Abstract
McCune-Albright Syndrome (MAS) is a rare sporadic syndrome caused by post-zygotic mutations in the GNAS oncogene, leading to constitutional mosaicism for these alterations. Somatic activating GNAS mutations also commonly occur in several gastrointestinal and pancreatic neoplasms, but the spectrum of abnormalities in these organs in patients with MAS has yet to be systematically described. We report comprehensive characterization of the upper gastrointestinal tract in seven patients with MAS and identify several different types of polyps, including gastric heterotopia/metaplasia (7/7), gastric hyperplastic polyps (5/7), fundic gland polyps (2/7), and a hamartomatous polyp (1/7). In addition, one patient had an unusual adenomatous lesion at the gastroesophageal junction with high-grade dysplasia. In the pancreas, all patients had endoscopic ultrasound findings suggestive of intraductal papillary mucinous neoplasm (IPMN), but only 2 patients met the criteria for surgical intervention. Both of these patients had IPMNs at resection, one with low-grade dysplasia and one with high-grade dysplasia. GNAS mutations were identified in the majority of lesions analyzed, including both IPMNs and the adenomatous lesion from the gastroesophageal junction. These studies suggest that there is a broad spectrum of abnormalities in the gastrointestinal tract and pancreas in patients with MAS, and that patients with MAS should be evaluated for gastrointestinal pathology, some of which may warrant clinical intervention due to advanced dysplasia.
Keywords: McCune Albright Syndrome, GNAS, intraductal papillary mucinous neoplasm, gastric heterotopia
Introduction
McCune-Albright Syndrome (MAS) is defined by a clinical spectrum including skeletal, endocrine, and skin abnormalities–specifically, fibrous dysplasia of bone, hyperfunctioning endocrinopathies (such as precocious puberty), and café au lait macules [1]. This rare sporadic syndrome is caused by somatic (post-zygotic) activating mutations in GNAS, the gene coding for the G protein-α stimulatory subunit, leading to ligand-independent signaling, elevated cAMP levels, and activation of downstream signaling pathways [2]. The same GNAS mutations are also found as somatic alterations in a number of sporadic tumor types.
Somatic activating mutations at codon 201 (the same codon targeted in patients with MAS) have been reported in several gastrointestinal and pancreatic neoplasms, including intraductal papillary mucinous neoplasms (IPMNs) of the pancreas and pyloric gland adenomas (PGAs) of the stomach [3,4]. This genetic similarity raises the possibility that patients with MAS are also at increased risk for gastrointestinal and pancreatic neoplasms, which has been confirmed by reports of gastrointestinal and pancreatic abnormalities in patients with MAS. A small series of four patients with MAS revealed hamartomatous duodenal polyps, and pancreatic abnormalities were identified radiologically in approximately 20% of patients with MAS, with most believed to be IPMNs [5,6].
In the current study, we report thorough upper endoscopic examination of seven patients with MAS, all selected for evaluation due to pancreatic cysts on magnetic resonance cholangiopancreatography (MRCP). Examination revealed a variety of polyps throughout the esophagus, stomach, and small intestine. In addition, all patients underwent endoscopic ultrasound (EUS) of their pancreas, revealing that all patients had EUS abnormalities suggestive of IPMN, and two of these patients underwent resection of IPMN due to worrisome radiologic features.
Methods
Patients and Samples
As part of a screening protocol at the National Institutes of Health, patients with MAS were screened for pancreatic lesions using magnetic resonance cholangiopancreatography (MRCP). All patients gave informed consent, and the protocol was approved by the Institutional Review Board of the National Institute of Dental and Craniofacial Research. The seven patients with pancreatic cysts on MRCP were referred to the Johns Hopkins Hospital for endoscopic evaluation. The clinical features of MAS in the patient cohort are described in Table 1. At upper endoscopy, biopsies were taken from the normal esophagus, stomach, and duodenum, as well as from any visible lesions. Endoscopic ultrasound (EUS) was performed, and cyst fluid was sampled for cysts >1.5cm. Hematoxylin-and-eosin stained sections of all biopsies were reviewed by two gastrointestinal pathologists (LDW and EAM) in order to reach a consensus diagnosis. Resection of gastrointestinal and pancreatic lesions was performed as clinically indicated, and all sections from resected specimens were reviewed.
Table 1.
Clinical manifestations of McCune-Albright Syndrome in patient cohort
| Patient | Fibrous Dysplasia | Café Au Lait Macules | Precocious Puberty | Hyperthyroidism | Hypophosphatemia | Neonatal Cushing’s Syndrome | GNAS Mutation for Initial Diagnosis |
|---|---|---|---|---|---|---|---|
| 1 | Yes | Yes | No | Yes | Yes | No | R201C |
| 2 | Yes | Yes | Yes | No | Yes | No | Not done |
| 3 | Yes | Yes | Yes | No | No | No | Not done |
| 4 | Yes | Yes | No | Yes | No | No | Not done |
| 5 | Yes | Yes | No | Yes | No | Yes | R201H |
| 6 | Yes | Yes | Yes | Yes | Yes | Yes | Not done |
| 7 | Yes | Yes | No | No | No | No | R201C |
Immunohistochemistry
A single section of each IPMN was analyzed for expression of MUC1, MUC2, MUC5, and CDX2 in order to determine the direction of differentiation. Immunostaining with MUC1, MUC2 and MUC5 antibodies was performed on an automated instrument (XT BenchMark, ROCHE-Ventana Medical Systems, Inc. Tucson, AZ). Sections were deparaffinized and hydrated and antigen retrieval with a high pH buffer (CC1 standard) was performed. Incubation with primary antibody was performed, followed by a biotin labeled secondary antibody and chromogenic substrate as per manufacturer’s instructions (I-View detection, 790-091. ROCHE-Ventana Medical Systems, Inc. Tucson, AZ). Immunostaining for CDX2 was developed on Bond-Leica automated instrument (Leica Microsystems, Bannockburn, IL). Following deparaffinization and heat induced antigen retrieval, primary and secondary antibody incubation steps were performed. Immunoreactivity was visualized using DAB chromogen and a hematoxylin counterstain was applied to all tissue sections. Sections were incubated with the anti-MUC1 and anti-MUC5 antibodies (Vector Lab, Burlingame, CA) for 44 minutes at 1:100 dilution, with the anti-MUC2 antibody (NCL, LEICA, Bannockburn, IL) for 32 minutes at 1:50 dilution and with the anti-CDX2 antibody (Agilent Technologies, pre-diluted) for 15 minutes.
GNAS Pyrosequencing
On the basis of previously published findings, GNAS mutational analysis was restricted to the codon 201 hot spot contained within exon 8 [7]. Mutations in codon 227 have been described in less than 5% of fibrous dysplasia cases [8]. For small lesions, 10 serial sections of formalin-fixed paraffin-embedded (FFPE) tissue were cut onto membrane slides. After deparaffinization in xylene, rehydration in decreasing concentrations of ethanol and staining with hematoxylin, lesional cells were captured by laser capture microdissection (LMD7000, Leica, Wetzlar, Germany). Tissue from larger lesions was cut onto coated glass-slides, deparaffinized, rehydrated and the lesional cells were scraped from these slides under a dissection microscope. When sufficient neighboring normal tissue was present, this was also selected for further analysis. DNA extraction was performed using the QIAamp DNA FFPE Tissue Kit (Qiagen, Valencia, CA, USA) according the manufacturer’s instructions. Mutations in codon 201 of GNAS were assessed by pyrosequencing according the manufacturer’s instructions as previously described [9]. In brief, extracted DNA was amplified on the Veriti Thermal Cycler (Applied Biosystems, Foster City, CA, USA), with the following primers: forward primer, 5′-CCAGACCTTTGCTTTAGATTGG- 3′ and reverse primer, 5′-biotin- TCCACCTGGAACTTGGTCTC-3′ and using the PCR SuperMix High Fidelity (Invitrogen, Carlsbad, CA, USA). PCR conditions were as follows: 95°C × 15 minutes; 38 cycles of 95°C × 20 seconds, 53°C × 30 seconds, 72°C × 20 seconds; 72°C × 5 minutes. Biotinylated PCR products were bound to streptavidin-coated sepharose beads (Streptavidin Sepharose High Performance, GE Health Care Bio-Sciences Corp, Piscataway, NJ, USA). After washing, the beads with PCR-products were released in wells with the sequencing primer, 5′-TTTGTTTCAGGACCTGCTTCGC- 3′, and annealing buffer. Sequencing was done with the PyroMark Q24 (Qiagen), using the Pyromark Gold reagents (Qiagen) [10].
