Abstract
BACKGROUND/OBJECTIVE
Although Angelica keiskei (AK) has widely been utilized for the purpose of general health improvement among Asian, its functionality and mechanism of action. The aim of this study was to determine the protective effect of ethanol extract of AK (AK-Ex) on acute hepatotoxicity induced by acetaminophen (AAP) in HepG2 human hepatocellular liver carcinoma cells and HepaRG human hepatic progenitor cells.
MATERIALS/METHODS
AK-Ex was prepared HepG2 and HepaRG cells were cultured with various concentrations and 30 mM AAP. The protective effects of AK-Ex against AAP-induced hepatotoxicity in HepG2 and HepaRG cells were evaluated using 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide, lactate dehydrogenase (LDH) assay, flow cytometry, and Western blotting.
RESULTS
AK-Ex, when administered prior to AAP, increased cell growth and decreased leakage of LDH in a dose-dependent manner in HepG2 and HepaRG cells against AAP-induced hepatotoxicity. AK-Ex increased the level of Bcl-2 and decreased the levels of Bax, Bok and Bik decreased the permeability of the mitochondrial membrane in HepG2 cells intoxicated with AAP. AK-Ex decreased the cleavage of poly (ADP-ribose) polymerase (PARP) and the activation of caspase-9, -7, and -3.
CONCLUSIONS
These results demonstrate that AK-Ex downregulates apoptosis via intrinsic and extrinsic pathways against AAP-induced hepatotoxicity. We suggest that AK could be a useful preventive agent against AAP-induced apoptosis in hepatocytes.
Keywords: Liver, hepatocyte, apoptosis, functional food
INTRODUCTION
Angelica keiskei (AK) is a large perennial herb belongings to the Umbelliferae family. It grows mainly along the Pacific coast of Asia and is known in Japan as ashitaba and in Korea as sinsuncho [1]. AK is widely cultivated as a functional food and beverage ingredients. AK green tea is widely consumed in Asian countries including China, India, and Japan as a health-related drink. AK leaves have been consumed as food and medicine in Japan and Korea [2]. Several studies have shown that AK possesses a wide range of pharmacologic and health-promoting properties including anti-inflammatory [2,3], anti-adiposity [4], anti-diabetic, anti-hypertensive [5], and anti-tumor effects [6,7]. Many of these properties have been described as resulting from various active chalcones, coumarines and flavonoids in AK [1,2,6]. In addition, AK has widely been utilized among Koreans that believe it can protect against hepatotoxicity. Noh et al. [8] recently reported that an AK extract supplement can improve liver function in heavy drinkers. However, the effects of AK on hepatotoxicity and its mechanisms of action have yet to be elucidated.
Worldwide, acetaminophen (AAP) is widely prescribed for treatment of mild pain and fever. AAP is generally considered a safe and effective anesthetic and antipyretic drug at therapeutic doses; however, an AAP overdose can result in severe liver injury through mitochondrial dysfunction and damage [9,10]. AAP overdose is the most common cause of acute liver failure in most western countries [11]. Several studies have revealed that AAP causes apoptosis in hepatocytes and then exhibits hepatotoxicity under in vitro and in vivo conditions [11]. N-acetyl-p-benzoquinone imine, the toxic metabolite of AAP, inhibits mitochondrial oxidative phosphorylation, depletes adenosine triphosphate (ATP), produces selective mitochondrial oxidant stress [12], decreases mitochondrial membrane potential, increases Ca2+ levels, changes membrane permeability transition, and releases pro-apoptotic factors into the cytosol [13]. These actions are accompanied by caspase-3 activation, DNA fragmentation, and apoptotic cell death [14]. AAP-induced hepatotoxicity has been used as a useful model in hepatoprotective study.
In this study, we attempted to investigate the protective effects of an AK ethanol extract (AK-Ex) against AAP-induced liver injury in HepG2 human hepatocellular liver carcinoma cells and HepaRG human hepatic progenitor cells. We also examined the possible molecular mechanisms of their activities.
MATERIALS AND METHODS
Materials
Anti-β-actin antibody and 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Dulbecco's modified Eagle's medium (DMEM) was purchased from Gibco BRL (Gaithersburg, MD, USA). Fetal bovine serum (FBS), trypsin/EDTA and penicillin/streptomycin were purchased from Cambrex Bio Technology (Walkersville, MD, USA). Antibodies against cleaved caspase-3, cleaved caspase-7, cleaved caspase-9, and cleaved poly (ADP-ribose) polymerase (PARP) were purchased from Cell Signal Technology (Beverly, MA, USA). Antibodies against Bcl-2, Bcl-xL, Bax, Bak, Bok, and Bik were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Preparation of extract
Dried AK cultivated in Chuncheon, Korea was purchased from an herbal medicine store in Seoul, Korea. Dried AK was pulverized by using a blender. The powder (1 kg) was refluxed in 1 L of 70% ethanol at 100℃ for 8 h. The supernatant solution was filtered through Whatman filter paper #2, after which the filtrate was evaporated in a rotary vacuum evaporator and subsequently freeze-dried at −70℃. The resulting powder was used as an ethanol extract of AK (AK-Ex) and preserved at −20℃ for use. The yield of AK-Ex was 241.6 g per kilogram of dried AK powder.
Cell culture
The HepG2 human hepatoma cell line was obtained from the American Type Culture Collection (Manassas, VA, USA). Cells were cultured in DMEM supplemented with 100 mL/L FBS, 100,000 U/L penicillin, and 100 mg/L streptomycin. HepaRG human hepatic progenitor cells were obtained from Life Technology (Carlsbad, CA, USA). HepaRG cells were cultured in Williams' medium E (WME) supplemented with 200 mL/L HepaRG™ Tox medium supplement and 10 mL/L GlutaMAX™ supplement according to the recommendations of the manufacturer (Life Technology). HepG2 and HepaRG cells were maintained in an incubator at 37℃ in a humidified atmosphere of 5% CO2 and 95% air.
Cell viability assay
In order to assess the effect of AK-Ex on the growth of HepG2 and HepaRG cells, cells were plated in multi-well plates with their respective culture media. After incubation for 24 h, we replaced the respective culture media with various concentrations of AK-Ex and/or 30 mM AAP as indicated. Viable cell numbers were estimated via MTT assays, as previously described [15].
Lactate dehydrogenase assay
Leakage of lactate dehydrogenase (LDH) was measured as an index of cell membrane damage [16]. HepG2 cells were plated at a density of 5 × 104 cells/well in a 24-well plate and incubated for 24 h. HepaRG cells were plated at a density of 1.2 × 105 cells/well in a 24-well plate and incubated for 72 h. Cells were treated for 48 h with various concentration of AK-Ex and then treated with 30 mM AAP for an additional 24 h. The 24 h-conditioned media were collected for use in LDH activity assays. The LDH activity was measured by using an LDH cytotoxicity detection assay kit (Takara Bio Inc., Osaka, Japan) according to the manufacturer's instructions.
Quantification of apoptotic cells and measurement of mitochondrial membrane depolarization
HepG2 cells and HepaRG cells were treated with AK-Ex and/or AAP, respectively, as described above. Apoptotic cells were quantified by using a cell death detection ELISAPLUS assay kit (Roche Applied Science, Switzerland) in accordance with the manufacturers' instructions. To measure mitochondrial membrane depolarization, a dual-emission, potential-sensitive probe JC-1 was utilized as described previously [17].
