Abstract
Aim
Major depression is associated with hippocampal volume changes, especially in late-life depression. These changes usually consist of volume reductions, but depression-related increases in hippocampal volume have also been reported. Subfield analysis has identified structural changes primarily in the CA1, CA2–3, and subiculum of the hippocampus in individuals with major depression; however, it is unclear whether lower levels of depressive symptoms are also associated volume reduction, or if depressive symptoms interact with age to impact hippocampal subfields. The current study addressed these questions.
Methods
Forty-three community-dwelling older adults completed the Center for Epidemiologic Studies Depression Scale (CES-D) and underwent magnetic resonance imaging. Hippocampal subfield segmentation was performed using an automated procedure, and left and right volumes from CA1, CA2–3, and the subiculum served as outcome measures. Multiple hierarchical regressions were conducted with age, CES-D scores, and their interaction as the independent variables, and sex and total intracranial volume as covariates.
Results
Higher CES-D scores were associated with less age-related volumetric decreases in the right subiculum and right CA1.
Conclusions
Age-related atrophy in the hippocampus may be counteracted by depressive symptom-related enlargement of CA1 and the subiculum. More research is needed to better understand the functional significance of this relationship.
Keywords: aging, brain volume, depressive symptoms, hippocampus, MRI
Introduction
Major depression (MDD) is the most common psychiatric disorder seen in community-dwelling older adults1. Depression can be thought of as a continuum of symptoms that range from milder conditions, such as elevated depressive symptoms, to more severe forms of major depression. Elevated depressive symptoms are even more common than major depression in older adults, with an estimated prevalence of 7–15%2. These subthreshold depressive symptoms are of critical concern, as they are associated with similar cognitive and fronto-subcortical neural dysfunction and adverse health outcomes as major depression3, 4, but are often undiagnosed and therefore untreated.
For outcomes such as brain changes, the impact of subthreshold depressive symptoms may be greater in older adults compared to young adults due to the cumulative effect of depressive symptoms and normal age-related changes. In particular, depression-related hippocampal alterations may be more pronounced in older adults compared to their younger counterparts due to the cumulative effect of depression5 and age-related hippocampal atrophy6. Older age is associated with hippocampal volume reduction, but findings in major and subthreshold depression vary, with many studies reporting smaller hippocampal volume7, 8, but other studies reporting no differences9, 10 or larger hippocampal volume for at least some subgroups of depressed individuals11.
Inconsistencies in the depression literature may be due to heterogeneity within subregions of the hippocampus that is obscured when the hippocampus is examined globally. The hippocampus comprises histologically distinct functional and structural subfields, including cornu ammonis (CA) 1–4, subiculum, and dentate gyrus (DG)12, that have different associations with memory and other functions and may also be differentially related to both depressive disorders and non-pathological aging. Findings for the relationships between hippocampal subfields, depression, and aging are heterogeneous, with differing results for the subfield most affected. With respect to depression, some studies indicate smaller CA1, CA2–3, and subiculum volume in individuals with late-life depression13, 14 and less DG volume as a function of multiple depressive episodes in young to middle-aged adults15. In contrast, there is also evidence of larger volume of CA1 and portions of the subiculum bilaterally in unmedicated young to middle-aged depressed adults16. Similarly, findings on the effect of age on hippocampal subfields varies, with some studies showing age effects on volume in the subiculum and relative sparing of CA1 and other subfields17, while others show age effects on volumes in CA2–3 and CA4-DG18. Less is known about the potentially interactive effect of age and elevated depressive symptoms on hippocampal subfield volume.
The purpose of the current study was to determine whether or not age effects on volume of hippocampal subfields are modified by elevated depressive symptoms in older adults. Based on CA1, CA2–3, and the subiculum being most consistently related to late-life depression13, we focused on these regions. We predicted that older age would be associated with smaller volume in these hippocampal subfields and that this association would be more pronounced at higher levels of depressive symptoms.
