Abstract
In Liu et al., the authors present a 2.5-Å structure of the Trypanosoma cruzi 60S ribosomal subunit and propose a model for the stepwise assembly of the large subunit ribosomal RNA (rRNA). Based on this study, we discuss how the unique features of trypanosomatid ribosome assembly offer potential drug targets.
Keywords: Trypanosomatids, rRNA processing, ribosome assembly, ribosome structure
The trypanosomatids are a family of parasitic protozoans, which include the pathogenic species Trypanosoma brucei, Trypanosoma cruzi, and Leishmania spp. These organisms cause devastating diseases that affect many individuals worldwide with an at risk population of half a billion [1]. Current treatments against these diseases are expensive, difficult to administer, and have significant side effects. Thus, better therapeutic targets are still strongly needed. A unique cellular factor that has been a target for drug development is the ribosome. Ribosomes are the cellular machines that perform protein synthesis and are therefore essential in all life forms. Studies of the bacterial and eukaryotic ribosomes have highlighted unique differences between these ribosomes and advances in structural studies of these ribosomes have paved the way for structure-based drug design [2]. Structural studies of the trypanosomatid ribosomes have revealed trypanosomatid-specific features that differentiate these ribosomes from the eukaryotic ribosome of other organisms. These include the presence of trypanosome-specific insertions known as expansion segments (ES) of the ribosomal RNAs (rRNA), large extensions of the ribosomal proteins, and also the presence of a trypanosome-specific ES, the kinetoplastid domain (KSD), as part of the large subunit (LSU) rRNA [3, 4]. Another distinctive feature of the trypanosomatid ribosomes is that the large subunit rRNA, which is equivalent to the yeast 25S rRNA, is processed into six fragments known as LSU-α, LSU-β, and srRNAs (small rRNA) 1–4 [3, 5].
A pathway of assembly of these fragments and of the other 60S rRNAs has now been postulated in detail by Liu et al., [5] based on the 2.5-Å structure of the T. cruzi 60S subunit that they report. This structure shows that the T. cruzi 60S subunit is similar to that of yeast; however, the T. cruzi structure is much larger due to the larger size of the ESs, extensions of ribosomal proteins, and the addition of trypanosome-specific ESs. The authors were able to attribute the six fragments of the large subunit rRNA to the equivalent domains of the yeast 25S rRNA. Then the authors postulated how these six fragments are assembled in order to form the 60S rRNA scaffold. Liu et al., [5] show that the rRNAs that form the backbone of the 60S rRNA scaffold are 5.8S rRNA, LSU-α, and LSU-β which interact through two specific sets of contacts. The first is formed by interactions between 5.8S rRNA and nucleotides in the 5′ end of LSU-α and the second contact is formed by interactions between the nucleotides in the 3′ end of LSU-α and 5′ end of LSU-β. These two sets of rRNA contacts are conserved in yeast but what is unique in T.cruzi is that the interaction between the 3′ end of LSU-α and the 5′ end of LSU-β is mediated by ES3L of 5.8S rRNA.
Next, the authors examined the structure and interactions that stabilize the srRNAs to the rRNA scaffold. They showed that srRNA1 is anchored to the rRNA scaffold through interactions with srRNA2 and with two anchoring proteins eL19 and eL34. SrRNA2 is anchored to the scaffold through interactions with both LSU-α and LSU-β and also with uL3 protein. The T. cruzi srRNA3 corresponds to ES39L in yeast. This srRNA is really unique in T.cruzi in that it lacks two of the three helices present in the yeast ES39L rRNA. Instead, the space that these helices would occupy is occupied by trypanosome-specific ES42L (location of ES39 helix 1) and by the KSD stretch, the C-terminal extension portion of eL14, and the N-terminal domain of eL33 (location of ES39L helix 3). Both eL33 protein, and ES7L anchor srRNA3 to the rRNA scaffold. srRNA4 contains an ES (srRNA4-ES) and makes contact with the scaffold rRNA through nucleotides of srRNA2, and positively charged residues of uL3. From these arrangements of the rRNAs and interactions with their anchoring proteins, Liu et al., [5] were able to model the likely order of assembly of the 60S rRNAs (Figure 1). They propose that after the rRNA scaffold is formed, srRNA2 and srRNA3 are positioned onto the scaffold by their anchoring proteins. SrRNA1 and srRNA4 are then assembled to the scaffold through strong contacts with srRNA2. ES31L then stabilizes this growing scaffold and the trypanosome-specific ESs, ES42L and the KSD, stabilize srRNAs2-4. Upon completion of the assembly of the 60S rRNA scaffold, conformational changes of the 5S rRNA-containing ribonucleoprotein takes place (at the central protuberance), which enables the subsequent maturation steps of the 60S subunit.
Figure 1. A model of the assembly of the 60S ribosomal RNAs (rRNA) in Trypanosoma cruzi.