Western blot analysis
Cells were lysed as described previously [18]. The protein contents of the cell lysates were measured by using a BCA protein assay kit (Pierce, Rockford, IL, USA). Western blot analyses were conducted as described previously [18]. Blots were developed by using Immobilon™ Western chemiluminescent HRPsubstrate (Pierce). The intensity of each band was quantified by using the Bio-profile Bio-1D application (Vilber, Eberhadzell, Germany). The expressions were normalized to β-actin.
Statistical analysis
Results are expressed as means ± SEM values. Significant differences between means were determined by performing analysis of variance. Differences between groups were determined by using Duncan's multiple range test. Analyses utilized the SAS statistical analysis software package (SAS Institute, Cary, NC, USA). Differences were regarded significant at P < 0.05.
RESULTS
Effect of an ethanol extract of Angelica keiskei (AK-Ex) on viable cell numbers against acetaminophen (AAP)-induced hepatotoxicity in HepG2 and HepaRG cells
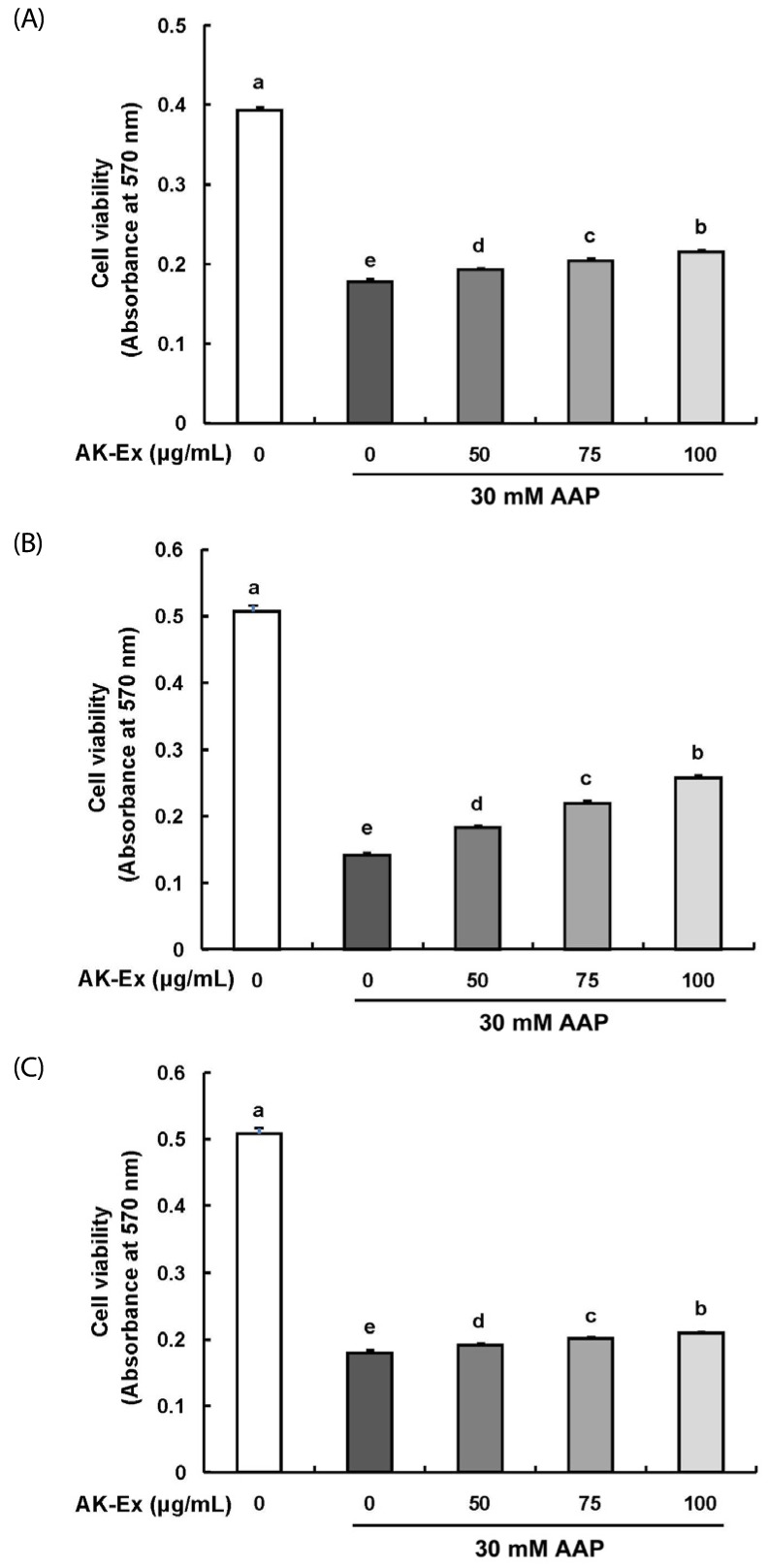
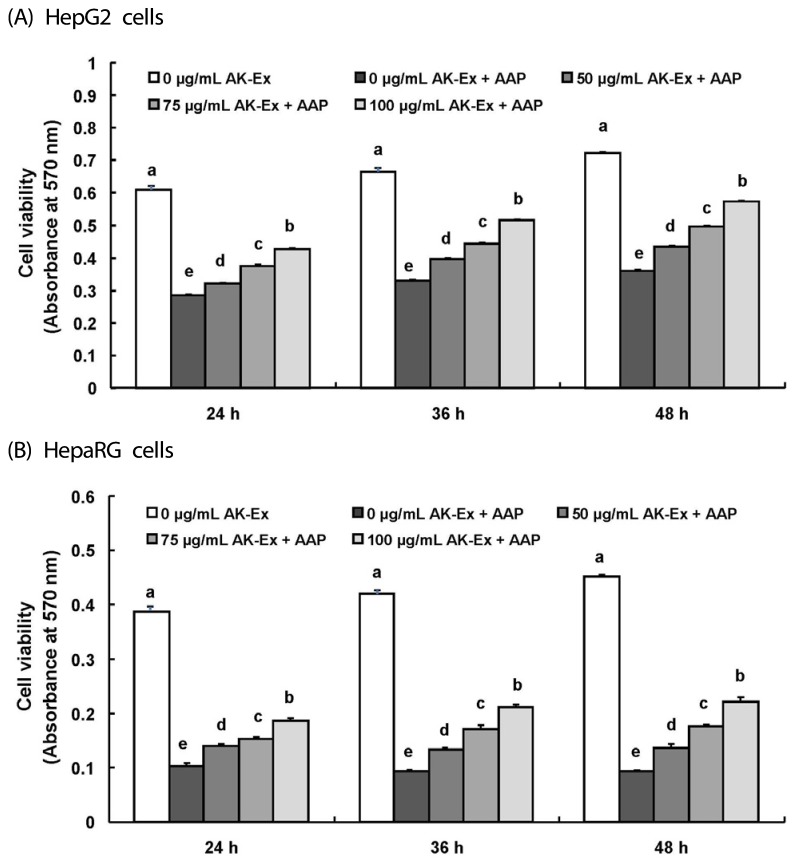
We first assessed the effect of various concentrations (0, 50, 75, 100 µg/mL) of AK-Ex on cell growth against AAP-induced hepatotoxicity in HepG2 cells by estimating the total number of viable cells by performing MTT assays. As shown in Fig. 1, according to the concentration of AK-Ex and under AAP overdose treatment, the effects on cell growth against AAP-induced hepatotoxicity were different. When treating AK-Ex and AAP simultaneously or when inducing hepatotoxicity first by treating with of AAP overdose and subsequently treating AK-Ex, there were significant differences in the number of viable cells. However, there was a remarkably marked increase in the number of viable cells when treating AK-Ex first and then inducing AAP-hepatotoxicity (Fig. 1). Therefore, AK-Ex seems to be effective in preventing AAP-induced hepatotoxicity when HepG2 cell are pretreated with AK-Ex. To examine the effect on protecting liver cell against AAP-induced hepatotoxicity according to AK-Ex pretreatment time, HepG2 cells in culture media containing various concentrations of AK-Ex were treated for 24, 36, and 48 h followed by 30 mM AAP to induce hepatotoxicity. The number of viable cells were determined after 24 h. The results show that AK-Ex increased the number of viable cell numbers in a time-dependent manner (Fig. 2A).
