Abstract
In generating a point-to-point movement, the brain does more than produce the transient commands needed to move the body part; it also produces the sustained commands that are needed to hold the body part at its destination. In the oculomotor system, these functions are mapped onto two distinct circuits: a premotor circuit that specializes in generating the transient activity that displaces the eyes and a “neural integrator” that transforms that transient input into sustained activity that holds the eyes. Different parts of the cerebellum adaptively control the motor commands during these two phases: the oculomotor vermis participates in fine tuning the transient neural signals that move the eyes, monitoring the activity of the premotor circuit via efference copy, whereas the flocculus participates in controlling the sustained neural signals that hold the eyes, monitoring the activity of the neural integrator. Here, I review the oculomotor literature and then ask whether this separation of control between moving and holding is a design principle that may be shared with other modalities of movement. To answer this question, I consider neurophysiological and psychophysical data in various species during control of head movements, arm movements, and locomotion, focusing on the brain stem, motor cortex, and hippocampus, respectively. The review of the data raises the possibility that across modalities of motor control, circuits that are responsible for producing commands that change the sensory state of a body part are distinct from those that produce commands that maintain that sensory state.
Keywords: brain stem, cerebellum, motor cortex, reaching, saccades
Desire itself is movement.
Not in itself desirable…only the cause and end of movement.
T. S. Eliot
as david robinson (1970) measured the activity of motoneurons that innervated the extraocular muscles, he noted that during a saccadic eye movement, the motoneurons of the agonist muscles exhibited a burst, and once the movement ended, the same motoneurons maintained a sustained level of discharge. This steady firing, coupled with firing in the antagonist motoneurons, allowed the eyes to remain still during fixation. In contrast, the premotor neurons that innervated these motoneurons only exhibited a burst during the movement but were essentially silent during the hold phase. Robinson was puzzled, because as he wrote, the act of holding still was “just as much an active process as movement.” Why should the inputs to a motoneuron be separated into two branches with one specializing in the movement phase and the other providing control of the hold phase?
In the decades that followed, experiments demonstrated that the commands that were needed to make goal-directed eye movements were generated by two distinct circuits. A premotor circuit housed in the brain stem provided the activity needed to move the eyes, whereas another circuit, also housed in the brain stem, sat downstream from the move circuit, accumulated its transient activity, and then sustained it to produce the activity needed during the hold period. Interestingly, activities in these two brain stem circuits were monitored and supported by two separate regions of the cerebellum. The brain not only needed the cerebellum to learn to move accurately, but it also needed the cerebellum to learn to hold still after the movement had ended. Learning to move depended on the oculomotor vermis region of the cerebellum, whereas learning to hold depended on the flocculus region. As a result, the acts of moving and holding the eyes were functions of two distinct neural systems within the brain stem and supported by two distinct circuits within the cerebellum.
Is this separation of control a design feature that is shared across the various motor systems? For example, when we reach, a motoneuron may be activated to engage a muscle that produces some of the forces needed to displace the arm. During the hold period that follows, the same motoneuron may also be activated to produce the forces needed to hold the arm. Are the activations during these two periods coming from distinct neural circuits?
Here, I consider head movements, arm movements, and locomotion, focusing on the activity in the brain stem structures that are involved in control of head movements, the activity in the primary motor cortex (M1) and spinal interneurons that are involved in control of arm movements, and the activity in the hippocampus regions responsible for representing position of the body during locomotion. By considering the results together, a scenario emerges in which regardless of modality of action, there are separate controllers for generating the motor commands during movement and holding still.
THE OCULOMOTOR NEURAL INTEGRATOR
To move the eyes from one location to another, the motoneurons that innervate the extraocular muscles produce a burst of activity (a pulse), but to hold the eyes at that location, the motoneurons produce a steady discharge (a step). Robinson (1970) illustrated this by recording from motoneurons of the inferior rectus, the muscle that pulls the eye downward (Fig. 1A). He observed that a typical motoneuron gave a strong burst, ~10 ms before onset of a downward saccade (Fig. 1A), followed by a plateau of activity after the saccade had completed. When the saccade was in the upward direction (Fig. 1A), the motoneuron stopped firing but then increased its firing to a plateau after the saccade had ended. As a result, the discharge of the motoneuron during the hold phase scaled linearly with position of the eye: the greater the downward position of the eye during fixation, the greater the discharge of the neuron.
Fig. 1.
