Abstract
Adipose tissue (AT) is no longer regarded as an inert lipid storage, but as an important central regulator in energy homeostasis and in immunity. Three parasite species are uniquely associated with AT during part of their life cycle: Trypanosoma cruzi, the causative agent of Chagas disease, Trypanosoma brucei, the cause of African sleeping sickness and Plasmodium spp, the cause of malaria. In the AT, T. cruzi resides inside adipocytes, T. brucei is found in the interstitial spaces between adipocytes, while Plasmodium spp. infect red blood cells that may adhere to the blood vessels supplying AT. We will discuss how each parasite species adapts to this tissue environment and what the implications are for pathogenesis, clinical manifestations and therapy.
Keywords: adipocyte, adipose tissue, fat, malaria, trypanosoma, lipid
Parasites find a home in adipose tissue
Microbes show incredible diversity with respect to which environment they preferentially colonize or invade. Among microbes that infect mammals, some live inside host cells, while others are extracellular; some remain in the blood, while others penetrate and accumulate into tissues; and sometimes they accumulate in certain reservoirs for at least a period of time. The localization of microbes has major implications on the type and efficiency of the immune response, on disease pathology, potential of transmission and ultimately survival of the host.
Adipose tissue (AT), once regarded as a relatively inert and static organ specialized in lipid storage and release, is now appreciated as the largest endocrine organ that releases a vast number of bioactive peptides and larger proteins, critical metabolites and signaling lipids [1]. The physiological roles go beyond being a central player in systemic energy homeostasis. AT is involved both in innate and adaptive immune responses and is a critical player in stromal interactions with tumors [2]. Most importantly, its ability to store and neutralize lipotoxic lipids make it essential to maintain normal cellular homeostasis in many other critical organs such as the liver, kidney and the heart [3]. In mammals, the majority of AT is white adipose tissue (WAT), which is functionally distinct from brown adipose tissue (BAT) and the more recently described beige adipose tissue [4]. Very little is known about the specific involvement of brown and beige adipose tissues that would set them apart from conventional WAT in the context of infection, so we focus our discussion here predominantly on WAT.
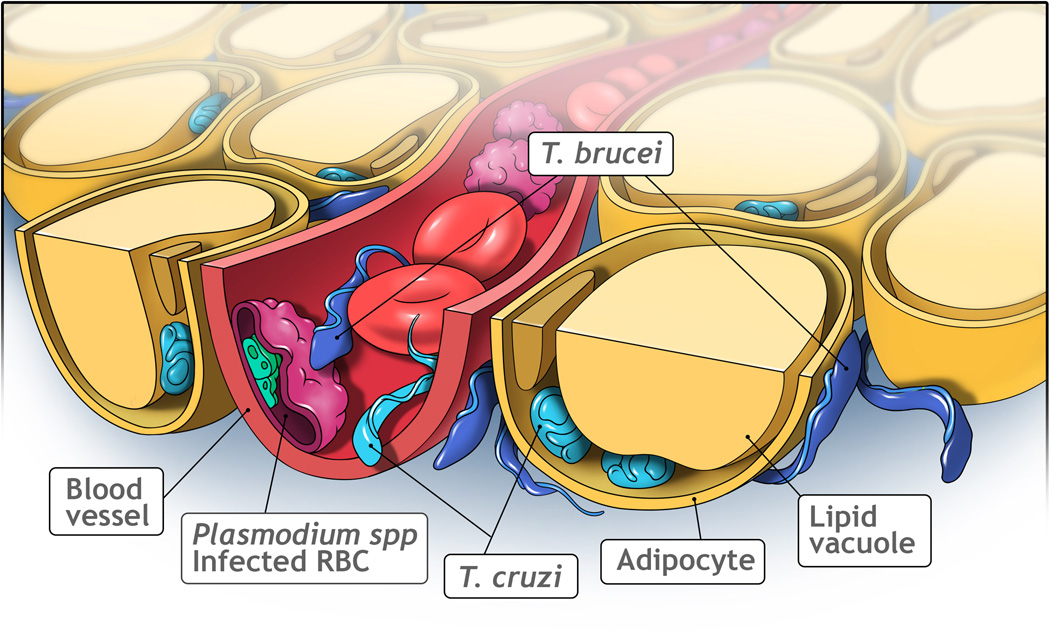
The main constituent of AT is the adipocyte. In WAT, the adipocyte contains a large lipid vacuole that takes up most of the space in the cell and in which triglycerides are stored (Figure 1). The nucleus and other organelles are often ‘squeezed’ against the plasma membrane. But AT is heterogeneous, containing many relevant cell types beyond the adipocyte. Endothelial cells, a vast array of immune cells, adipocyte-precursor cells, as well as fibroblasts and myofibroblasts constitute the stromal vascular fraction of AT; each of these cell types play indispensable roles for the normal physiology of AT [5]. Dysfunctions in the AT that lead to an impairment in the metabolic flexibility of this tissue (i.e. its ability to adapt effectively to feeding and fasting conditions) has, therefore, widespread functional consequences [6,7]. Chronic overnutrition as well as infectious agents that target adipocytes specifically or constituents of the stromal vascular fraction can lead to both acute, as well as chronic impairments to proper functionality.
