Abstract
Activity-dependent remodeling of neuronal connections is critical to nervous system development and function. These processes rely on the ability of synapses to detect neuronal activity and translate it into the appropriate molecular signals. One way to convert neuronal activity into downstream signaling is the proteolytic cleavage of cell adhesion molecules (CAMs). Here we review studies demonstrating the mechanisms by which proteolytic processing of CAMs direct the structural and functional remodeling of excitatory glutamatergic synapses during development and plasticity. Specifically, we examine how extracellular proteolytic cleavage of CAMs switches on or off molecular signals to 1) permit, drive, or restrict synaptic maturation during development and 2) strengthen or weaken synapses during adult plasticity. We will also examine emerging studies linking improper activity-dependent proteolytic processing of CAMs to neurological disorders such as schizophrenia, brain tumors, and Alzheimer’s disease. Together these findings suggest that the regulation of activity-dependent proteolytic cleavage of CAMs is vital to proper brain development and lifelong function.
Keywords: Proteolytic cleavage, cell adhesion molecules, neuronal activity, synaptic development, synaptic plasticity
1. Introduction
Neuronal activity is at the heart of information transfer and processing in the brain. Neuronal activity, in the form of synaptic transmission, also regulates synaptic development, strength, and remodeling. An important question is how neuronal activity is detected and then converted into the molecular signals that regulate synaptic connectivity and function. One answer to this question is the proteolytic cleavage of Cell Adhesion Molecules (CAMs). Proteolytic cleavage is the process by which proteins are cut into fragments in a rapid and sequence-specific manner by enzymes known as proteases. The two major classes of extracellular proteases that have been heavily linked to brain development and function are Matrix Metalloproteinases (MMPs) and A Disintegrin and Metalloproteinases (ADAMs). A number of in vivo studies demonstrate the clear importance of these proteases in both synapse development and function (Nagy et al., 2006; Michaluk et al., 2011; Zhuang et al., 2015; reviewed in Sonderegger and Matsumoto-Miyai, 2014).
There are several reasons why proteolytic cleavage of CAMs is an effective regulator of activity-dependent signaling. 1) Numerous CAMs undergo proteolytic cleavage in an activity-dependent manner in the developing as well as the adult brain (Table 1). 2) Proteolytic cleavage can activate or inactivate CAM-mediated signaling and also generate novel, bioactive fragments to influence a broad range of signaling pathways. 3) In response to synaptic transmission, activity-dependent CAM cleavage can occur rapidly (seconds to a few minutes) and in a spatially restricted manner (Conant et al., 2010; Peixoto et al., 2012). In this review, we will discuss the role of proteolytic cleavage of CAMs during synaptic development and then in the adult brain. In particular, we will focus on the different yet coordinated ways by which activity-dependent proteolytic cleavage can permit, drive and then restrict the maturation of active synapses during development. Then, we will survey the mechanisms by which proteolytic cleavage can strengthen or weaken synapses in response to neuronal activity during synaptic plasticity in the adult brain. We will also discuss how unregulated cleavage can result in neurological disorders such as schizophrenia, brain tumors, and Alzheimer’s disease. Finally, we will examine the possible mechanisms by which neuronal activity regulates the proteolytic cleavage of CAMs.
TABLE 1.
Comparison of CAMs that undergo activity-dependent proteolytic cleavage and what the cleaved fragments do at the synapse during development and adulthood. Here we list molecules that undergo activity-dependent cleavage, the type of activity that has been shown to cleave them, and the protease(s) responsible for this cleavage. The possible roles of cleaved proteins during development versus adulthood are also compared.
| Family | Molecule | Types of activity | Developmental function(s) | Adult function(s) | Protease(s) | Reference |
|---|---|---|---|---|---|---|
| Transmembrane protein | ||||||
| SIRP | SIRPα | Glutamate receptor activation, depolarization | Cleaved ectodomain drives presynaptic maturation | Unknown | MMP | Umemori & Sanes, 2008, Toth et al., 2013 |
| Neuroligin | Neuroligin-1 | Glutamate receptor activation, seizures, light exposure, depolarization | Decrease in neurotransmitter release probability | Synaptic homeostasis? | MMP-9, ADAM-10 | Suzuki et al., 2012; Peixoto et al., 2012 |
| Neuroligin-3 | Optogenetic stimulation | Unknown | Unknown | Unknown | Venkatesh et al., 2015 | |
| Cadherin | N-Cadherin | NMDA receptor activation | Unknown | LTP? Regulation of spine size? | MMP-9, ADAM-10 | Reiss et al., 2005, Uemura et al., 2006 |
| ICAM | ICAM-5 | Glutamate receptor activation, seizures, LTP | Cleavage permits postsynaptic maturation | LTP? AMPAR membrane insertion? | MMP-2, MMP-9 | Tian et al., 2007; Conant et al. 2010, Lonskaya et al., 2013 |
| SynCAM | SynCAM-2 | Unknown | Unknown | Unknown | MMP-9 | Tanabe et al., 2008, Bajor et al., 2012 |
| Nectin | Nectin-1 | NMDA receptor activation | Unknown | Spine maintenance | ADAM-10 | Kim et al., 2010, Lim et al., 2012 |
| Nectin-3 | NMDA receptor activation, Chronic stress | Unknown | Unknown | MMP-9 | van der Kooij et al., 2014 | |
| APP | APP | Varied | Unknown | Hebbian plasticity | α-secretase | Kamenetz et al., 2003, Hsieh et al., 2006, Shankar et al., 2008 |
| Dystroglycan | β-Dystroglycan | Glutamate receptor activation, seizures | Unknown | Unknown | MMP-9 | Michaluk et al., 2007, Dziembowska et al., 2012 |
| NCAM | NCAM | LTP induction | Cleaved fragment decreases excitatory synapse number | Unknown | MMP-9 | Fazeli et al., 1994, Pillai-Nair et al., 2005, Hinkle et al., 2006 |
| L1-CAM | L1-CAM | NMDA receptor activation | Unknown | LTP? | Neuropsin | Matsumoto-Miyai et al., 2003 |
| Netrin Ligand | G-NGL-3 | LTD induction | Unknown | Unknown | MMP | Lee et al., 2014 |
| Neurexin | Neurexin-3β | Glutamate receptor activation, depolarization | Unknown | Unknown | α-secretase | Bot et al., 2011, Saura et al., 2011 |
| Extracellular matrix proteins | ||||||
| Collagen | Multiplexin (Drosophila) | Glutamate receptor suppression | Unknown | Homeostatic plasticity | Unknown | Wang et al., 2014 |
| Agrin | Agrin | Concurrent activation of pre & postsynaptic terminals | Unknown | Formation of dendritic filopodia | Neurotrypsin | Matsumoto-Miyai et al., 2009 |
2. Proteolytic Cleavage in the Developing Brain
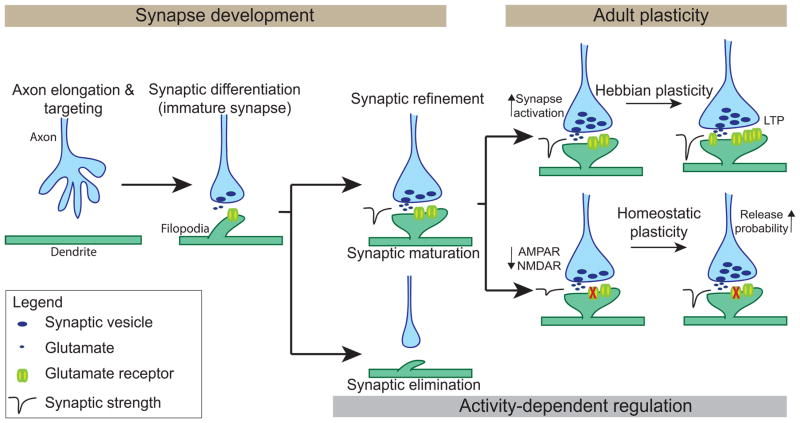
Synapses develop via multiple stages: 1) axon elongation and targeting, 2) synaptic differentiation, and 3) synaptic refinement, which includes both the maturation of active synapses and the elimination of inactive ones (Sanes and Lichtman, 1999; Fox and Umemori, 2006; Johnson-Venkatesh and Umemori, 2010). The first two stages are thought to be largely activity independent, but activity is critical for the synaptic refinement stage (Figure 1). Proteolytic cleavage regulates each of these stages of synaptic development. The regulation of axon elongation and targeting by proteolytic cleavage is well established. The ectodomain cleavage of proteins such as DCC, Robo, and Ephrin A has been shown to be a critical step during axon elongation and targeting (reviewed in Bai and Pfaff, 2011). The cleavage of these proteins acts as a mechanism to switch between the attraction and repulsion signals required to guide an axon to its appropriate target. Blocking proteolytic cleavage during this stage results in axon guidance defects such as the aberrant outgrowth and improper midline crossing of axons (Galko and Tessier-Lavigne, 2000; Hattori et al., 2000; Nguyen Ba-Charvet et al., 2001).
