Abstract
BACKGROUND/OBJECTIVES
A pivotal role of oxidative stress has been emphasized in the pathogenesis as well as in the disease progression of Parkinson's disease (PD). We aimed at investigating serum levels of antioxidant vitamins and elucidating whether they could be associated with the pathogenesis and progression of PD.
MATERIALS/METHODS
Serum levels of retinol, α- and γ-tocopherols, α- and β-carotenes, lutein, lycopene, zeaxanthin and β-cryptoxanthin were measured and compared between 104 patients with idiopathic PD and 52 healthy controls matched for age and gender. In order to examine the relationship between antioxidant vitamins and the disease progression, multiple group comparisons were performed among the early PD (Hoehn and Yahr stage I and II, N = 47), advanced PD (stage III and IV, N = 57) and control groups. Separate correlation analyses were performed between the measured antioxidant vitamins and clinical variables, such as Hoehn and Yahr stage and Unified Parkinson's Disease Rating Scale (UPDRS) motor score.
RESULTS
Compared to controls, PD patients had lower levels of α- and β-carotenes and lycopene. α-carotene, β-carotene and lycopene levels were significantly reduced in advanced PD patients relative to early PD patients and were negatively correlated with Hoehn and Yahr stage and UPDRS motor score in PD patients. No significant differences were found in serum levels of retinol, α- and γ-tocopherols, and other carotenoids between PD patients and controls. No significant correlations were found between these vitamin levels and clinical variables in PD patients.
CONCLUSIONS
We found that serum levels of some carotenoids, α-carotene, β-carotene and lycopene, were lower in PD patients, and that these carotenoids inversely correlated with clinical variables representing disease progression. Our findings suggest that decreases in serum α-carotene, β-carotene and lycopene may be associated with the pathogenesis as well as progression of PD.
Keywords: Parkinson disease, oxidative stress, carotenoids, disease progression
INTRODUCTION
Parkinson's disease (PD) is the second most prevalent neurodegenerative disorder after Alzheimer's disease [1]. The incidence of PD rises steeply with increasing age, reaching 93.1 in 100,000 person-years between 70 and 79 years [1]. With elongation of life expectancy, a rapid increase in the prevalence of PD is predicted in Korea [2].
PD is a slowly progressive, debilitating neurodegenerative disorder. Its pathologic hallmark is the preferential loss of dopaminergic neurons in the substantia nigra pars compacta in association with the presence of ubiquinated protein deposits in the cytoplasm of neurons, namely Lewy bodies [3]. Although the mechanisms responsible for nigral dopaminergic cell degeneration remain obscure, a growing body of evidence suggests that degeneration of substantia nigra neurons may be caused by oxidative stress and damage from increased formation or insufficient scavenging of cytotoxic reactive oxygen species [4].
Carotenoids that are found in detectable levels in brain tissues such as frontal and occipital lobes [5], are known to be effective lipophilic antioxidants quenching singlet oxygen and scavenging other reactive oxygen species [6]. Like carotenoids, vitamin E is an essential antioxidant that has neuroprotective functions [7]. As more evidence points to the role of oxidative stress in PD, many investigators have examined antioxidant vitamin E and carotenoids in PD cohorts, with inconsistent findings across the studies. Several studies have shown that higher intakes of vitamin E [8,9,10,11] or carotenoids [10,12,13] may be associated with a low risk of the PD development, whereas other studies have found no significant association between intakes of vitamin E [12,14,15,16] and carotenoids [11,12,16] and the risk of PD. Some observational studies have found no difference between PD patients and healthy controls in serum, plasma or erythrocyte vitamin E [17,18,19,20,21,22,23] and carotenoids [22,24].
In the current study, we compared serum levels of retinol, carotenoids and tocopherols between patients with early and advanced PD and controls. Possible correlations were also delineated between these vitamins and clinical variables representing disease severity or progression.
MATERIALS AND METHODS
Patients and controls
Consecutive 104 patients with idiopathic PD (63 females; mean age, 66.9 ± 8.2 years) and 52 healthy control subjects (37 females; mean age, 65.4 ± 8.4 years) were recruited from the outpatient clinic in the Department of Neurology at Korea University Guro Hospital. Control subjects were mostly spouses and relatives of the patients. Unaffected spouses may be a good source of control in studies on neurodegenerative disorders due to time- and cost-efficiency [25,26]. All subjects gave written informed consent prior to their inclusion and the local Ethics Committee approved the study (KUGH0543).
The following exclusion criteria were applied to all subjects: (a) history of ischemic or hemorrhagic stroke or severe head trauma, (b) cognitive impairment as assessed by Mini-Mental State Examination score of 24 or less, (c) regular intake of antioxidant drugs or vitamin supplements in the last 6 months, (d) heavy alcohol consumption, (e) atypical dietary habits (diets constituted exclusively of one type of foodstuff, such as vegetables, fruits, meat, or others), and (f) history of chronic systemic diseases such as liver cirrhosis or chronic renal failure. All patients, but not control subjects, underwent MRI examination, and patients with significant structural lesions, such as hydrocephalus, brain tumor, or severe leukoaraiosis, were further excluded. The clinical diagnosis of idiopathic PD was made by two experienced neurologists (J.H.K. and S.B.K.), based on the established criteria [27]. Demographic data and clinical variables such as age of onset, duration of illness, antiparkinsonian medications at the time of study, Hoehn and Yahr (H-Y) stage [28] and motor subset score of Unified Parkinson's Disease Rating Scale (UPDRS) in “off state” were obtained in all patients. The PD patients consisted of patients under medical treatment (N = 89) and de novo untreated patients (N = 15). Of the 89 treated patients, 70 patients received levodopa (10 patients with levodopa alone and 60 with levodopa plus dopamine agonists as add-on drugs) and 19 patients were on monotherapy with dopamine agonists (ropirinole or pramipexole). Other add-on drugs included amantadine in 45 patients and COMT inhibitor in 8. In order to examine possible relationships between the oxidative stress parameters and disease progression, patients were divided into two groups according to H-Y stage: early PD group comprising patients with stage I and II (N = 47), and advanced PD group comprising patients with stage III and IV (N = 57).