Next Generation Sequencing
Digital next generation sequencing on duodenal fluid samples was performed on the Ion Torrent PGM as previously described [11]. Targeted next generation sequencing was performed on cyst fluid samples. Briefly, DNA was extracted from 200uL of cyst fluid, and 4ng of cyst fluid DNA was analyzed in duplicate. The target region was amplified using Ampliseq reagents according to the manufacturer’s instructions. The gene panel contained the hotspot regions of KRAS, GNAS, BRAF, PIK3CA as well as the entire coding regions of TP53, CDKN2A, SMAD4, RNF43, ARID1A, FBXW7, TGFBR2, VHL. Variants were identified by the NextGENe software (SoftGenetics) as previously described [11].
Results
The MAS patients in our cohort were selected for endoscopic evaluation due to the presence of pancreatic abnormalities on magnetic resonance cholangiopancreatography (MRCP). All seven patients had multiple branch duct IPMNs (>5), and more than ten cysts were identified in five of the seven patients scattered throughout the head, body and tail of the pancreas (average size of the largest cyst: 22mm, range: 9–30 mm). In addition, five of seven patients also had main duct IPMNs–one had diffuse involvement of the main duct, while the other four had segmental involvement (average size of the pancreatic duct with main duct IPMN: 5.4 mm, range: 5–9 mm). Pancreatic glandular atrophy was present in four of the five patients with main duct IPMNs. There were no mural nodules or pancreatic masses seen on contrast enhanced MRI and MRCP. Pancreatic EUS was performed at the time of upper endoscopy and also demonstrated multiple pancreatic cysts in each of the seven patients.
In the upper gastrointestinal tract of all seven patients polyps were identified (Table 2). The most common lesion was heterotopic gastric mucosa (polypoid zones of non-neoplastic appearing oxyntic and/or foveolar type mucin outside of the stomach), present in at least one location in the esophagus or small bowel in all seven patients. Of note, the absence of criteria to definitively distinguish gastric heterotopia from gastric metaplasia did not allow us to make this distinction in these samples, although the lack of mucosal injury in the adjacent tissues near the biopsied sites suggested heterotopia rather than metaplasia. In addition, gastric hyperplastic-type polyps were also common, occurring in 5/7 (71%) patients. They were unusual in that they were not associated with gastritis of either the environmental or autoimmune type. Fundic gland polyps were identified in two of the seven (29%) patients, while a single patient (14%) had a hamartomatous polyp of the ampulla of Vater presenting features similar but not identical to those of Peutz Jeghers polyps, with cords of smooth muscle partitioning off nests of mucosa with lamina propria (Figure 1). This lesion differed from a classic Peutz Jeghers polyp as it featured less smooth muscle and more inflammation than a typical Peutz Jeghers polyp. One patient (14%) had intestinal metaplasia at the gastroesophageal junction.
Table 2.
Gastrointestinal and pancreatic lesions in patients with McCune-Albright Syndrome
| Patient | Age* | Location | Diagnosis | GNAS-lesional tissue | GNAS-normal tissue |
|---|---|---|---|---|---|
| 1 | 20 | Proximal esophagus | Gastric heterotopia/metaplasia | R201C | – |
| Stomach body | Fundic gland polyp | WT | – | ||
| Major papilla | Hamartomatous-type polyp | WT | WT | ||
| Minor papilla | Gastric heterotopia/metaplasia | WT | – | ||
|
| |||||
| 2 | 22 | Proximal stomach | Gastric hyperplastic polyps | WT | – |
| Duodenal bulb | Gastric heterotopia/metaplasia | R201C | WT | ||
| Duodenum | Gastric heterotopia/metaplasia | WT | WT | ||
| Ampulla | Gastric heterotopia/metaplasia | WT | – | ||
|
| |||||
| 3 | 46 | Gastroesophageal junction | Gastric hyperplastic polyp | R201H | – |
| Duodenum | Gastric heterotopia/metaplasia | R201H | – | ||
|
| |||||
| 4 | 55 | Proximal esophagus | Gastric heterotopia/metaplasia | WT | WT |
| Proximal stomach | Multiple adenomatous lesions with high grade dysplasia# | R201C | – | ||
| Stomach | Fundic gland polyps | WT | – | ||
| Stomach body | Foveolar hyperplasia | R201C | – | ||
| Duodenum | Gastric heterotopia/metaplasia | WT | – | ||
| Ampulla | Gastric heterotopia/metaplasia | R201C | – | ||
| Pancreas | Intraductal papillary mucinous neoplasm with low grade dysplasia | R201C | WT | ||
|
| |||||
| 5 | 27 | Duodenum | Gastric heterotopia/metaplasia with adjacent neuroendocrine hyperplasia | R201H+ | WT |
| Pancreas | Intraductal papillary mucinous neoplasm with high grade dysplasia | R201H | – | ||
|
| |||||
| 6 | 19 | Proximal esophagus | Gastric heterotopia/metaplasia | R201C | – |
| Proximal stomach | Gastric hyperplastic polyps | WT | – | ||
| Proximal duodenum | Gastric heterotopia/metaplasia | R201C | – | ||
| Duodenum | Gastric heterotopia/metaplasia | R201C | – | ||
| Ampulla | Gastric heterotopia/metaplasia | R201C | WT | ||
|
| |||||
| 7 | 50 | Proximal esophagus | Gastric heterotopia/metaplasia | R201C | – |
| Gastroesophageal junction | Intestinal metaplasia | WT | – | ||
| Distal esophagus | Gastric hyperplastic polyp | WT | – | ||
| Duodenum | Gastric heterotopia/metaplasia | R201C | – | ||
| Duodenum | Gastric heterotopia/metaplasia | R201C | R201C | ||
at first procedure at JHH;
see text for more complete description of these unusual lesions;
GNAS R201H mutation identified in gastric heterotopia but not in separately analyzed neuroendocrine hyperplasia
Figure 1.