Figure 1. Localization of parasites in adipose tissue.
Adipocytes are the major constituent of adipose tissue, which contain a large lipid vacuole. Trypanosoma cruzi (light blue) can live in the blood as a trypomastigote or in the cytoplasm of cells (such as adipocytes) as an amastigote. Trypanosoma brucei (dark blue) can be found in the blood and in interstitial spaces of several tissues. In the adipose tissue, they reside between adipocytes. Plasmodium species (green) live inside red blood cells (RBC), which adhere to the blood vessel when they are infected. Image generated by VisuallyMedical.com.
Many organisms have been shown to persist in AT during infection, from virus, to bacteria and parasites (Table 1). One of the best studied examples is Trypanosoma cruzi, which causes Chagas disease, an important cause of morbidity and some mortality in endemic areas of Mexico, Central and South America. Vectorial transmission has also been reported in the United States and is becoming increasingly an issue [8]. Due to immigration, chronic Chagas disease and mother to child vertical transmission have been described in non-endemic areas of the world. Prior to 2005, there were some scattered reports of T. cruzi being found in AT. In a mouse model, T. cruzi was seen in brown adipose tissue (BAT) [9] and Andrade and Silva published electron micrographs of amastigotes of T. cruzi within adipocytes [10]. Combs et al. eventually demonstrated that T. cruzi trypomastigotes readily infect AT and adipocytes [11]. Both BAT and WAT are targets of early infection [12] and a reservoir during the chronic phase in mice and humans [11,13].
Table 1.
Examples of pathogens that persist in adipose tissue.
| Species | Type of pathogen |
Disease | Host | Localization in Adipose Tissue |
Refs |
|---|---|---|---|---|---|
|
Trypanosoma cruzi |
Parasite | Chagas disease |
Mouse, humans |
Cytoplasm of adipocytes | [9] |
|
Trypanosoma brucei |
Parasite | African trypanosomia sis |
Mouse, humans |
Interstitial spaces of visceral and subcutaneous AT |
[17] [18] [20] |
|
Plasmodium berghei |
Parasite | Malaria | Mouse | Inside vessels | [29] |
|
Rickettsia prowazekii |
Bacteria | Epidemic typhus |
Mouse | Cytoplasm of adipocytes | [59] |
|
Coxiella burnetii |
Bacteria | Q fever | Mouse | Intracellular: in adipocytes and macrophages |
[60] |
|
Mycobacteriu m tuberculosis |
Bacteria | Tuberculosis | Mouse, humans |
Adipocytes and stromal vascular fraction |
[61] |
| HIV & SIV | Virus | AIDS & SAIDS |
Humans, macaques |
Stromal vascular fraction (including immune cells) |
[54] |
Trypanosoma brucei is a unicellular and extracellular parasite that causes African trypanosomiasis, a neglected tropical disease restricted to Sub-Saharan Africa [14]. The human form of this disease, human African trypanosomiasis (HAT), also known as sleeping sickness is believed to be fatal most of the times. It is transmitted to mammalian hosts by the bite of a tsetse fly. T. brucei parasites live in the blood and in interstitial spaces of many different tissues of the mammalian host [15]. When parasites penetrate the brain, they cause neuropsychiatric and sleep abnormalities [16–20]. Tropism has recently been described to the skin and testis, which is important for transmission by the tsetse and sexually, respectively [17–19,21]. T. brucei parasites were also found in the skin of 0.5% patients screened for Onchocerca microfilaria at the Democratic Republic of Congo, suggesting that undiagnosed individuals may be a previously unknown source of transmission [17]. In an independent study in mice, Trindade et al. recently showed by quantitative PCR that the AT harbors around 10-fold more parasites than the average number found in the blood in chronic stages of the disease [20]. Interestingly, in the two studies describing tropism for the skin, many parasites were found in the vicinity of subcutaneous adipocytes [17,18]. Although it remains to be compared the relative number of parasites in skin, visceral adipose tissue and testis from the same mouse, there is no doubt that the adipose tissue is one of the major parasite reservoirs. The implications of these extravascular parasite reservoirs in terms of pathogenesis, transmission, treatment and diagnosis are just beginning to be investigated.
Plasmodium spp. are obligate intracellular parasites and the causative agents of malaria. While five species can cause malaria in humans, Plasmodium falciparum is responsible for the majority of malaria deaths globally and is the most prevalent species in sub-Saharan Africa. The remaining species are not typically as life threatening as P. falciparum. Erythrocytes infected with P. falciparum are known to cytoadhere to endothelial cells lining blood vessels of different organs, and this feature has been associated, in part, with the severity of the malaria pathology. Examination of post-mortem tissue obtained from individuals who died of malaria revealed that P. falciparum infected red blood cells (iRBCs) sequester in differing amounts in tissues of a variety of organs. While the lung and the spleen are recognized as the main sites for accumulation of P. falciparum, the AT has also been identified as a site of P. falciparum iRBC sequestration [22,23].