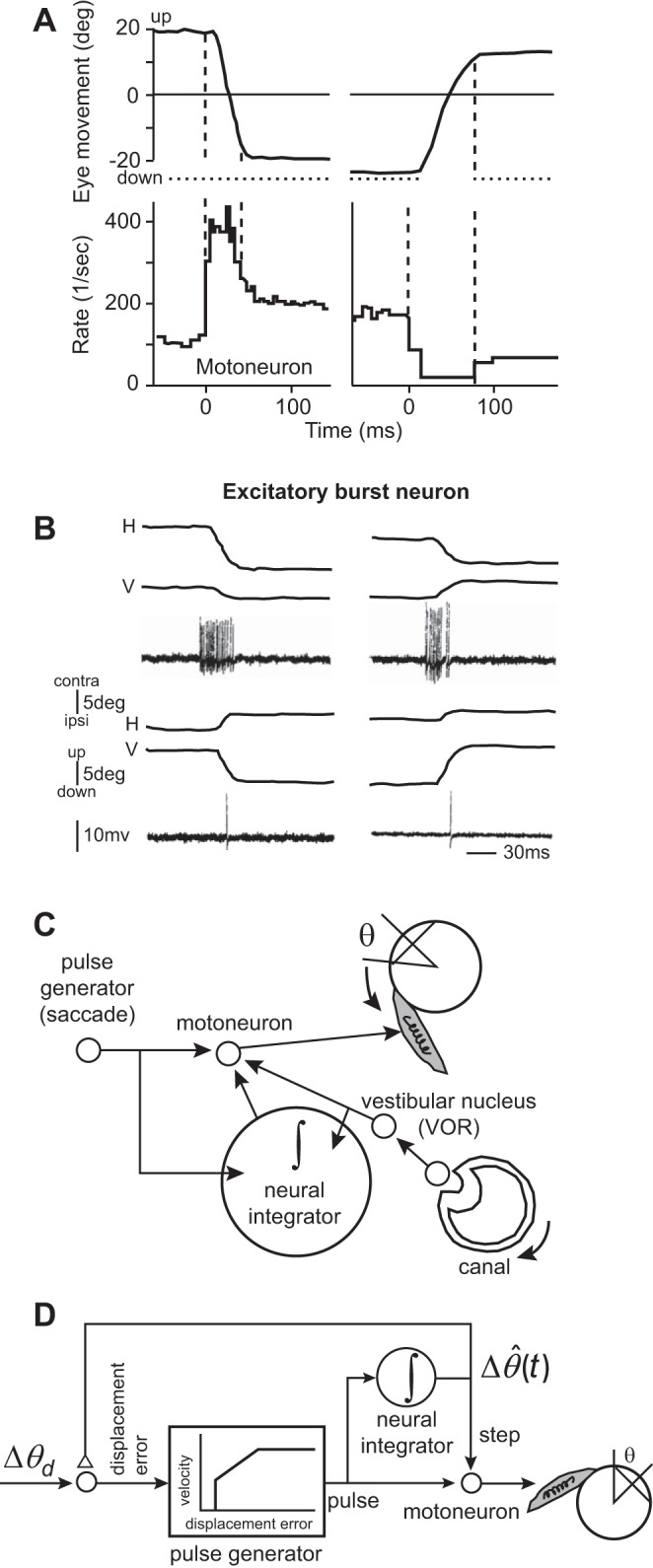
Activity of motoneurons that innervate the eye muscles includes information regarding moving the eyes, as well as holding the eyes. However, activity of some premotor neurons that project to these motoneurons includes only information about moving the eyes. A: activity of a single motoneuron that (probably) innervates the inferior rectus muscle. The motoneuron has a pulse-step pattern of activity. The left column is for a downward saccade, and the right column is for an upward saccade. Vertical, dashed lines indicate onset and offset of the saccade. From Robinson (1970), with permission. B: activity of an excitatory burst neuron (EBN) in the left brain stem for leftward (top) and rightward (bottom) saccades (both have a vertical component). The EBN cell excites ipsilateral (ipsi) abducens motoneuron and fires prominently during ipsilateral saccades but has no activity during the hold period. contra, contralateral; H, horizontal; V, vertical. From Strassman et al. (1986), with permission. C: the neural integrator and the Robinson (1973) model of eye movements. In the vestibulo-ocular reflex (VOR), the afferents in the head canal sense head-rotation velocity, providing a pulse-like input to the vestibular nucleus neurons. These neurons drive the eyes (in the opposite direction of the head movement), but their pulse is aided by the neural integrator, which also receives the pulse and sustains it through its input to the motoneurons. To generate a saccade, the burst generators produce a pulse, activating the motoneurons and moving the eyes to one side. The neural integrator receives this pulse and produces a step that sustains the motoneuron activity beyond the duration of the pulse, maintaining the eyes at the displaced position after the pulse has ended. D: Robinson (1973) hypothesized that control of saccades benefited from internal feedback of the neural integrator. The pulse generator received a desired displacement signal (Δθd), which it transformed into a pulse that depended on a real-time estimate of the current difference [] between the position of the eye and desired position. The neural integrator provided an estimate of current position and fed this signal back to the input of the pulse generator. When the pulse had pulled the eye to the desired location, the integrator’s output matched the desired position, which ended the pulse.