FIGURE 1.
Stages of excitatory synaptic development and two forms of adult synaptic plasticity. The synaptic development stage occurs in distinct steps: 1) axon elongation and targeting, 2) synaptic differentiation, and 3) synaptic refinement. Synaptic refinement is regulated by neuronal activity and includes both the maturation of active synapses and the elimination of inactive ones. In the adult, synapses undergo further activity-dependent modification primarily via two forms of synaptic plasticity: Hebbian plasticity and homeostatic plasticity. Non-activity dependent cleavage of CAMs contributes to the first two stages of synaptic development. Then, activity-dependent proteolytic cleavage of CAMs regulates synaptic maturation and adult plasticity.
Proteolytic cleavage, specifically the cleavage of Collagens, a family of Extracellular Matrix (ECM) proteins, is implicated in the synaptic differentiation stage as well. Many Collagens undergo proteolytic cleavage to generate bioactive fragments (termed matricryptins; Davis et al., 2000; reviewed in Ricard-Blum and Vallet, 2016). At the neuromuscular junction (NMJ), Collagen IV-derived matricryptins direct the assembly of motor nerve terminals (Fox et al., 2007). In the cerebellum, the cleaved product of Collagen XVIII is both necessary and sufficient to drive the differentiation of climbing fiber synapses (Su et al., 2012). In the neocortex, the C-terminal peptide from Collagen XIX promotes inhibitory nerve terminal formation (Su et al., 2016). Proteolytic cleavage during these two initial stages of synaptic development is activity-independent.
During the final, activity-dependent stage of synaptic development, the synaptic refinement stage, activity-dependent proteolytic cleavage occurs. Cleavage of CAMs, in response to neuronal activity, plays three distinct, but coordinated roles in orchestrating the structural and functional maturation of active synapses during synaptic refinement. Namely, proteolytic cleavage 1) creates a permissive environment for maturation by switching off signals that prevent maturation; 2) drives maturation by switching on maturation-promoting signals and; 3) restricts synaptic maturation to maintain synapses at a stable state once the synapse is sufficiently mature (Figure 2).
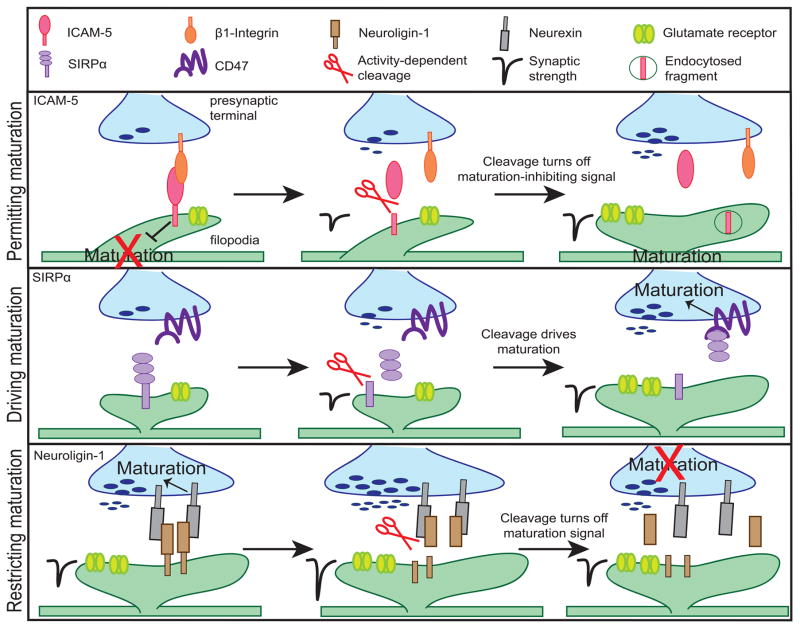
FIGURE 2.
The activity-dependent proteolytic cleavage of CAMs’ extracellular domains play specific, but coordinated roles in orchestrating synaptic maturation. First, activity-dependent cleavage of ICAM-5 removes the postsynaptic maturation-inhibiting signal. Thus, upon cleavage, postsynaptic maturation can begin. Second, the activity-dependent cleavage of SIRPα drives presynaptic maturation of active synapses by enabling the interaction between SIRPα ectodomain and CD47. Finally, when the synapse is mature, Neuroligin-1 is cleaved to restrict maturation and maintain the synapse at a stable state by disrupting its interaction with Neurexin.
2.1. Permitting synaptic maturation
During the refinement process, active and functional synapses undergo maturation, while inactive ones are eliminated. During synaptic maturation, immature synapses undergo structural and functional reorganization to form mature synapses. Relative to the immature synapse, the mature synapse is characterized by increases in the number of glutamatergic vesicles in the presynaptic active zone and neurotransmitter receptors in the postsynaptic density (PSD). Additionally, both the active zone and PSD widen, and immature, dendritic filopodia morph into mature, mushroom-like spines (Fiala et al., 1998; Li and Sheng, 2003; Yuste and Bonhoeffer, 2004). Synaptic maturation can also involve an increase in the ratio of AMPA to NMDA receptors, changes to NMDA receptor subunits, and changes to the types of presynaptic calcium channels (Reviewed in Yasuda and Umemori, 2009).
Given that only active synapses may undergo "activity-dependent" maturation, there likely are signals that keep the synapse in an immature state until that synapse is able to detect neuronal activity. Once activity is detected, the brake on maturation is removed, allowing synaptic maturation to occur. Full-length ICAM-5 (also known as Telencephalin) is one signal maintaining the synapse in an immature state (Figure 2, top). At immature synapses, full-length ICAM-5-mediated signaling has been shown to be a negative regulator of postsynaptic maturation (Benson et al., 1998; Matsuno et al., 2006). ICAM-5 is enriched in dendritic filopodia, the immature form of a dendritic spine, and is excluded from mature spines. Overexpression of ICAM-5 increases the number of immature dendritic filopodia, while the loss of ICAM-5 drives spine maturation in vitro (Matsuno et al., 2006). ICAM-5 inhibits maturation via its interaction with presynaptic β1-integrin. ICAM-5 co-immunoprecipitates with β1-integrin, and the application of a β1-integrin blocking antibody to cultured neurons demonstrates enhanced spine maturation similar to the effect of blocking ICAM-5 function (Ning et al., 2013). When the synapse is ready for maturation, ICAM-5 is likely eliminated from active, functional filopodia via the disruption of the ICAM-5 and β1-integrin interaction followed by the activity-driven proteolytic cleavage of ICAM-5. The pharmacological activation of NMDA receptors results in increased MMP-9-driven cleavage of the ICAM-5 extracellular domain (Tian et al., 2007; Ning et al., 2013). In MMP-9 null animals, ICAM-5 is not reduced at spines even after the age of maturation, supporting the idea that activity-driven cleavage diminishes filopodial ICAM-5 (Kelly et al., 2014). The extracellular domain cleavage of ICAM-5 may turn off the maturation-inhibiting signal in a number of ways. One likely way is that the extracellular domain cleavage may be followed by a subsequent internalization or removal of the remaining intracellular domain thereby shutting off ICAM-5 signaling to the filopodia. Evidence for this hypothesis comes from experiments indicating that the activity-driven, intracellular dissociation of ICAM-5 and α-actinin, an actin binding protein, is rescued via the blockade of MMP activity. The cleavage-driven dissociation of ICAM-5 from α-actinin permits spine maturation (Ning et al., 2015). Additionally, the shed ICAM-5 ectodomain may help initiate synapse maturation through the interaction with postsynaptic β1-integrins. This interaction can drive actin polymerization, a process involved in spine enlargement, as well as an increase in AMPA receptors (Wang et al., 2008; Conant et al., 2011; Lonskaya et al., 2013).