In this review, we discuss how and why these three different parasites (T. cruzi, T. brucei and Plasmodium species) have found a home in AT. Most of the knowledge was obtained using well-established mouse model of the infections, although we will also mention clinical studies whenever appropriate. The data is still scarce when it comes to Plasmodium, but we will include it in the discussion when relevant.
Within the adipose tissue, where do parasites live?
T. brucei is an extracellular parasite and, in the visceral adipose tissue, parasites can be found in the interstitial spaces between adipocytes. Replicating parasites have been found both in the AT and in the skin, suggesting these tissue environments provide enough nutrients to support parasite growth [17,18,20]. A large number of non-dividing transmissible forms have also been found in the two tissues [15], suggesting that transmission can happen from extra-vascular sites [17,20].
T. brucei displays waveforms that seem best adapted to move in confined viscous environments such as [17,20] tissue spaces [24]. Live imaging revealed that indeed some T. brucei slender parasites present a fast persistent movement, while others are less mobile [17]. Conversely by scanning electron microscopy, parasites appear to tightly interact with subcutaneous adipocytes, with entanglement by reticular fibers and embedment between collagen bundles [17,18,20]. It remains unknown if the parasites interact with adipocytes to propel themselves forward, or as a means of slowing down at least temporarily. We also do not know if slender and stumpy forms show any different behavior in terms of physical interaction with host cells.
T. cruzi trypomastigotes infect virtually any cell in the body comprising pre-adipocytes and adipocytes [25,26]. Trypomastigotes transform into intracellular amastigotes, which escape the parasitophorous vacuole and lie free in the cytoplasm. There, they multiply by binary fission and transform back to trypomastigotes, which escape the host cells and then invade adjacent uninfected cells. Alternatively, they enter the blood and lymphatic vessels and begin another infection cycle.
The adherence of Plasmodium iRBCs to host tissue, also known as sequestration, occurs in small capillaries and post-capillary venules of many organs. In AT, this is no different. Plasmodium iRBCs remain in blood vessels. Through the use of in vitro binding assays, a number of host molecules that are expressed on the surface of endothelial cells have been identified as playing a role in P. falciparum iRBC adherence. CD36 and chondroitin sulfate A (CSA) are however the only two receptors that maintain a stable stationary adherence to iRBCs [27,28]. The application of in vivo imaging techniques in laboratory animals that either express or lack CD36 revealed that CD36 has a major role in the rodent malaria parasite Plasmodium berghei sequestration, specifically in AT and lungs [29]. In animals deficient in CD36, the sequestration of iRBCs in both the lungs and AT is significantly reduced [29].
How do parasites enter/invade the adipose tissue?
Trypomastigotes of both T. cruzi and T. brucei reside extracellularly. Therefore, they must cross the capillary wall to reach inside a tissue. We can envision several non-exclusive possibilities for how parasites enter the parenchyma of the adipose tissue:
First, these parasites may cross the vessels into and out of all tissues, but only the AT provides the conditions to replicate or to avoid being eliminated by the immune system.
Second, the entry may be specific: it could involve the recognition and binding to a specific receptor that is enriched in the capillaries of the AT. How this process exactly occurs has not been extensively examined. However, AT endothelium can be targeted specifically [30], and it is possible that the trypomastigotes gain entry through one of these uniquely enriched markers. Indeed, this has been shown for the entry of T. brucei across the blood brain barrier, which happens only in vessels where the endothelial basement membrane contains the laminin α4 chain [31].
A third possibility is that the vasculature in AT is constantly undergoing remodeling, more so than any other tissue in the system. This is based on the observation that AT is most susceptible to the effects of angiogenesis inhibitors in the absence of a tumor mass [32] and easily becomes leaky with small changes in vascular endothelial growth factor (VEGF) even within the physiological range [7,33], even though there is no direct evidence for this process. This high level of constant remodeling with intermittent stages of vascular leakage may also enable parasites to gain easy access to adipocytes.
A fourth possibility is that entry is actually favored by the immune response mounted against these parasites when they are in the blood. The passage of T. brucei parasites into the brain is facilitated by the presence of a number of components of the immune system (such as interferon γ (IFNγ)) [31,34]. IFN-γ is abundantly expressed during a T. brucei infection and it promotes parasite growth [35]. However, there are no experimental data at this point substantiating if parasite entry into the brain and other tissues shares similar features.
Once in the interstitial space, T. cruzi continues its journey into the adipocyte. Here, another set of receptors, including the low density lipoprotein receptor (LDLr), serve as the primary target for the parasites to gain access to the intracellular milieu [25]. T. cruzi invasion via the LDLr may exert important effects on intracellular cholesterol homeostasis [26].
Do parasites adapt to living in the adipose tissue?