Robinson (1970) asked whether the activities during the move and hold phases could have been due to two different inputs: one that produced the phasic discharge that displaced the eyes and another that produced the tonic discharge needed to hold the eyes. In his 1970 paper (Robinson 1970), he wrote the following: “the presynaptic fibers [to the motoneurons] might carry discharge that are variously related to eye position and eye velocity, … influenced by two central structures, one concerned with position and the other with velocity.” This was a critical concept, because the focus of research in motor control at the time (and still today) was on the neural system that generated the movement. Robinson (1970) proposed that the premotor circuitry that drove the motoneurons was composed of two distinct circuits: one that generated a displacement, roughly encoding the velocity and duration of the movement, providing the pull that the eyes needed to move, and one that monitored the displacement and accumulated it, acting as a neural integrator, providing the tonic discharge that the eye needed to hold it in place (Robinson 1973).
He justified this idea by not considering saccades, which involve very fast movements of the eyes, but rather, a reflex that generates much slower eye movements, the vestibulo-ocular reflex (VOR). VOR refers to the observation that when the head is rotated to one side, the eyes rotate in the opposite direction. For example, when you are walking and your heel strikes the ground, the impact is translated from your legs through your torso, reaching your head and pulling it slightly down. This head rotation engages the vestibular afferents in the semicircular canals, where they report the motion by discharging linearly with respect to head-rotation velocity. The afferents engage neurons in the vestibular nucleus, which in turn generates a velocity-like discharge to drive the motoneurons of the eye, pulling it in the opposite direction of motion of the head. As a result, the VOR responds to the head movement through a compensatory motion of the eye, keeping the visual image stable on the retina, giving you the ability to read the signs on the street while walking (Leigh and Zee 2015).
During such relatively slow movements of the eye, motoneuron discharge is dominated by the position of the eye and not its velocity (Sylvestre and Cullen 1999). However, firing rates of vestibular neurons are dominated by head velocity. This was the clue that Robinson (1973) used to predict that there must exist a “neural integrator” between the vestibular nucleus and the motoneurons, as shown in Fig. 1C. He then generalized this conjecture to the control of saccades, producing the model shown in Fig. 1D.
In Robinson’s (1973) saccade model (Fig. 1D), the movement began with a representation of desired displacement of the eye (Δθd). A pulse generator transformed a position error into a burst of activity that resembled a velocity-like signal, driving the eye toward its goal. That pulse was integrated in real time to produce an estimate of how far the eye had been displaced, resulting in . The integration of the pulse was a step, and the sum of the pulse and step was the driving input to the motoneuron.
Importantly, Robinson (1973) went beyond the idea of two separate neural circuits for generating the pulse and step: he imagined a feedback system that would control the movement as it unfolded. In Robinson’s (1973) model, the output of the neural integrator not only provided the signal needed to hold the eye after the saccade completed, but it was also fed back and compared in real time with the desired displacement. In this way, the real-time difference between desired displacement and estimated displacement was the error signal that drove the pulse generator. Once the estimated displacement reached the desired displacement, the pulse stopped, and the eye stopped moving and was held in place by the step.