2.2. Driving synaptic maturation
While the cleavage of ICAM-5 switches off a signal that inhibits maturation, cleavage can also drive maturation specifically at active synapses by sending a maturation-promoting signal. The latter is a role of SIRPα, a member of the immunoglobulin superfamily. SIRPα has been identified as a target-derived synaptic organizer necessary to drive synaptic maturation of excitatory synapses (Figure 2, middle). The extracellular domain of SIRPα is cleaved and released from neurons in response to neuronal activation. The bath application of the soluble, extracellular fragment of SIRPα to cultured hippocampal or motor neurons is sufficient to increase presynaptic vesicle clustering and the frequency of miniature excitatory postsynaptic currents (Umemori and Sanes, 2008; Toth et al., 2013). Synapse maturation by SIRPα requires both neuronal activity and proteolytic cleavage, because 1) the overexpression of the cleavable, full-length SIRPα in cultured neurons drives synapse maturation while the overexpression of a non-cleavable mutant does not and 2) the blockade of neuronal activity deters the maturation-promoting effect of SIRPα overexpression. These suggest that the cleavage of SIRPα ectodomain by neuronal activity is necessary for SIRPα-dependent synaptic maturation (Toth et al., 2013). Proteolytic cleavage of SIRPα may be necessary for SIRPα to bind its receptor CD47 because of the physical distance between the two molecules. SIRPα is expressed postsynaptically, while CD47 is primarily expressed presynaptically. Crystal structure modeling of SIRPα-CD47 demonstrates that the size of this complex is smaller than the width of the synaptic cleft suggesting that direct interaction between the two molecules across the cleft is unlikely (Hatherley et al., 2008). Therefore, SIRPα can only bind CD47 after it is cleaved and released, allowing it to translocate across the synaptic cleft. This process allows SIRPα-CD47 to send maturation signals specifically at active, functional synapses where neuronal activity cleaves the extracellular domain of SIRPα. Other members of the SIRP family, such as SIRPβ and SIRPγ, could serve a similar role at other synapses. Both SIRPβ and SIRPγ extracellular domain fragments show presynaptic organizing activity in vitro, but whether they are cleaved by neuronal activity is still unknown (Umemori and Sanes, 2008).
2.3. Restricting synaptic maturation
Proteolytic cleavage can also regulate synaptic maturation by turning off synaptic growth-promoting signals and allowing the mature synapse to be maintained at equilibrium. An example of this process is the activity-dependent cleavage of Neuroligin-1, which terminates Neuroligin-1/Neurexin signaling by destabilizing the interaction between these two proteins (Figure 2, bottom). Full-length postsynaptic Neuroligin-1 drives the maturation of excitatory presynaptic terminals via its interaction with the presynaptically expressed Neurexin. Thus, full-length Neuroligin-1 acts as a retrograde synaptic organizer that drives excitatory synapse maturation and increases neurotransmitter release (Scheiffele et al., 2000; Dean et al., 2003; Varoqueaux et al., 2006; Futai et al., 2007; Wittenmeyer et al., 2009;). However, Neuroligin-1 is also cleaved in an activity-dependent manner. Increases in neuronal activity both in vitro and in vivo results in greater amounts of Neuroligin-1 extracellular domain cleavage. Contrary to full-length Neuroligin-1 signaling, the cleavage of Neuroligin-1 results in decreased neurotransmitter release, EPSC frequency and amplitude (Suzuki et al., 2012; Peixoto et al., 2012). Furthermore, the application of the soluble Neuroligin-1 fragment blocks the synaptogenic effect of Thrombospondin-1 in cultured hippocampal neurons likely by interrupting the interaction of Thrombospondin-1 with full-length Neuroligin-1 (Xu et al., 2010). Together, these data suggest that Neuroligin-1 cleavage not only turns off Neurexin-mediated synaptogenic signaling but may also block the synaptogenic signaling mediated by other molecules. Thus, while the physiological role of Neuroligin-1 cleavage still requires elucidation, we speculate that the activity-dependent cleavage of Neuroligin-1 acts to restrict the maturation of already mature synapses. The balance between full-length Neuroligin-1 signaling and Neuroligin-1 cleavage offers a mechanism for fine-tuning synaptic maturation, thus, allowing the synapse to be maintained in an appropriately mature state.
2.4. When proteolytic cleavage goes amiss in the developing brain - Schizophrenia and brain tumors
Since activity-dependent proteolytic cleavage plays crucial roles in synaptogenesis, improper proteolytic processing can result in neurodevelopmental diseases that are characterized by impaired synaptic development. One such case is the psychiatric disorder, schizophrenia. Schizophrenia is characterized by alterations to synaptic circuitry, where the spine density of cortical pyramidal neurons is markedly lower in schizophrenic patients relative to healthy controls (Garey et al., 1998; Glantz and Lewis, 2000; Glausier and Lewis, 2013). Interestingly, levels of cleaved NCAM are increased in the prefrontal cortex and hippocampus of human schizophrenia patients (Vawter et al., 2001). However, the effect or function of these cleaved NCAM fragments in the human brain is still unknown.
To determine the role of NCAM cleavage in schizophrenia, a mouse model with increased secretion of cleaved NCAM fragments has been created. These mice demonstrate schizophrenia-like behaviors such as attenuated paired-pulse inhibition and hyperactivity. At a cellular level, these mice also demonstrate decreases in cortical dendritic spine density, mimicking schizophrenic patients (Pillai-Nair et al., 2005). While the signaling pathways altered by NCAM cleavage still remain unknown, the fact that the proteolytic cleavage of NCAM can be regulated by neuronal activity is known. High frequency stimulation of the rat hippocampus leads to an increase in the cleavage and release of an 115kDa NCAM fragment. This fragment is similar in size to the cleaved NCAM fragment detected in schizophrenic patients (Fazeli et al., 1994; Vawter et al., 2001). Therefore, further understanding the mechanisms through which neuronal activity regulates NCAM cleavage and what the functional consequences are for this cleavage as it pertains to schizophrenia are important questions for future research.
Proteolytic cleavage has also been incriminated in the regulation of gliomas. Gliomas are a leading cause of brain tumors in children as well as adults. Increases in neuronal activity have been shown to drive the proliferation of human gliomas. This activity-dependent increase in proliferation requires the proteolytic cleavage and ectodomain secretion of a Neuroligin family member, Neuroligin-3 (Venkatesh et al., 2015). Activation of neurons in cultured cortical slices results in an increase in cleaved Neuroligin-3 in the media. Furthermore, the application of recombinant Neuroligin-3 protein is sufficient to drive the proliferation of gliomas. Mechanistically, the secreted Neuroligin-3 may activate the growth-promoting PI3K-mTOR signaling pathway causing the increase in glioma proliferation. Interestingly, the application of Neuroligin-3 results in a feed-forward increase in Neuroligin-3 expression in glioma cells (Venkatesh et al., 2015). This feed-forward cycle may explain the unchecked growth of tumors in the brain.
3. Proteolytic Cleavage in the Adult Brain
In the adult brain, synaptic connections still undergo constant activity and experience-dependent strengthening and weakening. This synaptic remodeling is thought to underlie learning, memory, and certain aspects of behavior. There are two general forms of synaptic plasticity known as Hebbian and homeostatic plasticity (Figure 1). Both these forms of plasticity consist of neuronal activity impacting synaptic structure and strength. Recent studies implicate activity-dependent proteolytic cleavage of CAMs in both Hebbian and homeostatic plasticity.
3.1. Hebbian plasticity
Hebbian plasticity consists of the well-known forms of plasticity, long-term potentiation (LTP) and long-term depression (LTD). These forms of plasticity result in long-term changes in synaptic strength. LTP can be triggered by the correlated firing of the presynaptic terminal and activation of the postsynaptic terminal resulting in synaptic strengthening; whereas, LTD can result from uncorrelated pre and postsynaptic activity, leading to synaptic weakening. Structurally, dendritic spines of potentiated synapses become enlarged following LTP. Functionally, potentiated synapses are strengthened through a number of mechanisms. The most prominent of these are increases in postsynaptic surface AMPA receptor number and presynaptic release probability (Reviewed in Herring and Nicoll, 2016).
The first evidence linking proteolytic cleavage to the regulation of Hebbian plasticity came from studies of proteases. LTP-induction increases the levels of proteases (Qian et al., 1993). Furthermore, the magnitude and duration of LTP is attenuated in MMP-9 null mice (Nagy et al., 2006). Similarly, the inhibition of MMPs diminishes the magnitude of induced LTD in hippocampal slices (Meighan et al., 2007). However, how affecting protease activity can result in impaired LTP and LTD remains largely unknown. What we do know is that some CAMs such as N-Cadherin, ICAM-5, NCAM, and L1-CAM can be cleaved during the induction of LTP, and disruptions to the function of these CAMs can result in impaired LTP (Lüthl et al., 1994; Fazeli et al., 1994; Reiss et al., 2005; Conant et al., 2010; Niedringhaus et al., 2012). Another synaptic protein, NGL-3, undergoes cleavage following an LTD-inducing stimulus (Lee et al., 2014). Although, the precise role of the CAM cleavage in LTP and LTD is still unknown, activity-driven cleavage of CAMs may regulate Hebbian plasticity.