The bloodstream is populated by two forms of T. brucei parasites that correspond to the two life cycle stages (the replicative slender form and the cell-cycle arrested transmissible stumpy forms). These two stages are functionally different, as demonstrated by their different gene expression signatures, including the upregulation in stumpy forms of mitochondrial components [36,37]. Are slender and stumpy forms in the AT phenotypically identical to these forms in the blood? Trindade et al. used RNA-seq as a proxy to test this hypothesis. The authors demonstrated that parasites in AT have a gene expression signature that is different from that of parasites in the blood, [20] suggesting that the phenotypic diversity of parasites in the host is probably much greater than previously anticipated [20]. Many of the differentially expressed genes are related to metabolism, which suggests that parasites may adapt to the tissue environment. A biochemical assay confirmed the activation of fatty acid beta-oxidation, a pathway in which fatty acids are used as a carbon source. Although, we do not know the source of these lipids/fatty acids, it is possible that they are either imported from the AT interstitial space where there are high levels of lipid trafficking between feeding and fasting periods.
How are adipocytes affected by infection?
When mice are infected by T. cruzi, in the early stage of infection (15 days post infection - dpi), AT displayed a widespread macrophage invasion and a reduction in lipid accumulation and adipocyte size [12]. Of interest, there was a reduction in fat mass associated with increased expression of lipolytic enzymes. Many of the increases in pro-inflammatory mediators described at 30 and 90 dpi were already present at very early stages of infection. Both BAT and WAT obtained from T. cruzi-infected mice display increased markers of oxidative stress, findings consistent with the persistent inflammatory state observed [38]. T. cruzi infection of AT alters the expression and function of the connexin-43 gap junction protein, which may have widespread implications for intercellular communication in AT [39]. Caradonna and colleagues have demonstrated that the pre-replication phase of the T. cruzi infection cycle is predominantly affected by host cell signaling pathways and cytoskeletal regulators. On the other hand, factors in the host that foster T. cruzi amastigote growth are highly enriched for metabolic functions [40].
Although infections caused by T. brucei and P. berghei are typically associated with weight loss and cachexia [41,42], the consequences of these infections on adipocytes has not been characterized.
What is the immune response in AT and is it weaker than in blood?
There is no accepted literature that argues that adipose tissue is immune-depleted. In fact, the more we learn about immunometabolism, the more complex the situation seems to be in adipose tissue. T. cruzi hides inside adipocytes [43]. By definition, intracellular residency in a long-living cell is one of the most effective ways to escape the immune response, Nevertheless, infection of adipocytes by T. cruzi results in increased expression of pro-inflammatory cytokines and chemokines and prompts a migration of F4/80+ macrophages into AT as early as 15 dpi [12]. These events are accompanied by a reduction in adiponectin serum levels and adiponectin expression in AT (an important insulin-sensitizing adipokine) [11]. Adiponectin is also a potent negative regulator of inflammation and a critical secretory adipokine required to maintain normal AT function during dietary challenges [44]. The increased expression of pro-inflammatory cytokines in AT was still evident well into the chronic stage in surviving mice [11].
AT is highly enriched in a vast number of immune cells. With respect to macrophage populations, there is a balance between pro-inflammatory M1 macrophages, and macrophages expressing a gene expression profile more consistent with a role in remodeling (M2 macrophages) [45]. During a T. cruzi mouse infection, a shift towards M2 macrophages has been described, which could help maintaining tissue homeostasis or promoting parasite survival [46]. However, it remains unknown if the M1 response is muted. Further studies are necessary to clarify the role of inflammatory mechanisms in adipose tissue during a T. cruzi infection.
The local immune response against T. brucei and P. berghei has not been studied yet and it is unknown if in the AT there is an immune suppressive environment that could explain the persistence of the parasites in the tissue.
Is the AT a nutrient rich environment?
Why would T. cruzi opt to reside in adipocytes during the chronic stage of the infection? We can only speculate, since specific data are missing at this point. One possible explanation is the extreme longevity of adipocytes, both in humans [47] as well as in mice [48]. Adipocytes have a half-life on average of approximately 10 years, so this may allow parasites to remain in a quiescent state for prolonged periods of time without the need to go through another infectious cycle. A second possibility is that the adipocyte intracellularly offers a constant source of nutrients, in particular fatty acids that can easily be mobilized from the lipid droplet through recruitment of specific intracellular lipases, such as adipose triglyceride lipase (ATGL), hormone-sensitive lipase (HSL) and monoacyl glycerol lipase (MGL) [49]. Indeed, T. cruzi amastigotes are in close proximity to lipid vacuole, which ensures a continuous supply of fatty acids from the adipocyte to the parasite [11]. Furthermore, postprandial surges of insulin ensure an influx of glucose on a regular basis. So both carbohydrate and lipid needs for the parasites in a quiescent state are readily met by the adipocyte.
It is not so obvious how T. brucei and P. berghei could benefit from the nutrients available inside the adipocytes. It is possible that these parasites directly or indirectly induce adipocytes to release stored nutrients (such as free fatty acids and glycerol), which could be subsequently taken up by the parasites outside.
