Abstract
Objective
The gold standard for measuring oral contraceptive (OC) pharmacokinetics is the 24-hour steady-state area-under-the-curve (AUC). We conducted this study to assess whether limited sampling at steady state or measurements following use of one or two OCs could provide an adequate proxy in epidemiological studies for the progestin 24-hour steady-state AUC of a particular OC.
Study Design
We conducted a 13-sample, 24-hour pharmacokinetic study on both day 1 and day 21 of the first cycle of a monophasic OC containing 30 μg ethinyl estradiol and 150 μg levonorgestrel (LNG) in 17 normal-weight healthy white women, and a single-dose 9-sample study of the same OC after a one-month washout. We compared the 13-sample steady-state results with several steady-state and single-dose results calculated using parsimonious sampling schemes.
Results
The 13-sample steady-state 24-hour LNG AUC was highly correlated with the steady-state 24-hour trough value (r = 0.95; 95% CI [0.85, 0.98]) and with the steady-state 6, 8, 12 and 16-hour values (0.92 ≤ r ≤ 0.95). The trough values after one or two doses were moderately correlated with the steady-state 24-hour AUC value (r = 0.70; 95% CI [0.27, 0.90] and 0.77; 95% CI [0.40, 0.92], respectively).
Conclusions
Single time-point concentrations at steady-state and after administration of one or two OCs gave highly to moderately correlated estimates of steady-state LNG AUC. Using such measures could facilitate prospective pharmaco-epidemiologic studies of the OC and its side effects.
Keywords: oral contraceptives, pharmacokinetics, levonorgestrel, ethinyl estradiol, single-dose, steady-state
Introduction
Levonorgestrel (LNG) is the most widely used progestin in combined oral contraceptives (OCs). The pharmacokinetics (PK) of orally administered LNG are well-explored and show that LNG does not undergo significant first-pass metabolism [1, 2], but women using the same OC exhibit substantial variation in their LNG serum concentrations; PK studies report a 1.6-fold increase in LNG concentration from the 25th to the 75th percentile level [3, 4].
Many studies have described OC side effects as being due to their progestin and estrogen components [5-8]. These studies consider administered hormone dose when describing prevalence of side effects, but wide inter-individual variation in systemic exposure to the hormones limits the statistical power of such studies [9]. Investigating side-effect prevalence based on a user' individually measured systemic exposure may lead to a better understanding of the relationship with side effects.
The gold standard for evaluating systemic estrogen and progestin exposure from an OC is a steady-state area-under-the-curve (AUC) calculation using samples collected at multiple time points over 24 hours. The main objective of this study was to evaluate whether more parsimonious sampling schemes may provide proxies, for use in epidemiological studies, for the usual resource-demanding approach of measuring steady-state AUC. This approach has previously been employed in the pharmacokinetics literature to establish proxy measurements for systemic exposure [10].
We have previously published PK results for ethinyl estradiol (EE2) during the use of a particular OC (30 μg EE2, 150 μg LNG) [11]. Here we report our findings regarding LNG from the same study.
Material and Methods
This single-arm, open-label pilot clinical trial took place at Columbia University Medical Center, New York, NY, USA after Institutional Review Board approval. Participants were 18-35 years old, self-identified as white, and provided written informed consent before enrollment. We excluded women with medical contraindications to use of combined hormonal contraception [12]. Additional exclusion criteria included hysterectomy or oophorectomy, irregular cycles or cycles > 35 days, childbirth within 6 months, breastfeeding, current smoker, body mass index ≥ 30.0 kg/m2, and use of an OC within 1 month or injectable contraception within 6 months of entering the study.
The study OC contained 30 μg EE2 and 150 μg LNG packaged with 21 active and 7 placebo pills (Portia; Teva, Philadelphia, USA). Treatment began within 7 days of the start of menses. After 21 active pills, participants had a one-cycle, OC-free washout period. Each participant returned within 7 days of the start of her next spontaneous menses to take a single OC tablet. A study coordinator directly observed OC intake on days 1, 2, 3, 4, 7, 21, and 60 and instructed participants to take each OC at the same time using daily reminders.