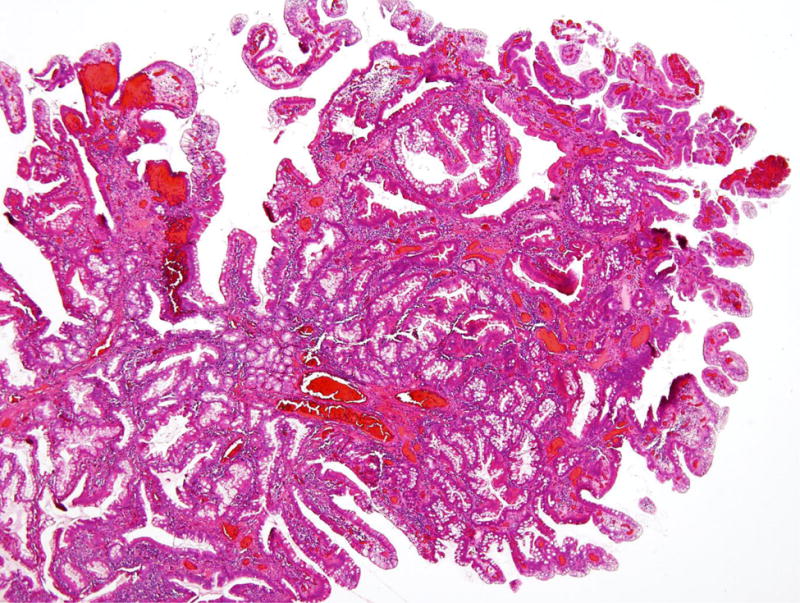
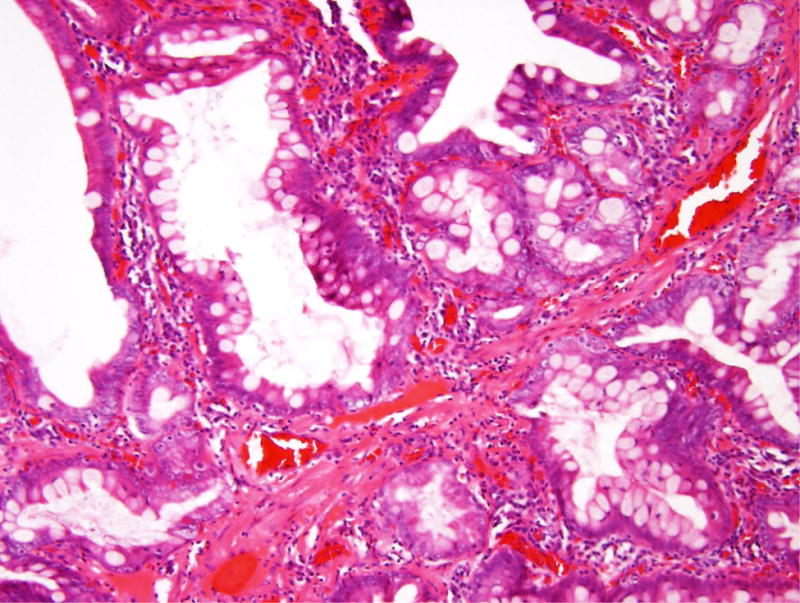
Hamartomatous polyp in patient 1. (A) This ampullary polyp is characterized by nests of small intestinal mucosa partitioned by bands of smooth muscle. (B) The smooth muscle bands are not as prominent as those typically seen in Peutz Jeghers polyps.
These small non-dysplastic lesions noted above encompassed the disease spectrum in five of the seven patients, who were only examined with endoscopic biopsies of the gastrointestinal tract. In addition, although all had abnormalities on pancreatic EUS suggestive of IPMN (confirming the radiologic impression on MRCP), none of these five had sufficiently concerning features on EUS to meet criteria for surgical intervention to address pancreatic neoplasia.
The remaining two patients (patients 4 and 5 in Table 1) had additional unusual lesions, and both underwent resection of pancreatic and gastrointestinal lesions. In addition to fundic gland polyps and gastric heterotopia, patient 4 had multiple dysplastic lesions in the proximal stomach and gastroesophageal junction (Figure 2). These lesions displayed a variety of directions of differentiation, including gastric foveolar, pyloric, oxyntic and intestinal, and at resection were found to have high-grade dysplasia but no invasive carcinoma. Resection of one radiologically worrisome IPMN in the pancreatic tail showed an IPMN with low-grade dysplasia (Figure 3). Immunohistochemistry revealed that the neoplastic IPMN cells strongly expressed MUC5, while expression of MUC1, MUC2, and CDX2 was weak and focal. Although the morphology and immunohistochemistry was most similar to a gastric-type IPMN, the MUC2 and CDX2 labeling raise the possibility of a mixed-type IPMN with some intestinal features [12].
