support.15,25 Given the widespread adoption of LVAD therapy and the increasingly comorbid status of LVAD recipients, preoperative nutritional interventions warrant prospective evaluation. Serum cholesterol may be a useful biomarker of the catabolic state. As demonstrated in this study, many advanced HF patients remain on statins at the time of LVAD implantation and beyond, despite relatively low cholesterol levels. A potential role of statin therapy cessation in select advanced HF patients warrants prospective evaluation.
Study Limitations
Our study is limited by its retrospective design and reliance on lipid results obtained at the discretion of healthcare providers during routine clinical care. Patients with extremes in cholesterol values or greater clinical instability may be biased toward greater frequency of lipid level collection. In addition, patients who did not survive the early postoperative period would not have lipid data available for the analyses based on the 3-month time point, which would likely bias the impact on mortality toward the null. The analytic cohort represents only a subset of the total cohort of patients receiving LVADs at the 2 centers, because more than half of the patients did not have lipid results at the specified time points for inclusion in the retrospective cohort. It is possible that the noncholesterol lipid analyses, particularly the Cox model for LDL recovery, were underpowered and would have shown significant associations if the sample size were larger.
This cohort represents the experience of only 2 tertiary care centers, and it is possible that our findings may not be applicable to all continuous-flow LVAD recipients. In addition, our analyses are restricted to patients who were both willing and medically approved for LVAD implantation, which may have introduced a selection bias that excludes the most cachectic HFrEF patients, patients with poorly controlled diabetes mellitus or morbidly obese individuals.
Conclusions
The initiation of continuous-flow LVAD support in advanced systolic HF is associated with significant recovery of total cholesterol, LDL, HDL, and triglyceride levels, and a reversal of the lipid paradox. A greater cholesterol change from baseline to 3 months and a higher absolute cholesterol level at 3 months post implantation were both associated with greater survival during LVAD support. This is despite the absence of a negative effect of low preoperative cholesterol in this cohort. This novel finding is important both in providing insights into the mechanisms underlying the positive impact of LVAD implantation on survival in advanced HF and also in further refining patient selection and optimization for LVAD support.
Supplementary Material
CLINICAL PERSPECTIVE.
The analyses in this article build on earlier observations about improvements in insulin resistance after left ventricular assist device implantation to further highlight the metabolic recovery that occurs when patients with advanced heart failure (HF) are mechanically supported. Although the hemodynamic goals of augmented cardiac output and reduced left ventricular end-diastolic pressure are thought to be responsible for the morbidity and mortality gains seen post left ventricular assist device implantation, the reversal of the disordered metabolism of advanced HF may also contribute to the clinical effect of left ventricular assist device support. These cholesterol recovery observations also raise practical considerations when managing patients with advanced HF. These data again remind us of the association between low lipid levels and poorer clinical outcomes in the population with systolic HF and raise the question of whether statins may be at best unnecessary, or at worst even harmful, in advanced HF patients with falling cholesterol levels. Conversely, these data also highlight the phenomenon of the patient with baseline hyperlipidemia, whose cholesterol levels fall during their progression to advanced HF, only to sharply increase again after left ventricular assist device implantation. This may have a significant clinical implication if the patient is bridging to transplantation; it may be clinically appropriate to restart lipid-lowering therapy to control the low-density lipoprotein before the administration of steroids and a calcineurin inhibitor post-transplant, to potentially reduce the risk of early coronary artery vasculopathy development.
Acknowledgments
Dr Vest was a consultant for Sunshine Heart Inc, reviewing patient eligibility for COUNTER-HF (C-Pulse System: A Heart Assist Device Clinical Study).
Footnotes
Disclosures
The other authors report no conflicts.
Contributor Information
Amanda R. Vest, Division of Cardiology, Department of Medicine, Tufts Medical Center, Boston, MA.
Peter J. Kennel, Division of Cardiology, Department of Medicine, Columbia University Medical Center, New York.
Dawn Maldonado, Division of Cardiology, Department of Medicine, Columbia University Medical Center, New York.
James B. Young, Kaufman Center for Heart Failure, Cardiovascular Medicine Department, Heart and Vascular Institute, Cleveland Clinic, OH.
Maria M. Mountis, Kaufman Center for Heart Failure, Cardiovascular Medicine Department, Heart and Vascular Institute, Cleveland Clinic, OH.
Yoshifumi Naka, Division of Cardiothoracic Surgery, Department of Surgery, Columbia University Medical Center, New York.
Paolo C. Colombo, Division of Cardiology, Department of Medicine, Columbia University Medical Center, New York.
Donna M. Mancini, Division of Cardiology, Department of Medicine, Mount Sinai Icahn School of Medicine, New York.
Randall C. Starling, Kaufman Center for Heart Failure, Cardiovascular Medicine Department, Heart and Vascular Institute, Cleveland Clinic, OH.