Fig. 1. Effects of an ethanol extract of Angelica keiskei (AK-Ex) on viable cell numbers in HepG2 cells.
Cells were plated at a density of 5 × 104 cells/well in 24-well plates. One day later, (A) cells were co-treated with the indicated concentration of AK-Ex and 30 mM AAP for 48 h. (B) Cells were treated with various concentration of AK-Ex for 24 h and then treated with 30 mM AAP for 24 h. (C) Cells were treated with 30 mM AAP for 24 h and then treated with various concentration of AK-Ex for 24 h. Cell numbers were estimated by using the MTT assay. Each bar represents mean ± SEM values (n = 4). Means without a common letter differ significantly, P < 0.05.
Fig. 2. Effect of AK-Ex pretreatment period on viable cell numbers in HepG2 and HepaRG cells.
(A) HepG2 cells and (B) HepaRG cells were plated at a density of 5 × 104 cells/well in 24-well plates. After 24 h, cells were pretreated with the indicated concentration of AK-Ex for 24, 36, or 48 h, respectively. Subsequently, cells were incubated with 30 mM AAP for 24 h. Cell numbers were estimated by using the MTT assay. Each bar represents mean ± SEM values (n = 4). Means without a common letter differ significantly, P < 0.05.
The HepG2 cell line originated from a human hepatocellular carcinoma and has been a useful human cellular liver model for toxicity testing. One of the reasons for its usefulness is that it is easy to cultivate HepG2 cells [19]. Although the HepG2 cell line indicates many liver-specific functions, it also has some disadvantages, the most distinct being that in comparison to primary human hepatocytes, HepG2 cells differ in the expression and activity levels of many xenobiotic metabolizing enzymes and transporters [19,20,21,22]. Therefore, we examined the effects of AK-Ex on cell growth in HepaRG cells, the primary human hepatocyte. It is hard to use HepaRG for study because of its need for difficult culture conditions. However, HepaRG may represent a more promising liver model than HepG2 when evaluating hepatic drug metabolism under in vitro conditions because HepaRG possesses the metabolic capacity characteristics of primary human hepatocytes [23,24,25].
As shown in Fig. 2B, after 24, 36, 48 h of Ak-Ex pretreatment in HepaRG cells, we induced hepatotoxicity by using 30 mM AAP and measured the number of viable cells after 24 h. As a result, the number of viable cell significantly increased in HepaRG cells, as was also observed in HepG2 cells, as pretreatment time increased. Therefore, AK-Ex can reduce AAP-induced hepatotoxicity in HepaRG cells, as well as in HepG2 cells.
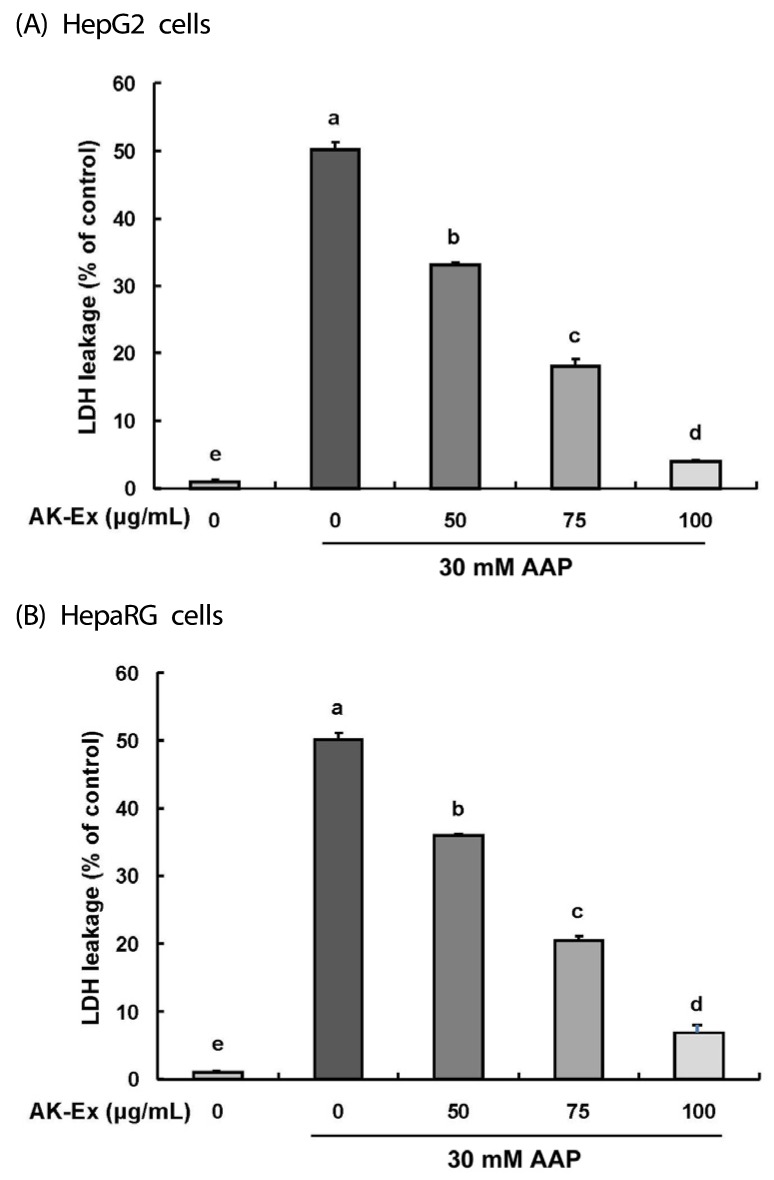
Effect of AK-Ex on lactate dehydrogenase release in HepG2 and HepaRG cells intoxicated with AAP
LDH was found in all cells and its release was regarded as a biomarker of cell cytotoxicity, and such relases increases mitochondrial permeability and damage [26]. In this study, in order to determine whether AK-Ex changes the LDH concentration in culture media, we measured the LDH concentration of HepG2 and HepaRG cells by culturing with various concentrations of AK-Ex for 48 h and then culturing for another 24 h after adding 30 mM AAP. As shown in Fig. 3A and 3B, AK-Ex reduced the membrane permeability resulting in AAP-induced hepatotoxicity in HepG2 and HepaRG cells in a dose-dependent manner. These results show that pretreatment of HepG2 and HepaRG cells with AK-Ex can prevent AAP-induced hepatotoxicity.