Figure 2.
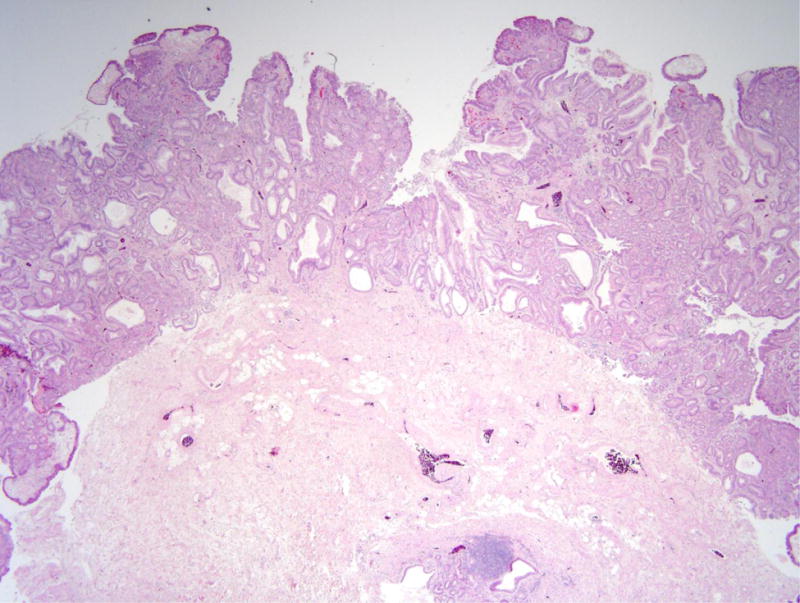
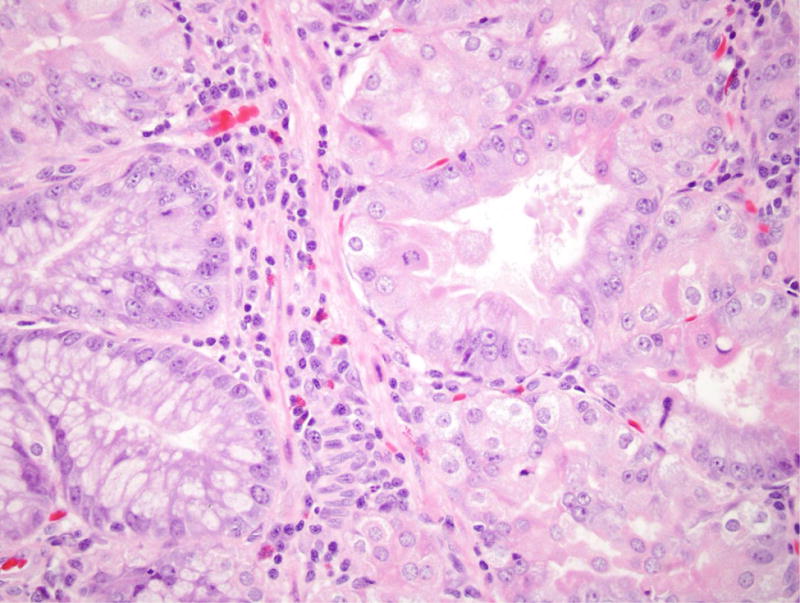
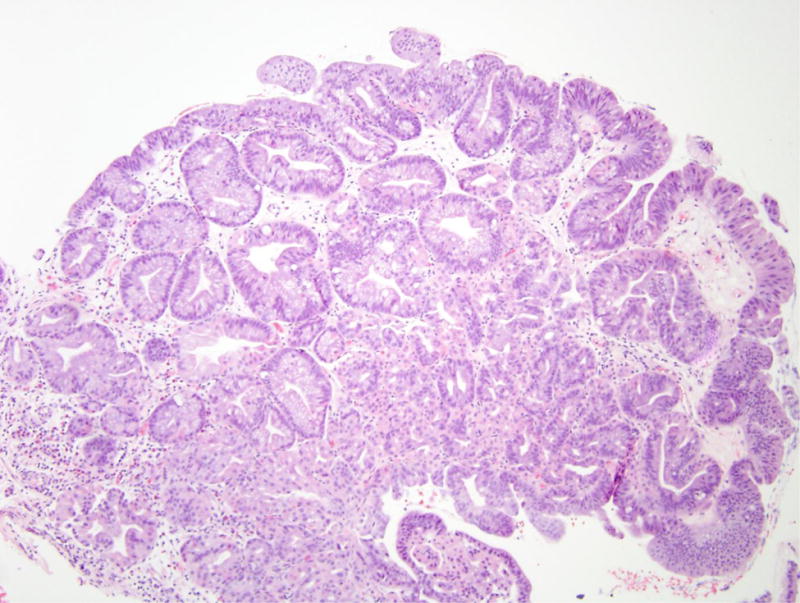
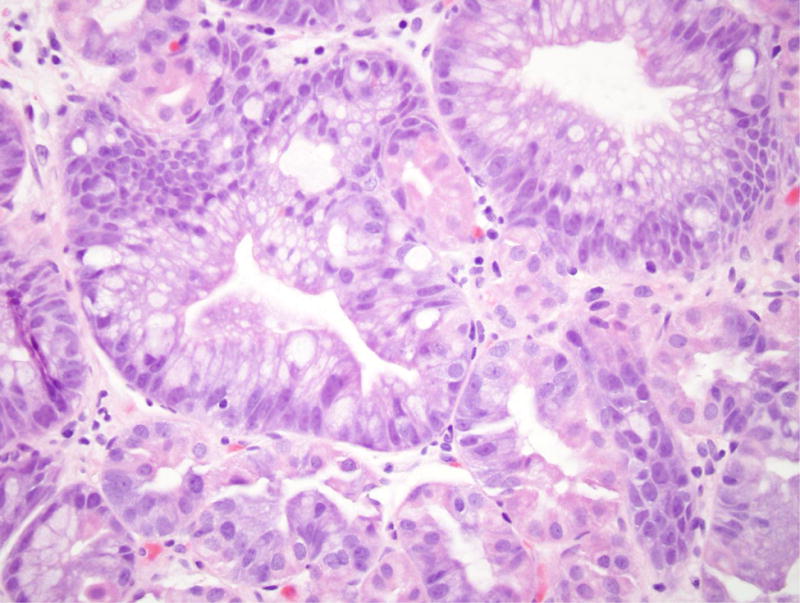
Gastric adenoma with high-grade dysplasia in patient 4. (A) This large glandular lesion at the gastroesophageal junction showed multiple directions of differentiation. (B) High-grade dysplasia was identified throughout the lesion, exhibiting irregularly fused glands, rounded nuclei with striking loss of nuclear polarity, and diffuse nuclear atypia. (C) Areas of pyloric and oxyntic differentiation were prominent. (D) Focal intestinal differentiation was also present.
Figure 3.
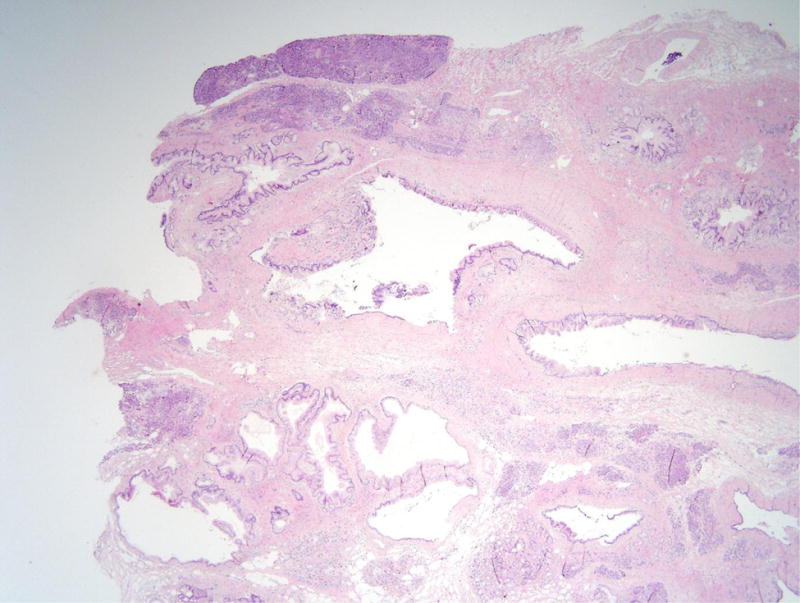
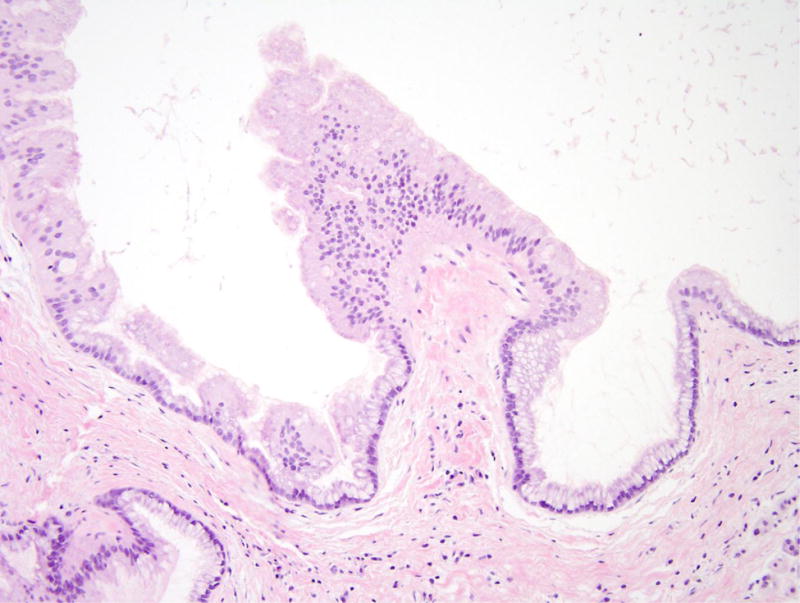
Intraductal papillary mucinous neoplasm in patient 4. (A) This cystic lesion was located in the pancreatic tail and clearly involves the pancreatic duct system. (B) The epithelium lining the cyst is mostly gastric-type with low-grade dysplasia.
Patient 5 underwent a Whipple resection for an IPMN in the pancreatic head raising clinical concern. The resection specimen revealed a 2.3 cm IPMN with high-grade dysplasia (Figure 4). By immunohistochemistry, the neoplastic IPMN cells strongly expressed CDX2 and MUC2, while MUC5 was only weakly and focally expressed. Immunohistochemistry for MUC1 was negative in this lesion. Overall, the morphological and immunohistochemical findings were most consistent with an intestinal-type IPMN in this patient [12]. In addition, the duodenum showed multiple areas of nodular gastric heterotopia with associated neuroendocrine proliferation bordering on well-differentiated neuroendocrine tumors (Figure 5).
Figure 4.