One possible mechanistic example of activity-dependent proteolytic cleavage in Hebbian plasticity is that of ICAM-5. ICAM-5 is cleaved by LTP-inducing stimuli such as chemical LTP and tetanic stimulation of adult hippocampal slices (Conant et al., 2010; Niedringhaus et al., 2012). The treatment of cultured neurons with the soluble ICAM-5 fragment causes an increase in the surface localization of GluA1 subunit-containing AMPA receptors (Lonskaya et al., 2013). These observations suggest a possible model for activity-dependent ICAM-5 cleavage in LTP, where the induction of LTP at the synapse leads to an increase in ICAM-5 cleavage. This cleaved fragment then increases the number of AMPA receptors at that synapse, resulting in potentiation.
3.2. Homeostatic plasticity
In contrast to Hebbian plasticity, homeostatic plasticity consists of compensatory changes in synaptic strength as a way to maintain synaptic stability. Thus, during periods of high activity, homeostatic plasticity works to decrease synaptic strength, and, during periods of low activity, homeostatic plasticity works to increase synaptic strength. Proteolytic cleavage of CAMs could either act to prevent synaptic silencing by increasing synaptic strength, or prevent hyperactivity by decreasing synaptic strength.
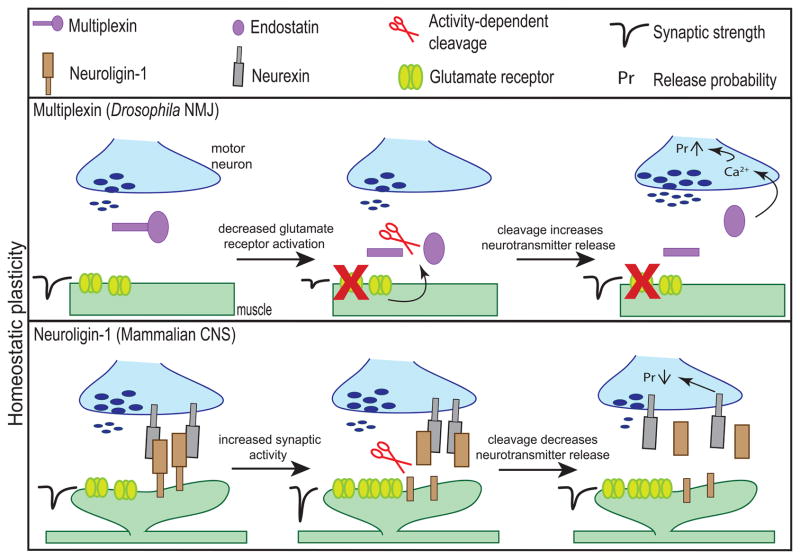
Following the attenuation of postsynaptic receptor activity, one way to maintain synaptic strength is to increase presynaptic neurotransmitter release. In Drosophila, the activity (or more accurately, suppressed activity)-dependent proteolytic cleavage of Multiplexin, a member of the Collagen family of ECM proteins, acts as a feedback mechanism to maintain the efficacy of mature synaptic connections; where, the cleaved proteolytic product, Endostatin, turns on a signaling pathway to increase presynaptic activity to maintain synaptic homeostasis (Figure 3). Experiments at the Drosophila NMJ suggest that when neurotransmission is impeded via the blockade of postsynaptic glutamate receptors, Multiplexin is cleaved to form Endostatin. Endostatin is then shown to genetically interact with presynaptic voltage-gated calcium channel, CaV2.1, leading to an increase in presynaptic calcium influx. This increase of presynaptic calcium likely contributes to an increase in neurotransmitter release, thereby offsetting the impeded neurotransmission (Wang et al., 2014). The proteolytic cleavage of Multiplexin into Endostatin offers a way of coordinating postsynaptic responses and presynaptic changes to maintain synaptic homeostasis.
FIGURE 3.
Activity-dependent proteolytic cleavage of CAMs in the adult brain regulates homeostatic plasticity. Suppression of glutamate receptor activity at the Drosophila NMJ results in a presynaptic increase of neurotransmitter release to maintain homeostasis. This plasticity requires the cleavage of Multiplexin to form Endostatin that results in an increase in presynaptic calcium levels. Contrarily, when synaptic activity is elevated, the cleavage of Neuroligin-1 can return the synapse to a stable state by weakening the synapse. At overactive synapses, the cleavage of Neuroligin-1 decreases presynaptic release via the perturbation of Neuroligin-1/Neurexin signaling.
Multiplexin and its mammalian homolog, Collagen, are ubiquitously expressed in the nervous system. In mammals, matricryptins, the cleaved fragments of Collagens, have been implicated as synaptic organizers involved in the induction of pre and postsynaptic terminals during development (see above; Fox et al., 2007; Su et al., 2012, 2016), but little is known about the mechanisms underlying this cleavage. Therefore, testing whether these (and other) molecules are cleaved in an activity-dependent manner and act to regulate homeostatic plasticity will be an interesting direction for future research.
In contrast to the proteolytic cleavage of Multiplexin, cleavage could also decrease the activity of hyperactive synapses. For example, the cleaved extracellular domain of Neuroligin-1 is detected in brain lysates even after synaptic development and well into adulthood. Furthermore, this adult cleavage is driven by neuronal activity, as indicated by the increased levels of cleaved Neuroligin-1 extracellular domain detected following pilocarpine-induced seizures in the adult mouse brain (Suzuki et al., 2012; Peixoto et al., 2012). While the function of this adult Neuroligin-1 proteolytic cleavage is unknown, the activity-dependent cleavage of Neuroligin-1 could depress synaptic transmission and return the synapse to a stable state (Figure 3, also see Figure 2, bottom). However, future studies will need to confirm this hypothesis.
3.3. When proteolytic cleavage goes amiss in the adult brain - Alzheimer’s disease
Perhaps the most studied protein that undergoes improper proteolytic processing in the brain is Amyloid Precursor Protein (APP), an important molecule in the devastating neurodegenerative disorder, Alzheimer’s disease. APP is a single-pass transmembrane protein with a large extracellular domain. Very little is known about the physiological function of APP; however, the mechanism of APP cleavage is well characterized. The improper cleavage of APP to Amyloid-β (Aβ) is affected by aberrant levels of neuronal activity both in humans and animal models. Some patients with elevated brain activity due to epilepsy show earlier accumulation of Aβ plaques (Mackenzie and Miller, 1994). In mouse models, the treatment of hippocampal slices with picrotoxin, a GABA receptor antagonist, to raise activity, increases the amount of Aβ detected. In contrast, blocking neuronal activity via the application of tetrodotoxin decreases the amount of Aβ (Kamenetz et al., 2003; Wu et al., 2011). Accordingly, whisker stimulation increases the amount of Aβ in the mouse interstitial fluid, while whisker deprivation decreases the amount of Aβ (Bero et al., 2011).
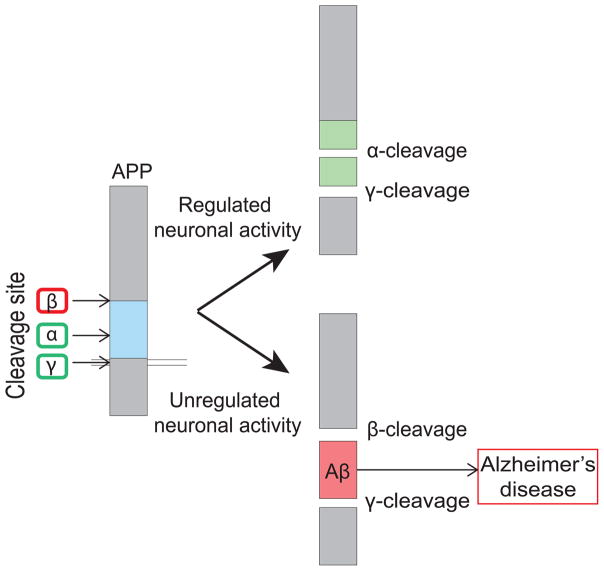
In a healthy individual, APP is normally cleaved via α-secretase. However, in Alzheimer’s disease, APP is incorrectly cleaved by β-secretase and γ-secretase to generate Aβ (Figure 4; reviewed in Haass et al., 2012). This improper cleavage of APP to form Aβ leads to a variety of synaptotoxic effects that ultimately causes neurodegeneration. The application of soluble Aβ to hippocampal slices and to cultured neurons has been shown to cause impaired LTP, increased LTD, and decreased synapse density (Hsieh et al., 2006; Shankar et al., 2008). These alterations to synaptic function and plasticity are believed to underlie the degenerative pathophysiology of Alzheimer’s disease.
FIGURE 4.
Improper proteolytic processing of APP in Alzheimer’s disease. Under physiological conditions, APP is cleaved via α-secretase (α) followed by γ-secretase (γ) to generate non-amyloidogenic fragments. However, when neuronal activity is altered, APP is incorrectly cleaved by β-secretase (β) and γ-secretase to generate amyloidogenic Amyloid-β (Aβ), which leads to Alzheimer’s disease.