Fig. 3. Effect of AK-Ex on LDH leakage in HepG2 and HepaRG cells.
(A) HepG2 cells were plated at a density of 5 × 104 cells/well in a 24-well plate and incubated for 24 h. (B) HepaRG cells were plated at a density of 1.2 × 105 cells/well in a 24-well plate and incubated for 72 h. Cells were treated for 48 h with various concentration of AK-Ex and then treated with 30 mM AAP for an additional 24 h. The 24 h-conditioned media were collected for LDH activity assay. LDH activity was measured by using an LDH cytotoxicity detection assay kit. Each bar represents the mean ± SEM values (n = 4). Means without a common letter differ significantly, P < 0.05.
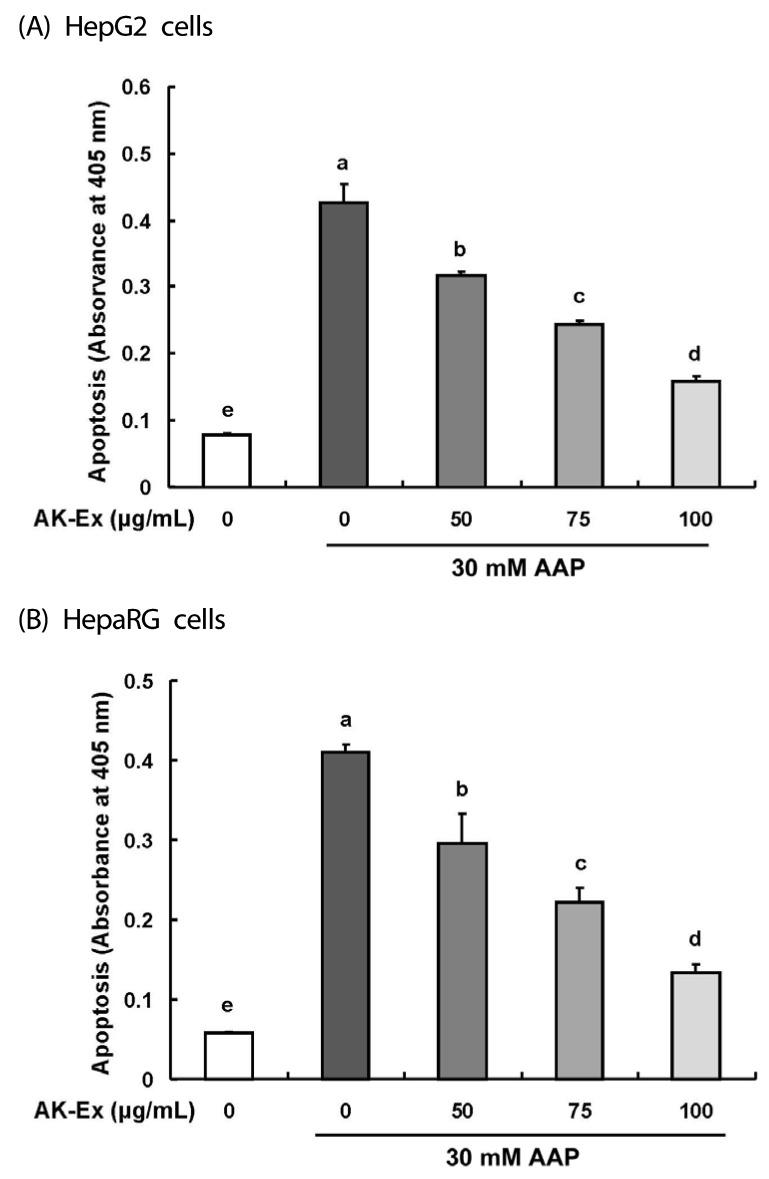
Effect of AK-Ex on apoptosis in HepG2 and HepaRG cells intoxicated with AAP
To investigate the effect of AK-Ex on apoptosis in hepatocytes, we examined the effect of AK-Ex on DNA fragmentation. HepG2 or HepaRG cells were treated with 0, 50, 75, 100 µg/mL AK-Ex and then cytotoxicity was induced by 30 mM AAP treatment. DNA fragmentation, a morphometric characteristics of apoptosis was detected via a cell death detection ELISAPLUS assay kit. As shown in Fig. 4A and 4B, the number of apoptotic cell increased following AAP overdose in HepG2 and HepaRG cell lines. However, when the cells were pretreated with AK-Ex for 48 h, AAP-induced apoptosis significantly decreased in a dose-dependent manner. Thus, AK-Ex pretreatment can reduce AAP-induced hepatotoxicity.
Fig. 4. Effect of AK-Ex on apoptosis in HepG2 and HepaRG cells.
(A) HepG2 and (B) HepaRG cells were treated with AK-Ex and/or AAP, as described in Fig. 3. The number of apoptotic cells was assayed by using a cell death detection ELISAPLUS assay kit. Each bar represents mean ± SEM values (n = 4). Means without a common letter differ significantly, P < 0.05.
Effect of AK-Ex on depolarization of mitochondria in HepG2 cells intoxicated with AAP
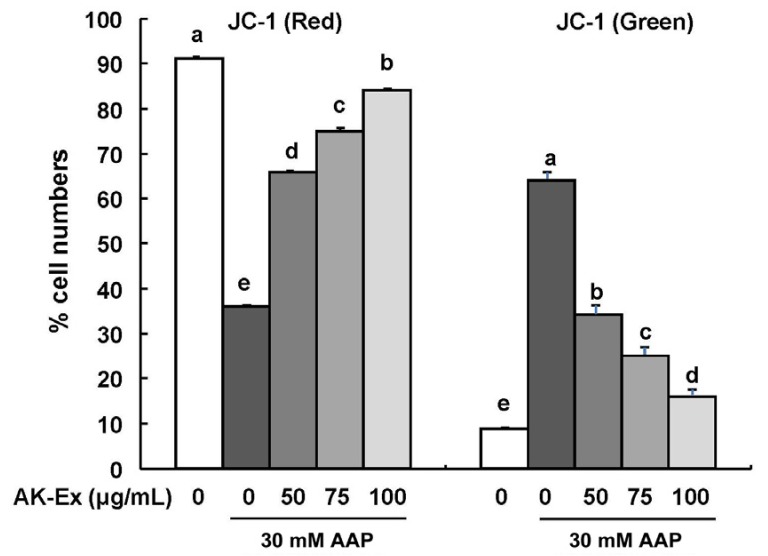
To determine whether AK-Ex-reduced apoptosis against AAP-induced cytotoxicity was mediated via a mitochondrial-dependent pathway, we used JC-1 as a mitochondrial depolarization marker. JC-1 was used because of its capability to detect changes in the mitochondrial membrane potential of living cells. As shown in Fig. 5, AK-Ex increased the percentage of cells with intact mitochondria (red positive and green negative) and decreased the percentage of cells with depolarized mitochondrial membranes (green positive and red negative) in an AK-Ex concentration-dependent manner. The results show that mitochondrial membrane permeability decreases in HepG2 cells against AAP-induced hepatotoxicity by pretreatment of AK-Ex in a dose-dependent manner.
Fig. 5. Effect of AK-Ex on mitochondrial membrane potential in HepG2 and HepaRG cells.