Robinson’s (1973) model introduced two new ideas. First, he proposed that the neural circuitry that moved the eye was different than the circuitry that held it in place. The circuitry that held the eye in place was an integrator that accumulated the activity of the circuitry that moved the eye. This gave birth to the idea of a neural integrator. Second, he proposed that all eye movements, including saccades that were only a few tens of milliseconds in duration, were controlled by an internal feedback circuitry. The feedback circuitry estimated the real-time progress that had been made in moving the body part and fed it back to the system that was moving it, telling it when to stop. As we will see, this idea was true in principle but incorrect in part. The mechanism for monitoring progress of the movement was not via feedback from the integrator but via a dedicated circuit in the cerebellum—a circuit that today we call a forward model.
MOVING THE EYES
According to Fig. 1D, there is a neural circuit responsible for generating the pulse-like activity that the motoneurons need to displace the eyes. For horizontal saccades, this input to the motoneurons comes from the burst generations in the brain stem: premotor neurons, called the excitatory burst neurons (EBNs) and inhibitory burst neurons (IBNs), located in two small regions of the reticular formation. In contrast, the step component of the input comes from the prepositus, another brain stem region that appears to integrate the input mathematically from the burst generators.
The paramedian pontine reticular formation (PPRF) is a brain stem region rostral to the abducens nucleus (Fig. 2A). This region houses the EBNs, which make an excitatory synapse on the ipsilateral abducens motoneurons. EBNs burst ~10 ms before ipsiversive saccades and then become silent after the saccade (Fig. 1C). As a result, the EBN discharge does not have the step component present in the activity of motoneurons, just the pulse (Strassman et al. 1986). EBNs are strongly directional and only fire occasionally for movements to the opposite side. Their distribution of preferred direction (PD) is mostly along the horizontal axis. In their on direction, the duration of discharge is correlated with duration of saccades. Sparks and colleagues (2002) found that the number of spikes in the discharge of the EBNs increased with the amplitude of the saccade, and the peak spike rate during the saccade increased with peak velocity of the saccade.
Fig. 2.
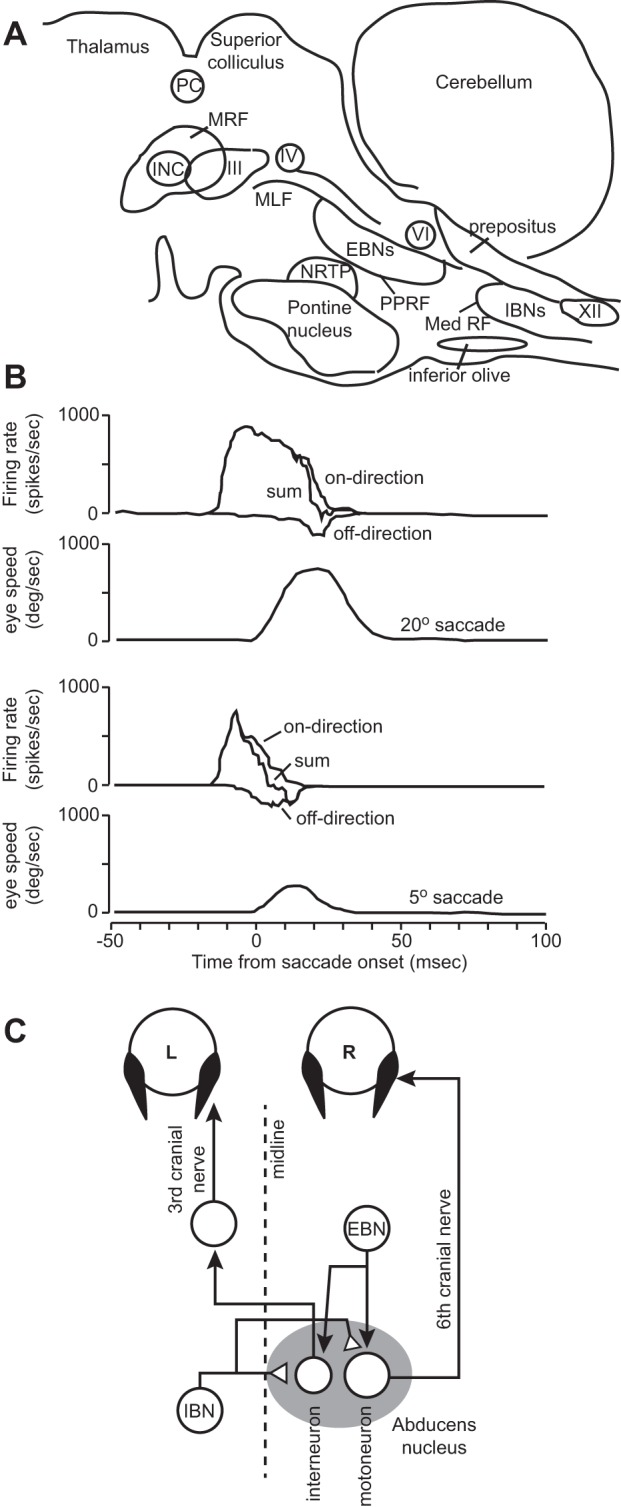
A: a sagittal schematic view of the monkey brain stem showing the locations of the important regions for generation of saccades and gaze holding. MRF, mesencephalic reticular formation; PPRF, paramedian pontine reticular formation; Med RF, medullary reticular formation; III, oculomotor nucleus, housing motoneurons for the medial rectus, superior rectus, and inferior rectus muscles; IV, troclear nucleus; VI, abducens nucleus; XII, 12th nerve. INC, interstitial nucleus of Cajal; NRTP, nucleus reticularis tegmenti pontis; PC, posterior commissure; MLF, medial longitudinal fasciculus; IBNs, inhibitory burst neurons. B: activity of an IBN cell during saccades. The cell has a strong burst for a saccade in the on direction [in this case, the cell is to the left (L) of the midline, and the on direction is a saccade to the right (R)] and a small burst for a saccade in the off direction. The off-direction discharge is depicted as a negative changing value with respect to baseline. The trace labeled sum is the addition of the positive and negative going values. From Van Gisbergen et al. (1981), with permission. C: a rightward saccade is due to excitation received at the motoneurons from EBNs and inhibition received from the contralateral IBNs.