Sample collection and preservation
After overnight fasting, approximately 10 mL of venous blood was collected through the antecubital puncture and injected into a plain Vacutainer tube with aluminum foil to avoid photooxidation. Patients were given the last dose of antiparkinsonian medications in the early morning of the same day. Blood samples were immediately put on ice and, within 1 hr of collection, were centrifuged at 3,000 g for 10 min at 4℃ to obtain serum. Aliquots of serum were immediately added to the Eppendorf tubes and stored at −70℃ until analyzed. Baseline blood tests were performed for hemoglobin, protein, albumin and total cholesterol.
Levels of carotenoids, retinol and tocopherols
All-trans β-carotene (type IV), α-carotene, lycopene, retinol, α-tocopherol and γ-tocopherol were purchased from Sigma Chemical (St. Louis, MO). Lutein was purchased from Kemin Industries (Des Moines, IA). Zeaxanthin, cryptoxanthin, 13-cis β-carotene and 9-cis β-carotene were gifts from Hoffmann-La Roche (Nutley, NJ). Solutions of carotenoids were prepared under red light before use. All high performance lipid chromatography (HPLC) solvents were obtained from J. T. Baker Chemical (Philipsburg, NJ) and were filtered through a 0.2-µm membrane filter before use.
Serum carotenoids, retinol and tocopherols were extracted using a modified enzyme extraction method reported earlier [29]. Echinenone, retinyl acetate and tocol were added as internal standards for the analysis of carotenoids, retinoids and tocopherols, respectively. The samples were extracted with 3 mL of ether/hexane (2:1, stabilized with 1% ethanol) twice, and diluted with 2 mL H2O and 2 mL ethanol. After centrifugation for 5 min at 800 g, the organic layer was collected. The extract was evaporated under N2 in a 40℃ water bath and resuspended with 100 µL of ethanol, and 50 µL was injected onto the HPLC system. The extracted sample was analyzed for serum carotenoids, retinol and tocopherols by using a reverse-phase, gradient HPLC system. The HPLC system consisted of a Series 410 LC pump (Perkin-Elmer, Norwalk, CT), a Waters 717 plus autosampler (Millipore, Milford, MA), a C30 carotenoid column (3 µm, 150 × 4.6 mm, YMC, Wilmington, NC), an HPLC Column Temperature Controller (Model 7950 Column Heater/Chiller, Jones Chromatography, Lakewood, CO) and a Waters 840 Digital 350 data station. The Waters 994 programmable photodiode array detector was set at 450 and 475 nm for carotenoids and 340 nm for retinoids. A fluorescence detector (excitation at 292 nm, emission at 330 nm; Waters 470; Millipore) was connected in series for tocopherol analysis. The HPLC mobile phase was methanol/methyl-tert-butyl ether/water (83:15:2, v/v/v, with 1.5% ammonium acetate in the water; solvent A) and methanol/methyl-tert-butyl ether/water (8:90:2, v/v/v, with 1% ammonium acetate in the water; solvent B). The gradient procedure at a flow rate of 1 mL/min (16℃) was as follows: 1) 100% solvent A for 1 min; 2) a 7-min linear gradient to 70% solvent A; 3) a 5-min hold at 70% solvent A; 4) a 9-min linear gradient to 45% solvent A; 5) a 2-min hold at 45 % solvent A; 6) a 10-min linear gradient to 95% solvent B; 7) a 4-min hold at 95% solvent B; and 8) a 2-min gradient back to 100% solvent A. Total running time for HPLC separation of both carotenoids and tocopherols was 40 min. Retention time of lutein was 11.86 min, zeaxanthin 13.40 min, cryptoxanthin 10.13 min, 13-cis β-carotene 24.61 min, α-carotene 25.57 min, all-trans β-carotene 26.70 min, 9-cis β-cahrotene 27.52 min, 9-cis lycopene 37.41 min, trans lycopene 37.65 min, γ-tocopherol 10.30 min and α-tocopherol 11.85 min, respectively. Using this method, lutein, zeaxanthin, cryptoxanthin (α-cryptoxanthin and β-cryptoxanthin eluted as a single peak), α-carotene, 13-cis β-carotene, all-trans β-carotene, 9-cis β-carotene and trans- and cis-lycopenes were adequately separated. 9-cis β-Carotene was monitored at both 450 and 475 nm and confirmed through diode-array spectra because 9-cis β-carotene coelutes with ζ-carotene (λmax = 400) in our system. A fluorescence detector (Ex:292 nm, Em:330 nm; Waters 470; Millipore) was connected for tocopherol analysis. Carotenoids, retinol and tocopherols were quantified by determining peak areas in the HPLC chromatograms, calibrated against known amounts of standards. The lower limits of detection were 0.2 pmol for carotenoids, 2.0 pmol for retinol and 2.7 pmol for tocopherols. β-Carotene levels were calculated as the sum of 13-cis β-carotene, all-trans β-carotene and 9-cis β-carotene. Results were reported as micromole per liter (µmol/L).