(A) HepG2 and (B) HepaRG cells were treated with AK-Ex and/or AAP, as described in Fig. 3. Cells were loaded with JC-1 and then analyzed by performing flow cytometry. The numbers of cells with normally polarized mitochondrial membranes (red) or with depolarized mitochondrial membranes (green) are expressed as percentages of the total cell number. Each bar represents the mean ± SEM values (n = 4). Means without a common letter differ significantly, P < 0.05.
Effect of AK-Ex on the levels of Bcl-2 family proteins in HepG2 cells intoxicated with AAP
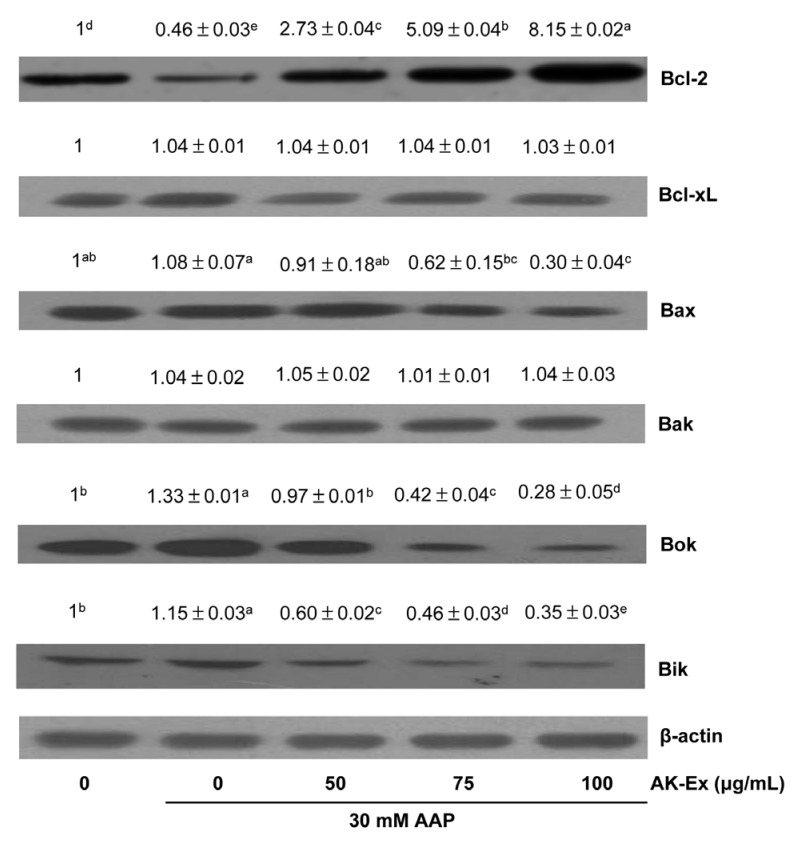
To explain the molecular mechanism of AK-Ex associated with the suppression of apoptosis, we examined the effects of AK-Ex on the expression of Bcl-2 family proteins in AAP-induced hepatotoxic HepG2 cells. Bcl-2 family proteins regulate apoptosis by controlling mitochondrial membrane permeability and cytochrome c release [27]. Cells were treated with various concentrations of AK-Ex for 48 h and then treated with 30 mM AAP, after which total cell lysates were prepared for western blot analysis. We analyzed the protein expressions of Bax, Bok, Bik, and Bak as proapoptotic markers and Bcl-2 and Bcl-xL as anti-apoptotic markers. AK-Ex increased the levels of the anti-apoptotic Bcl-2 protein but did not affect Bcl-xL expression in AAP-induced hepatotoxic HepG2 cells. AK-Ex did not affect the levels of the proapoptotic protein Bak, but Bax, Bok, and Bik expressions were decreased in cells treated with AK-Ex (Fig. 6).
Fig. 6. Effect of AK-Ex on the protein levels of Bal-2 family proteins in HepG2 cells.
Cells were plated at a density of 1 × 106 cells/dish in 100 mm dishes and treated with AK-Ex and/or AAP, as described in Fig. 3. Cell lysates were analyzed by western blotting with the indicated antibodies. Photographs of the chemiluminescent detection of the blots, which are representative of three independent experiments, are shown. Relative abundance of each band to their own β-actin level was quantified and the control levels were set at 1. The adjusted mean ± SEM values (n = 3) of each band are shown above each blot. Means without a common letter differ significantly, P < 0.05.
Effect of AK-Ex on activation of caspases and PARP cleavage in HepG2 and HepaRG cells intoxicated with AAP
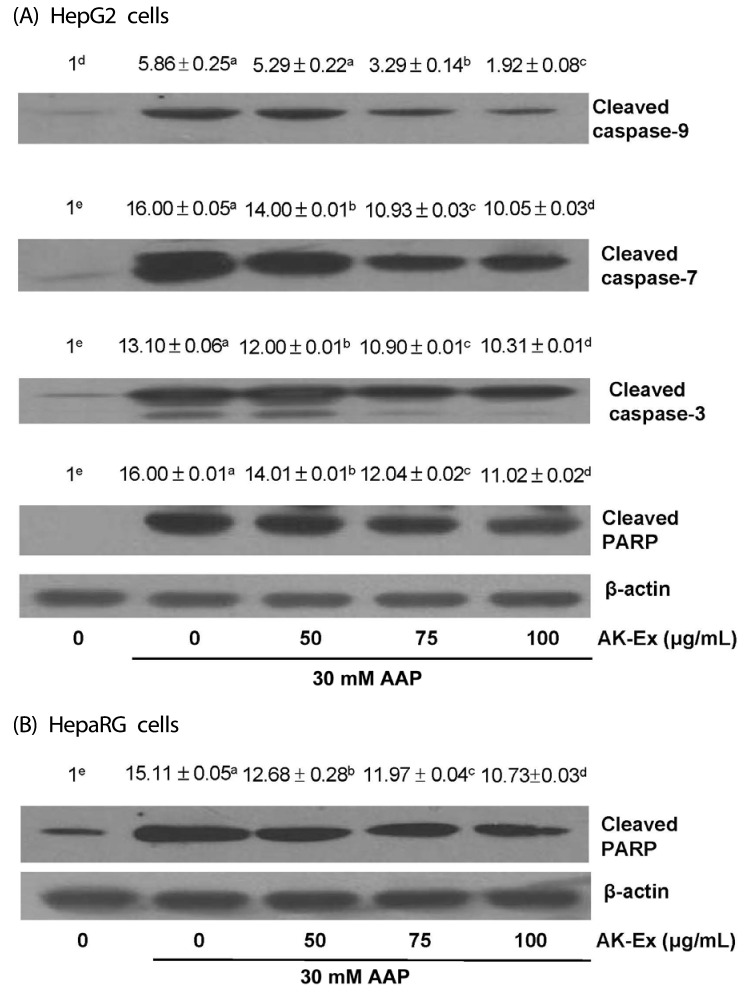
We also determined whether AK-Ex activates caspases. To that end, we performed western blotting with antibodies that detect cleaved forms of the enzymes. AK-Ex decreased the cleaved caspase-9, -7 and -3 levels in concentration-dependent manners. In addition, the PARP cleavage product significantly decreased with increasing concentrations of AK-Ex (Fig. 7A). We then tried to determine whether AK-Ex affects the PARP cleavage in HepaRG cells. The results showed that the PARP cleavage product significantly decreased with increasing concentrations of AK-Ex (Fig. 7B).