Methods
Participants
Forty-eight community-dwelling older adults (mean age = 68.88 ± 7.21 years) were recruited for this study. All participants were right-handed, native English speakers with at least 8 years of education. Participants were required to have a score of greater than 30 on the Telephone Interview for Cognitive Status (TICS)19 and a score of greater than 24 on the Mini-Mental State Examination (MMSE)20, which are the suggested cut-offs for cognitive impairment, respectively. Exclusionary criteria included self-reported history of major neurological or other medical illness, head trauma, learning disorders, current epileptic or antipsychotic medication usage, language comprehension difficulties, and magnetic resonance imaging (MRI) contraindications. Participants with MDD were not excluded in order to increase the range of depressive symptom severity in the sample. Two participants met criteria for MDD per clinical interview. Both were taking antidepressant medication, as were five additional subjects who did not meet criteria for depression. Five subjects were excluded from analyses due to either missing data, MRI evidence of past stroke, current substance abuse, or a learning disorder diagnosis. Thus, our final sample comprised 43 individuals (9 young-old (aged 55–64), 24 middle-old (aged 65–74), and 10 old-old (aged 75+)). Demographic data for this sample is presented in Table 1. All procedures were reviewed and approved by the University of Florida’s Institutional Review Board and all participants provided verbal and written informed consent.
Table 1.
Sample Demographic Characteristics
| Mean | SD | Range | |
|---|---|---|---|
| Total Sample (n = 43) | |||
| Age (years) | 68.79 | 7.00 | 55–81 |
| Sex (% female) | 69.76 | -- | -- |
| Education (years) | 15.07 | 2.53 | 10–20 |
| MMSE total | 28.91 | 1.25 | 25–30 |
| CES-D total | 7.84 | 8.90 | 0–45 |
| Those Using Antidepressants (n = 7) | |||
| Age (years) | 62.57 | 6.78 | 56–72 |
| Sex (% female) | 71.46 | -- | -- |
| Education (years) | 15.57 | 2.64 | 12–19 |
| MMSE total | 29.42 | 0.79 | 28–30 |
| CES-D total | 17.29 | 16.09 | 1–45 |
Notes. SD = standard deviation; MMSE = Mini-Mental State Examination; CES-D = Center for Epidemiologic Studies Depression Scale.
Measures
Participants completed the Center for Epidemiologic Studies Depression Scale (CES-D)21, which consists of 20 self-report questions assessing the frequency and severity of depressive symptoms over the previous week.
MRI Data Acquisition
MRI data was collected within one week of completing the CES-D at the University of Florida’s McKnight Brain Institute on the Advanced Magnetic Resonance Imaging and Spectroscopy (AMRIS) facility’s Philips (Amsterdam, Netherlands) 3-Tesla scanner using a Philips 8-channel radio-frequency coil. A high resolution, T1-weighted turbo field echo anatomical scan was collected using the following parameters: TR = 81 ms, TE = 3.7 ms, 170 slices acquired in a sagittal orientation, flip angle = 8 degrees, 1 mm cubic resolution. To minimize noise while in the scanner, participants were given headphones and earplugs. Head movement was minimized via cushions positioned inside the head coil.
Hippocampal Subfield Measurement
The Freesurfer image analysis suite (version 5.3, http://surfer.nmr.mgh.harvard.edu) was used to quantify brain volumes22. Briefly, processing included motion correction, removal of non-brain tissue, automated Talairach transformation, segmentation of the gray and white matter tissue, and cortical surface inflation. Each image was also manually inspected for errors in the automatic processing by one of two raters. A two-way mixed effects model calculated the interclass correlation coefficient (ICC) for manual volume adjustments. ICC between raters was extremely high (0.99), likely reflecting the minimal manual adjustments needed following the automatic processing. Volumes of the bilateral hippocampi were obtained using an automated procedure for volumetric measurement of brain structure, which uses Bayesian inference and a probabilistic atlas of hippocampal formation based on manual delineations of subfields in ultra-high-T1-weighted MRI scans from a number of subjects23. The left and right hippocampi were segmented into 7 subfields: CA1, CA2–3, CA4-dentate gyrus, subiculum, presubiculum, fimbria, and hippocampal fissure. Average dice coefficients of approximately 0.7 for CA2–3 and subiculum were reported for overlap between manual and automated segmentation methods23. Regions of interest for the current study included left and right volumes from CA1, CA2–3, and the subiculum.