The medullary reticular formation is a region caudal and ventral to the abducens motor nuclei (Fig. 2A). This region houses the IBNs, which make inhibitory connections with contralateral motoneurons. When a saccade is made in the direction ipsiversive to the location of IBNs, IBNs burst, inhibiting the motoneurons on the contralateral side, resulting in their pause (Fig. 1A). That is, for an ipsiversive saccade, the EBNs excite the motoneurons that are the agonist for the movement, whereas the IBNs inhibit the motoneurons that are the antagonist.
Indeed, a typical IBN bursts during ipsiversive saccades and discharges only a few spikes during contraversive saccades. Their on direction is typically for saccades along the horizontal axis. Scudder et al. (1988) found that similar to EBNs, the IBN discharge exhibited a strong correlation between duration of burst and saccade duration, a strong correlation between peak burst magnitude and peak saccade velocity, and a strong correlation between number of spikes in the burst and the amplitude of the saccade.
EBNs and IBNs have a burst during the movement phase of the saccade but are essentially silent during the hold phase (Fig. 1B). To see how the EBNs and IBNs together drive the motoneurons during the move phase, consider the drawing in Fig. 2C. In the abducens nucleus, one finds motoneurons that innervate the lateral rectus of the ipsilateral eye and interneurons that project to the motoneurons that innervate the medial rectus of the contralateral eye. To make a saccade to the right, EBNs on the right and IBNs on the left combine their excitatory and inhibitory activities to drive the abducens nucleus on the right, producing contraction in the lateral rectus muscle of the right eye and the medial rectus muscle of the left eye. For the EBN neuron on the right, this is an on-direction saccade (ipsiversive), whereas for the IBN neuron on the left, this is an off-direction saccade (contraversive). In this way, burst of activity in the right EBNs drives the two eyes together to the right, producing a conjugate movement.
Because EBN and IBN discharge is similar to each other during an on-direction saccade (as well as during an off-direction saccade), one can measure the EBN discharge on the right of the midline for a rightward saccade (ipsiversive) and then imagine that during that saccade the IBN neurons on the left of the midline fire a discharge equivalent to EBN discharge on the right of the midline for a leftward saccade. This is the insight that was described by Van Gisbergen et al. (1981). They recorded from a region caudal to the abducens (likely from IBNs). An example of discharge of a cell in the on and off directions is shown in Fig. 2B (off-direction activity is drawn as negative going, even though it is, of course, a positive firing rate). They imagined that the on-direction activity is analogous to the EBN discharge, and the off-direction activity is analogous to the IBN discharge. The activity in the on direction minus the activity in the off direction is the total input to the motoneuron. We see that the EBN activity accelerates the eye, whereas the IBN activity comes late in the saccade, acting as a breaking signal to decelerate and terminate the movement. Importantly, there is no activity in these premotoneurons during the hold period.