Clinical relevance and drug treatment
The possible clinical importance of AT in Chagas disease or in sleeping sickness remains unclear. Recent studies demonstrated that T. cruzi-infected mice fed a high fat diet displayed a reduction in parasitemia and in myocardial pathology, associated with an increase in parasite load in WAT, suggesting that WAT acts as a ‘sponge’ for the parasite and perhaps protects the heart from an increased parasite load [50,51]. In contrast, T. cruzi infection of obese and diabetic leptin-receptor deficient mice db/db mice lead to increased mortality compared with lean genetically modified db/db mice [52]. Another study showed that in infected mice, there is an impairment of insulin release from the pancreas [53], but there are no definitive data that there is an increase in the incidence of diabetes in humans. Thus, at present there is no reliable data that suggest that T. cruzi infection causes obesity or that obese individuals are more likely to become infected or that the parasite load is greater in obese patients [11–13]. As far as we know, no epidemiological study has been undertaken in patients with HAT to assess whether the number or severity of HAT cases is correlated with body mass index prior to infection. In mice, a T. brucei infection in obese and diabetic leptin-receptor deficient mice (db/db) resulted in decreased mortality compared to wild-type, suggesting a protective role of AT [51]. Other mouse mutants, drugs and diets need to be tested in infection mouse models to understand if there is a causal relationship between adipose tissue mass and infectivity.
Since the AT is a lipid rich environment and most drugs are hydrophilic, it may pose some limitations to the efficacy of drug treatments. Indeed, this has been observed for HIV treatment: the AT acts as an HIV reservoir that prevents full treatment [54]. Recently, it was demonstrated that the failure of posaconazole to cure T. cruzi in a mouse model was due to the failure to eliminate the parasite from its protected environment in AT during the acute phase [55]. Trindade et al. have shown, using transplantation experiments, that parasites can egress from AT and repopulate the blood [20]. Thus, it is possible that AT contributes to the relapses also observed in the treatment of African trypanosomiasis, which are typically less than 5–10% after treatment [56,57].
Importantly, the gene expression adaptation of parasites to tissues means that there is a much greater phenotypic diversity of parasites within the host than previously anticipated. A specific drug target may be expressed at different levels in each tissue type (i.e. AT, brain), which would result in different susceptibilities to drug treatment depending on the tissue where parasites reside. Such a scenario could also contribute to the inefficacy of drug treatment and to relapses.
Concluding remarks
Tissues and blood are very different at various levels, including (i) biochemically (such as the concentration of nutrients and oxygen), (ii) biomechanically (tissues are more viscous and crowded environments), (iii) immunogenically (different types of immune response are triggered in tissues), (iv) interface with the exterior environment and possible transmission vectors. In recent years there has been an increased appreciation in the role of AT in the pathogenesis of infectious agents [58] but we still have a very limited understanding about this topic. Many questions remain to be answered to ultimately understand the selective advantage of parasite tropism to adipose tissue (see Outstanding Questions box). Parasites may have access to different nutrients, benefit from a weaker immune response, or the AT may act as a reservoir of replicative forms. When parasites are in the skin, they may also benefit from some of these advantages due to the presence of subcutaneous adipose tissue. In the skin, however, the most obvious selective advantage is an increased chance of transmission by the tsetse vector.
OUTSTANDING QUESTIONS BOX.
Do all parasites adapt to every tissue they occupy?
What is the selective advantage to persist in adipose tissue?
Is elimination of parasites in adipose tissue less efficient?
How do parasites sense they are in adipose tissue?
Do parasites manipulate adipocytes to redirect nutrients for their own benefit?
Is there a causal relationship between persistence in adipose tissue and pathogenesis?
Is the chronic presence of parasites in adipose tissue lead to a higher chance of pathological sequelae, such as diabetes and chronic kidney disease?
Conversely, it is also possible that the adipose tissue is the “lesser of two evils”, i.e. perhaps infection of the adipose tissue causes less damage to the host than infection of other organs, such as the liver or brain. Because the survival of the host is important for pathogens to ensure their replication and transmission, there may have been a selective pressure for pathogens that were best adapted to live in this tissue.
Another interesting question is how to integrate the accumulation of parasites in different tissues. Plasmodium species accumulate not only in adipose tissue, but also in the lungs. T. brucei also infects testis, skin and brain. Is the tropism for one tissue independent from the others? Is there a common mechanism for entry and adaptation? Can the presence of parasites in one tissue have repercussions of parasites in another, because for example the metabolism of the host is systemically affected?
T. brucei is capable of sensing and adapting to the tissue environment. What is the nature of the factor that triggers this adaption? It could be a nutrient or may be a mechanical signal. In the future, it will be interesting to study whether these adaptations change with time of infection and/or between tissues or organs. For example, given that the brain is a lipid-rich environment, do T. brucei parasites undergo an adaptation similar to when they are in the adipose tissue? It will also be interesting to study if other parasites also adapt to their surrounding tissue. For example, are Plasmodium infected erythrocytes phenotypically identical when they are sequestered in lungs and adipose tissue?