Statistical Analysis
All analyses were conducted using SPSS 22.0 software (Armonk, NY). Separate hierarchical regression analyses were conducted for the left and right CA1, CA2–3, and subiculum, with age, CES-D scores, and their interaction as the independent variables, and sex and total intracranial volume as covariates. Education and antidepressant usage were initially entered as covariates, but were removed from final analyses due to lack of statistical significance. CES-D scores were highly skewed; therefore we applied a square root transformation to this data to ensure a more normal distribution. All variables besides sex were continuous measures in the models. Age and CES-D scores were mean-centered and multiplied to create the interaction terms. We used a statistical significance threshold of α ≤ 0.05. Due to the relatively small sample size, correcting for multiple comparisons would result in a highly stringent threshold for significance and may increase the chance of type II error. We therefore present uncorrected results, but indicate when results met significance after Bonferroni multiple comparison correction.
Results
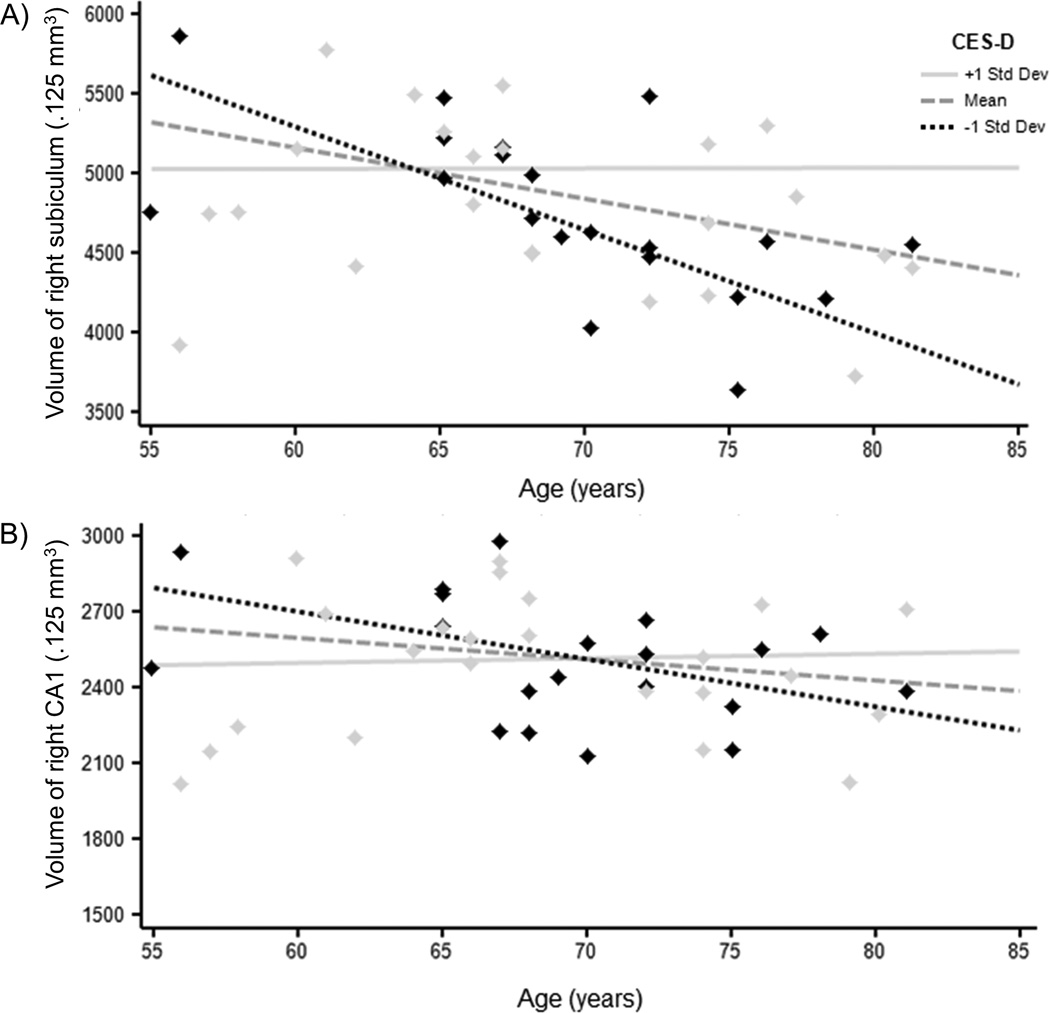
Results are summarized in Table 2 and Figure 1. With respect to the subiculum, there was a significant main effect of age, such that older age was associated with smaller volume of the subiculum bilaterally (right: p = 0.003, left: p = 0.008; both significant after Bonferroni correction). This was further qualified by a significant age × CES-D interaction for the right subiculum (p = 0.001; significant after Bonferroni correction), suggesting that age effects on volume were greater in individuals with lower CES-D scores, but minimized in individuals with