Statistical analysis
Normally distributed variables are presented as mean ± standard deviation, and non-normally distributed variables are presented as median and interquartile range (25-75th percentile). Comparisons between patient and control groups were performed by using two-sample student t-test, Mann-Whitney U test, or χ2 test, when appropriate. Normally distributed variables in multiple groups (early PD group, advanced PD group and control group) were compared using one-way analysis of variance (ANOVA) followed by Tukey's post hoc test for multiple pairwise comparisons. Non-normally distributed variables in multiple groups were compared using Kruskal-Wallis ANOVA followed by Dunn's post hoc test for multiple pairwise comparisons. Correlation analyses between the oxidative stress parameters and clinical variables in PD patients (disease duration, UPDRS motor score, H-Y stage, levodopa dosage) were performed using Spearman correlation test. A P value of less than 0.05 was considered to indicate a statistically significant difference in all tests. Data storage and statistical analyses were performed using SPSS (version 20.0, IBM, Armonk, NY) and Prism (version 5; GraphPad Software, Inc., San Diego, CA).
RESULTS
Characteristics of the study subjects
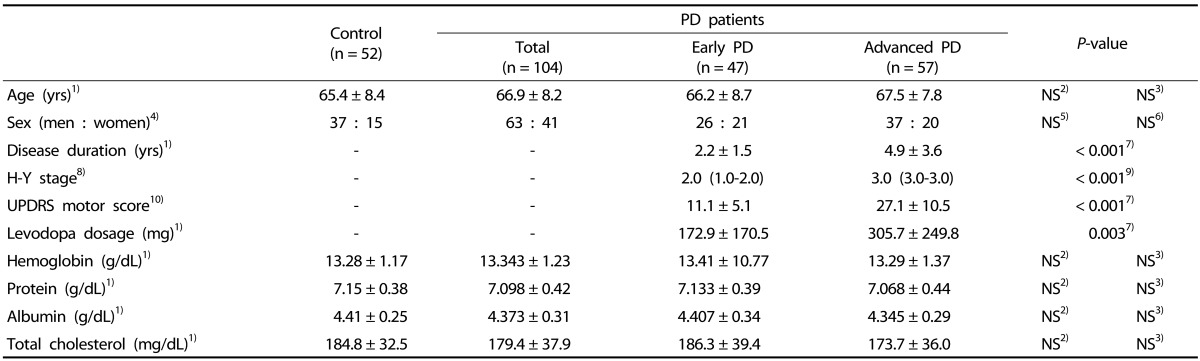
One hundred and fifty-six subjects were finally recruited for the present study, comprising 104 PD patients and 52 healthy controls. Table 1 shows demographics and clinical variables as well as baseline blood laboratory tests in controls and patients with early PD (H-Y stage I or II) and advanced PD (H-Y stage III or IV). Three groups did not differ in age, sex and levels of hemoglobin, protein, albumin and total cholesterol (all P > 0.05). Compared to early PD group, advanced PD group had longer duration of disease (4.9 ± 3.6 years vs. 2.2 ± 1.5 years in early PD group; P < 0.001), higher H-Y stage (median, 3.0 vs. 2.0 in early PD group; P < 0.001), higher UPDRS motor score (27.1 ± 10.5 vs. 11.1 ± 5.1 in early PD group; P < 0.001) and higher daily levodopa dosage (305.7 ± 249.8 mg vs. 172.9 ± 170.5 mg in early PD group; P = 0.003).
Table 1. General characteristics of the subjects.
PD, Parkinson's disease; NS, non-significant differences.
1) Mean ± SD.
2) Student's t-test between control and total PD patient groups.
3) ANOVA followed by Tukey's post hoc test between control, early PD patient and advanced PD patient groups.
4) Number of the subjects.
5) Chi-square tests for control and total PD patient groups.
6) Chi-square tests for control, early PD patient and advanced PD patient groups.
7) Student's t-test between early and advanced PD patient groups.
8) Hoehn and Yahr stage; median (25-75th percentile).
9) Mann-Whitney U test between early and advanced PD patient groups.
10) Motor subset score of Unified Parkinson's Disease Rating Scale; mean ± SD.
Serum levels of carotenoids, retinol and tocopherols
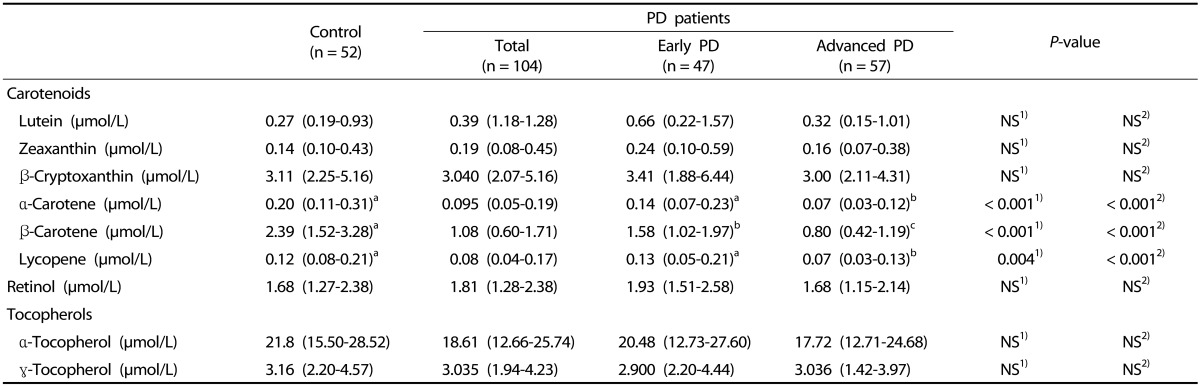
Serum levels of carotenoids, retinol and tocopherols are detailed in Table 2. Compared to controls, PD patients had significantly lower levels of lipophilic antioxidant micronutrients: α-carotene (P < 0.001), β-carotene (P < 0.001) and lycopene (P = 0.004). No between-group differences were found in levels of lutein, zeaxanthin, β-cryptoxanthin, retinol, α-tocopherol and γ-tocopherol. Advanced PD group had lower α-carotene (P < 0.001) and lycopene (P < 0.001) levels than did control and early PD groups. There were no significant differences between control and early PD groups in α-carotene and lycopene levels. Significant differences were observed between all pairs of groups in serum β-carotene level (P < 0.001). β-Carotene level in early and advanced PD groups was lower than in control group respectively, and this variable was also lower in early PD group than in advanced PD group.