We hope this paper may spur others to investigate other roles of AT in the setting of infection, immunity and metabolism.
TRENDS BOX.
Several pathogens accumulate in the adipose tissue
Within the adipose tissue, pathogens occupy different niches, which means different access to nutrients and different exposure to the immune system
Parasites adapt their gene expression to the tissue environment, which increases the phenotypic diversity of parasites within the host
The exceptional longevity of adipocytes along with a nutrient-dense microenvironment offers an ideal long-term environment for parasites in the chronic stage of the infection
Because most drugs are designed to be hydrophylic, persistence of parasites in adipose tissue may compromise efficacy of drug treatment
Acknowledgments
HBT was supported by the National Institutes of Health (NIH) (R21-AI124000). PES was supported by the NIH (R01-DK55758, R01-DK099110 and P01-DK088761) as well as a grant from the Cancer Prevention and Research Institute of Texas (CPRIT RP140412). MMM was supported by the European Research Council (ERC-2012-StG_311502) and Fundação para a Ciência e Tecnologia (FCT) (FCT/EXCL/IMI-MIC/0056/2012). We thank Sandra Trindade for suggestions on the manuscript. LMF was supported by Howard Hughes Medical Institute (55007419) and FCT (PTDC/BIM-MET/4471/2014).
GLOSSARY
- Adipocyte
the main cell that composes the adipose tissue
- Adipose tissue (AT)
also known, as fat, comprises 10–50 % of the body of healthy human. It is an essential tissue to maintain metabolism homeostasis.
- Lipid vacuole
a storage structure found in the adipocytes that contains neutral lipids (triglycerides).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Scherer PE. The Multifaceted Roles of Adipose Tissue-Therapeutic Targets for Diabetes and Beyond: The 2015 Banting Lecture. Diabetes. 2016;65:1452–1461. doi: 10.2337/db16-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park J, et al. Obesity and cancer--mechanisms underlying tumour progression and recurrence. Nat Rev Endocrinol. 2014;10:455–465. doi: 10.1038/nrendo.2014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stern JH, et al. Adiponectin, Leptin, and Fatty Acids in the Maintenance of Metabolic Homeostasis through Adipose Tissue Crosstalk. Cell Metab. 2016;23:770–784. doi: 10.1016/j.cmet.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu Y, et al. Connexin 43 Mediates White Adipose Tissue Beiging by Facilitating the Propagation of Sympathetic Neuronal Signals. Cell Metab. 2016;24:420–433. doi: 10.1016/j.cmet.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rutkowski JM, et al. The cell biology of fat expansion. J Cell Biol. 2015;208:501–512. doi: 10.1083/jcb.201409063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asterholm IW, Scherer PE. Enhanced metabolic flexibility associated with elevated adiponectin levels. Am J Pathol. 2010;176:1364–1376. doi: 10.2353/ajpath.2010.090647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asterholm IW, et al. Lack of “immunological fitness” during fasting in metabolically challenged animals. J Lipid Res. 2012;53:1254–1267. doi: 10.1194/jlr.M021725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia MN, et al. Historical Perspectives on the Epidemiology of Human Chagas Disease in Texas and Recommendations for Enhanced Understanding of Clinical Chagas Disease in the Southern United States. PLoS Negl Trop Dis. 2015;9:e0003981. doi: 10.1371/journal.pntd.0003981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shoemaker JP, et al. Trypanosoma cruzi: preference for brown adipose tissue in mice by the Tulahuen strain. Exp Parasitol. 1970;27:403–407. doi: 10.1016/0014-4894(70)90045-7. [DOI] [PubMed] [Google Scholar]
- 10.Andrade ZA, Silva HR. Parasitism of adipocytes by Trypanosoma cruzi. Mem Inst Oswaldo Cruz. 1995;90:521–522. doi: 10.1590/s0074-02761995000400018. [DOI] [PubMed] [Google Scholar]
- 11.Combs TP, et al. The adipocyte as an important target cell for Trypanosoma cruzi infection. J Biol Chem. 2005;280:24085–24094. doi: 10.1074/jbc.M412802200. [DOI] [PubMed] [Google Scholar]
- 12.Nagajyothi F, et al. Response of adipose tissue to early infection with Trypanosoma cruzi (Brazil strain) J Infect Dis. 2012;205:830–840. doi: 10.1093/infdis/jir840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferreira AV, et al. Evidence for Trypanosoma cruzi in adipose tissue in human chronic Chagas disease. Microbes Infect. 2011;13:1002–1005. doi: 10.1016/j.micinf.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franco JR, et al. Epidemiology of human African trypanosomiasis. Clin Epidemiol. 2014;6:257–275. doi: 10.2147/CLEP.S39728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Losos GJ, Ikede BO. In: Veterinary Pathology. Dodd DC, editor. S. Karger; 1972. pp. 1–71. [Google Scholar]
- 16.Kennedy PG. Human African trypanosomiasis-neurological aspects. J Neurol. 2006;253:411–416. doi: 10.1007/s00415-006-0093-3. [DOI] [PubMed] [Google Scholar]