higher CES-D scores. A similar age × CES-D interaction was found for right CA1 subfield volume (p = 0.023). There were no other significant main effects or age × CES-D interactions for the other regions of interest. This pattern of results was unchanged when the two participants with MDD were excluded.
Table 2.
Effects of Age and CES-D Scores on Hippocampal Subfield Volumes, adjusted for Total Intracranial Volume and Sex
| Total ICV | Sex | Age | CES-D | Age × CES-D | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | t | p | β | t | p | β | t | p | β | t | p | β | t | p | |
| RIGHT | |||||||||||||||
|
| |||||||||||||||
| CA1 | 0.361 | 2.514 | 0.016* | −0.204 | −1.452 | 0.155 | −0.214 | −1.612 | 0.116 | −0.024 | −0.155 | 0.877 | 0.356 | 2.375 | 0.023* |
| CA2–3 | 0.320 | 2.063 | 0.046* | −0.248 | −1.632 | 0.111 | −0.271 | −1.893 | 0.066 | −0.099 | −0.594 | 0.556 | 0.113 | 0.698 | 0.490 |
| Subiculum | 0.151 | 1.107 | 0.276 | −0.246 | −1.844 | 0.073 | −0.408 | −3.236 | 0.003** | 0.209 | 1.418 | 0.165 | 0.497 | 3.489 | 0.001** |
|
| |||||||||||||||
| LEFT | |||||||||||||||
|
| |||||||||||||||
| CA1 | 0.456 | 2.875 | 0.007** | −0.096 | −0.619 | 0.540 | −0.206 | −1.407 | 0.168 | −0.081 | −0.474 | 0.638 | 0.024 | 0.148 | 0.883 |
| CA2–3 | 0.269 | 1.739 | 0.090 | −0.316 | −2.089 | 0.044* | −0.209 | −1.463 | 0.152 | −0.156 | −0.933 | 0.357 | 0.104 | 0.644 | 0.523 |
| Subiculum | 0.360 | 2.467 | 0.018* | −0.177 | −1.238 | 0.223 | −0.376 | −2.783 | 0.008** | 0.166 | 1.050 | 0.301 | 0.277 | 1.818 | 0.077 |
Notes. ICV = Intracranial Volume; CES-D = Center for Epidemiologic Studies Depression Scale; CA = cornu ammonis. Men were coded as 0; women were coded as 1.
p < 0.05.
significant after Bonferroni correction (p ≤ 0.008).
Figure 1.