Table 2. Serum antioxidant vitamins in PD patients and controls.
Median (25-75th percentile); PD, Parkinson's disease; NS, non-significant differences; Numbers with different letters in the same row are significantly different from the others.
1) Mann-Whitney U test between control and total PD patient groups.
2) Kruskal-Wallis ANOVA followed by Dunn's post hoc test between control, early PD patient and advanced PD patient groups.
Correlations between serum carotenes and disease severity parameters
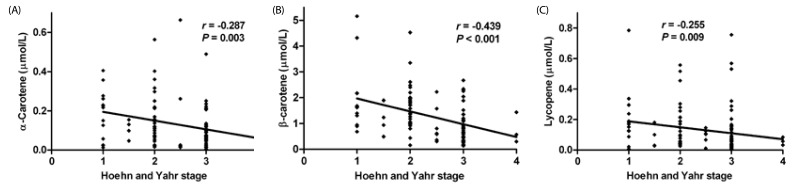
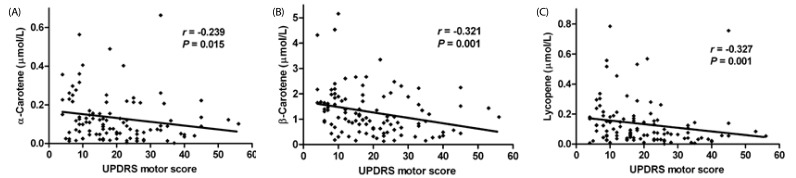
H-Y stages in PD patients (N = 104) negatively correlated with serum levels of α-carotene (r = −0.287; P = 0.003), β-carotene (r = −0.439; P < 0.001) and lycopene (r = −0.255; P = 0.009) (Fig. 1). UPDRS motor scores in PD patients (N= 104) negatively correlated with serum levels of α-carotene (r = −0.239; P = 0.015), β-carotene (r = −0.321; P = 0.001) and lycopene (r = −0.327; P = 0.001) (Fig. 2).
Fig. 1. Significant correlations between Hoehn and Yahr stages and serum carotenoids.
Spearman's test, P < 0.05; A, α-carotene; B, β-carotene; C, lycopene.
Fig. 2. Significant correlations between UPDRS motor scores and serum carotenoids.
Spearman's test, P < 0.05; A, α-carotene; B, β-carotene; C, lycopene.
DISCUSSION
We investigated serum vitamins including retinol, carotenoids and tocopherols in PD patients and controls. We found that serum β-carotene, α-carotene and lycopene concentrations were lower in PD patients relative to controls, and that these carotenoids were correlated with clinical variables representing disease progression. Our results support the notion that carotenes may play an important role in the pathogenesis and progression of PD.
The brain is especially vulnerable to free radical attacks because of its relatively high concentrations of polyunsaturated fatty acids and metabolic activity [30], and needs for lipophilic antioxidants [31] capable of penetrating the blood-brain barrier to enter the brain parenchyma [32]. PD is a common neurodegenerative disorder characterized by the loss of dopaminergic neurons in the nigrostriatal system [3] that are susceptible to oxidative stress and damage [4]. Carotenoids are effective lipid-soluble antioxidants [6], which are found in clearly detectable concentrations in the frontal and occipital lobes of the human brain [5]. Serum carotenoid concentrations are known to positively correlate with those in the brain, implying that serum carotenoids can be a biomarker to predict the brain carotenoid concentrations [33]. Peripheral blood levels of carotenoids in PD were examined in only two studies [22,24]. Jiménez-Jiménez et al. [24] reported that serum levels of carotenoids including β-carotene in 61 PD patients did not differ from those of controls, and that serum carotenoids did not correlate with disease duration or H-Y stage. Foy et al. [22] also failed to find any differences in blood levels of β-carotene, α-carotene and lycopene between 41 PD patients without dementia and controls. We observed significant reductions in serum levels of α- and β-carotenes and lycopene in 104 PD patients compared to controls. Multiple group comparisons also showed that β- and α-carotenes and lycopene were significantly lower in advanced stage PD patients than in early stage PD patients and controls. Levels of β- and α-carotenes and lycopene inversely correlated with the clinical variables representing disease severity or progression (i.e., UPDRS motor score and H-Y stage), strongly implicating involvement of these carotenoids in the risk and progression of PD. A large cross-sectional study showed that β- and α-carotenes are less closely correlated with fruit and vegetable intakes compared to other carotenoids [34]. β- and α-Carotenes are the main precursors of retinol [35], the major circulating retinoid in the human body including the brain. Decreases in serum β- and α-carotene levels in PD patients and no between-group difference in retinol in our study could be, in part, explained by activated biochemical metabolism such as a rapid uptake of these carotenes into the brain or increased conversion to retinol in compensation for dopaminergic degeneration in PD, because the dopaminergic system of the brain is one of the best-established targets for retinoid actions [36] including gene expression, neuronal differentiation and neural tube patterning [37]. The plasma retinol concentration may not exactly reflect nutritional status [38] because of strict homeostatic control [39]. Lycopene is not a precursor of retinol, and has the greatest singlet oxygen quenching ability among the biologically occurring carotenoids, with twice as much as that of β-carotene and four times as much as that of lutein [40]. Lower lycopene level in our PD patients is assumed to be related with negative influence on the antioxidant systems in PD.