Participants made 10 study visits over 9 weeks. The 5 study visits of interest here occurred on OC cycle day 1 (single-dose 1 [SD1]), cycle days 2 and 3, day 21 (steady-state [SS]), and the visit for a single OC tablet at approximately day 60 after study entry (single-dose 2 [SD2]). Participants underwent multiple timed venous blood collections on days 1, 21, and 60. With the use of an indwelling catheter, 13 samples were collected at 0, 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 12, 16, and 24 hours (t0, t0.5, … t24) after OC administration on day 1 and day 21. On day 60, we collected the first 8 specimens up to 4 hours and additional specimens at 24, 48, and 72 hours following the OC. Each OC administration occurred immediately after the t0 blood draws. Samples were allowed to clot for 30 minutes at room temperature, centrifuged at 3000 rpm at 4°C for 10 minutes, and stored in 1 mL aliquots at -80°C.
Corticosteroid-binding globulin (CBG) was measured to assess long-term participant compliance with OC use during the study [13]. LNG is tightly bound to sex-hormone-binding globulin (SHBG). We measured SHBG in specimens collected at t0 on days 1, 21, and 60 to study whether the PK of non-SHBG-bound LNG might differ significantly from the PK of total LNG.
Laboratory Methods
The Irving Institute for Clinical and Translational Research (IICTR) laboratory measured serum LNG by ultra-performance liquid chromatography-tandem mass spectrometry after liquid-liquid extraction. The in-house developed method, using deuterated LNG as internal standard, is described in detail in the Appendix. Intra- and inter-assay coefficients of variation are 3% and 6%, respectively. The assay is linear between 0.05 and 100 ng/mL and has a lower limit of quantification of 0.05 ng/mL.
CBG was measured using a radioimmunoassay kit (IBL-America, Minneapolis, MN, USA). Assay sensitivity is 0.26 μg/mL with intra- and inter-assay coefficients of variation of 8.6% and 8.7%, respectively. Normal range is 40-154 μg/mL. We measured SHBG using a chemiluminescence immunoassay (CLIA) on an automated immunochemistry analyzer (Immulite 1000, Siemens Healthcare Diagnostics Inc., Deerfield, IL, USA). Assay sensitivity is 0.2 nmol/L with intra- and inter-assay coefficients of variation of 2.4% and 2.5%, respectively. Normal range is 13-114 nmol/L.
Pharmacokinetic Analysis
PK analyses were conducted using the STATA14 (Stata Corporation, College Station, TX, USA) non-compartmental analysis procedure, pkexamine, with the trapezoidal rule [11]. The AUC estimates at single-dose 1 and steady-state using all samples are noted as AUCSD1, 0-24 and AUCSS, 0-24. The 9-sample AUCs at SD1, SD2 and SS ignore samples taken at time points > 4 and < 24 hours, and are noted as AUCSD1, 0-4-24, AUCSD2, 0-4-24 and AUCSS, 0-4-24 respectively. The 6-sample AUCs used values from t0, t1, t2, t3, t4, and t24. LNG concentrations are denoted as C0, … C24 at t0, … t24. As our aim was to determine whether calculations from limited samples could serve as a proxy for systemic exposure, we chose these time points for their ease of use in larger studies.
We calculated non-SHBG-bound LNG concentration using Södergard' method [14] based on the law of mass action with the SHBG and albumin binding affinities given by Nilsson [15]. Non-SHBG-bound LNG concentration is a multiple of calculated free LNG concentration [14]. Using this method of estimating non-SHBG-bound estradiol was highly correlated with measured values in large collaborative studies [16].
Statistical Methods
The validity of steady-state (day 21) PK parameter estimates depends on whether previous pills were taken close to 24 and 48 hours before taking the day 21 pill. At steady-state, LNG values immediately before taking the day 21 pill (C0) should be roughly equal to the values obtained 24 hours later (C24); thus, we excluded three women whose LNG and EE2 [11] concentrations at C0 were substantially different from their concentrations at C24. The exclusions were based on a standard Z-score approach with the resistant median absolute deviation estimate of the underlying variability (drastically reducing the effect of any outliers); a modified z-score > 4 was considered an outlier [17]. We used this approach because we did not directly observe the time of all tablet intake and elevated hormone concentrations strongly suggested [11] that these participants had not been compliant in timing their prior tablet. To evaluate the possible consequences of these exclusions, we repeated the correlation analyses using all 17 participants.