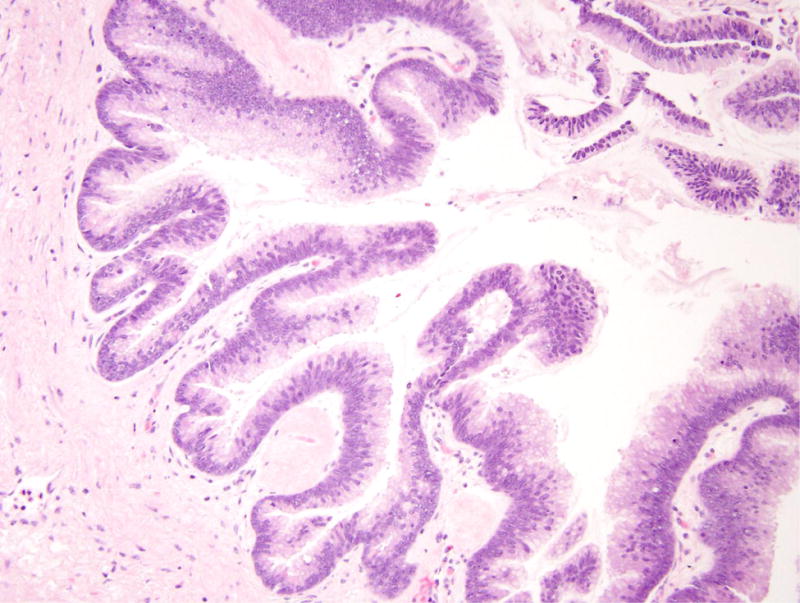
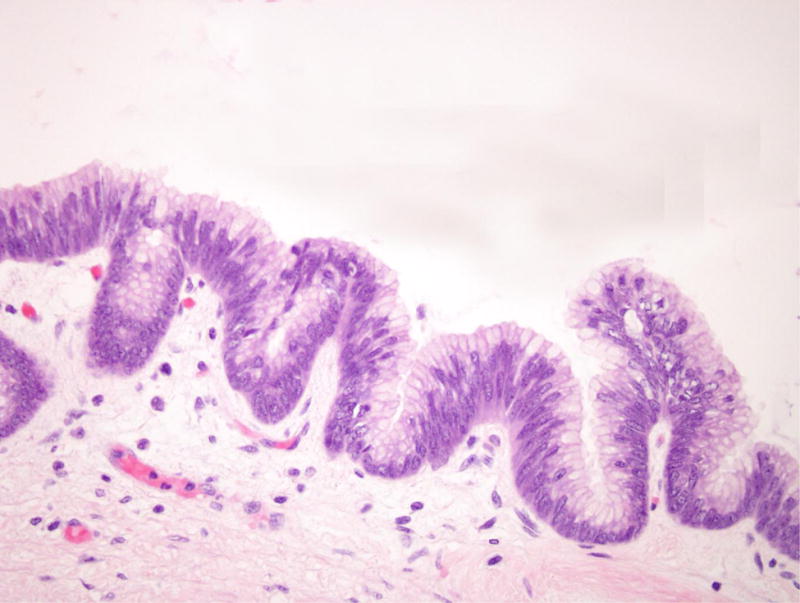
Intraductal papillary mucinous neoplasm in patient 5. (A) This cystic lesion was located in the pancreatic head and shows prominent papillae. (B) The epithelial lining shows intestinal differentiation and focal high-grade dysplasia, with elongated pencillate nuclei and areas with complete loss of nuclear polarity.
Figure 5.
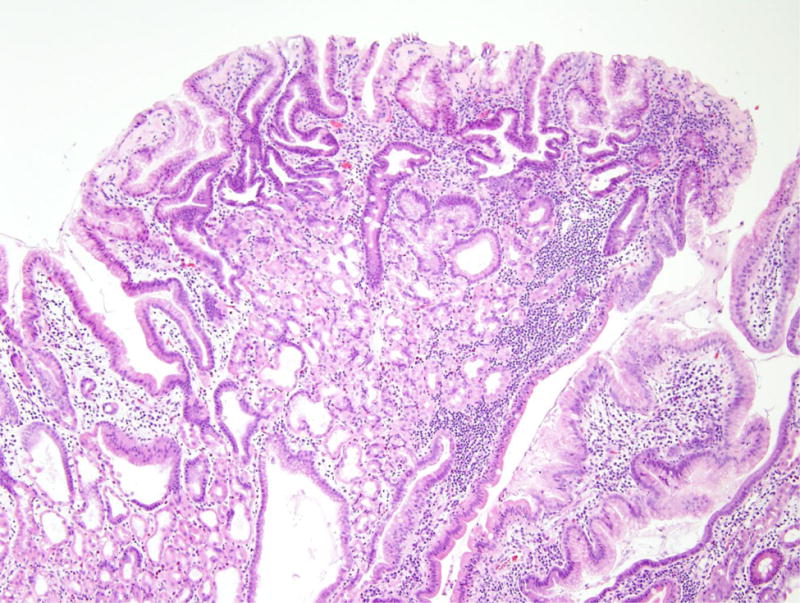
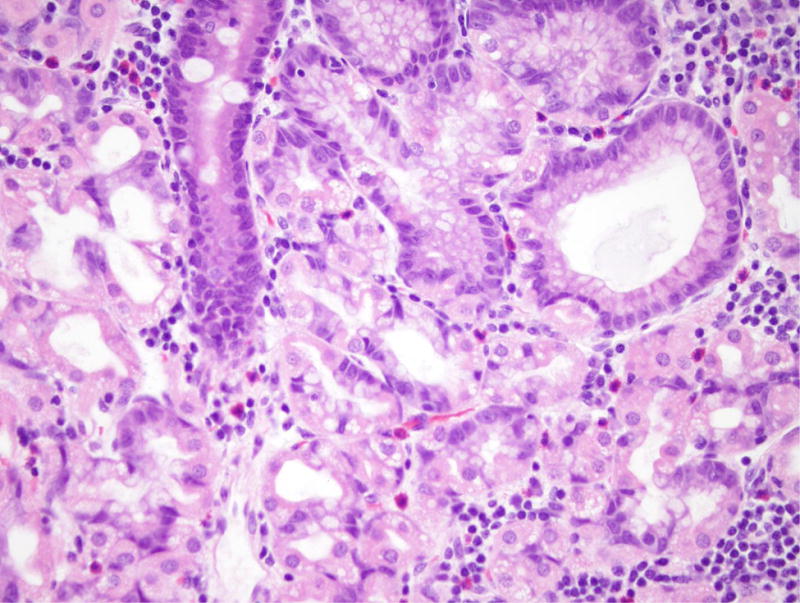
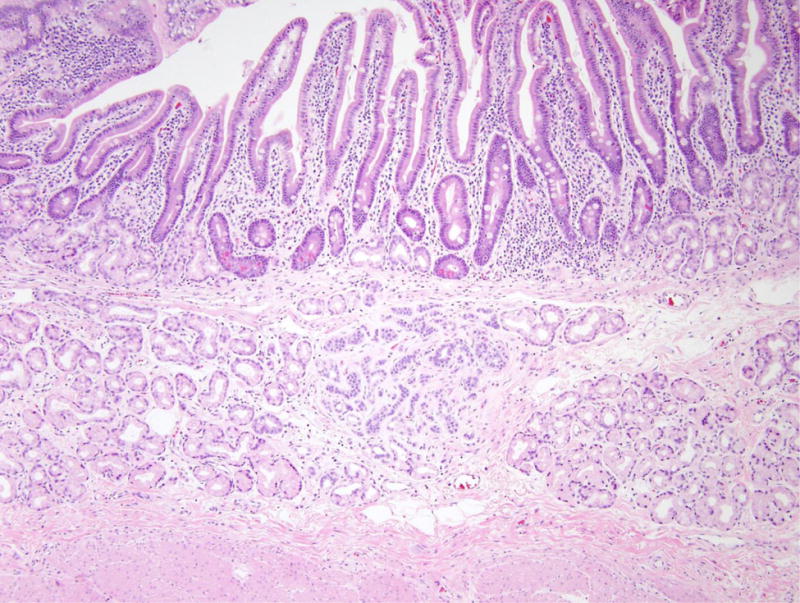
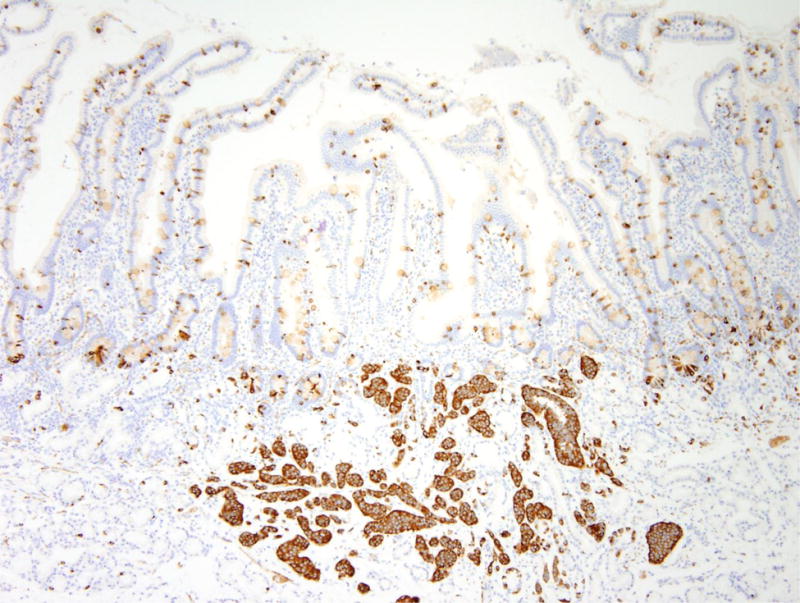
Nodular gastric heterotopia and neuroendocrine proliferations in patient 5. (A) At the luminal surface, nodular gastric heterotopia in the duodenum show nodules of gastric-type epithelium. (B) No dysplasia is evident in the gastric-type epithelium. The presence of parietal cells suggests gastric heterotopia rather than gastric metaplasia. (C) Several areas of nodular gastric heterotopia had nearby underlying neuroendocrine cell proliferations. It is not clear whether these represent hyperplasia or neoplasia. (D) Synaptophysin stain confirms neuroendocrine differentiation of these cells. The largest neuroendocrine proliferation measures 10 mm.