Carotenoids are a class of naturally occurring pigments that are synthesized by red, orange and yellow colored fruits and vegetables. It is generally accepted that intakes of specific fruits and vegetables are reliable predictors of individual plasma carotenoid levels [34]. Carotenoids consist of two subclasses: xanthophylls (lutein, zeaxanthin and β-cryptoxanthin) and carotenes (α-carotene, β-carotene and lycopene). Among the xanthophylls found in green leafy vegetables and brightly colored fruits, lutein has been proposed as a biomarker for dietary intake of the Cruciferae family such as broccoli, Brussels sprouts, cabbage and kale [41]. In our study, significant group differences were found in carotenes, but not in xanthophylls, which may account for the similarity in dietary carotenoid intakes between patients and controls. Lutein and zeaxanthin are the dominant carotenoids in human brain tissue, accounting for 66-77% of total carotenoid concentrations [5,33], and they are not the precursors for retinoids. To our knowledge, there is no currently available study that assessed blood or brain levels of xanthophylls such as lutein, zeaxanthin and cryptoxanthin. Although it could be hypothesized that a large intake of these xanthophylls may slow the progression of PD, there have been only a few studies investigating the protective role of dietary lutein, zeaxanthin and cryptoxanthin in PD cohorts, with inconsistent findings across the studies [11,13,42]. A cohort study showed a non-significant inverse association of dietary intakes of lutein and zeaxanthin with the risk of PD [11], while some case-control studies reported a statistically significant positive correlation between intake of xanthophylls including lutein and PD risk [13,42].
Given that oxidative stress plays a central role in the pathogenesis and progression of PD [43], the role of antioxidant vitamin E has been the issue of great interest in both clinical and laboratory investigations. Intakes of vitamin E or foods rich in vitamin E are shown to be associated with a neuroprotective effect that attenuate the risk of PD in some case-control studies [8,9,10,11], but not in others [12,14,15,16]. Vitamin E protects membranes from lipid peroxidation by scavenging lipid peroxyl radicals at high partial pressure of oxygen [44] at an early stage of lipid peroxidation, thus protecting cells from oxidative damage [45], while β-carotene is one of the most potent scavengers of singlet oxygen at low partial pressure of oxygen [46]. We found no differences in serum α- and γ-tocopherol levels between patients and controls. Moreover, we did not observe any correlations between tocopherol levels and clinical variables, suggesting that serum tocopherols do not seem to be associated with the risk or progression of PD. Our findings might be explained by limited passage of tocopherols across the blood-brain barrier [47], unlike carotenoids [5,33]. Postmortem studies showed that α-tocopherol level was not altered in brains of the PD patients [48], and most studies failed to show any difference in cerebrospinal fluid [49,50] or blood [17,18,19,20,21,22,23] levels of α-tocopherol between PD patients and control subjects.
Our study has several limitations that should be addressed. First, dietary intake survey was not carried out. Since serum or plasma concentrations of carotenoids are markers of recent fruit and vegetable intakes [35], further studies with complete nutritional assessment are needed to clarify whether lower levels in some carotenoid vitamins found in PD are caused by reduced intake of these vitamins or altered metabolism such as increased oxidative stress in the pathophysiological process of PD. Second, our case-control data are limited by the use of spouses or relatives as controls, which can lead to differences in the prevalence of the sexes and similarity of lifestyle including dietary habits between cases and controls, though they have easy accessibility and a lower dropout rate compared to population controls [25,26]. Finally, enzymatic antioxidant capacity including superoxide dismutase and catalase, and water-soluble nonenzymatic antioxidants such as ascorbate and urate were not assessed to determine systemic antioxidant state in PD.
In summary, our findings of reduced serum levels of α-carotene, β-carotene and lycopene in PD patients, and inverse relationships between these carotenoids and disease severity parameters suggest that lower serum carotenes may be associated with the pathogenesis as well as progression of PD. Further studies incorporating a larger cohort and detailed nutritional assessment are required to elucidate the exact mechanisms of tissue-specific uptake of carotenes and their biological actions.
Footnotes
CONFLICT OF INTEREST: The authors declare no potential conflicts of interests.