Ninety-five percent confidence intervals (95% CIs) and 2-sided significance values (P values) of the Pearson correlation coefficients (r values) were calculated based on Fisher' z transformation of r.
Results
Seventeen normal-weight (mean BMI 22.6 kg/m2) white women ages 18-34 (mean 24.9) participated in the study [11]; one participant missed the day 60 visit while others completed all scheduled visits. All participants experienced at least a 45 μg/mL increase in CBG from baseline (geometric mean, ± SD: 48.2 μg/mL, [33.0, 70.4]) to day 21 (138.4 μg/mL, [114.4, 167.5]) and a return to baseline concentrations at day 60 (58.6 μg/mL, [52.8, 65.0]), consistent with good protocol compliance [13].
Table 1 shows the PK results for the 14 timing-compliant participants. As expected, AUC, Cmax, and C24 estimates were all higher at steady-state than after SD1. Trough levels (C24) between single-doses (SD1 and SD2) were correlated (r = 0.74; 95% CI [0.34, 0.91]), and AUCSD1, 0-24 was highly correlated with AUCSD2, 0-4-24 (r = 0.84; 95% CI [0.56, 0.95]).
Table 1. Oral contraceptive LNG pharmacokinetic parameter estimates of single-dose vs. steady-state (n = 14).
| Parametera | Single-dose 1 | Single-dose 2b | Steady-statec | Notes |
|---|---|---|---|---|
| AUC0-24, ng h/Ml | 14.8 (10.1, 21.8) | ———— | 59.4 (43.5, 81.1) | AUC from t0–t24 using 13 measurements: geometric mean ± standard deviation |
| AUC0-inf, ng h/mL | 29.9 (16.3, 54.9) | ———— | ———— | AUC from t0-tinf using 13 measurements: geometric mean ± standard deviation |
| AUC0-4-24, ng h/mL | 18.9 (11.9, 30.2) | 18.2 (12.8, 25.9) | 66.1 (49.8, 87.8) | AUC from t0–t24 using 9 measurements: geometric mean ± standard deviation |
| Cmax, ng/mL | 2.1 (1.4, 3.3) | 2.0 (1.3, 3.2) | 5.0 (3.8, 6.6) | Maximum LNG concentration: geometric mean ± standard deviation |
| Time at Cmax, h | 1.75 (1, 3) | 1.5 (1, 4) | 1.5 (1, 2.5) | Time at maximum concentration: median and interquartile range |
| Elimination half-life, h | 27.2 (15.8, 47.1) | ———— | 43.1 (16.8, 110.9) | Elimination half-life calculated using concentrations at 12, 16, and 24 hours: geometric mean ± standard deviation |
| C24, ng/mL at t24 | 0.3X (0.2X, 0.6X) | 0.3X (0.2X, 0.5X) | 1.75 (1.1X, 2.8X) | Concentration at 24 hours: geometric mean ± standard deviation |
Table 2 shows correlations between AUCSS, 0-24 and several PK calculations using reduced sampling strategies at steady state. We found strong correlations (r = 0.94; 95% CI [0.82, 0.98]) for AUCSS calculations made using 9 or 6 measurements. However, the single steady-state trough concentration, C24, was just as highly correlated with AUCSS, 0-24 (r = 0.95; 95% CI [0.85, 0.98]; Figure 1A), as were correlations from single time-point concentrations at 6, 8, 12, and 16 hours (Table 2).
Table 2. Correlation between reduced-sampling estimates of LNG PK parameters at steady state and 13-sample steady-state AUCSS, 0-24.
| Parameters | na | kb | r (95% Confidence Interval)c | P value |
|---|---|---|---|---|
| AUCd | ||||
| Steady-state AUCSS, 0-4-24 | 14 | 9 | 0.94 (0.82—0.98) | <.0001 |
| Steady-state AUCSS, 0-4-24 | 14 | 6 | 0.94 (0.82—0.98) | <.0001 |
| Steady-state C6 | 14 | 1 | 0.94 (0.82—0.98) | <.0001 |
| Steady-state C8 | 14 | 1 | 0.92 (0.76—0.97) | <.0001 |
| Steady-state C12 | 14 | 1 | 0.95 (0.85—0.98) | <.0001 |
| Steady-state C16 | 14 | 1 | 0.93 (0.79—0.98) | <.0001 |
| Steady-state C24 | 14 | 1 | 0.95 (0.85—0.98) | < .0001 |
No. of participants used in calculation.