P. Christian Schulze, Division of Cardiology, Department of Medicine, Columbia University Medical Center, New York; Department of Internal Medicine I, Division of Cardiology, Angiology, Pneumology and Intensive Medical Care, University Hospital Jena, Friedrich-Schiller-University Jena, Germany.
References
- 1.Swan JW, Anker SD, Walton C, Godsland IF, Clark AL, Leyva F, Stevenson JC, Coats AJ. Insulin resistance in chronic heart failure: relation to severity and etiology of heart failure. J Am Coll Cardiol. 1997;30:527–532. doi: 10.1016/s0735-1097(97)00185-x. [DOI] [PubMed] [Google Scholar]
- 2.Velagaleti RS, Massaro J, Vasan RS, Robins SJ, Kannel WB, Levy D. Relations of lipid concentrations to heart failure incidence: the Framingham Heart Study. Circulation. 2009;120:2345–2351. doi: 10.1161/CIRCULATIONAHA.109.830984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang J, Sarnola K, Ruotsalainen S, Moilanen L, Lepistö P, Laakso M, Kuusisto J. The metabolic syndrome predicts incident congestive heart failure: a 20-year follow-up study of elderly Finns. Atherosclerosis. 2010;210:237–242. doi: 10.1016/j.atherosclerosis.2009.10.042. [DOI] [PubMed] [Google Scholar]
- 4.Voulgari C, Tentolouris N, Dilaveris P, Tousoulis D, Katsilambros N, Stefanadis C. Increased heart failure risk in normal-weight people with metabolic syndrome compared with metabolically healthy obese individuals. J Am Coll Cardiol. 2011;58:1343–1350. doi: 10.1016/j.jacc.2011.04.047. [DOI] [PubMed] [Google Scholar]
- 5.Rauchhaus M, Clark AL, Doehner W, Davos C, Bolger A, Sharma R, Coats AJ, Anker SD. The relationship between cholesterol and survival in patients with chronic heart failure. J Am Coll Cardiol. 2003;42:1933–1940. doi: 10.1016/j.jacc.2003.07.016. [DOI] [PubMed] [Google Scholar]
- 6.Horwich TB, Hernandez AF, Dai D, Yancy CW, Fonarow GC. Cholesterol levels and in-hospital mortality in patients with acute decompensated heart failure. Am Heart J. 2008;156:1170–1176. doi: 10.1016/j.ahj.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Afsarmanesh N, Horwich TB, Fonarow GC. Total cholesterol levels and mortality risk in nonischemic systolic heart failure. Am Heart J. 2006;152:1077–1083. doi: 10.1016/j.ahj.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 8.Kahn MR, Kosmas CE, Wagman G, Serrao GW, Fallahi A, Grady KM, Vittorio TJ. Low-density lipoprotein levels in patients with acute heart failure. Congest Heart Fail. 2013;19:85–91. doi: 10.1111/chf.12006. [DOI] [PubMed] [Google Scholar]
- 9.Charach G, George J, Roth A, Rogowski O, Wexler D, Sheps D, Grosskopf I, Weintraub M, Keren G, Rubinstein A. Baseline low-density lipoprotein cholesterol levels and outcome in patients with heart failure. Am J Cardiol. 2010;105:100–104. doi: 10.1016/j.amjcard.2009.08.660. [DOI] [PubMed] [Google Scholar]
- 10.Mehra MR, Uber PA, Lavie CJ, Milani RV, Park MH, Ventura HO. High-density lipoprotein cholesterol levels and prognosis in advanced heart failure. J Heart Lung Transplant. 2009;28:876–880. doi: 10.1016/j.healun.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 11.Greene SJ, Vaduganathan M, Lupi L, Ambrosy AP, Mentz RJ, Konstam MA, Nodari S, Subacius HP, Fonarow GC, Bonow RO, Gheorghiade M, EVEREST Trial Investigators Prognostic significance of serum total cholesterol and triglyceride levels in patients hospitalized for heart failure with reduced ejection fraction (from the EVEREST Trial) Am J Cardiol. 2013;111:574–581. doi: 10.1016/j.amjcard.2012.10.042. [DOI] [PubMed] [Google Scholar]
- 12.Levy WC, Mozaffarian D, Linker DT, Sutradhar SC, Anker SD, Cropp AB, Anand I, Maggioni A, Burton P, Sullivan MD, Pitt B, Poole-Wilson PA, Mann DL, Packer M. The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation. 2006;113:1424–1433. doi: 10.1161/CIRCULATIONAHA.105.584102. [DOI] [PubMed] [Google Scholar]
- 13.Kjekshus J, Apetrei E, Barrios V, Böhm M, Cleland JG, Cornel JH, Dunselman P, Fonseca C, Goudev A, Grande P, Gullestad L, Hjalmarson A, Hradec J, Jánosi A, Kamenský G, Komajda M, Korewicki J, Kuusi T, Mach F, Mareev V, McMurray JJ, Ranjith N, Schaufelberger M, Vanhaecke J, van Veldhuisen DJ, Waagstein F, Wedel H, Wikstrand J, CORONA Group Rosuvastatin in older patients with systolic heart failure. N Engl J Med. 2007;357:2248–2261. doi: 10.1056/NEJMoa0706201. [DOI] [PubMed] [Google Scholar]