References
- 1.de Rijk MC, Breteler MM, Graveland GA, Ott A, Grobbee DE, van der Meché FG, Hofman A. Prevalence of Parkinson's disease in the elderly: the Rotterdam study. Neurology. 1995;45:2143–2146. doi: 10.1212/wnl.45.12.2143. [DOI] [PubMed] [Google Scholar]
- 2.Kim HS, Lee KS, Bae HJ, Koh IS, Chang SH, Im DH, Heo JH. Changes in length of stay for neurological geriatric diseases in Korea between 2003 and 2007. J Clin Neurol. 2011;7:148–155. doi: 10.3988/jcn.2011.7.3.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalia LV, Lang AE. Parkinson's disease. Lancet. 2015;386:896–912. doi: 10.1016/S0140-6736(14)61393-3. [DOI] [PubMed] [Google Scholar]
- 4.Jenner P, Olanow CW. Oxidative stress and the pathogenesis of Parkinson's disease. Neurology. 1996;47:S161–S170. doi: 10.1212/wnl.47.6_suppl_3.161s. [DOI] [PubMed] [Google Scholar]
- 5.Craft NE, Haitema TB, Garnett KM, Fitch KA, Dorey CK. Carotenoid, tocopherol, and retinol concentrations in elderly human brain. J Nutr Health Aging. 2004;8:156–162. [PubMed] [Google Scholar]
- 6.Fiedor J, Burda K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients. 2014;6:466–488. doi: 10.3390/nu6020466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ulatowski L, Parker R, Warrier G, Sultana R, Butterfield DA, Manor D. Vitamin E is essential for Purkinje neuron integrity. Neuroscience. 2014;260:120–129. doi: 10.1016/j.neuroscience.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Rijk MC, Breteler MM, den Breeijen JH, Launer LJ, Grobbee DE, van der Meché FG, Hofman A. Dietary antioxidants and Parkinson disease. The Rotterdam study. Arch Neurol. 1997;54:762–765. doi: 10.1001/archneur.1997.00550180070015. [DOI] [PubMed] [Google Scholar]
- 9.Golbe LI, Farrell TM, Davis PH. Follow-up study of early-life protective and risk factors in Parkinson's disease. Mov Disord. 1990;5:66–70. doi: 10.1002/mds.870050116. [DOI] [PubMed] [Google Scholar]
- 10.Miyake Y, Fukushima W, Tanaka K, Sasaki S, Kiyohara C, Tsuboi Y, Yamada T, Oeda T, Miki T, Kawamura N, Sakae N, Fukuyama H, Hirota Y, Nagai M Fukuoka Kinki Parkinson's Disease Study Group. Dietary intake of antioxidant vitamins and risk of Parkinson's disease: a case-control study in Japan. Eur J Neurol. 2011;18:106–113. doi: 10.1111/j.1468-1331.2010.03088.x. [DOI] [PubMed] [Google Scholar]
- 11.Zhang SM, Hernán MA, Chen H, Spiegelman D, Willett WC, Ascherio A. Intakes of vitamins E and C, carotenoids, vitamin supplements, and PD risk. Neurology. 2002;59:1161–1169. doi: 10.1212/01.wnl.0000028688.75881.12. [DOI] [PubMed] [Google Scholar]
- 12.Logroscino G, Marder K, Cote L, Tang MX, Shea S, Mayeux R. Dietary lipids and antioxidants in Parkinson's disease: a population-based, case-control study. Ann Neurol. 1996;39:89–94. doi: 10.1002/ana.410390113. [DOI] [PubMed] [Google Scholar]
- 13.Johnson CC, Gorell JM, Rybicki BA, Sanders K, Peterson EL. Adult nutrient intake as a risk factor for Parkinson's disease. Int J Epidemiol. 1999;28:1102–1109. doi: 10.1093/ije/28.6.1102. [DOI] [PubMed] [Google Scholar]
- 14.Morens DM, Grandinetti A, Waslien CI, Park CB, Ross GW, White LR. Case-control study of idiopathic Parkinson's disease and dietary vitamin E intake. Neurology. 1996;46:1270–1274. doi: 10.1212/wnl.46.5.1270. [DOI] [PubMed] [Google Scholar]
- 15.Anderson C, Checkoway H, Franklin GM, Beresford S, Smith-Weller T, Swanson PD. Dietary factors in Parkinson's disease: the role of food groups and specific foods. Mov Disord. 1999;14:21–27. doi: 10.1002/1531-8257(199901)14:1<21::aid-mds1006>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 16.Hellenbrand W, Boeing H, Robra BP, Seidler A, Vieregge P, Nischan P, Joerg J, Oertel WH, Schneider E, Ulm G. Diet and Parkinson's disease. II: a possible role for the past intake of specific nutrients. Results from a self-administered food-frequency questionnaire in a case-control study. Neurology. 1996;47:644–650. doi: 10.1212/wnl.47.3.644. [DOI] [PubMed] [Google Scholar]
- 17.Nicoletti G, Crescibene L, Scornaienchi M, Bastone L, Bagalà A, Napoli ID, Caracciolo M, Quattrone A. Plasma levels of vitamin E in Parkinson's disease. Arch Gerontol Geriatr. 2001;33:7–12. doi: 10.1016/s0167-4943(01)00092-9. [DOI] [PubMed] [Google Scholar]