No. of LNG measures used per participant.
Pearson correlation coefficient.
Figure 1.
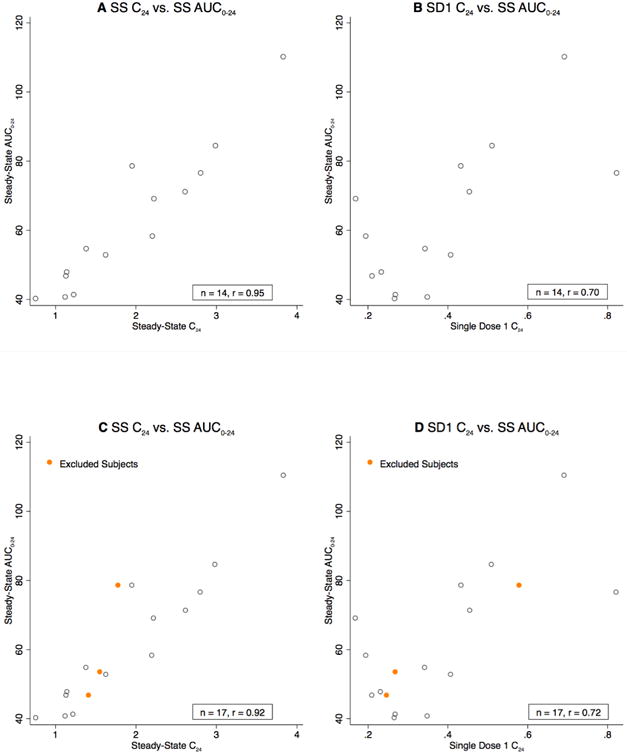
Title- Correlations between trough values (C24) and steady-state AUC Captions- 1A. Steady-state trough values (C24) versus steady-state AUCSS, 0-24 values (timing compliant). 1B. Single-dose trough values (C24) versus steady-state AUCSS, 0-24 values (timing compliant). 1C. Steady-state trough values (C24) versus steady-state AUCSS, 0-24 values (all women). 1D. Single-dose trough values (C24) versus steady-state AUCSS, 0-24 values (all women).
Table 3 shows correlations between AUCSS, 0-24 and several PK values after a single dose. We found only a moderate correlation for AUCSD1, 0-24 (r = 0.58; 95% CI [0.07, 0.85]) using all 13 measurements. The correlation was improved by using only the trough value (C24) after single-dose 1 (r = 0.70; 95% CI [0.27, 0.90]; Figure 1B). The correlation using single-dose 2 trough concentration was 0.67 (95% CI [0.22, 0.89]) and averaging trough levels from single-dose 1 and single-dose 2 showed a similar correlation (r = 0.73; 95% CI [0.33, 0.91]). Trough concentrations measured after administration of two consecutive pills produced a slightly increased correlation: r = 0.77 (95% CI [0.40, 0.92]). We also examined other limited sampling strategies to calculate AUC including taking averages of LNG values later in the SD1 curve, but none of these improved on SD1 trough values alone (data not shown).
Table 3. Correlation between single-dose reduced-sampling estimates of LNG PK parameters and 13-sample steady-state AUCSS, 0-24.
| Parameters | N | k | r (95% CI) | P value |
|---|---|---|---|---|
| Single-dose 1 AUCSD1, 0-24 | 14 | 13 | 0.58 (0.07—0.85) | 0.028 |
| Single-dose 1 C24 | 14 | 1 | 0.70 (0.27—0.90) | 0.0040 |
| Single-dose 2 C24 | 14 | 1 | 0.67 (0.22—0.89) | 0.0092 |
| Single-doses 1 & 2 C24a | 14 | 2 × 1 | 0.73 (0.33—0.91) | 0.0021 |
| Trough after two doses | 14 | 1 | 0.77 (0.40—0.92) | 0.0007 |
Mean of estimated parameters.