In a subset of lesions, selected for the availability of adjacent normal tissue, we performed pyrosequencing of the mutational hotspot in GNAS on formalin-fixed paraffin-embedded (FFPE) tissue from each lesion and adjacent normal tissue (Table 2). Both IPMNs had hotspot GNAS mutations, as did the unusual gastric adenomatous lesion in patient 4. In addition, GNAS mutations were also identified in 12 of 17 (71%) gastric heterotopic/metaplastic lesions. Intriguingly, in the duodenum of patient 5, a GNAS mutation was identified in the nodular gastric heterotopia, but the nearby neuroendocrine cell proliferation was wild-type. Of five gastric foveolar hyperplasia/gastric hyperplastic polyps, only two (40%) had GNAS mutations. Both fundic gland polyps were wild-type for GNAS, as was the ampullary hamartomatous-type polyp in patient 1. In all but one analyzed sample, the adjacent normal tissue was GNAS wild-type, even if the epithelium sampled was immediately adjacent to a lesion that had a GNAS mutation.
Pancreatic juice from patient 1 was collected from the duodenum following secretin stimulation and sequenced using digital next generation sequencing (NGS) for pancreatic cancer driver genes. As described by Yu and colleagues, this technique necessitates 96 separate NGS reactions for each patient, greatly increasing the sensitivity and ability to discriminate true point mutations from false positives compared to traditional NGS [11]. However, copy number alterations are not accurately assessed due to the low prevalence of mutant alleles in these samples. In patient 1, digital NGS revealed the expected oncogenic hotspot mutation in GNAS (R201C) with a digital NGS score of 29, as well as a missense mutation in RNF43 (R343H) with a digital NGS score of 10 [11]. In addition, non-digital targeted NGS of the same driver genes was performed on aspirated cyst fluid from two patients. Analysis of cyst fluid from patient 4 revealed the predicted mutation in GNAS (R201C) with a mutant allele frequency of 40.3%, as well as hotspot mutations in KRAS (Q61H) with a mutant allele frequency of 4.3% and PIK3CA (Q546R) with a mutant allele frequency of 1.6%. Similarly, cyst fluid from patient 7 contained the predicted GNAS mutation (R201C) with a mutant allele frequency of 9.1%, as well as an inactivating mutation in CDKN2A (V115fs) with a mutant allele frequency of 2.6%. Of note, all of these NGS analyses were performed on either pancreatic juice collected from the duodenum or aspirated pancreatic cyst fluid–pyrosequencing of the GNAS hotspot as described above was the only molecular analysis performed on tissue samples.
Discussion
The current study augments previous findings of gastrointestinal and pancreatic manifestations of MAS. In a study of four MAS patients, Zacharin and colleagues reported only hamartomatous polyps of the duodenum [6]. However, in that study, only relatively large polyps were examined histologically, while numerous small polyps of the stomach and small bowel were noted endoscopically but not biopsied. It is possible that these small unsampled polyps included the frequent gastric heterotopia/metaplasia noted in our cohort. Moreover, while the previous study reported significant histologic overlap with the hamartomatous polyps similar to those described in Peutz Jeghers syndrome, the smooth muscle proliferation in the hamartomatous polyp we examined was less striking than typically encountered in Peutz Jeghers syndrome. In addition, all patients in the previous study were in their teens or twenties, while our cohort had a broader age range, up to 55 years old, which allowed us to observe more advanced gastrointestinal manifestations of the syndrome. While our cohort is too small to make any statistically robust correlations between age and progression of the lesions, it is intriguing that the dysplastic gastric lesion manifested in the oldest patient, supporting the hypothesis that dysplasia takes decades to develop, even in patients with embryonic GNAS mutations. Overall, our study greatly broadens the spectrum of gastrointestinal findings in patients with MAS, adding frequent heterotopias as well as infrequent glandular dysplasia.
Gaujoux and colleagues previously reported imaging findings in hepatic and pancreatobiliary lesions in 19 MAS patients [5]. Four of these patients had pancreatic lesions, three of which were radiologically consistent with IPMNs. In addition, in a study of 272 unselected IPMN patients, Parvanescu and colleagues report one patient with an intestinal type IPMN associated with an invasive colloid carcinoma who had clinical features strongly suggestive of MAS [13]. In our cohort, all patients were selected for referral because they had pancreatic abnormalities at MRCP suggestive of IPMN. These findings were confirmed by EUS, and at resection two patients had a histologically proven IPMN. Although one patient had high-grade dysplasia (the patient was only 27 years old at diagnosis of pancreatic lesions), we did not find invasive carcinoma in either of the resected pancreata. By providing histologic confirmation of these IPMNs in patients with known MAS, our study confirms the association of IPMNs and MAS. However, it is not possible to determine the prevalence of IPMNs in unselected MAS patients from our study, as our cohort was biased by the criteria for referral to The Johns Hopkins Hospital for endoscopic evaluation–all patients in our study had pancreatic cysts on MRCP.
The spectrum of neoplasms reported here in MAS patients fits well with those known to have somatic GNAS mutations in sporadic lesions. For example, 60–80% of sporadic IPMNs have activating GNAS mutations [3,14]. Both of the IPMNs in this study had features of intestinal differentiation, and GNAS mutations have been reported to be more frequent in intestinal type IPMNs [3,9]. In addition, activating GNAS mutations have also been reported in several gastrointestinal lesions, namely pyloric gland adenomas (PGAs), oxyntic gland adenomas (OGAs), gastric heterotopia and gastric mucin cell metaplasia [4,15,16]. The occurrence of GNAS mutations in sporadic cases of ectopic gastric mucosa fits well with our finding of this ectopic mucosa in all MAS patients analyzed. In addition, although we did not find any PGAs or OGAs, the dysplastic lesion in patient 4 exhibited oxyntic and pyloric differentiation so may be a variant of this process.
The previously reported gastric lesions that harbor GNAS mutations, both sporadic and syndromic, have a spectrum of overlapping morphology. Gastric tumors that have been described as oxyntic gland adenomas and pyloric gland adenomas have differentiation along the lines of chief and parietal cells (oxyntic gland adenomas) and antral/cardiac type glands (pyloric gland adenoma), respectively. Additionally, some gastric polyps that arise in patients with familial adenomatous polyposis (FAP) can be classified as pyloric gland adenomas and also harbor GNAS mutations despite the history of FAP and its associated germline APC mutations [17,18].