- 18.Sohmiya M, Tanaka M, Tak NW, Yanagisawa M, Tanino Y, Suzuki Y, Okamoto K, Yamamoto Y. Redox status of plasma coenzyme Q10 indicates elevated systemic oxidative stress in Parkinson's disease. J Neurol Sci. 2004;223:161–166. doi: 10.1016/j.jns.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 19.Ahlskog JE, Uitti RJ, Low PA, Tyce GM, Nickander KK, Petersen RC, Kokmen E. No evidence for systemic oxidant stress in Parkinson's or Alzheimer's disease. Mov Disord. 1995;10:566–573. doi: 10.1002/mds.870100507. [DOI] [PubMed] [Google Scholar]
- 20.Sudha K, Rao AV, Rao S, Rao A. Free radical toxicity and antioxidants in Parkinson's disease. Neurol India. 2003;51:60–62. [PubMed] [Google Scholar]
- 21.Férnandez-Calle P, Molina JA, Jiménez-Jiménez FJ, Vázquez A, Pondal M, García-Ruiz PJ, Urra DG, Domingo J, Codoceo R. Serum levels of alpha-tocopherol (vitamin E) in Parkinson's disease. Neurology. 1992;42:1064–1066. doi: 10.1212/wnl.42.5.1064. [DOI] [PubMed] [Google Scholar]
- 22.Foy CJ, Passmore AP, Vahidassr MD, Young IS, Lawson JT. Plasma chain-breaking antioxidants in Alzheimer's disease, vascular dementia and Parkinson's disease. QJM. 1999;92:39–45. doi: 10.1093/qjmed/92.1.39. [DOI] [PubMed] [Google Scholar]
- 23.Buhmann C, Arlt S, Kontush A, Möller-Bertram T, Sperber S, Oechsner M, Stuerenburg HJ, Beisiegel U. Plasma and CSF markers of oxidative stress are increased in Parkinson's disease and influenced by antiparkinsonian medication. Neurobiol Dis. 2004;15:160–170. doi: 10.1016/j.nbd.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Jiménez-Jiménez FJ, Molina JA, Fernández-Calle P, Vázquez A, Cabrera-Valdivia F, Catalán MJ, García-Albea E, Bermejo F, Codoceo R. Serum levels of beta-carotene and other carotenoids in Parkinson's disease. Neurosci Lett. 1993;157:103–106. doi: 10.1016/0304-3940(93)90653-3. [DOI] [PubMed] [Google Scholar]
- 25.Verhage BA, Aben KK, Straatman H, Verbeek AL, Beaty TH, Kiemeney LA. Spouse controls in family case-control studies: a methodological consideration. Fam Cancer. 2003;2:101–108. doi: 10.1023/a:1025737025219. [DOI] [PubMed] [Google Scholar]
- 26.Smith DS, Fillenbaum GG. Comparison of spouse and nonkin controls: the experience of the Consortium to Establish a Registry for Alzheimer's disease (CERAD) Aging (Milano) 1994;6:151–157. doi: 10.1007/BF03324230. [DOI] [PubMed] [Google Scholar]
- 27.Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol. 1999;56:33–39. doi: 10.1001/archneur.56.1.33. [DOI] [PubMed] [Google Scholar]
- 28.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 29.Yeum KJ, Ahn SH, Rupp de Paiva SA, Lee-Kim YC, Krinsky NI, Russell RM. Correlation between carotenoid concentrations in serum and normal breast adipose tissue of women with benign breast tumor or breast cancer. J Nutr. 1998;128:1920–1926. doi: 10.1093/jn/128.11.1920. [DOI] [PubMed] [Google Scholar]
- 30.Sopher BL, Fukuchi K, Kavanagh TJ, Furlong CE, Martin GM. Neurodegenerative mechanisms in Alzheimer disease. A role for oxidative damage in amyloid beta protein precursor-mediated cell death. Mol Chem Neuropathol. 1996;29:153–168. doi: 10.1007/BF02814999. [DOI] [PubMed] [Google Scholar]
- 31.Kontush K, Schekatolina S. Vitamin E in neurodegenerative disorders: Alzheimer's disease. Ann N Y Acad Sci. 2004;1031:249–262. doi: 10.1196/annals.1331.025. [DOI] [PubMed] [Google Scholar]
- 32.Delanty N, Dichter MA. Antioxidant therapy in neurologic disease. Arch Neurol. 2000;57:1265–1270. doi: 10.1001/archneur.57.9.1265. [DOI] [PubMed] [Google Scholar]
- 33.Johnson EJ, Vishwanathan R, Johnson MA, Hausman DB, Davey A, Scott TM, Green RC, Miller LS, Gearing M, Woodard J, Nelson PT, Chung HY, Schalch W, Wittwer J, Poon LW. Relationship between serum and brain carotenoids, alpha-tocopherol, and retinol concentrations and cognitive performance in the oldest old from the Georgia Centenarian Study. J Aging Res. 2013;2013:951786. doi: 10.1155/2013/951786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Al-Delaimy WK, Ferrari P, Slimani N, Pala V, Johansson I, Nilsson S, Mattisson I, Wirfalt E, Galasso R, Palli D, Vineis P, Tumino R, Dorronsoro M, Pera G, Ocké MC, Bueno-de-Mesquita HB, Overvad K, Chirlaque M, Trichopoulou A, Naska A, Tjønneland A, Olsen A, Lund E, Alsaker EH, Barricarte A, Kesse E, Boutron-Ruault MC, Clavel-Chapelon F, Key TJ, Spencer E, Bingham S, Welch AA, Sanchez-Perez MJ, Nagel G, Linseisen J, Quirós JR, Peeters PH, van Gils CH, Boeing H, van Kappel AL, Steghens JP, Riboli E. Plasma carotenoids as biomarkers of intake of fruits and vegetables: individual-level correlations in the European Prospective Investigation into Cancer and Nutrition (EPIC) Eur J Clin Nutr. 2005;59:1387–1396. doi: 10.1038/sj.ejcn.1602252. [DOI] [PubMed] [Google Scholar]