We performed a sensitivity analysis of our outlier exclusions using all 17 women and found all correlations calculated using the full sample were equal to or greater than those calculated using only 14 timing-compliant women (data not shown). Figures 1C and 1D show correlations between steady-state trough level, single-dose 1 trough level, and AUCSS, 0-24 for all 17 women.
Table 4 shows the correlations between AUC estimates, trough concentrations, and SHBG values at t0 of day 1 (SD1) and at t0 of day 21 (SS). Baseline SHBG concentrations (geometric mean, ± SD: 44.8 nmol/L, [32.7, 61.4]) were highly correlated with both LNG AUCSD1, 0-24 and LNG SD1 C24, but moderately correlated with LNG AUCSS, 0-24 and LNG SS C24. Steady-state SHBG (77.5 nmol/L, [55.7, 107.7]) was moderately correlated with LNG AUCSS, 0-24 and LNG SS C24. SHBG geometric mean concentrations increased by 65% during one treatment cycle (from SD1 to SS), but the within-woman correlation between SD1 and SS SHBG values was weak and not statistically significant (r = 0.44; 95% CI [-0.12, 0.79]). We reran all PK analyses presented in Tables 2 and 3 using calculated non-SHBG-bound LNG values (using SHBG at baseline and at steady state) rather than total LNG values; we found no substantial differences in the correlations.
Table 4. Correlation between PK parameters and SHBG concentrations.
| Parameters | SHBG at Baseline | SHBG at Steady-State |
|---|---|---|
| LNG Single Dose 1 AUCSD1, 0-24 | 0.95 (0.85—0.98) | ———— |
| LNG Single Dose 1 C24 | 0.81 (0.49—0.94) | ———— |
| LNG Steady-state AUCSS, 0-24 | 0.53 (-0.01—0.83) | 0.61 (0.12—0.86) |
| LNG Steady-state C24 | 0.47 (-0.08—0.80) | 0.61 (0.12—0.86) |
Discussion
This study shows that LNG single time-point concentrations at steady state are highly correlated with the ‘gold-standard’ 13-measurement AUCSS, 0-24 (0.92 ≤ r ≤ 0.95). These correlations are comparable to correlations for the immunosuppressive drug tacrolimus in stable solid organ transplantation patients for whom these correlations are used clinically to assess systemic exposure in order to prevent rejection and side effects [18, 19]. These correlations are comparable to the relationship shown between LNG trough values and AUC in a prior study on the effect of smoking on LNG absorption [20]. Additionally, using steady-state data from a previous PK study of the same OC formulation in normal-weight and obese women [21], we found that the C24 trough values were highly correlated with the AUC values for both normal weight (r = 0.96; 95% CI [0.87, 0.99]; n = 13) and obese women (r = 0.98; 95% CI [0.94, 0.99]; n = 15), and the relationship was the same in both groups.
As previously argued [11], a single well-timed value at steady-state as a proxy for systemic exposure can facilitate large pharmaco-epidemiologic studies with oral contraceptives by drastically reducing the number of blood samples necessary and eliminating overnight stays although at a cost of requiring an increase in number of subjects (see below). Because PK parameters are highly sensitive to accurate timing of drug administration, administering the penultimate as well as the steady-state OC tablet to study participants would in all likelihood yield more highly correlated results when using single time-point values as a proxy for systemic exposure.
The correlation of single-dose trough values with full curve, 13-sample SS AUC measurements indicates one can estimate steady-state LNG exposure sufficient for use in epidemiological studies without prolonged administration of an OC to reach steady state. This may facilitate large practical studies, particularly to study women who have had a serious adverse event during past OC use. This approach would permit estimation of steady-state exposure after administration of no more than two tablets, which may allow safe study of women who should not receive ongoing OC exposure [22].