- 17.Capewell P, et al. The skin is a significant but overlooked anatomical reservoir for vector-borne African trypanosomes. Elife. 2016;5:e17716. doi: 10.7554/eLife.17716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caljon G, et al. The Dermis as a Delivery Site of Trypanosoma brucei for Tsetse Flies. PLoS Pathog. 2016;12:e1005744. doi: 10.1371/journal.ppat.1005744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Claes F, et al. Bioluminescent imaging of Trypanosoma brucei shows preferential testis dissemination which may hamper drug efficacy in sleeping sickness. PLoS Negl Trop Dis. 2009;3:e486. doi: 10.1371/journal.pntd.0000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trindade S, et al. Trypanosoma brucei Parasites Occupy and Functionally Adapt to the Adipose Tissue in Mice. Cell Host Microbe. 2016;19:837–848. doi: 10.1016/j.chom.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biteau N, et al. Trypanosoma brucei gambiense Infections in Mice Lead to Tropism to the Reproductive Organs, and Horizontal and Vertical Transmission. PLoS Negl Trop Dis. 2016;10:e0004350. doi: 10.1371/journal.pntd.0004350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller LH. Distribution of mature trophozoites and schizonts of Plasmodium falciparum in the organs of Aotus trivirgatus, the night monkey. Am J Trop Med Hyg. 1969;18:860–865. doi: 10.4269/ajtmh.1969.18.860. [DOI] [PubMed] [Google Scholar]
- 23.Wilairatana P, et al. Prognostic significance of skin and subcutaneous fat sequestration of parasites in severe falciparum malaria. Southeast Asian J Trop Med Public Health. 2000;31:203–212. [PubMed] [Google Scholar]
- 24.Bargul JL, et al. Species-Specific Adaptations of Trypanosome Morphology and Motility to the Mammalian Host. PLoS Pathog. 2016;12:e1005448. doi: 10.1371/journal.ppat.1005448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagajyothi F, et al. Trypanosoma cruzi utilizes the host low density lipoprotein receptor in invasion. PLoS Negl Trop Dis. 2011;5:e953. doi: 10.1371/journal.pntd.0000953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johndrow C, et al. Trypanosoma cruzi infection results in an increase in intracellular cholesterol. Microbes Infect. 2014;16:337–344. doi: 10.1016/j.micinf.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fried M, Duffy PE. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science. 1996;272:1502–1504. doi: 10.1126/science.272.5267.1502. [DOI] [PubMed] [Google Scholar]
- 28.Berendt AR, et al. Intercellular adhesion molecule-1 is an endothelial cell adhesion receptor for Plasmodium falciparum. Nature. 1989;341:57–59. doi: 10.1038/341057a0. [DOI] [PubMed] [Google Scholar]
- 29.Franke-Fayard B, et al. Murine malaria parasite sequestration: CD36 is the major receptor, but cerebral pathology is unlinked to sequestration. Proc Natl Acad Sci U S A. 2005;102:11468–11473. doi: 10.1073/pnas.0503386102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kolonin MG, et al. Reversal of obesity by targeted ablation of adipose tissue. Nat Med. 2004;10:625–632. doi: 10.1038/nm1048. [DOI] [PubMed] [Google Scholar]
- 31.Masocha W, et al. Cerebral vessel laminins and IFN-gamma define Trypanosoma brucei brucei penetration of the blood-brain barrier. J Clin Invest. 2004;114:689–694. doi: 10.1172/JCI22104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rupnick MA, et al. Adipose tissue mass can be regulated through the vasculature. Proc Natl Acad Sci U S A. 2002;99:10730–10735. doi: 10.1073/pnas.162349799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun K, et al. Dichotomous effects of VEGF-A on adipose tissue dysfunction. Proc Natl Acad Sci U S A. 2012;109:5874–5879. doi: 10.1073/pnas.1200447109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amin DN, et al. Expression and role of CXCL10 during the encephalitic stage of experimental and clinical African trypanosomiasis. J Infect Dis. 2009;200:1556–1565. doi: 10.1086/644597. [DOI] [PubMed] [Google Scholar]
- 35.Bonfanti C, et al. Increased levels of antibodies to IFN-gamma in human and experimental African trypanosomiasis. Scand J Immunol. 1995;41:49–52. doi: 10.1111/j.1365-3083.1995.tb03532.x. [DOI] [PubMed] [Google Scholar]