- 35.Al-Delaimy WK, Slimani N, Ferrari P, Key T, Spencer E, Johansson I, Johansson G, Mattisson I, Wirfalt E, Sieri S, Agudo A, Celentano E, Palli D, Sacerdote C, Tumino R, Dorronsoro M, Ocké MC, Bueno-De-Mesquita HB, Overvad K, Chirlaque MD, Trichopoulou A, Naska A, Tjonneland A, Olsen A, Lund E, Skeie G, Ardanaz E, Kesse E, Boutron-Ruault MC, Clavel-Chapelon F, Bingham S, Welch AA, Martinez-Garcia C, Nagel G, Linseisen J, Quirós JR, Peeters PH, van Gils CH, Boeing H, van Kappel AL, Steghens JP, Riboli E. Plasma carotenoids as biomarkers of intake of fruits and vegetables: ecological-level correlations in the European Prospective Investigation into Cancer and Nutrition (EPIC) Eur J Clin Nutr. 2005;59:1397–1408. doi: 10.1038/sj.ejcn.1602253. [DOI] [PubMed] [Google Scholar]
- 36.Borrelli E, Chambon P. Control of transcription and neurological diseases. Mol Psychiatry. 1999;4:112–114. doi: 10.1038/sj.mp.4000491. [DOI] [PubMed] [Google Scholar]
- 37.Tafti M, Ghyselinck NB. Functional implication of the vitamin A signaling pathway in the brain. Arch Neurol. 2007;64:1706–1711. doi: 10.1001/archneur.64.12.1706. [DOI] [PubMed] [Google Scholar]
- 38.Ascherio A, Stampfer MJ, Colditz GA, Rimm EB, Litin L, Willett WC. Correlations of vitamin A and E intakes with the plasma concentrations of carotenoids and tocopherols among American men and women. J Nutr. 1992;122:1792–1801. doi: 10.1093/jn/122.9.1792. [DOI] [PubMed] [Google Scholar]
- 39.Goodman DS. Overview of current knowledge of metabolism of vitamin A and carotenoids. J Natl Cancer Inst. 1984;73:1375–1379. [PubMed] [Google Scholar]
- 40.Di Mascio P, Murphy ME, Sies H. Antioxidant defense systems: the role of carotenoids, tocopherols, and thiols. Am J Clin Nutr. 1991;53:194S–200S. [PubMed] [Google Scholar]
- 41.Martini MC, Campbell DR, Gross MD, Grandits GA, Potter JD, Slavin JL. Plasma carotenoids as biomarkers of vegetable intake: the University of Minnesota Cancer Prevention Research Unit Feeding Studies. Cancer Epidemiol Biomarkers Prev. 1995;4:491–496. [PubMed] [Google Scholar]
- 42.Scheider WL, Hershey LA, Vena JE, Holmlund T, Marshall JR, Freudenheim JL. Dietary antioxidants and other dietary factors in the etiology of Parkinson's disease. Mov Disord. 1997;12:190–196. doi: 10.1002/mds.870120209. [DOI] [PubMed] [Google Scholar]
- 43.Anglade P, Vyas S, Hirsch EC, Agid Y. Apoptosis in dopaminergic neurons of the human substantia nigra during normal aging. Histol Histopathol. 1997;12:603–610. [PubMed] [Google Scholar]
- 44.Niki E. Role of vitamin E as a lipid-soluble peroxyl radical scavenger: in vitro and in vivo evidence. Free Radic Biol Med. 2014;66:3–12. doi: 10.1016/j.freeradbiomed.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 45.Herrera E, Barbas C. Vitamin E: action, metabolism and perspectives. J Physiol Biochem. 2001;57:43–56. [PubMed] [Google Scholar]
- 46.Stahl W, Sies H. Antioxidant activity of carotenoids. Mol Aspects Med. 2003;24:345–351. doi: 10.1016/s0098-2997(03)00030-x. [DOI] [PubMed] [Google Scholar]
- 47.Pappert EJ, Tangney CC, Goetz CG, Ling ZD, Lipton JW, Stebbins GT, Carvey PM. Alpha-tocopherol in the ventricular cerebrospinal fluid of Parkinson's disease patients: dose-response study and correlations with plasma levels. Neurology. 1996;47:1037–1042. doi: 10.1212/wnl.47.4.1037. [DOI] [PubMed] [Google Scholar]
- 48.Dexter DT, Ward RJ, Wells FR, Daniel SE, Lees AJ, Peters TJ, Jenner P, Marsden CD. Alpha-tocopherol levels in brain are not altered in Parkinson's disease. Ann Neurol. 1992;32:591–593. doi: 10.1002/ana.410320420. [DOI] [PubMed] [Google Scholar]
- 49.Jiménez-Jiménez FJ, de Bustos F, Molina JA, Benito-León J, Tallón-Barranco A, Gasalla T, Ortí-Pareja M, Guillamón F, Rubio JC, Arenas J, Enríquez-de-Salamanca R. Cerebrospinal fluid levels of alpha-tocopherol (vitamin E) in Alzheimer's disease. J Neural Transm (Vienna) 1997;104:703–710. doi: 10.1007/BF01291887. [DOI] [PubMed] [Google Scholar]
- 50.Tohgi H, Abe T, Saheki M, Hamato F, Sasaki K, Takahashi S. Reduced and oxidized forms of glutathione and α-tocopherol in the cerebrospinal fluid of parkinsonian patients: comparison between before and after L-dopa treatment. Neurosci Lett. 1995;184:21–24. doi: 10.1016/0304-3940(94)11158-f. [DOI] [PubMed] [Google Scholar]