4. How Does Neuronal Activity Regulate Proteolytic Processing?
Clearly the activity-dependent proteolytic cleavage of CAMs is an important regulator of synaptic function. However, not much is known about the molecular pathways guiding the interplay between neuronal activity and the proteolytic cleavage. Various methods of stimulating neuronal activity have been shown to regulate proteolytic cleavage of CAMs both in vitro and in vivo (Table 1). A closer look at these different forms of stimulation reveals that proteolytic cleavage of CAMs often requires the activation of postsynaptic glutamate receptors, especially the activation of NMDA receptors. In the case of N-Cadherin and Nectin-1, activation of neurons using NMDA, but not AMPA, leads to increases in extracellular domain cleavage. Furthermore, the cleavage of N-Cadherin, Nectin-1, Neuroligin-1, and Nectin-3 can be inhibited by the application of APV or MK801 alone, both specific antagonists of NMDA receptors (Uemura et al., 2006; Kim et al., 2010; Suzuki et al., 2012; Peixoto et al., 2012; van der Kooij et al., 2014). This preference for NMDA receptor-dependent activity suggests that perhaps a postsynaptic influx of calcium is necessary for the activation of signaling pathways that may regulate cleavage. Accordingly, the activation of CaMK is often necessary for activity-dependent proteolytic cleavage. For example, the application of KN93, a CaMK inhibitor greatly diminishes the cleavage of Neuroligin-1 and SIRPα (Peixoto et al., 2012; Toth et al., 2013). However, what lies downstream of this CaMK signaling leading to cleavage remains unknown.
To regulate proteolytic cleavage, neuronal activity could directly regulate proteases, their cleavage substrates or both. In the case of proteases, studies suggest that the expression, activation, and transport of proteases can be regulated by neuronal activity. For example, MMP-9, an extracellular protease implicated in the cleavage of Neuroligin-1, ICAM-5, β-Dystroglycan, Nectin-3, and SynCAM2, is regulated by neuronal activity. MMP-9 is rapidly activated following LTP induction, and more MMP-9 protein is expressed in vivo following kainate-induced seizures (Szklarczyk et al., 2002; Nagy et al., 2006). Also, MMP-9 mRNA seems to be transported to and locally translated at specific synapses in an activity-dependent manner (Dziembowska et al., 2012). This local regulation could provide a mechanism by which proteolytic cleavage can be regulated in a synapse-specific manner; whereby, cleavage only occurs at active synapses. Localized, activity-dependent cleavage is known to occur where the activation of a single spine via glutamate uncaging results in the cleavage of Neuroligin-1 almost exclusively at the activated spine (Peixoto et al., 2012).
As for the regulation of cleavage substrates, synaptic transmission could influence proteolytic cleavage through the control of protein-protein interactions that may block or expose specific cleavage sites on CAMs that are recognized by proteases. For example, the binding of ICAM-5 and β1-integrin may block the cleavage of ICAM-5 by masking the cleavage site detected by proteases. Disrupting the ICAM-5 and β1-integrin interaction using blocking antibodies results in greater ICAM-5 cleavage, while enhancing the interaction results in less ICAM-5 cleavage (Ning et al., 2013). While how neuronal activity factors into the disruption of ICAM-5 and β1-integrin binding is unclear, it is clear that protein-protein interaction is another regulatory mechanism for proteolytic processing.
Activity could also regulate the synaptic or surface localization of these CAMs. The surface localization of Neuroligin-1 is regulated by neuronal activity. Mechanistically, synaptic transmission activates CaMK that then phosphorylates the intracellular domain of Neuroligin-1. This phosphorylation directs the surface localization of Neuroligin-1. The inhibition of this phosphorylation event attenuates the activity-driven increase of surface Neuroligin-1 expression (Bemben et al., 2014). The activity-regulated increase in surface localization of Neuroligin-1 may regulate cleavage by allowing greater exposure to extracellular proteases. Alternatively, by compelling the surface localization, activity may facilitate a protein-protein interaction that promotes cleavage. Evidence for the latter comes from an experiment showing that the interaction between Neuroligin-1 and its presynaptic partner, Neurexin can drive the cleavage of Neuroligin-1. The treatment of cultured neurons with soluble Neurexin-1α and soluble Neurexin-1β augmented levels of cleaved Neuroligin-1 detected in the culture media (Suzuki et al., 2012).
5. Concluding Remarks and Future Questions
Proteolytic cleavage of CAMs can be a deconstructive mechanism that turns off signaling pathways or drives protein turnover. Proteolytic cleavage can also play more constructive roles via the generation of functional protein fragments that activate a variety of signaling pathways. To date, the neuronal activity-driven cleavage of ICAM-5, SIRPα, and Neuroligin-1 during development have been best characterized. Studies suggest that the cleavage of these molecules govern unique stages of activity-dependent synaptic maturation. ICAM-5 creates a permissive environment for the maturation of active synapses by removing maturation-inhibiting signals. SIRPα is then cleaved to drive maturation of active synapses via CD47-mediated signaling. Finally, Neuroligin-1 is cleaved at active, maturated synapses, leading to the loss of a Neurexin-mediated maturation-promoting signal, allowing fine-tuning of the mature synapses. In the adult brain, proteolytic cleavage is linked to both Hebbian and homeostatic plasticity. The cleavage of ICAM-5 may contribute to LTP. While the cleavage of Multiplexin and Neuroligin-1 strengthen and weaken synapses, respectively, in a homeostatic manner. Together, these constructive and deconstructive roles of CAM cleavage allow the differential regulation of multiple molecular pathways that work in a coordinated manner to ensure the appropriate activity-dependent remodeling of synapses during development and plasticity. Nevertheless, we are only at the cusp of truly understanding how activity-dependent proteolytic cleavage orchestrates synaptic remodeling; thus, three key future questions stand out.
First, how do cleaved fragments of CAMs regulate synaptic remodeling? Thus far, the interactions between the cleaved ectodomains of ICAM-5, SIRPα, and Neuroligin-1 with β1-integrin, CD47, and Neurexin, respectively, have been described. However, in the case of many other CAMs, such as Multiplexin, Agrin, and NCAM, the receptors for and the signaling cascades regulated by the cleaved fragments remain largely unknown. Clearly delineating which signaling cascades are involved is important because the cleavage of different CAMs can perform distinct, yet coordinated roles in development and adult plasticity.
Secondly, is there a role for activity-dependent proteolytic cleavage of CAMs at other, non-glutamatergic synapses? In this review we specifically looked at the role of proteolytic cleavage in the development and function of excitatory glutamatergic synapses. However, growing evidence suggests that other synapses, especially inhibitory GABAergic synapses, also undergo activity-dependent remodeling (Lin et al., 2008; Flores et al., 2014). There is evidence that CAMs at GABAergic terminals, such as Neuroligin-2, Dystroglycan, and Agrin, undergo proteolytic cleavage (Suzuki et al., 2012; Pribiag et al., 2014). This suggests that cleavage could regulate the development and function of inhibitory and perhaps other types of synapses as well.
The third question is, mechanistically, what is the consequence of impaired proteolytic processing in neurological disorders? Here we looked at evidence showing that improper proteolytic cleavage of CAMs occurs in Schizophrenia, brain tumors and Alzheimer’s disease. However, many other studies have identified abnormal levels of protease expression and activity in various neurological disorders such as Fragile X, epilepsy, and addiction in both humans and well as mouse models (Brown et al., 2008; Suenaga et al., 2008; Janusz et al., 2013; Konopka et al., 2013; Sidhu et al., 2014). Yet, if and what proteins are subsequently cleaved erroneously, and what signaling pathways are altered in these diseases remains unclear. Tackling these questions will allow a more comprehensive understanding of how activity-dependent proteolytic cleavage guides synapse development and adult function, and how disrupting this cleavage can underlie various nervous system disorders.
Highlights.
Activity-dependent proteolytic cleavage of cell adhesion molecules (CAMs) can permit, drive and restrict synapse maturation
Activity-dependent proteolytic cleavage can regulate Hebbian and homeostatic plasticity
Improper proteolytic cleavage underlies schizophrenia, brain tumors and Alzheimer’s disease
Neuronal activity may control cleavage by regulating proteases, CAMs or both
Acknowledgments
Funding Sources
This work was supported by the NIH grant NS092578 (H.U.)