- 14.GISSI-HF Investigators. Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1231–1239. doi: 10.1016/S0140-6736(08)61240-4. [DOI] [PubMed] [Google Scholar]
- 15.Lietz K, Long JW, Kfoury AG, Slaughter MS, Silver MA, Milano CA, Rogers JG, Naka Y, Mancini D, Miller LW. Outcomes of left ventricular assist device implantation as destination therapy in the post-REMATCH era: implications for patient selection. Circulation. 2007;116:497–505. doi: 10.1161/CIRCULATIONAHA.107.691972. [DOI] [PubMed] [Google Scholar]
- 16.Dunlay SM, Park SJ, Joyce LD, Daly RC, Stulak JM, McNallan SM, Roger VL, Kushwaha SS. Frailty and outcomes after implantation of left ventricular assist device as destination therapy. J Heart Lung Transplant. 2014;33:359–365. doi: 10.1016/j.healun.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richartz BM, Radovancevic B, Frazier OH, Vaughn WK, Taegtmeyer H. Low serum cholesterol levels predict high perioperative mortality in patients supported by a left-ventricular assist system. Cardiology. 1998;89:184–188. doi: 10.1159/000006785. [DOI] [PubMed] [Google Scholar]
- 18.Uriel N, Naka Y, Colombo PC, Farr M, Pak SW, Cotarlan V, Albu JB, Gallagher D, Mancini D, Ginsberg HN, Jorde UP. Improved diabetic control in advanced heart failure patients treated with left ventricular assist devices. Eur J Heart Fail. 2011;13:195–199. doi: 10.1093/eurjhf/hfq204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan RS, Kato TS, Chokshi A, Chew M, Yu S, Wu C, Singh P, Cheema FH, Takayama H, Harris C, Reyes-Soffer G, Knöll R, Milting H, Naka Y, Mancini D, Schulze PC. Adipose tissue inflammation and adiponectin resistance in patients with advanced heart failure: correction after ventricular assist device implantation. Circ Heart Fail. 2012;5:340–348. doi: 10.1161/CIRCHEARTFAILURE.111.964031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chokshi A, Drosatos K, Cheema FH, Ji R, Khawaja T, Yu S, Kato T, Khan R, Takayama H, Knöll R, Milting H, Chung CS, Jorde U, Naka Y, Mancini DM, Goldberg IJ, Schulze PC. Ventricular assist device implantation corrects myocardial lipotoxicity, reverses insulin resistance, and normalizes cardiac metabolism in patients with advanced heart failure. Circulation. 2012;125:2844–2853. doi: 10.1161/CIRCULATIONAHA.111.060889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mebazaa A, Gayat E, Lassus J, Meas T, Mueller C, Maggioni A, Peacock F, Spinar J, Harjola VP, van Kimmenade R, Pathak A, Mueller T, Tavazzi L, Disomma S, Metra M, Pascual-Figal D, Laribi S, Logeart D, Nouira S, Sato N, Parenica J, Deye N, Boukef R, Collet C, Van den Berghe G, Cohen-Solal A, Januzzi JL, Jr, GREAT Network Association between elevated blood glucose and outcome in acute heart failure: results from an international observational cohort. J Am Coll Cardiol. 2013;61:820–829. doi: 10.1016/j.jacc.2012.11.054. [DOI] [PubMed] [Google Scholar]
- 22.Ardehali H, Sabbah HN, Burke MA, Sarma S, Liu PP, Cleland JGF, Maggioni A, Fonarow GC, Abel ED, Campia U, Gheorghiade M. Targeting myocardial substrate metabolism in heart failure: potential for new therapies. Eur J Heart Fail. 2014;14:120–129. doi: 10.1093/eurjhf/hfr173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tuunanen H, Engblom E, Naum A, Någren K, Scheinin M, Hesse B, Juhani Airaksinen KE, Nuutila P, Iozzo P, Ukkonen H, Opie LH, Knuuti J. Trimetazidine, a metabolic modulator, has cardiac and extracardiac benefits in idiopathic dilated cardiomyopathy. Circulation. 2008;118:1250–1258. doi: 10.1161/CIRCULATIONAHA.108.778019. [DOI] [PubMed] [Google Scholar]
- 24.Zhang L, Lu Y, Jiang H, Zhang L, Sun A, Zou Y, Ge J. Additional use of trimetazidine in patients with chronic heart failure: a meta-analysis. J Am Coll Cardiol. 2012;59:913–922. doi: 10.1016/j.jacc.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 25.Butler J, Howser R, Portner PM, Pierson RN., III Body mass index and outcomes after left ventricular assist device placement. Ann Thorac Surg. 2005;79:66–73. doi: 10.1016/j.athoracsur.2004.06.047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