Fig. 7. Effect of AK-Ex on the protein levels of cleaved caspases and cleaved PARP in HepG2 and HepaRG cells.
HepG2 and HepaRG cells were plated at a density of 1 × 106 cells/dish in 100 mm dishes and treated with AK-Ex and/or AAP, respectively, as described in Fig. 3. Cell lysates were analyzed by western blotting with the indicated antibodies. Photographs of the chemiluminescent detection of the blots, which are representative of three independent experiments, are shown. Relative abundance of each band to their own β-actin level was quantified and the control levels were set at 1. The adjusted mean ± SEM values (n = 3) of each band are shown above each blot. Means without a common letter differ significantly, P < 0.05.
DISCUSSION
AK has been used as a folk medicine and as a health-promoting herb. It has been reported that AK contains numerous bioactive substances such as chalcones, coumarines, flavonoids, saponins, and vitamins and exhibits anti-inflammatory [2,3,28], anti-adiposity [4,29], anti-oxidant [30], anti-diabetic [31,32], anti-hypertensive [5], anti-tumor [6,7], and anti-memory impairment [33] effects.
In the present study, we found that AK-Ex treatment, when executed prior to AAP treatment, increased the total viable cell numbers in HepG2 cells against AAP-induced hepatotoxicity (Fig. 1). Therefore, AK-Ex appears to effectively protect against future hepatotoxicity if, and only if, it is used as a supplement in advance of hepatic damage. Other researchers have also reported the hepatoprotective effect of AK [34]. An AK methanol extract, when administered prior to galactosamine, exerted protective effects against galactosamine-induced hepatotoxicity in rat [34]. These results suggest that AK has a hepatoprotective effects, but such effects are not to be equally expected as they depend on the hepatotoxines employed. In addition, our results indicate that AK-Ex reduces apoptosis against AAP-induced hepatotoxicity in HepG2 and HepaRG cells.
Apoptosis is an important process resulting in programmed cell death. Activation of apoptosis is initiated via two main pathways: intrinsic and/or extrinsic [35]. Mitochondria are an essential mediator of intrinsic apoptosis, and the Bcl-2 family of proteins, located in the outer mitochondrial membrane and consisting of proapoptotic (such as Bax, Bid, Bik, Bak, Bok) and antiapoptotic mediators (such as Bcl-2, Bcl-xL, Mcl-1), is important in modulating apoptosis by its ability to alter mitochondrial permeability to apoptotic mediators [36]. The permeability of the mitochondrial membrane is crucial in several apoptosis pathways. Apoptosis in mammalian cells has been shown to be regulated by Bcl-2 family of proteins [37]; thus, we determined whether AK-Ex has a protective effect on AAP-induced apoptosis in HepG2 cells and examined its association with the modulation of these proteins. AK-Ex, in a concentration-dependent manner, significantly reduced apoptosis and the percentage of cells with depolarized mitochondrial membranes, and it increased the percentage of cells with intact mitochondria (Fig. 4, 5). Western blotting results indicated that AK-Ex treatment increased the expression of Bcl-2 and decreased the expression of Bax, Bok, and Bik; however, it did not change the expressions of antiapoptotic Bcl-xL or pro-apoptotic Bak (Fig. 6). Bax and Bcl-2 are important regulators of cytochrome c release from mitochondria [37]. Internal damage to a cell causes a decrease in the Bcl-2 / Bax ratio, which results in holes in the mitochondrial membrane. These inter-mitochondrial apoptotic molecules are released into the cytosol upon various forms of signals, leading to changes in permeability of the mitochondrial membrane [38]. Cytochrome c is one of the pro-apoptotic molecules that, leads to activation of caspases and disrupts outer mitochondrial membrane permeability. In the present study, AK-Ex significantly increased the Bcl-2 / Bax ratio against AAP-induced cytotoxicity in HepG2 cells. Thus it seems that AK-Ex-inhibited apoptosis involves Bax and Bcl-2 signal transduction.
Activation of apoptosis via the extrinsic pathway occurs via the binding of apoptotic ligands to death receptors [39]. These death receptors have an intracellular domain that functions as a protein-binding module. Following the recruitment and signaling of various adaptor molecules, cleavage and activation of initiator caspase-8 -9, -10, and -12 occurs. In turn, this leads to activation of executioner caspase-3, -6, and -7 as well as effector caspases, culminating in DNA fragmentation [40]. In the present study, AK-Ex decreased cleaved caspase-9, -7, and -3 and cleaved PARP (Fig. 7). AK-Ex activates caspase-9, a cysteine protease, and caspase-9 activation lead to activation of caspase-7 and caspase-3, which are responsible for destroying the cell from within.
PARP found in the cell's nucleus, is a family of proteins involved in DNA repair and programmed cell death. PARP can be activated in cells experiencing stress and/or DNA damage, and PARP is inactivated by caspase-3 cleavage during programmed cell death. Cleaved PARP is a marker for apoptosis [41]. In this study, AK-Ex treatment decreased cleaved PARP in HepG2 and HepaRG cells intoxicated by AAP (Fig. 7). Therefore, the present study's results suggest that AK-Ex suppresses AAP-induced hepatocytotoxic apoptosis via both intrinsic and extrinsic pathways in HepG2 and HepaRG cells.
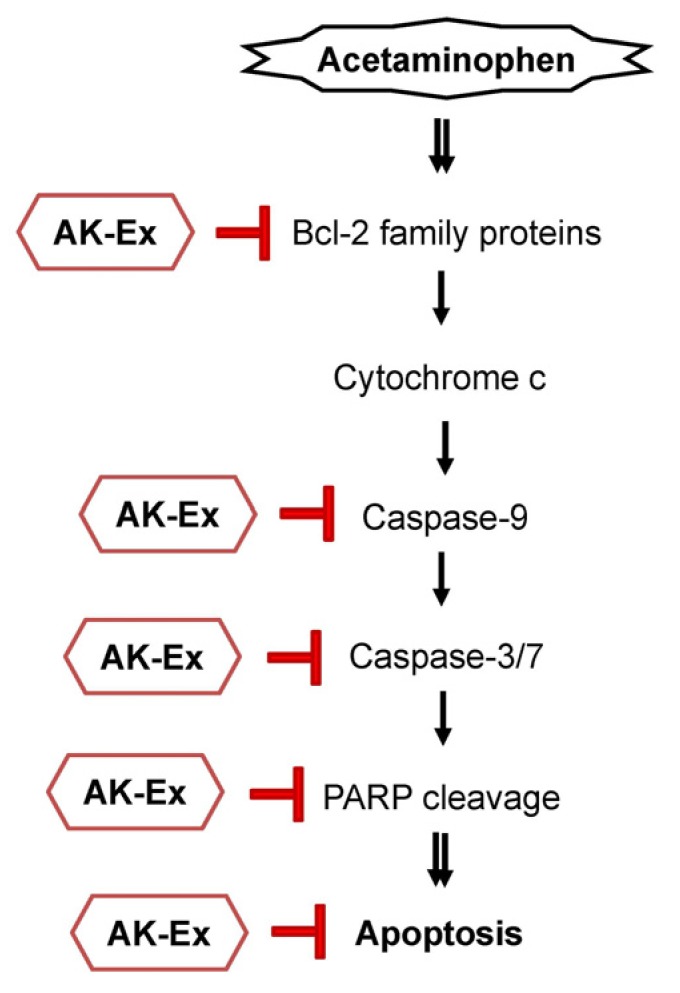
In the present study, we show that AK-Ex suppresses apoptosis; decreases mitochondrial membrane permeability; increases anti-apoptotic mediators such as Bcl-2, decreases pro-apoptotic mediators such as Bax, Bok, and Bik, and decreases cleaved caspase-9, -7, and -3, and cleaved PARP. Taken together, the present results suggest that AK-Ex suppresses AAP-induced hepatocytotoxic apoptosis via both intrinsic and extrinsic pathway in HepG2 and HepaRG cells (Fig. 8).