Pyloric gland adenomas consist of proliferations of closely packed tubules, each lined by a monolayer of round basally oriented nuclei with apically oriented eosinophilic ground glass cytoplasm [19–21 ]. They are most commonly detected in the stomach, where they are likely to arise in patients with autoimmune gastritis. Such patients have autoimmune loss of their parietal cells, and their gastric body mucosa is replaced by metaplastic glands with pyloric type differentiation as well as by glands showing intestinal metaplasia, the perfect soil for the development of pyloric gland adenomas and other types of gastric neoplasms [22]. In contrast, the patients with MAS in our series did not have gastritis, yet they had adenomas with pyloric gland differentiation as well as hyperplastic polyps, both of which are often associated with gastritis [23,20]. Autoimmune gastritis has a female predominance and is found in about 2% of gastric biopsies in a tertiary care center [24].
On the other hand, oxyntic gland adenomas (which have also been called chief cell adenomas) arise in normal oxyntic mucosa unaffected by gastritis and also harbor GNAS mutations. They consist of angulated glands that proliferate underneath the foveolar surface and show a variable mixture of cells that appear similar to chief cells punctuated by parietal cells. Their bland cytologic features raise the possibility that they are hamartomatous and indeed non-neoplastic, but their growth pattern is more in keeping with that of a neoplasm. In fact, colleagues in Japan regard lesions that we have termed oxyntic gland adenoma (chief cell adenoma) as low grade adenocarcinomas based on their architectural features, despite the lack of reports of metastases and their indolent growth [25]. Other sporadic gastric-type proliferations that lack dysplasia found in the duodenum can similarly harbor GNAS mutations [15].
It is of interest that the lesions detected in patient 4 showed several lines of differentiation, including GNAS mutation-associated gastric forms of differentiation as well as intestinal differentiation, the latter presumably a reflection of a more advanced lesion in keeping with the older age of patient 4. Indeed, superimposed intestinal differentiation in gastric pyloric gland adenomas can also be encountered in the setting of autoimmune gastritis, which features intestinal metaplasia. All of this suggests that GNAS mutations are insufficient in themselves to drive aggressive neoplasms even if they might initiate indolent ones.
Our GNAS mutation analysis demonstrated that the IPMNs and adenomatous gastric lesion had mutations in the hotspot known to underlie the phenotype in MAS. In addition, the majority of samples of gastric heterotopia/metaplasia also had GNAS mutations. Considering the previous data reporting GNAS mutation in similar sporadic lesions, our results suggest that the early post-zygotic GNAS mutations play a role in the formation of these lesions. However, the lack of GNAS mutations in the fundic gland polyps, hamartomatous-type polyps, and other polyps calls into question the role of the mosaic GNAS mutations in the development of these lesions, which could be sporadic and thus unrelated to the MAS in these patients.
Although we performed targeted next generation sequencing on samples from only a subset of the patients in our cohort, we identified mutations in several additional genes previously reported to be critical drivers of pancreatic tumorigenesis, including KRAS and CKDN2A [26,3]. Although more comprehensive sequencing will be necessary to fully describe the genomic landscape of pancreatic neoplasms in these patients, these preliminary findings suggest that they may be quite similar to sporadic IPMNs.
In summary, we report the pathologic spectrum of gastrointestinal and pancreatic lesions in patients with MAS. Overall, there is enrichment for lesions known to have GNAS mutations when they occur sporadically, including pancreatic IPMNs, gastric heterotopia, and gastric adenomas with pyloric gland and oxyntic differentiation. Although no patients in our series had invasive cancer, two had high-grade dysplasia, one in a gastric polyp and one in an IPMN. This raises the possibility of an increased risk for malignant transformation in MAS patients, particularly as they age. Although only a small series of patients is reported here since the condition is rare, these data suggest potential clinical utility in screening the gastrointestinal tract and pancreas in MAS patients in order to detect and remove high risk lesions. Further longitudinal study in MAS cohorts will be necessary to better understand the natural history of gastrointestinal and pancreatic neoplasms in these patients.
Acknowledgments
The authors acknowledge the following sources of support:
Intramural Research Program of the National Institute of Dental and Craniofacial Research; NIH/NCI P50 CA62924; NIH/NIDDK K08 DK107781; Sol Goldman Pancreatic Cancer Research Center; Buffone Family Gastrointestinal Cancer Research Fund; Kaya Tuncer Career Development Award in Gastrointestinal Cancer Prevention; AGA-Bernard Lee Schwartz Foundation Research Scholar Award in Pancreatic Cancer; Sidney Kimmel Foundation for Cancer Research Kimmel Scholar Award; AACR-Incyte Corporation Career Development Award for Pancreatic Cancer Research; Rolfe Pancreatic Cancer Foundation; Joseph C Monastra Foundation for Pancreatic Cancer Research; The Gerald O Mann Charitable Foundation (Harriet and Allan Wulfstat, Trustees); Sigma Beta Sorority; Tampa Bay Fisheries Inc; Dutch Digestive Foundation (MLDS CDG 14-02)
Footnotes
Conflict of Interest: Dr. Wood is a paid consultant for Personal Genome Diagnostics
References
- 1.Boyce AM, Collins MT. Fibrous Dysplasia/McCune-Albright Syndrome. In: Pagon RA, Adam MP, Ardinger HH, et al., editors. GeneReviews(R) University of Washington, Seattle; University of Washington, Seattle: 1993. All rights reserved., Seattle (WA) [PubMed] [Google Scholar]
- 2.Weinstein LS, Shenker A, Gejman PV, Merino MJ, Friedman E, Spiegel AM. Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. The New England journal of medicine. 1991;325(24):1688–1695. doi: 10.1056/nejm199112123252403. [DOI] [PubMed] [Google Scholar]
- 3.Wu J, Matthaei H, Maitra A, Dal Molin M, Wood LD, Eshleman JR, Goggins M, Canto MI, Schulick RD, Edil BH, Wolfgang CL, Klein AP, Diaz LA, Jr, Allen PJ, Schmidt CM, Kinzler KW, Papadopoulos N, Hruban RH, Vogelstein B. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Science translational medicine. 2011;3(92):92ra66. doi: 10.1126/scitranslmed.3002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsubara A, Sekine S, Kushima R, Ogawa R, Taniguchi H, Tsuda H, Kanai Y. Frequent GNAS and KRAS mutations in pyloric gland adenoma of the stomach and duodenum. The Journal of pathology. 2013;229(4):579–587. doi: 10.1002/path.4153. [DOI] [PubMed] [Google Scholar]
- 5.Gaujoux S, Salenave S, Ronot M, Rangheard AS, Cros J, Belghiti J, Sauvanet A, Ruszniewski P, Chanson P. Hepatobiliary and Pancreatic neoplasms in patients with McCune-Albright syndrome. The Journal of clinical endocrinology and metabolism. 2014;99(1):E97–101. doi: 10.1210/jc.2013-1823. [DOI] [PubMed] [Google Scholar]