Significant results for the age × CES-D interactions on volumes in the A) right subiculum, and B) right CA1. Raw scores are presented for ease of display, but age and CES-D scores were entered as continuous variables in the statistical models and were centered around the mean in all analyses. CES-D = Center for Epidemiologic Studies Depression Scale; CA = cornu ammonis; mm = millimeters.
Discussion
This study examined the interrelationships between depressive symptoms, age, and hippocampal subfield volumes. Previous work has generally shown smaller volumes in the subiculum and CA1–3 subfields in both midlife and late-life depression, as well as smaller dentate gyrus volume in young depressed adults13, 15. We add to this limited literature by investigating the interaction of age and depressive symptom severity in older adults with mostly subthreshold symptoms. This focus is important considering the high prevalence of subthreshold depressive symptoms in older adults2 and the impact of non-pathological aging on hippocampal subfield volumes18, which raises the possibility of a cumulative effect of aging and depressive symptoms on hippocampal structure.
Our finding of greater age effects on volume in individuals with lower depressive symptoms and less of an age effect at higher depressive symptom severity is contrary to our hypothesis. Nonetheless, results are not completely unexpected in the context of previous reports of larger volumes in the hippocampus. At least one study found larger hippocampal regions analogous to CA1 and the subiculum bilaterally in patients with MDD16, and depression-related enlargement of total hippocampal volume has also been reported11. In the present study, age effects on volume within the hippocampus may have been counteracted by depressive symptom-related enlargement of CA1 and the subiculum.
While the functional significance of larger hippocampal volumes, particularly in CA1 and the subiculum, in individuals with elevated depressive symptoms remains unclear, it may be that CA1 and the subiculum are particularly vulnerable to the effects of depression, as our study and others13, 16 have found alterations in these subfields. Post-mortem studies of individuals with mood disorders have also provided evidence of disproportionate structural changes in CA1 and the subiculum24. CA1 projects to the subiculum, which in turn provides the main output of the hippocampal formation to structures involved in mood regulation, including the entorhinal cortex, amygdala, ventromedial prefrontal cortex, and striatum25. The subiculum is suggested to be integral to hippocampal interactions with the hypothalamic-pituitary-adrenal (HPA) axis25. HPA axis dysfunction is thought to play a role in the pathophysiology of MDD, with persistent elevation of glucocorticoids leading to hippocampal atrophy26.
The mechanisms underlying larger, rather than smaller, hippocampal volume in relation to elevated depressive symptoms are unclear. Some researchers have argued that the early stages of depression are marked by a compensatory inflammatory response27, which may modulate neurogenesis in the hippocampus via activation of proinflammatory cytokines. In addition to increased hippocampal volumes, increased blood flow to the hippocampus has been seen in acutely depressed patients28, suggesting that these changes may reflect early or acute stages of depression. It may only be through prolonged duration of depressive symptoms that hippocampal atrophy becomes evident29. Most of our participants had subthreshold depressive symptoms, and results were unchanged when excluding two participants with MDD. Combined with evidence that subthreshold depressive symptoms are often a precursor to MDD2, this suggests our findings may reflect neurobiological changes that increase the risk for future clinical depression, which may subsequently lead to smaller hippocampal volumes if untreated.
The impact of depression treatment on hippocampal volumes has been highlighted by other investigations. There is evidence that longer duration of untreated depression is related to hippocampal volume reduction30, while antidepressant treatment is associated with increased volume over time31. Additional clinical variables may impact the relationship between depression and volume in the hippocampus. For example, morphological abnormalities were found in the left anterior subiculum and lateral CA1 in late-onset compared to early-onset depression in one study13. Other studies have found differences in first-episode compared to recurrent depression15, 32, including evidence of a positive relationship between total and subfield hippocampal volumes and severity of depression in first-episode MDD. Moreover, comorbid symptoms of anxiety may also play a role in increased hippocampal volume, as research has suggested a positive relationship between increased anxiety and larger hippocampal volumes33. There is some suggestion from the pediatric depression literature that anxiety influences the ratio of hippocampal volumes to volumes in the amygdala34. The amygdala is a closely connected structure that is important for emotional expression and, together with the hippocampus, has a role in the formation of emotion-related memories35–37. Larger studies are needed to investigate individual variability in anxiety and other clinical moderators and their relationship to depression-related brain changes as possible methods for better understanding the underlying mechanisms of depression and improving intervention strategies.