Assessing steady state systemic exposure using limited sampling strategies has a cost in precision; such studies would need to have more participants than those using 13-measures at steady state: To achieve the same statistical precision, the sample size would need to be increased by a factor of 1/r2 [11]. The higher the correlation, the smaller the necessary increase in sample size. Using the trough value after two tablets (r = 0.77) rather than after a single tablet (r = 0.70) would require a 69% increase in required sample size (1 - 1/0.772) versus a 104% increase in required sample size (1 – 1/0.702).
Baseline SHBG concentrations were highly correlated with SD1 LNG AUC (r = 0.95), but this correlation decreased during continued use of the OC and further analyses of the relationship between EE2 AUC and LNG AUC did not identify SHBG as having an important role in explaining the lack of association. Further research is necessary to understand how concurrently administered LNG and EE2 in this formulation affect each other' metabolism through SHBG.
Limitations of this study include its relatively small sample size and the narrow sample characteristics. We expect studies of other narrowly defined groups would yield comparable results; however, heterogeneous samples would require further study [23]. Another limitation was that we did not observe all pill intakes which presumably led to the observed deviations and may have contributed to the variability in the results. The correlations reported here might be specific to this OC formulation and dose, and require confirmation before they can be generally accepted. We also lack data to determine the intra-subject variability in AUC at steady state, which may be relevant to accurately assess overall systemic exposure.
No studies, to the best of our knowledge, have evaluated the prevalence of OC side effects according to a user' individually measured systemic exposure to the estrogen and progestin. Given the wide variation in bioavailability of these contraceptive hormones, evaluating risk based on systemic exposure may lead to a better understanding of the factors that lead to adverse outcomes. Using full 24-hour steady-state pharmacokinetic assessment is impractical when investigating larger numbers of women in pharmaco-epidemiologic studies. Use of a highly correlated proxy, such as trough level at steady-state (SS C24; r = 0.95; 95% CI [0.85, 0.98]) presents a more efficient alternative. Furthermore, use of correlated trough levels after just two doses of combined oral contraceptives may permit the safe estimation of systematic LNG exposure at steady state for women who are at a high risk of serious adverse events.
Implications.
A single time-point LNG concentration at steady state is an excellent proxy for complete and resource-intensive steady-state AUC measurement. The trough level after two single doses is a fair proxy for steady-state AUC. These results provide practical tools to facilitate large studies to investigate the relationship between systemic LNG exposure and side effects in a real-life setting.
Acknowledgments
We thank the following colleagues at CUMC: Biomarkers Core Laboratory staff at the IICTR for serum analyses, in particular, May Huang, Susan Pollack, Tiffany Thomas, and Roseann Zott; Mary-Jane McEneaney, DNP, Arielle Rodman, MD, and Da Li for assistance with study visits; and Rosalind Tang, Monica Sull, and Marianne DiNapoli for their prior pharmacokinetic work estimating ethinyl estradiol exposure from this study.
Financial support: This pilot study was funded by the Howard Solomon Research Fund and an Irving Institute for Clinical and Translational Research (IICTR) Collaborative and Multidisciplinary Pilot Research (CaMPR) Award. The Biomarkers Core Laboratory of the IICTR supported the assays. M.C.P. is partially supported by the NCI under award number P30CA008748 (PI: C. Thompson) to Memorial Sloan Kettering Cancer Center. This study was also supported by the National Center for Advancing Translational Sciences, National Institutes of Health (NIH), through Grant Number UL1 TR000040, formerly the National Center for Research Resources, Grant Number UL1 RR024156. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Appendix: Levonogestrel Liquid Chromatography – Tandem Mass Spectrometry (LC-MS/MS)
Materials
Levonorgestrel and deuterated levonorgestrel were purchased from Toronto Research Chemicals (Toronto, Ontario, Canada). All the solvents used for LC-MS/MS analysis were of LCMS grade.
Sample preparation
Levonorgestrel was extracted from serum samples using liquid-liquid extraction by mixing 250 μL of serum with 6 mL of hexane: dichloromethane mixture (3:2), vortexed for 10 min at 2000 rpm followed by centrifugation for 10 min at 2500 rpm. The organic layer was decanted and evaporated after the aqueous phase was frozen in a liquid nitrogen bath. The extract was resuspended in 1 mL of dichloromethane, transferred to a LCMS vial, evaporated under nitrogen stream and resuspended in 45% methanol. Calibration standards and QC samples were prepared by spiking blank human serum with levonorgestrel spanning a range of 50 pg/mL to 100 ng/mL and extracted same as the samples. Deuterated (D7) levonorgestrel was used as the internal standard and was spiked at a level of 5 ng/mL in all the calibration standards and samples prior to extraction.