Abbreviations
- Aβ
Amyloid-β
- ADAM
A disintegrin and metalloproteinase
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazoleproprionic acid
- APP
Amyloid precursor protein
- CAM
Cell adhesion molecule
- CaMK
Calcium-calmodulin dependent kinase
- DCC
Deleted in colorectal cancer
- ECM
Extracellular matrix
- GABA
γ-aminobutyric acid
- ICAM
Intercellular adhesion molecule
- LTD
Long term depression
- LTP
Long term potentiation
- MMP
Matrix metalloproteinase
- mTOR
Mechanistic target of Rapamycin
- NCAM
Neural cell adhesion molecule
- NGL
Netrin G-ligand
- NMDA
N-methyl-D-aspartate
- NMJ
Neuromuscular junction
- PI3K
Phosphoinositide 3-kinase
- PSD
Postsynaptic density
- SIRP
Signal regulatory protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bai G, Pfaff SL. Protease Regulation: The Yin and Yang of Neural Development and Disease. Neuron. 2011;72(1):9–21. doi: 10.1016/j.neuron.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajor M, Michaluk P, Gulyassy P, Kekesi AK, Juhasz G, Kaczmarek L. Synaptic cell adhesion molecule-2 and collapsin response mediator protein-2 are novel members of the matrix metalloproteinase-9 degradome. J Neurochem. 2012;122(4):775–788. doi: 10.1111/j.1471-4159.2012.07829.x. [DOI] [PubMed] [Google Scholar]
- Bemben MA, Shipman SL, Hirai T, Herring BE, Li Y, Badger JD, Nicoll RA, Diamond JS, Roche KW. CaMKII phosphorylation of neuroligin-1 regulates excitatory synapses. Nat Neurosci. 2014;17(1):56–64. doi: 10.1038/nn.3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson DL, Yoshihara Y, Mori K. Polarized distribution and cell type-specific localization of telencephalin, an intercellular adhesion molecule. J Neurosci Res. 1998;52:43–53. doi: 10.1002/(SICI)1097-4547(19980401)52:1<43::AID-JNR5>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Bero AW, Yan P, Roh JH, Cirrito JR, Stewart FR, Raichle ME, Lee JM, Holtzman DM. Neuronal activity regulates the regional vulnerability to amyloid-β deposition. Nat Neurosci. 2011;14(6):750–756. doi: 10.1038/nn.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bot N, Schweizer C, Halima S Ben, Fraering PC. Processing of the synaptic cell adhesion molecule neurexin-3β by Alzheimer disease α- and γ-secretases. J Biol Chem. 2011;286(4):2762–2773. doi: 10.1074/jbc.M110.142521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TE, Forquer MR, Harding JW, Wright JW, Sorg BA. Increase in matrix metalloproteinase-9 levels in the rat medial prefrontal cortex after cocaine reinstatement of conditioned place preference. Synapse. 2008;62:886–889. doi: 10.1002/syn.20562. [DOI] [PubMed] [Google Scholar]
- Conant K, Wang Y, Szklarczyk A, Dudak A, Mattson MP, Lim ST. Matrix metalloproteinase-dependent shedding of intercellular adhesion molecule-5 occurs with long-term potentiation. Neuroscience. 2010;166(2):508–521. doi: 10.1016/j.neuroscience.2009.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conant K, Lonskaya I, Szklarczyk A, Krall C, Steiner J, Maguire-Zeiss K, Lim ST. Methamphetamine-associated cleavage of the synaptic adhesion molecule intercellular adhesion molecule-5. J Neurochem. 2011;118:521–532. doi: 10.1111/j.1471-4159.2010.07153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis GE, Bayless KJ, Davis MJ, Meininger GA. Regulation of tissue injury responses by the exposure of matricryptic sites within extracellular matrix molecules. Am J Pathol. 2000;156:1489–1498. doi: 10.1016/S0002-9440(10)65020-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C, Scholl FG, Choih J, DeMaria S, Berger J, Isacoff E, Scheiffele P. Neurexin mediates the assembly of presynaptic terminals. Nat Neurosci. 2003;6:708–716. doi: 10.1038/nn1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziembowska M, Milek J, Janusz A, Rejmak E, Romanowska E, Gorkiewicz T, Tiron A, Bramham CR, Kaczmarek L. Activity-dependent local translation of matrix metalloproteinase-9. J Neurosci. 2012;32(42):14538–14547. doi: 10.1523/JNEUROSCI.6028-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazeli MS, Breen K, Errington ML, Bliss TVP. Increase in extracellular NCAM and amyloid precursor protein following induction of long-term potentiation in the dentate gyrus of anaesthetized rats. Neurosci Lett. 1994;169:77–80. doi: 10.1016/0304-3940(94)90360-3. [DOI] [PubMed] [Google Scholar]
- Fiala JC, Feinberg M, Popov V, Harris KM. Synaptogenesis via dendritic filopodia in developing hippocampal are CA1. J Neurosci. 1998;18(21):8900–8911. doi: 10.1523/JNEUROSCI.18-21-08900.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores CE, Nikonenko I, Mendez P, Fritschy J-M, Tyagarajan SK, Muller D. Activity-dependent inhibitory synapse remodeling through gephyrin phosphorylation. Proc. Natl. Acad. Sci. U.S.A. 2014:E65–72. doi: 10.1073/pnas.1411170112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MA, Umemori H. Seeking long-term relationship: axon and target communicate to organize synaptic differentiation. J Neurochem. 2006;97:1215–1231. doi: 10.1111/j.1471-4159.2006.03834.x. [DOI] [PubMed] [Google Scholar]
- Fox MA, Sanes JR, Borza DB, Eswarakumar VP, Fässler R, Hudson BG, John SWM, Ninomiya Y, Pedchenko V, Pfaff SL, Rheault MN, Sado Y, Segal Y, Werle MJ, Umemori H. Distinct target-derived signals organize formation, maturation and maintenance of motor nerve terminals. Cell. 2007;129:179–193. doi: 10.1016/j.cell.2007.02.035. [DOI] [PubMed] [Google Scholar]
- Futai K, Kim MJ, Hashikawa T, Scheiffele P, Sheng M, Hayashi Y. Retrograde modulation of presynaptic release probability through signaling mediated by PSD-95-neuroligin. Nat Neurosci. 2007;10:186–195. doi: 10.1038/nn1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galko MJ, Tessier-Lavigne M. Function of an axonal chemoattractant modulated by metalloproteases activity. Science. 2000;289:1365–1367. doi: 10.1126/science.289.5483.1365. [DOI] [PubMed] [Google Scholar]
- Garey LJ, Ong WY, Patel TS, Kanani M, Davis A, Mortimer AM, Barnes TRE, Hirsch SR. Reduced dendritic spine density of cerebral cortical pyramidal neurons in schizophrenia. J Neurol Neursurg Psychiatry. 1998;65:446–453. doi: 10.1136/jnnp.65.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- Glausier JR, Lewis DA. Dendritic spine pathology in Schizophrenia. Neuroscience. 2013;251:90–107. doi: 10.1016/j.neuroscience.2012.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C, Kaether C, Thinakaran G, Sisodia S. Trafficking and Proteolytic Processing of APP. Cold Spring Harbor Perspectives in Medicine. 2012:1–26. doi: 10.1101/cshperspect.a006270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatherly D, Graham SC, Turner J, Harlos K, Stuart DI, Barclay AN. Paired receptor specificity explained by structures of signal regulators proteins alone and complexed with CD47. Mol Cell. 2008;31:266–277. doi: 10.1016/j.molcel.2008.05.026. [DOI] [PubMed] [Google Scholar]
- Hattori M, Osterfield M, Flanagan JG. Regulated cleavage of a contact-mediated axon repellent. Science. 2000;289:1360–1365. doi: 10.1126/science.289.5483.1360. [DOI] [PubMed] [Google Scholar]
- Herring BE, Nicoll RA. Long-term potentiation: From CaMKII to AMPA receptor trafficking. Annu Rev Physiol. 2016;78:351–365. doi: 10.1146/annurev-physiol-021014-071753. [DOI] [PubMed] [Google Scholar]
- Hinkle CL, Diestel S, Lieberman J, Maness PF. Metalloprotease-induced ectodomain shedding of neural cell adhesion molecule (NCAM) J Neurobiol. 2006:1378–1395. doi: 10.1002/neu.20257. [DOI] [PubMed] [Google Scholar]