- 6.Zacharin M, Bajpai A, Chow CW, Catto-Smith A, Stratakis C, Wong MW, Scott R. Gastrointestinal polyps in McCune Albright syndrome. Journal of medical genetics. 2011;48(7):458–461. doi: 10.1136/jmg.2010.086330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lumbroso S, Paris F, Sultan C, European Collaborative S Activating Gsalpha mutations: analysis of 113 patients with signs of McCune-Albright syndrome–a European Collaborative Study. The Journal of clinical endocrinology and metabolism. 2004;89(5):2107–2113. doi: 10.1210/jc.2003-031225. [DOI] [PubMed] [Google Scholar]
- 8.Idowu BD, Al-Adnani M, O’Donnell P, Yu L, Odell E, Diss T, Gale RE, Flanagan AM. A sensitive mutation-specific screening technique for GNAS1 mutations in cases of fibrous dysplasia: the first report of a codon 227 mutation in bone. Histopathology. 2007;50(6):691–704. doi: 10.1111/j.1365-2559.2007.02676.x. [DOI] [PubMed] [Google Scholar]
- 9.Dal Molin M, Matthaei H, Wu J, Blackford A, Debeljak M, Rezaee N, Wolfgang CL, Butturini G, Salvia R, Bassi C, Goggins MG, Kinzler KW, Vogelstein B, Eshleman JR, Hruban RH, Maitra A. Clinicopathological correlates of activating GNAS mutations in intraductal papillary mucinous neoplasm (IPMN) of the pancreas. Annals of surgical oncology. 2013;20(12):3802–3808. doi: 10.1245/s10434-013-3096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen G, Olson MT, O’Neill A, Norris A, Beierl K, Harada S, Debeljak M, Rivera-Roman K, Finley S, Stafford A, Gocke CD, Lin MT, Eshleman JR. A virtual pyrogram generator to resolve complex pyrosequencing results. The Journal of molecular diagnostics : JMD. 2012;14(2):149–159. doi: 10.1016/j.jmoldx.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu J, Sadakari Y, Shindo K, Suenaga M, Brant A, Almario JA, Borges M, Barkley T, Fesharakizadeh S, Ford M, Hruban RH, Shin EJ, Lennon AM, Canto MI, Goggins M. Digital next-generation sequencing identifies low-abundance mutations in pancreatic juice samples collected from the duodenum of patients with pancreatic cancer and intraductal papillary mucinous neoplasms. Gut. 2016 doi: 10.1136/gutjnl-2015-311166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adsay NV, Merati K, Basturk O, Iacobuzio-Donahue C, Levi E, Cheng JD, Sarkar FH, Hruban RH, Klimstra DS. Pathologically and biologically distinct types of epithelium in intraductal papillary mucinous neoplasms: delineation of an “intestinal” pathway of carcinogenesis in the pancreas. The American journal of surgical pathology. 2004;28(7):839–848. doi: 10.1097/00000478-200407000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Parvanescu A, Cros J, Ronot M, Hentic O, Grybek V, Couvelard A, Levy P, Chanson P, Ruszniewski P, Sauvanet A, Gaujoux S. Lessons from McCune-Albright syndrome-associated intraductal papillary mucinous neoplasms: : GNAS-activating mutations in pancreatic carcinogenesis. JAMA surgery. 2014;149(8):858–862. doi: 10.1001/jamasurg.2014.535. [DOI] [PubMed] [Google Scholar]
- 14.Amato E, Molin MD, Mafficini A, Yu J, Malleo G, Rusev B, Fassan M, Antonello D, Sadakari Y, Castelli P, Zamboni G, Maitra A, Salvia R, Hruban RH, Bassi C, Capelli P, Lawlor RT, Goggins M, Scarpa A. Targeted next-generation sequencing of cancer genes dissects the molecular profiles of intraductal papillary neoplasms of the pancreas. The Journal of pathology. 2014;233(3):217–227. doi: 10.1002/path.4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsubara A, Ogawa R, Suzuki H, Oda I, Taniguchi H, Kanai Y, Kushima R, Sekine S. Activating GNAS and KRAS mutations in gastric foveolar metaplasia, gastric heterotopia, and adenocarcinoma of the duodenum. British journal of cancer. 2015;112(8):1398–1404. doi: 10.1038/bjc.2015.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kushima R, Sekine S, Matsubara A, Taniguchi H, Ikegami M, Tsuda H. Gastric adenocarcinoma of the fundic gland type shares common genetic and phenotypic features with pyloric gland adenoma. Pathology international. 2013;63(6):318–325. doi: 10.1111/pin.12070. [DOI] [PubMed] [Google Scholar]
- 17.Wood LD, Salaria SN, Cruise MW, Giardiello FM, Montgomery EA. Upper GI tract lesions in familial adenomatous polyposis (FAP): enrichment of pyloric gland adenomas and other gastric and duodenal neoplasms. The American journal of surgical pathology. 2014;38(3):389–393. doi: 10.1097/pas.0000000000000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hashimoto T, Ogawa R, Matsubara A, Taniguchi H, Sugano K, Ushiama M, Yoshida T, Kanai Y, Sekine S. Familial adenomatous polyposis-associated and sporadic pyloric gland adenomas of the upper gastrointestinal tract share common genetic features. Histopathology. 2015;67(5):689–698. doi: 10.1111/his.12705. [DOI] [PubMed] [Google Scholar]
- 19.Vieth M, Kushima R, Borchard F, Stolte M. Pyloric gland adenoma: a clinico-pathological analysis of 90 cases. Virchows Archiv : an international journal of pathology. 2003;442(4):317–321. doi: 10.1007/s00428-002-0750-6. [DOI] [PubMed] [Google Scholar]
- 20.Vieth M, Montgomery EA. Some observations on pyloric gland adenoma: an uncommon and long ignored entity! Journal of clinical pathology. 2014;67(10):883–890. doi: 10.1136/jclinpath-2014-202553. [DOI] [PubMed] [Google Scholar]
- 21.Chen ZM, Scudiere JR, Abraham SC, Montgomery E. Pyloric gland adenoma: an entity distinct from gastric foveolar type adenoma. The American journal of surgical pathology. 2009;33(2):186–193. doi: 10.1097/PAS.0b013e31817d7ff4. [DOI] [PubMed] [Google Scholar]
- 22.Park JY, Cornish TC, Lam-Himlin D, Shi C, Montgomery E. Gastric lesions in patients with autoimmune metaplastic atrophic gastritis (AMAG) in a tertiary care setting. The American journal of surgical pathology. 2010;34(11):1591–1598. doi: 10.1097/PAS.0b013e3181f623af. [DOI] [PubMed] [Google Scholar]
- 23.Abraham SC, Singh VK, Yardley JH, Wu TT. Hyperplastic polyps of the stomach: associations with histologic patterns of gastritis and gastric atrophy. The American journal of surgical pathology. 2001;25(4):500–507. doi: 10.1097/00000478-200104000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Pittman ME, Voltaggio L, Bhaijee F, Robertson SA, Montgomery EA. Autoimmune Metaplastic Atrophic Gastritis: Recognizing Precursor Lesions for Appropriate Patient Evaluation. The American journal of surgical pathology. 2015;39(12):1611–1620. doi: 10.1097/pas.0000000000000481. [DOI] [PubMed] [Google Scholar]
- 25.Ueyama H, Yao T, Nakashima Y, Hirakawa K, Oshiro Y, Hirahashi M, Iwashita A, Watanabe S. Gastric adenocarcinoma of fundic gland type (chief cell predominant type): proposal for a new entity of gastric adenocarcinoma. The American journal of surgical pathology. 2010;34(5):609–619. doi: 10.1097/PAS.0b013e3181d94d53. [DOI] [PubMed] [Google Scholar]
- 26.Wu J, Jiao Y, Dal Molin M, Maitra A, de Wilde RF, Wood LD, Eshleman JR, Goggins MG, Wolfgang CL, Canto MI, Schulick RD, Edil BH, Choti MA, Adsay V, Klimstra DS, Offerhaus GJ, Klein AP, Kopelovich L, Carter H, Karchin R, Allen PJ, Schmidt CM, Naito Y, Diaz LA, Jr, Kinzler KW, Papadopoulos N, Hruban RH, Vogelstein B. Whole-exome sequencing of neoplastic cysts of the pancreas reveals recurrent mutations in components of ubiquitin-dependent pathways. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(52):21188–21193. doi: 10.1073/pnas.1118046108. [DOI] [PMC free article] [PubMed] [Google Scholar]