Fig. 8. Schematic representation of a possible mechanism for the hepatoprotective effect of AK-Ex in HepG2 and HepaRG cells.
In conclusion, our data demonstrated that AK-Ex protects againsted AAP-induced growth inhibition and apoptosis induction in HepG2 and HepaRG cells. Moreover, AK-Ex modulates the expression of Bcl-2 family proteins and decreases the levels of cleaved caspase-9, -7, and -3, and cleaved PARP under AAP-induced hepatotoxicity. Based on these observations, we suggest that AK could be a useful hepatoprotective agent against AAP-induced apoptosis in hepatocytes. Future in vivo studies of animal hepatotoxic models are needed to examine whether AK-Ex could be a useful hepatoprotective treatment agent against hepatotoxicity.
Footnotes
This work was supported by the Ministry of Trade, Industry and Energy (MOTIE) and Korea Institute for Advancement of Technology (KIAT) through the Research and Promoting Regional Specialized Industry (R0005131) and the Center for Efficacy Assessment and Development of Functional Foods and Drugs at Hallym University (B0008864), Korea.
CONFLICT OF INTEREST: The authors declare no potential conflicts of interests.
References
- 1.Maronpot RR. Toxicological assessment of Ashitaba Chalcone. Food Chem Toxicol. 2015;77:111–119. doi: 10.1016/j.fct.2014.12.021. [DOI] [PubMed] [Google Scholar]
- 2.Chang HR, Lee HJ, Ryu JH. Chalcones from Angelica keiskei attenuate the inflammatory responses by suppressing nuclear translocation of NF-kappaB. J Med Food. 2014;17:1306–1313. doi: 10.1089/jmf.2013.3037. [DOI] [PubMed] [Google Scholar]
- 3.Yasuda M, Kawabata K, Miyashita M, Okumura M, Yamamoto N, Takahashi M, Ashida H, Ohigashi H. Inhibitory effects of 4-hydroxyderricin and xanthoangelol on lipopolysaccharide-induced inflammatory responses in RAW264 macrophages. J Agric Food Chem. 2014;62:462–467. doi: 10.1021/jf404175t. [DOI] [PubMed] [Google Scholar]
- 4.Zhang T, Yamashita Y, Yasuda M, Yamamoto N, Ashida H. Ashitaba (Angelica keiskei) extract prevents adiposity in high-fat diet-fed C57BL/6 mice. Food Funct. 2015;6:135–145. doi: 10.1039/c4fo00525b. [DOI] [PubMed] [Google Scholar]
- 5.Ogawa H, Okada Y, Kamisako T, Baba K. Beneficial effect of xanthoangelol, a chalcone compound from Angelica keiskei, on lipid metabolism in stroke-prone spontaneously hypertensive rats. Clin Exp Pharmacol Physiol. 2007;34:238–243. doi: 10.1111/j.1440-1681.2007.04578.x. [DOI] [PubMed] [Google Scholar]
- 6.Kim E, Choi J, Yeo I. The effects of Angelica keiskei Koidz on the expression of antioxidant enzymes related to lipid profiles in rats fed a high fat diet. Nutr Res Pract. 2012;6:9–15. doi: 10.4162/nrp.2012.6.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akihisa T, Kikuchi T, Nagai H, Ishii K, Tabata K, Suzuki T. 4-Hydroxyderricin from Angelica keiskei roots induces caspasedependent apoptotic cell death in HL60 human leukemia cells. J Oleo Sci. 2011;60:71–77. doi: 10.5650/jos.60.71. [DOI] [PubMed] [Google Scholar]
- 8.Noh HM, Ahn EM, Yun JM, Cho BL, Paek YJ. Angelica keiskei Koidzumi extracts improve some markers of liver function in habitual alcohol drinkers: a randomized double-blind clinical trial. J Med Food. 2015;18:166–172. doi: 10.1089/jmf.2014.3222. [DOI] [PubMed] [Google Scholar]
- 9.Ni HM, Bockus A, Boggess N, Jaeschke H, Ding WX. Activation of autophagy protects against acetaminophen-induced hepatotoxicity. Hepatology. 2012;55:222–232. doi: 10.1002/hep.24690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Labbe G, Pessayre D, Fromenty B. Drug-induced liver injury through mitochondrial dysfunction: mechanisms and detection during preclinical safety studies. Fundam Clin Pharmacol. 2008;22:335–353. doi: 10.1111/j.1472-8206.2008.00608.x. [DOI] [PubMed] [Google Scholar]
- 11.Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, Reisch JS, Schiødt FV, Ostapowicz G, Shakil AO, Lee WM Acute Liver Failure Study Group. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42:1364–1372. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- 12.Jaeschke H. Glutathione disulfide formation and oxidant stress during acetaminophen-induced hepatotoxicity in mice in vivo: the protective effect of allopurinol. J Pharmacol Exp Ther. 1990;255:935–941. [PubMed] [Google Scholar]
- 13.Holownia A, Braszko JJ. Acetaminophen alters microsomal ryanodine Ca2+ channel in HepG2 cells overexpressing CYP2E1. Biochem Pharmacol. 2004;68:513–521. doi: 10.1016/j.bcp.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 14.Kon K, Kim JS, Jaeschke H, Lemasters JJ. Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology. 2004;40:1170–1179. doi: 10.1002/hep.20437. [DOI] [PubMed] [Google Scholar]
- 15.Kim EJ, Holthuizen PE, Park HS, Ha YL, Jung KC, Park JH. Trans-10, cis-12-conjugated linoleic acid inhibits Caco-2 colon cancer cell growth. Am J Physiol Gastrointest Liver Physiol. 2002;283:G357–G367. doi: 10.1152/ajpgi.00495.2001. [DOI] [PubMed] [Google Scholar]
- 16.Thomas JP, Geiger PG, Girotti AW. Lethal damage to endothelial cells by oxidized low density lipoprotein: role of selenoperoxidases in cytoprotection against lipid hydroperoxide- and iron-mediated reactions. J Lipid Res. 1993;34:479–490. [PubMed] [Google Scholar]
- 17.Jung JI, Lim SS, Choi HJ, Cho HJ, Shin HK, Kim EJ, Chung WY, Park KK, Park JH. Isoliquiritigenin induces apoptosis by depolarizing mitochondrial membranes in prostate cancer cells. J Nutr Biochem. 2006;17:689–696. doi: 10.1016/j.jnutbio.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 18.Cho HJ, Kim WK, Kim EJ, Jung KC, Park S, Lee HS, Tyner AL, Park JH. Conjugated linoleic acid inhibits cell proliferation and ErbB3 signaling in HT-29 human colon cell line. Am J Physiol Gastrointest Liver Physiol. 2003;284:G996–1005. doi: 10.1152/ajpgi.00347.2002. [DOI] [PubMed] [Google Scholar]