- Hsieh H, Boehm J, Sato C, Iwatsubo T, Tomita T, Sisodia S, Malinow R. AMPA-R removal underlies Aβ-induced synaptic depression and dendritic spine loss. Neuron. 2006;52:831–843. doi: 10.1016/j.neuron.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janusz A, Milek J, Perycz M, Pacini L, Bagni C, Kaczmarek L. The Fragile X mental retardation protein regulates matrix metalloproteinase 9 mRNA at synapses. J Neurosci. 2013;33:18234–18241. doi: 10.1523/JNEUROSCI.2207-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Venkatesh EM, Umemori H. Secreted factors as synaptic organizers. Eur J Neurosci. 2010;32:181–190. doi: 10.1111/j.1460-9568.2010.07338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamenetz F, Tomita T, Hsieh H, Seabrook G, Borchelt D, Iwatsubo T, Sisodia S, Malinow R. APP Processing and Synaptic Function. Neuron. 2003;37:925–937. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- Kelly EA, Tremblay M, Gahmberg CG, Tian L, Majewska AK. Subcellular Localization of Intercellular Adhesion Molecule-5 (Telencephalin) in the Visual Cortex Is Not Developmentally Regulated in the Absence of Matrix. J Comp Neurol. 2014;5:676–688. doi: 10.1002/cne.23440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Lilliehook C, Dudak A, Prox J, Saftig P, Federoff HJ, Lim ST. Activity-dependent α-cleavage of nectin-1 is mediated by a disintegrin and metalloprotease 10 (ADAM10) J Biol Chem. 2010;285(30):22919–22926. doi: 10.1074/jbc.M110.126649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka A, Grajkowska W, Ziemiańska K, Roszkowski M, Daszkiewicz P, Rysz A, Marchel A, Koperski L, Wilczyński GM, Dzwonek J. Matrix metalloproteinase-9 (MMP-9) in human intractable epilepsy cause by focal cortical dysplasia. Epilepsy Res. 2013;104(1–2):45–58. doi: 10.1016/j.eplepsyres.2012.09.018. [DOI] [PubMed] [Google Scholar]
- Lee H, Lee EJ, Song YS, Kim E. Long-term depression-inducing stimuli promote cleavage of the synaptic adhesion molecule NGL-3 through NMDA receptors, matrix metalloproteinases and presenilin/γ-secretase. Philos T Roy Soc B. 2014;369(1633):20130158. doi: 10.1098/rstb.2013.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Sheng M. Some assembly required: The development of neuronal synapses. Nat Rev Mol Cell Biol. 2003;4:833–841. doi: 10.1038/nrm1242. [DOI] [PubMed] [Google Scholar]
- Lim ST, Chang A, Giuliano RE, Federoff HJ. Ectodomain shedding of nectin-1 regulates the maintenance of dendritic spine density. J Neurochem. 2012;120(5):741–751. doi: 10.1111/j.1471-4159.2011.07592.x. [DOI] [PubMed] [Google Scholar]
- Lin Y, Bloodgood BL, Hauser JL, Lapan AD, Koon AC, Kim TK, Hu LS, Malik AN, Greenberg ME. Activity-dependent regulation of inhibitory synapse development by Npas4. Nature. 2008;455:1198–1204. doi: 10.1038/nature07319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonskaya I, Partridge J, Lalchandani RR, Chung A, Lee T, Vicini S, Hoe HS, Lim ST, Conant K. Soluble ICAM-5, a product of activity dependent proteolysis, increases mEPSC frequency and dendritic expression of GluA1. PLOS ONE. 2013;8(7):1–12. doi: 10.1371/journal.pone.0069136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüthl A, Laurent JP, Figurov A, Muller D, Schachner M. Hippocampal long-term potentiation and neural cell adhesion molecules L1 and NCAM. Nature. 1994;372:777–779. doi: 10.1038/372777a0. [DOI] [PubMed] [Google Scholar]
- Mackenzie IR, Miller LA. Senile plaques in temporal lobe epilepsy. Acta Neuropathol. 1994;87:504–510. doi: 10.1007/BF00294177. [DOI] [PubMed] [Google Scholar]
- Matsumoto-Miyai K, Ninomiya A, Yamasaki H, Tamura H, Nakamura Y, Shiosaka S. NMDA-dependent proteolysis of presynaptic adhesion molecule L1 in the hippocampus by neuropsin. J Neurosci. 2003;23(21):7727–36. doi: 10.1523/JNEUROSCI.23-21-07727.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto-Miyai K, Sokolowska E, Zurlinden A, Gee CE, Lüscher D, Hettwer S, Wölfel J, Ladner AP, Ster J, Gerber U, Rülicke T, Kunz B, Sonderegger P. Coincident pre- and postsynaptic activation induces dendritic filopodia via neurotrypsin-dependent Agrin cleavage. Cell. 2009;136(6):1161–1171. doi: 10.1016/j.cell.2009.02.034. [DOI] [PubMed] [Google Scholar]
- Matsuno H, Okabe S, Mishina M, Yanagida T, Mori K, Yoshihara Y. Telencephalin slows spine maturation. J Neurosci. 2006;26(6):1776–1786. doi: 10.1523/JNEUROSCI.2651-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meighan PC, Meighan SE, Davis CJ, Wright JW, Harding JW. Effects of matrix metalloproteinase inhibition on short- and long-term plasticity of schaffer collateral/CA1 synapses. J Neurochem. 2007;102:2085–2096. doi: 10.1111/j.1471-4159.2007.04682.x. [DOI] [PubMed] [Google Scholar]
- Michaluk P, Kolodziej L, Mioduszewska B, Wilczynski GM, Dzwonek J, Jaworski J, Gorecki DC, Ottersen OP, Kaczmarek L. β-Dystroglycan as a target for MMP-9, in response to enhanced neuronal activity. J Biol Chem. 2007;282(22):16036–16041. doi: 10.1074/jbc.M700641200. [DOI] [PubMed] [Google Scholar]
- Michaluk P, Wawrzyniak M, Alot P, Szczot M, Wyrembek P, Mercik K, Medvedev N, Wikczek E, De Roo M, Zuschratter W, Muller D, Wilczynski GM, Mozrzymas JW, Stewart MG, Kaczmarek L, Wlodarczyk J. Influence of matrix metalloproteinase MMP-9 on dendritic spine morphology. J Cell Sci. 2011;124:3369–3380. doi: 10.1242/jcs.090852. [DOI] [PubMed] [Google Scholar]
- Nagy V, Bozdagi O, Matynia A, Balcerzyk M, Okulski P, Dzwonek J, Costa RM, Silva AJ, Kaczmarek L, Huntley GW. Matrix Metalloproteinase-9 Is Required for Hippocampal Late-Phase Long-Term Potentiation and Memory. J Neurosci. 2006;26(7):1923–1934. doi: 10.1523/JNEUROSCI.4359-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen Ba-Charvet KT, Brose K, Ma L, Wang KH, Marillat V, Sotelo C, Tessier-Lavigne M, Chédotal A. Diversity and specificity of actions of Slit2 proteolytic fragments in axon guidance. J Neurosci. 2001;21(12):4281–4289. doi: 10.1523/JNEUROSCI.21-12-04281.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedringhaus M, Chen X, Dzakpasu R, Conant K. MMPs and soluble ICAM-5 increase neuronal excitability within in vitro networks of hippocampal neurons. PLOS ONE. 2012;7(8):1–9. doi: 10.1371/journal.pone.0042631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning L, Tian L, Smirnov S, Vihinen H, Llano O, Vick K, Davis RL, Rivera C, Gahmberg CG. Interactions between ICAM-5 and beta1 integrins regulate neuronal synapse formation. J Cell Sci. 2013;126(Pt 1):77–89. doi: 10.1242/jcs.106674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning L, Paetau S, Nyman-Huttunen H, Tian L, Gahmberg CG. ICAM-5 affects spine maturation by regulation of NMDA receptor binding to α-actinin. Biology Open. 2015;4(2):125–36. doi: 10.1242/bio.201410439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peixoto RT, Kunz PA, Kwon H, Mabb AM, Sabatini BL, Philpot BD, Ehlers MD. Transsynaptic Signaling by Activity-Dependent Cleavage of Neuroligin-1. Neuron. 2012;76(2):396–409. doi: 10.1016/j.neuron.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai-Nair N, Panicker AK, Rodriguiz RM, Gilmore KL, Demyanenko GP, Huang JZ, Wetsel WC, Maness PF. Neural cell adhesion molecule-secreting transgenic mice display abnormalities in GABAergic interneurons and alterations in behavior. J Neurosci. 2005;25(18):4659–4671. doi: 10.1523/JNEUROSCI.0565-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pribiag H, Peng H, Shah WA, Stellwagen D, Carbonetto S. Dystroglycan mediates homeostatic synaptic plasticity at GABAergic synapses. Proc. Natl. Acad. Sci. U.S.A. 2014;111:6810–6815. doi: 10.1073/pnas.1321774111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Z, Gilbert ME, Colicos MA, Kandel ER, Kuhl D. Tissue-plasminogen activator is induced as an immediate-early gene during seizure, kindling and long-term potentiation. Nature. 1993;361:453–457. doi: 10.1038/361453a0. [DOI] [PubMed] [Google Scholar]