The current findings should be interpreted in the context of limitations of the study, including the inherent limitations of the automated hippocampal segmentation program38, as well as our relatively small sample size. In addition, our sample included individuals taking antidepressants. While we did not find any differences in subfield volumes between the two groups, it has been shown that antidepressant use can affect hippocampal volume31 and that may have played a role in our findings. Furthermore, while all participants in this study had TICS scores greater than 30 and MMSE scores greater than 24, we cannot rule out the possibility that individuals with mild cognitive impairment were included, which may have affected the hippocampal subfield results. Moreover, information regarding anxiety symptoms was not available for all participants in our study; therefore, we were unable to determine the influence of anxiety on the present results. Nevertheless, the study adds to the literature by investigating depressive symptoms as a continuous measure and not as a dichotomous variable (MDD vs. healthy controls), as many other studies have previously done. Gaining a better understanding of the longitudinal relationship between depressive symptoms and age-related hippocampal volume change may increase our understanding of the pathophysiology of depression in older adults and provide potential targets for behavioral and pharmacological treatments.
Acknowledgments
This work was supported by the McKnight Brain Research Foundation; the National Institute on Aging (under Grants T32AG020499-11 and P30AG028740-01); the National Center for Advancing Translational Science (under Grants UL1TF000064 and KL2TR000065); and the Thomas H. Maren Foundation. Neuroimaging was performed at the Advanced Magnetic Resonance Imaging and Spectroscopy (AMRIS) facility in the McKnight Brain Institute of the University of Florida, which is supported by National Science Foundation Cooperative Agreement No. DMR-1157490 and the State of Florida.
SMS assisted with data collection and image processing, performed statistical analyses, and took primary responsibility for manuscript writing. MEM assisted with image processing and manuscript writing. AO assisted with image processing and manuscript writing. AJW supervised all image processing and assisted with manuscript writing. SDA assisted with data collection and manuscript writing. VMD designed the study, supervised data collection, and supervised statistical analyses and manuscript writing.
Footnotes
Disclosure Statement
The authors declare no conflict of interest.
References
- 1.Luijendijk HJ, van den Berg JF, Dekker MJ, et al. Incidence and recurrence of late-life depression. Arch Gen Psychiatry. 2008;65:1394–1401. doi: 10.1001/archpsyc.65.12.1394. [DOI] [PubMed] [Google Scholar]
- 2.Laborde-Lahoz P, El-Gabalawy R, Kinley J, Kirwin PD, Sareen J, Pietrzak RH. Subsyndromal depression among older adults in the USA: prevalence, comorbidity, and risk for new-onset psychiatric disorders in late life. Int J Geriatr Psychiatry. 2015;30:677–685. doi: 10.1002/gps.4204. [DOI] [PubMed] [Google Scholar]
- 3.Kumar A, Jin Z, Bilker W, Udupa J, Gottlieb G. Late-onset minor and major depression: early evidence for common neuroanatomical substrates detected by using MRI. Proc Natl Acad Sci U S A. 1998;95:7654–7658. doi: 10.1073/pnas.95.13.7654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cuijpers P, Vogelzangs N, Twisk J, Kleiboer A, Li J, Penninx BW. Differential mortality rates in major and subthreshold depression: meta-analysis of studies that measured both. Br J Psychiatry. 2013;202:22–27. doi: 10.1192/bjp.bp.112.112169. [DOI] [PubMed] [Google Scholar]