- 36.Capewell P, et al. Regulation of Total and Polysomal mRNA during Development within Its Mammalian Host. PLoS One. 2013;8:e67069. doi: 10.1371/journal.pone.0067069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.MacGregor P, et al. Trypanosomal immune evasion, chronicity and transmission: an elegant balancing act. Nat Rev Microbiol. 2012;10:431–438. doi: 10.1038/nrmicro2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wen JJ, et al. Markers of oxidative stress in adipose tissue during Trypanosoma cruzi infection. Parasitol Res. 2014;113:3159–3165. doi: 10.1007/s00436-014-3977-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burke S, et al. Adipocytes in both brown and white adipose tissue of adult mice are functionally connected via gap junctions: implications for Chagas disease. Microbes Infect. 2014;16:893–901. doi: 10.1016/j.micinf.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caradonna KL, et al. Host metabolism regulates intracellular growth of Trypanosoma cruzi. Cell Host Microbe. 2013;13:108–117. doi: 10.1016/j.chom.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Palmer JJ, et al. Syndromic algorithms for detection of gambiense human African trypanosomiasis in South Sudan. PLoS Negl Trop Dis. 2013;7:e2003. doi: 10.1371/journal.pntd.0002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lamb TJ, et al. Insights into the immunopathogenesis of malaria using mouse models. Expert Rev Mol Med. 2006;8:1–22. doi: 10.1017/S1462399406010581. [DOI] [PubMed] [Google Scholar]
- 43.Oishi Y, Manabe I. Integrated regulation of the cellular metabolism and function of immune cells in adipose tissue. Clin Exp Pharmacol Physiol. 2016;43:294–303. doi: 10.1111/1440-1681.12539. [DOI] [PubMed] [Google Scholar]
- 44.Holland WL, et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat Med. 2011;17:55–63. doi: 10.1038/nm.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun K, et al. Adipose tissue remodeling and obesity. J Clin Invest. 2011;121:2094–2101. doi: 10.1172/JCI45887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cabalén ME, et al. Chronic Trypanosoma cruzi infection potentiates adipose tissue macrophage polarization toward an anti-inflammatory M2 phenotype and contributes to diabetes progression in a diet-induced obesity model. Oncotarget. 2016;7:13400–13415. doi: 10.18632/oncotarget.7630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spalding KL, et al. Dynamics of fat cell turnover in humans. Nature. 2008;453:783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- 48.Wang QA, et al. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med. 2013;19:1338–1344. doi: 10.1038/nm.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nielsen TS, et al. Dissecting adipose tissue lipolysis: molecular regulation and implications for metabolic disease. J Mol Endocrinol. 2014;52:R199–R222. doi: 10.1530/JME-13-0277. [DOI] [PubMed] [Google Scholar]
- 50.Nagajyothi F, et al. High Fat Diet Modulates Trypanosoma cruzi Infection Associated Myocarditis. PLoS Negl Trop Dis. 2014;8:e3118. doi: 10.1371/journal.pntd.0003118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Amole BO, et al. Trypanosoma brucei: infection in murine diabetes. Exp Parasitol. 1985;60:342–347. doi: 10.1016/0014-4894(85)90040-2. [DOI] [PubMed] [Google Scholar]
- 52.Nagajyothi F, et al. Crucial role of the central leptin receptor in murine Trypanosoma cruzi (Brazil strain) infection. J Infect Dis. 2010;202:1104–1113. doi: 10.1086/656189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nagajyothi F, et al. Alterations in glucose homeostasis in a murine model of Chagas disease. Am J Pathol. 2013;182:886–894. doi: 10.1016/j.ajpath.2012.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Damouche A, et al. Adipose tissue is a neglected viral reservoir and an inflammatory site during chronic HIV and SIV infection. PLoS Pathog. 2015;11:e1005153. doi: 10.1371/journal.ppat.1005153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Francisco AF, et al. Limited Ability of Posaconazole To Cure both Acute and Chronic Trypanosoma cruzi Infections Revealed by Highly Sensitive In Vivo Imaging. Antimicrob Agents Chemother. 2015;59:4653–4661. doi: 10.1128/AAC.00520-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pepin J, Khonde N. Relapses following treatment of early-stage Trypanosoma brucei gambiense sleeping sickness with a combination of pentamidine and suramin. Trans R Soc Trop Med Hyg. 1996;90:183–186. doi: 10.1016/s0035-9203(96)90132-7. [DOI] [PubMed] [Google Scholar]
- 57.Richardson JB, et al. Whole genome sequencing shows sleeping sickness relapse is due to parasite regrowth and not reinfection. Evol Appl. 2016;9:381–393. doi: 10.1111/eva.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Desruisseaux MS, et al. Adipocyte, adipose tissue, and infectious disease. Infect Immun. 2007;75:1066–1078. doi: 10.1128/IAI.01455-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bechah Y, et al. Adipose tissue serves as a reservoir for recrudescent Rickettsia prowazekii infection in a mouse model. PLoS One. 2010;5:e8547. doi: 10.1371/journal.pone.0008547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bechah Y, et al. Persistence of Coxiella burnetii, the agent of Q fever, in murine adipose tissue. PLoS One. 2014;9:e97503. doi: 10.1371/journal.pone.0097503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Neyrolles O, et al. Is adipose tissue a place for Mycobacterium tuberculosis persistence? PLoS One. 2006;1:e43. doi: 10.1371/journal.pone.0000043. [DOI] [PMC free article] [PubMed] [Google Scholar]