- Reiss K, Maretzky T, Ludwig A, Tousseyn T, de Strooper B, Hartmann D, Saftig P. ADAM10 cleavage of N-cadherin and regulation of cell-cell adhesion and beta-catenin nuclear signalling. EMBO J. 2005;24(4):742–752. doi: 10.1038/sj.emboj.7600548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricard-Blum S, Vallet SD. Proteases decode the extracellular matric cryptome. Biochimie. 2016;122:300–313. doi: 10.1016/j.biochi.2015.09.016. [DOI] [PubMed] [Google Scholar]
- Sanes JR, Lichtman JW. Development of the vertebrate neuromuscular junction. Annu Rev Neurosci. 1999;2:791–805. doi: 10.1146/annurev.neuro.22.1.389. [DOI] [PubMed] [Google Scholar]
- Saura CA, Servián-Morilla E, Scholl FG. Presenilin/γ-secretase regulates Neurexin processing at synapses. PLOS ONE. 2011;6(4):e19430. doi: 10.1371/journal.pone.0019430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiffele P, Fan J, Choih J, Fetter R, Serafini T. Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell. 2000;101(6):657–69. doi: 10.1016/s0092-8674(00)80877-6. [DOI] [PubMed] [Google Scholar]
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008;14(8):837–42. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu H, Dansie LE, Hickmott PW, Ethell DW, Ethell IM. Genetic removal of matrix metalloproteinase 9 rescues the symptoms of fragile X syndrome in a mouse model. J Neurosci. 2014;34:9867–9879. doi: 10.1523/JNEUROSCI.1162-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonderegger P, Matsumoto-Miyai K. Activity-controlled proteolytic cleavage at the synapse. Trends Neurosci. 2014;38(8):413–423. doi: 10.1016/j.tins.2014.05.007. [DOI] [PubMed] [Google Scholar]
- Su J, Stenbjorn RS, Gorse K, Su K, Hauser KF, Ricard-Blum S, Pihlajaniemi R, Fox MA. Target-derived matricryptins organizer cerebellar synapse formation through a3b1 integrins. Cell Reports. 2012;2(2):223–230. doi: 10.1016/j.celrep.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su J, Chen J, Lippold K, Monavarfeshani A, Carrillo GL, Jenkins R, Fox MA. Collagen-derived matricryptins promote inhibitory nerve terminal formation in the developing neocortex. J Cell Biol. 2016;212(6):721–736. doi: 10.1083/jcb.201509085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suenaga N, Ichiyama T, Kubota M, Isumi H, Tohyama J, Furukawa S. Roles of matrix metalloproteinase-9 and tissue inhibitors of metalloproteinases 1 in acute encephalopathy following prolonged febrile seizures. J Neurol Sci. 2008;266:126–130. doi: 10.1016/j.jns.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Hayashi Y, Nakahara S, Kumazaki H, Prox J, Horiuchi K, Zeng M, Tanimura S, Nishiyama Y, Osawa S, Sehara-Fujisawa A, Saftig P, Yokoshima S, Fukuyama T, Matsuki N, Koyama R, Tomita T, Iwatsubo T. Activity-dependent cleavage of Neuroligin-1. Neuron. 2012;76(2):410–422. doi: 10.1016/j.neuron.2012.10.003. [DOI] [PubMed] [Google Scholar]
- Szklarczyk A, Lapinska J, Rylski M, Mckay RD, Kaczmarek L. Matrix metalloproteinase-9 undergoes expression and activation during dendritic remodeling in adult hippocampus. J Neurosci. 2002;22:920–930. doi: 10.1523/JNEUROSCI.22-03-00920.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe Y, Kasahara T, Momoi T, Fujita E. Neuronal RA175/SynCAM1 isoforms are processed by tumor necrosis factor-alpha-converting enzyme (TACE)/ADAM17-like proteases. Neurosci Lett. 2008;444(1):16–21. doi: 10.1016/j.neulet.2008.08.023. [DOI] [PubMed] [Google Scholar]
- Tian L, Stefanidakis M, Ning L, Van Lint P, Nyman-Huttunen H, Libert C, Itohara S, Mishina M, Rauvala H, Gahmberg CG. Activation of NMDA receptors promotes dendritic spine development through MMP-mediated ICAM-5 cleavage. J Cell Biol. 2007;178(4):687–700. doi: 10.1083/jcb.200612097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth AB, Terauchi A, Zhang LY, Johnson-Venkatesh EM, Larsen DJ, Sutton MA, Umemori H. Synapse maturation by activity-dependent ectodomain shedding of SIRPα. Nat Neurosci. 2013;16(10):1417–1425. doi: 10.1038/nn.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura K, Kihara T, Kuzuya A, Okawa K, Nishimoto T, Ninomiya H, Sugimoto H, Kinoshita A, Shimohama S. Characterization of sequential N-cadherin cleavage by ADAM10 and PS1. Neurosci Lett. 2006;402(3):278–283. doi: 10.1016/j.neulet.2006.04.018. [DOI] [PubMed] [Google Scholar]
- Umemori H, Sanes JR. Signal regulatory proteins (SIRPS) are secreted presynaptic organizing molecules. J Biol Chem. 2008;283:34053–34061. doi: 10.1074/jbc.M805729200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kooij MA, Fantin M, Rejmak R, Grosse J, Zanoletti O, Fournier C, et al. Role for MMP-9 in stress-induced downregulation of nectin-3 in hippocampal CA1 and associated behavioural alterations. Nat Commun. 2014;5:4995. doi: 10.1038/ncomms5995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varoqueaux F, Aramuni G, Rawson RL, Mohrmann R, Missler M, Gottmann K, Zhang W, Südhof TC, Brose N. Neuroligins determine synapse maturation and function. Neuron. 2006;51(6):741–754. doi: 10.1016/j.neuron.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Vawter MP, Usen N, Thatcher L, Ladenheim B, Zhang P, VanderPutten DM, Conant K, Herman MM, van Kammen DP, Sedvall G, Garver DL, Freed WJ. Characterization of human cleaved N-CAM and association with Schizophrenia. Experimental Neurology. 2001;172:29–46. doi: 10.1006/exnr.2001.7790. [DOI] [PubMed] [Google Scholar]
- Venkatesh HS, Johung TB, Caretti V, Noll A, Tang Y, Nagaraja S, Gibson EM, Mount CW, Polepalli J, Mitra SS, Woo PJ, Malenka RC, Vogel H, Bredel M, Mallick P, Monje M. Neuronal activity promotes glioma growth through Neuroligin-3 secretion. Cell. 2015;161:803–816. doi: 10.1016/j.cell.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Hauswirth AG, Tong A, Dickman DK, Davis GW. Endostatin is a trans -synaptic signal for homeostatic synaptic plasticity. Neuron. 2014;83(3):616–629. doi: 10.1016/j.neuron.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XB, Bozdagi O, Nikitczuk JS, Zhai ZW, Zhou Q, Huntley GW. Extracellular proteolysis by matrix metalloproteinase-9 drives dendritic spine enlargement and long-term potentiation coordinately. Proc Natl Acad Sci USA. 2008;105:19520–19525. doi: 10.1073/pnas.0807248105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenmayer N, Körber C, Liu H, Kremer T, Varoqueaux F, Chapman ER, Brose N, Kuner T, Dresbach T. Postsynaptic Neuroligin1 regulates presynaptic maturation. Proc Natl Acad Sci USA. 2009;106:13564–13569. doi: 10.1073/pnas.0905819106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Petralia RS, Kurushima H, Patel H, Jung MY, Volk L, Chowdury S, Shepherd JD, Dehoff M, Li Y, Kuhl D, Huganir RL, Price DL, Scannevin R, Troncoso JC, Wong PC, Worley PF. Arc/Arg3.1 regulates an endosomal pathway essential for activity-dependent β-Amyloid Generation. Cell. 2011;147(3):615–628. doi: 10.1016/j.cell.2011.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Xiao N, Xia J. Thrombospondin 1 accelerates synaptogenesis in hippocampal neurons through Neuroligin 1. Nat Neurosci. 2010;13:22–24. doi: 10.1038/nn.2459. [DOI] [PubMed] [Google Scholar]
- Yasuda M, Umemori H. Synapse formation in the mammalian central nervous system. In: Hortsch M, Umemori H, editors. The Sticky Synapse: Cell Adhesion Molecules and Their Role in Synapse Formation and Maintenance. Springer; New York: 2009. pp. 85–106. [Google Scholar]
- Yuste R, Bonhoeffer T. Genesis of dendritic spines: Insights from ultrastructural and imaging studies. Nature. 2004;5:24–34. doi: 10.1038/nrn1300. [DOI] [PubMed] [Google Scholar]
- Zhuang J, Wei Q, Lin Z, Zhou C. Effects of ADAM10 deletion on Notch-1 signaling pathway and neuronal maintenance in adult mouse brain. Gene. 2015;555:150–158. doi: 10.1016/j.gene.2014.10.056. [DOI] [PubMed] [Google Scholar]