- 5.Fotuhi M, Do D, Jack C. Modifiable factors that alter the size of the hippocampus with ageing. Nat Rev Neurol. 2012;8:189–202. doi: 10.1038/nrneurol.2012.27. [DOI] [PubMed] [Google Scholar]
- 6.Allen JS, Bruss J, Brown CK, Damasio H. Normal neuroanatomical variation due to age: the major lobes and a parcellation of the temporal region. Neurobiol Aging. 2005;26:1245–1260. doi: 10.1016/j.neurobiolaging.2005.05.023. discussion 79–82. [DOI] [PubMed] [Google Scholar]
- 7.Cole J, Costafreda SG, McGuffin P, Fu CH. Hippocampal atrophy in first episode depression: a meta-analysis of magnetic resonance imaging studies. J Affect Disord. 2011;134:483–487. doi: 10.1016/j.jad.2011.05.057. [DOI] [PubMed] [Google Scholar]
- 8.Spalletta G, Piras F, Caltagirone C, Fagioli S. Hippocampal multimodal structural changes and subclinical depression in healthy individuals. J Affect Disord. 2014;152–154:105–112. doi: 10.1016/j.jad.2013.05.068. [DOI] [PubMed] [Google Scholar]
- 9.Greenberg DL, Payne ME, MacFall JR, Steffens DC, Krishnan RR. Hippocampal volumes and depression subtypes. Psychiatry Res. 2008;163:126–132. doi: 10.1016/j.pscychresns.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dotson VM, Davatzikos C, Kraut MA, Resnick SM. Depressive symptoms and brain volumes in older adults: a longitudinal magnetic resonance imaging study. J Psychiatry Neurosci. 2009;34:367–375. [PMC free article] [PubMed] [Google Scholar]
- 11.Phillips JL, Batten LA, Tremblay P, Aldosary F, Blier P. A prospective, longitudinal study of the effect of remission on cortical thickness and hippocampal volume in patients with treatment-resistant depression. Int J Neuropsychopharmacol. 2015;18:1–9. doi: 10.1093/ijnp/pyv037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szabo K, Hennerici MG. The Hippocampus in Clinical Neuroscience. Switzerland: Karger; 2014. [Google Scholar]
- 13.Ballmaier M, Narr KL, Toga AW, et al. Hippocampal morphology and distinguishing late-onset from early-onset elderly depression. AJ Psychiatry. 2008;165:229–237. doi: 10.1176/appi.ajp.2007.07030506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim HK, Hong SC, Jung WS, et al. Automated hippocampal subfields segmentation in late life depression. J Affect Disord. 2012;143:253–256. doi: 10.1016/j.jad.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 15.Treadway MT, Waskom ML, Dillon DG, et al. Illness progression, recent stress, and morphometry of hippocampal subfields and medial prefrontal cortex in major depression. Biol Psychiatry. 2015;77:285–294. doi: 10.1016/j.biopsych.2014.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bearden CE, Thompson PM, Avedissian C, et al. Altered hippocampal morphology in unmedicated patients with major depressive illness. ASN Neuro. 2009;1:e00020. doi: 10.1042/AN20090026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.La Joie R, Fouquet M, Mezenge F, et al. Differential effect of age on hippocampal subfields assessed using a new high-resolution 3T MR sequence. Neuroimage. 2010;53:506–514. doi: 10.1016/j.neuroimage.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 18.Pereira JB, Valls-Pedret C, Ros E, et al. Regional vulnerability of hippocampal subfields to aging measured by structural and diffusion MRI. Hippocampus. 2014;24:403–414. doi: 10.1002/hipo.22234. [DOI] [PubMed] [Google Scholar]
- 19.Brandt J, Spencer M, Folstein M. The telephone interview for cognitive status. Neuropsychiatry Neuropsychol Behav Neurol. 1988;1:111–117. [Google Scholar]
- 20.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 21.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Appl Psych Meas. 1977;1:385–401. [Google Scholar]