Lc-Ms/Ms
LC-MS/MS analysis was performed on a platform comprising a triple quadrupole API 4000 mass spectrometer (SCIEX, Foster City, CA, USA) equipped with an electrospray ionization source operated in the positive ion mode and integrated with an Eksigent UltraLC system controlled by Analyst 1.6 (Sciex, Foster City, CA, USA). LC was performed on a Phenomenex Kinetex C18 (Torrance, CA, USA) column (50×2.1 mm, 1.7 μ, 100° A) maintained at 40°C. The flow rate was maintained at 300 μL/min. The initial flow conditions were 45% solvent A (water containing 0.1% formic acid) and 55% solvent B (Acetonitrile with 0.1% formic acid). Solvent B was raised to 95% linearly over 2 min and held for 30 sec and back to initial conditions by 3 min and held constant until 5 min. The operating conditions for the mass spectrometer were as follows: ion spray voltage: 3000V, capillary temperature: 500°C, nebulizer gas: 30 psi, and heater gas: 55 psi. For quantitation, multiple reaction monitoring (MRM) was utilized with the transition 313.22-109.00 (collision energy 35 V) for levonorgestrel. Data acquisition, peak integration and quantitation was performed with Analyst 1.6 software (Sciex, Foster City, CA, USA).
Footnotes
Competing Interest Statement: C.L.W. is a paid consultant to Merck and Bayer, both of which manufacture oral contraceptives; however, not the oral contraceptive evaluated in this study. The remaining authors report no financial relationships with any organizations that might have an interest in the submitted work and no other relationships or activities that could appear to have influenced the submitted work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Humpel M, Wendt H, Pommerenke G, Weib C, Speck U. Investigations of pharmacokinetics of LNG to specific consideration of a possible first-pass effect in women. Contraception. 1978;17:207–20. doi: 10.1016/0010-7824(78)90012-4. [DOI] [PubMed] [Google Scholar]
- 2.Back DJ, Bates M, Breckenridge AM, Hall JM, MacIver M, Orme ML, et al. The pharmacokinetics of levonorgestrel and ethynylestradiol in women—studies with Ovran and Ovranette. Contraception. 1981;23:229–39. doi: 10.1016/0010-7824(81)90045-7. [DOI] [PubMed] [Google Scholar]
- 3.Fotherby K. Pharmacokinetics of gestagens: some problems. Am J Obstet Gynecol. 1990;163:323–8. doi: 10.1016/0002-9378(90)90576-s. [DOI] [PubMed] [Google Scholar]
- 4.Fotherby K. Potency and pharmacokinetics of gestagens. Contraception. 1990;41:533–50. doi: 10.1016/0010-7824(90)90062-z. [DOI] [PubMed] [Google Scholar]
- 5.de Bastos M, Stegeman BH, Rosendaal FR, Van Hylckama Vlieg A, Helmerhorst FM, Stijnen T, et al. Combined oral contraceptives: venous thrombosis. Cochrane Database Syst Rev. 2014;3:CD010813. doi: 10.1002/14651858.CD010813.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Royer PA, Jones KP. Progestins for contraception: modern delivery systems and novel formulations. Clin Obstet Gynecol. 2014;57:644–58. doi: 10.1097/GRF.0000000000000072. [DOI] [PubMed] [Google Scholar]
- 7.Warren AM, Gurvich C, Worsley R, Kulkarni J. A systematic review of the impact of oral contraceptives on cognition. Contraception. 2014;90:111–6. doi: 10.1016/j.contraception.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 8.Lawrie TA, Helmerhorst FM, Maitra NK, Kulier R, Bloemenkamp K, Gulmezoglu AM. Types of progestogens in combined oral contraception: effectiveness and side-effects. Cochrane Database Syst Rev. 2011;5:CD004861. doi: 10.1002/14651858.CD004861.pub2. [DOI] [PubMed] [Google Scholar]