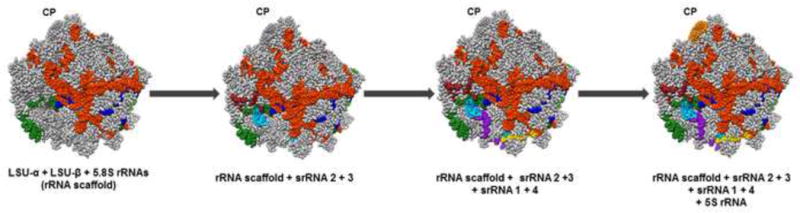
First, the rRNA scaffold is formed by the assembly of the large subunit (LSU)-α (orange red), LSU-β (green), and 5.8S (dark blue) rRNAs. Next, srRNA2 (light blue) and srRNA3 (brown) are assembled to the rRNA scaffold. Then srRNA1 (yellow) and srRNA4 (purple) are assembled. Finally, after the 60S rRNA scaffold is assembled, 5S rRNA (orange)-containing ribonucleoprotein, which is assembled at the central protuberance (CP), undergoes conformational changes that allows for subsequent processing steps of the large subunit. The full 60S subunit of T. cruzi (both proteins and rRNAs) is outlined in grey. Model structure from PDB (5T5H), structure prepared using Chimera [10].
An effective drug must be highly specific to the target and have low toxicity, if any, to the patient. A way to achieve this is to identify a pathogen-specific target and identify ligands that bind with high specificity to that target. This study by Liu et al., [5] is exciting in presenting the best structural resolution thus far for a trypanosomatid ribosomal subunit and in providing a detailed structural model of the assembly of the trypanosomatid 60S subunit. The advantage of such a high-resolution structure is that it allows for a more detailed understanding of the unique functional aspects of ribosome assembly; this allows future identification of critical interactions that may be susceptible to chemotherapeutic disruption. From these studies we now know when the trypanosome-specific ESs are incorporated into the rRNA scaffold. For instance the KSD and ES42L are assembled late to the rRNA scaffold. This information can be combined with studies of the 60S subunit ribosomal proteins hierarchy of assembly to identify late associating proteins that may interact with these trypanosome-specific ESs, in order to determine if they are druggable [6]. Adding to this work are other studies which have identified not only trypanosome-specific proteins essential for this process but also rRNA processing steps that are unique to these organisms [7–9]. These unique factors could also be potential drug targets. Future studies will be needed to correlate the interactions described in this study with their specific binding partners and to determine the effect of ligand inhibition on these parasite-specific assembly processes.
Acknowledgments
We would like to acknowledge Daniel Jaremko for help in preparing the structures used in the model figure.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rodrigues JCF, et al. Biology of Human Pathogenic Trypanosomatids: Epidemiology, Lifecycle and Ultrastructure. In: Santos ALS, Branquinha MH, d’Avila-Levy CM, Kneipp LF, Sodré CL, editors. Proteins and Proteomics of Leishmania and Trypanosoma. Springer; Netherlands: 2014. pp. 1–42. [DOI] [PubMed] [Google Scholar]
- 2.Wilson DN. Ribosome-targeting antibiotics and mechanisms of bacterial resistance. Nature Rev Microbiol. 2014;12:35–48. doi: 10.1038/nrmicro3155. [DOI] [PubMed] [Google Scholar]
- 3.Hashem Y, et al. High-resolution cryo-electron microscopy structure of the Trypanosoma brucei ribosome. Nature. 2013;494:385–9. doi: 10.1038/nature11872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shalev-Benami M, et al. 2.8-A Cryo-EM Structure of the Large Ribosomal Subunit from the Eukaryotic Parasite Leishmania. Cell Rep. 2016;16:288–94. doi: 10.1016/j.celrep.2016.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Z, et al. Structure and assembly model for the Trypanosoma cruzi 60S ribosomal subunit. PNAS, USA. 2016;113:12174–9. doi: 10.1073/pnas.1614594113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gamalinda M, et al. A hierarchical model for assembly of eukaryotic 60S ribosomal subunit domains. Genes Dev. 2014;28:198–210. doi: 10.1101/gad.228825.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hellman KM, et al. Two trypanosome-specific proteins are essential factors for 5S rRNA abundance and ribosomal assembly in Trypanosoma brucei. Eukaryot cell. 2007;6:1766–72. doi: 10.1128/EC.00119-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Das A, et al. A major tyrosine-phosphorylated protein of Trypanosoma brucei is a nucleolar RNA-binding protein. J Biol Chem. 1996;271:15675–81. doi: 10.1074/jbc.271.26.15675. [DOI] [PubMed] [Google Scholar]
- 9.Liang XH, et al. A genome-wide analysis of C/D and H/ACA-like small nucleolar RNAs in Trypanosoma brucei reveals a trypanosome-specific pattern of rRNA modification. RNA. 2005;11:619–45. doi: 10.1261/rna.7174805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pettersen EF, et al. UCSF Chimera–a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–12. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]