- 19.Jetten MJ, Kleinjans JC, Claessen SM, Chesne C, van Delft JH. Baseline and genotoxic compound induced gene expression profiles in HepG2 and HepaRG compared to primary human hepatocytes. Toxicol In Vitro. 2013;27:2031–2040. doi: 10.1016/j.tiv.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 20.Jennen DG, Magkoufopoulou C, Ketelslegers HB, van Herwijnen MH, Kleinjans JC, van Delft JH. Comparison of HepG2 and HepaRG by whole-genome gene expression analysis for the purpose of chemical hazard identification. Toxicol Sci. 2010;115:66–79. doi: 10.1093/toxsci/kfq026. [DOI] [PubMed] [Google Scholar]
- 21.Liguori MJ, Blomme EA, Waring JF. Trovafloxacin-induced gene expression changes in liver-derived in vitro systems: comparison of primary human hepatocytes to HepG2 cells. Drug Metab Dispos. 2008;36:223–233. doi: 10.1124/dmd.107.017608. [DOI] [PubMed] [Google Scholar]
- 22.Guo L, Dial S, Shi L, Branham W, Liu J, Fang JL, Green B, Deng H, Kaput J, Ning B. Similarities and differences in the expression of drug-metabolizing enzymes between human hepatic cell lines and primary human hepatocytes. Drug Metab Dispos. 2011;39:528–538. doi: 10.1124/dmd.110.035873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aninat C, Piton A, Glaise D, Le Charpentier T, Langouët S, Morel F, Guguen-Guillouzo C, Guillouzo A. Expression of cytochromes P450, conjugating enzymes and nuclear receptors in human hepatoma HepaRG cells. Drug Metab Dispos. 2006;34:75–83. doi: 10.1124/dmd.105.006759. [DOI] [PubMed] [Google Scholar]
- 24.Andersson TB. The application of HepRG cells in evaluation of cytochrome P450 induction properties of drug compounds. Methods Mol Biol. 2010;640:375–387. doi: 10.1007/978-1-60761-688-7_20. [DOI] [PubMed] [Google Scholar]
- 25.Ferreira A, Rodrigues M, Silvestre S, Falcão A, Alves G. HepaRG cell line as an in vitro model for screening drug-drug interactions mediated by metabolic induction: amiodarone used as a model substance. Toxicol In Vitro. 2014;28:1531–1535. doi: 10.1016/j.tiv.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 26.Danpure CJ. Lactate dehydrogenase and cell injury. Cell Biochem Funct. 1984;2:144–148. doi: 10.1002/cbf.290020305. [DOI] [PubMed] [Google Scholar]
- 27.Cory S, Huang DC, Adams JM. The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene. 2003;22:8590–8607. doi: 10.1038/sj.onc.1207102. [DOI] [PubMed] [Google Scholar]
- 28.Ohkura N, Nakakuki Y, Taniguchi M, Kanai S, Nakayama A, Ohnishi K, Sakata T, Nohira T, Matsuda J, Baba K, Atsumi G. Xanthoangelols isolated from Angelica keiskei inhibit inflammatory-induced plasminogen activator inhibitor 1 (PAI-1) production. Biofactors. 2011;37:455–461. doi: 10.1002/biof.187. [DOI] [PubMed] [Google Scholar]
- 29.Nagata J, Morino T, Saito M. Effects of dietary Angelica keiskei on serum and liver lipid profiles, and body fat accumulations in rats. J Nutr Sci Vitaminol (Tokyo) 2007;53:133–137. doi: 10.3177/jnsv.53.133. [DOI] [PubMed] [Google Scholar]
- 30.Aoki N, Muko M, Ohta E, Ohta S. C-geranylated chalcones from the stems of Angelica keiskei with superoxide-scavenging activity. J Nat Prod. 2008;71:1308–1310. doi: 10.1021/np800187f. [DOI] [PubMed] [Google Scholar]
- 31.Ohnogi H, Hayami S, Kudo Y, Deguchi S, Mizutani S, Enoki T, Tanimura Y, Aoi W, Naito Y, Kato I, Yoshikawa T. Angelica keiskei extract improves insulin resistance and hypertriglyceridemia in rats fed a high-fructose drink. Biosci Biotechnol Biochem. 2012;76:928–932. doi: 10.1271/bbb.110927. [DOI] [PubMed] [Google Scholar]
- 32.Kawabata K, Sawada K, Ikeda K, Fukuda I, Kawasaki K, Yamamoto N, Ashida H. Prenylated chalcones 4-hydroxyderricin and xanthoangelol stimulate glucose uptake in skeletal muscle cells by inducing GLUT4 translocation. Mol Nutr Food Res. 2011;55:467–475. doi: 10.1002/mnfr.201000267. [DOI] [PubMed] [Google Scholar]
- 33.Oh SR, Kim SJ, Kim DH, Ryu JH, Ahn EM, Jung JW. Angelica keiskei ameliorates scopolamine-induced memory impairments in mice. Biol Pharm Bull. 2013;36:82–88. doi: 10.1248/bpb.b12-00681. [DOI] [PubMed] [Google Scholar]
- 34.Choi SH, Park KH. Protective effects of Angelica keiskei extracts against D-galactosamine(GalN)-induced hepatotoxicity in rats. J Food Hyg Saf. 2011;26:235–241. [Google Scholar]
- 35.Huerta S, Goulet EJ, Huerta-Yepez S, Livingston EH. Screening and detection of apoptosis. J Surg Res. 2007;139:143–156. doi: 10.1016/j.jss.2006.07.034. [DOI] [PubMed] [Google Scholar]
- 36.Thomadaki H, Scorilas A. BCL2 family of apoptosis-related genes: functions and clinical implications in cancer. Crit Rev Clin Lab Sci. 2006;43:1–67. doi: 10.1080/10408360500295626. [DOI] [PubMed] [Google Scholar]
- 37.Boise LH, González-García M, Postema CE, Ding L, Lindsten T, Turka LA, Mao X, Nuñez G, Thompson CB. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993;74:597–608. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- 38.Nicholson DW. Caspase structure, proteolytic substrates, and function during apoptotic cell death. Cell Death Differ. 1999;6:1028–1042. doi: 10.1038/sj.cdd.4400598. [DOI] [PubMed] [Google Scholar]
- 39.Bürkle A, Brabeck C, Diefenbach J, Beneke S. The emerging role of poly(ADP-ribose) polymerase-1 in longevity. Int J Biochem Cell Biol. 2005;37:1043–1053. doi: 10.1016/j.biocel.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 40.Andrabi SA, Kim NS, Yu SW, Wang H, Koh DW, Sasaki M, Klaus JA, Otsuka T, Zhang Z, Koehler RC, Hurn PD, Poirier GG, Dawson VL, Dawson TM. Poly(ADP-ribose) (PAR) polymer is a death signal. Proc Natl Acad Sci U S A. 2006;103:18308–18313. doi: 10.1073/pnas.0606526103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Isabelle M, Moreel X, Gagné JP, Rouleau M, Ethier C, Gagné P, Hendzel MJ, Poirier GG. Investigation of PARP-1, PARP-2, and PARG interactomes by affinity-purification mass spectrometry. Proteome Sci. 2010;8:22. doi: 10.1186/1477-5956-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]