- 22.Fischl B. FreeSurfer. Neuroimage. 2012;62:774–781. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Leemput K, Bakkour A, Benner T, et al. Automated segmentation of hippocampal subfields from ultra-high resolution in vivo MRI. Hippocampus. 2009;19:549–557. doi: 10.1002/hipo.20615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosoklija G, Toomayan G, Ellis SP, et al. Structural abnormalities of subicular dendrites in subjects with schizophrenia and mood disorders: preliminary findings. Arch Gen Psychiatry. 2000;57:349–356. doi: 10.1001/archpsyc.57.4.349. [DOI] [PubMed] [Google Scholar]
- 25.O'Mara S. The subiculum: what it does, what it might do what neuroanatomy has yet to tell us. J Anat. 2005;207:271–282. doi: 10.1111/j.1469-7580.2005.00446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conrad CD. Chronic stress-induced hippocampal vulnerability: the glucocorticoid vulnerability hypothesis. Rev Neurosci. 2008;19:395–411. doi: 10.1515/revneuro.2008.19.6.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dowlati Y, Herrmann N, Swardfager W, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 28.Videbech P, Ravnkilde B, Pedersen AR, et al. The Danish PET/depression project: PET findings in patients with major depression. Psychol Med. 2001;31:1147–1158. doi: 10.1017/s0033291701004469. [DOI] [PubMed] [Google Scholar]
- 29.Bell-McGinty S, Butters MA, Meltzer CC, Greer PJ, Reynolds CF, 3rd, Becker JT. Brain morphometric abnormalities in geriatric depression: long-term neurobiological effects of illness duration. AJ Psychiatry. 2002;159:1424–1427. doi: 10.1176/appi.ajp.159.8.1424. [DOI] [PubMed] [Google Scholar]
- 30.Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. AJ Psychiatry. 2003;160:1516–1518. doi: 10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- 31.Frodl T, Jager M, Smajstrlova I, et al. Effect of hippocampal and amygdala volumes on clinical outcomes in major depression: a 3-year prospective magnetic resonance imaging study. J Psychiatry Neurosci. 2008;33:423–430. [PMC free article] [PubMed] [Google Scholar]
- 32.Cole J, Toga AW, Hojatkashani C, et al. Subregional hippocampal deformations in major depressive disorder. J Affect Disord. 2010;126:272–277. doi: 10.1016/j.jad.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rusch BD, Abercrombie HC, Oakes TR, Schaefer SM, Davidson RJ. Hippocampal morphometry in depressed patients and control subjects: relations to anxiety symptoms. Biol Psychiatry. 2001;50:960–964. doi: 10.1016/s0006-3223(01)01248-3. [DOI] [PubMed] [Google Scholar]
- 34.MacMillan S, Szeszko PR, Moore GJ, et al. Increased amygdala: hippocampal volume ratios associated with severity of anxiety in pediatric major depression. J Child Adolesc Psychopharmacol. 2003;13:65–73. doi: 10.1089/104454603321666207. [DOI] [PubMed] [Google Scholar]
- 35.Davis M. The role of the amygdala in fear-potentiated startle: implications for animal models of anxiety. Trends Pharmacol Sci. 1992;13:35–41. doi: 10.1016/0165-6147(92)90014-w. [DOI] [PubMed] [Google Scholar]
- 36.LeDoux JE. Emotional memory systems in the brain. Behav Brain Res. 1993;58:69–79. doi: 10.1016/0166-4328(93)90091-4. [DOI] [PubMed] [Google Scholar]
- 37.McGaugh JL, Introini-Collison IB, Nagahara AH, Cahill L, Brioni JD, Castellano C. Involvement of the amygdaloid complex in neuromodulatory influences on memory storage. Neurosci Biobehav Rev. 1990;14:425–431. doi: 10.1016/s0149-7634(05)80065-x. [DOI] [PubMed] [Google Scholar]
- 38.Wisse LE, Biessels GJ, Geerlings MI. A critical appraisal of the hippocampal subfield segmentation package in FreeSurfer. Front Aging Neurosci. 2014;6:261. doi: 10.3389/fnagi.2014.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]