- 9.Goldzieher JW, Stanczyk FZ. Oral Contraceptives and individual variability of circulating levels of ethinyl estradiol and progestins. Contraception. 2008;78:4–9. doi: 10.1016/j.contraception.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 10.Koup JR. Single-point prediction methods: a critical review. Drug Intell Clin Pharm. 1982;16:855–62. doi: 10.1177/106002808201601108. [DOI] [PubMed] [Google Scholar]
- 11.Westhoff CL, Pike MC, Tang R, DiNapoli MN, Sull M, Cremers S. Estimating systemic exposure to ethinyl estradiol from an oral contraceptive. Am J Obstet Gynecol. 2015;212:614.e1–614.e7. doi: 10.1016/j.ajog.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. Reproductive Health and Research. 4th. Geneva: World Health Organization; 2010. Medical eligibility criteria for contraceptive use; pp. 15–43. [Google Scholar]
- 13.Westhoff CL, Petrie KA, Cremers S. Using changes in binding globulins to assess oral contraceptive compliance. Contraception. 2013;87:176–81. doi: 10.1016/j.contraception.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Södergard R, Backstrom Y, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem. 1982;16:801–10. doi: 10.1016/0022-4731(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 15.Nilsson B, von Shoultz B. Binding of LNG, norethisterone and desogestrel to human sex hormone binding globulin and influence on free testosterone levels. Gynecol Obstet Invest. 1989;27:151–4. doi: 10.1159/000293644. [DOI] [PubMed] [Google Scholar]
- 16.Endogenous Hormones and Breast Cancer Collaborative Group. Free estradiol and breast cancer risk in postmenopausal women: comparison of measured and calculated values. Cancer Epidemiol Biomarkers Prev. 2003;12:1457–61. [PubMed] [Google Scholar]
- 17.Iglewicz B, Hoaglin DC. How to detect and handle outliers: the American Society for Quality Basic References in Quality Control: statistical techniques. Milwaukee, WI: Quality Press; 1993. [Google Scholar]
- 18.Braun F, Peters B, Schütz E, Lorf T, Undre N, Oellerich M, Ringe B. Therapeutic drug monitoring of tacrolimus early after liver transplantation. Transplant Proc. 2002;34:1538–9. doi: 10.1016/s0041-1345(02)03010-5. [DOI] [PubMed] [Google Scholar]
- 19.Scholten EM, Cremers SC, Schoemaker RC, Rowshani AT, van Kan EJ, de Hartigh J, et al. AUC-guided dosing of tacrolimus prevents progressive systemic overexposure in renal transplant recipients. Kidney Int. 2005;67:2440–7. doi: 10.1111/j.1523-1755.2005.00352.x. [DOI] [PubMed] [Google Scholar]
- 20.Kanarkowsi R, Tornatore KM, D'Ambrosio R, Gardner MJ, Jusko WJ. Pharmacokinetics of single and multiple doses of ethinyl estradiol and levonorgestrel in relation to smoking. Clin Pharmacol Ther. 1988;43:23–31. doi: 10.1038/clpt.1988.7. [DOI] [PubMed] [Google Scholar]
- 21.Westhoff CL, Torgal AH, Mayeda ER, Pike MC, Stanczyk FZ. Pharmacokinetics of a combined oral contraceptive in obese and normal-weight women. Contraception. 2010;81:474–80. doi: 10.1016/j.contraception.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bloemenkamp KWM, Rosendaal FR, Helmerhorst FM, Koster T, Bertina RM, Vandenbroucke JP. Hemostatic effects of oral contraceptives in women who developed deep-vein thrombosis while using oral contraceptives. Thromb Haemost. 1998;80:382–7. [PubMed] [Google Scholar]
- 23.Xie HG, Kim RB, Wood AJJ, Stein CM. Molecular basis of ethnic differences in drug disposition and response. Ann Rev Pharmacol Toxicol. 2001;41:815–50. doi: 10.1146/annurev.pharmtox.41.1.815. [DOI] [PubMed] [Google Scholar]