These ideas were later extended by Kojima et al. (2008), who recorded from IBNs. During ipsiversive saccades, all cells had a strong burst. During contraversive saccades, some IBN cells were silent, some cells gave occasional spikes (15/42), and the remaining cells consistently gave a few spikes (12/42 cells). Among these IBN cells with consistent spikes for the contraversive saccade (off-direction saccades), the number of spikes increased with saccade amplitude. Indeed, the timing of the first spike for a saccade in the contraversive direction came relatively late and became later as saccade amplitude increased. This was consistent with the idea that some of the IBNs contributed to stopping the saccade. To investigate this question directly, Kojima and colleagues (2008) considered an adaptation paradigm in which the monkey was presented with a 10° target but learned to make a smaller saccade. This type of adaptation is critically dependent on the cerebellum, particularly a region called the oculomotor vermis (lobules VI and VII). The oculomotor vermis of the cerebellum projects to an output nucleus of the cerebellum, called the fastigial, which in turn, projects to the IBNs. Normally, an IBN cell might have fired three spikes for the contraversive saccade. However, as adaptation took place, and amplitude of the saccade decreased, the same IBN cell now fired 12 spikes (Fig. 3A). This change was adaptation related, because normally, the IBNs fired less spikes for smaller saccades (here, the cell gave more spikes as the saccade got smaller). Furthermore, the spikes started progressively earlier as saccade amplitude decreased. Across all of the IBNs that had any spikes to begin with, gain-down adaptation produced an increased number of spikes as the contraversive saccades became smaller in amplitude during adaptation (Fig. 3B).
Fig. 3.
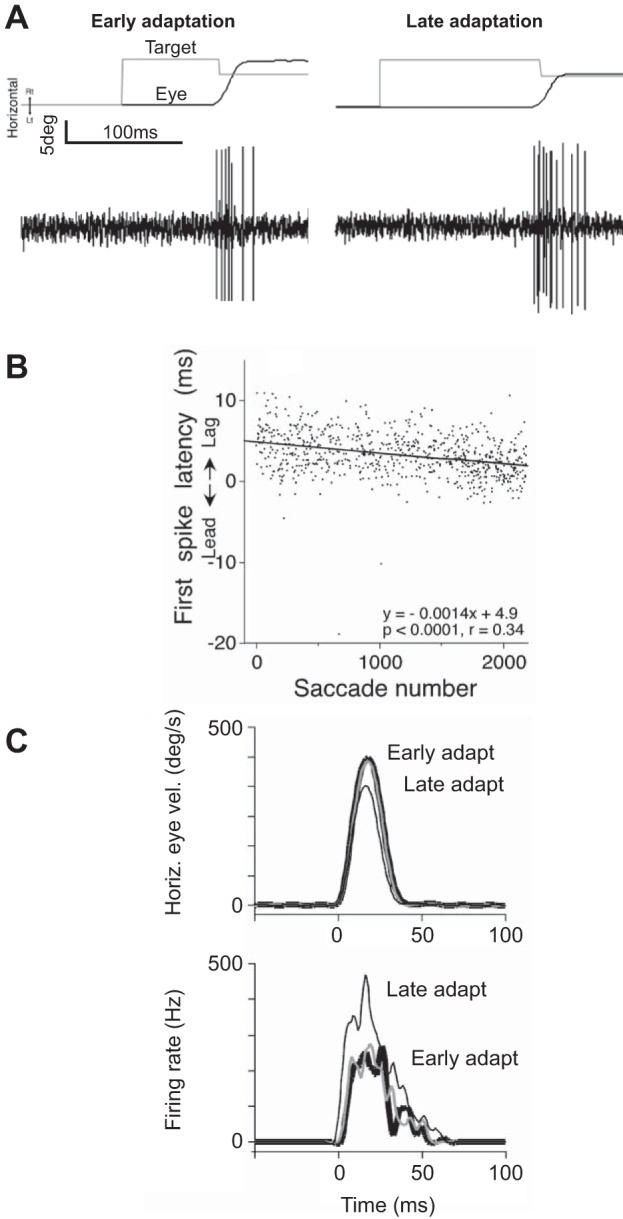
A: change in the discharge of an IBN cell in the left side of the brain stem. The monkey is engaged in a saccade adaptation paradigm in which it learns to produce a smaller than normal saccade. In the paradigm, a target is displayed (thin line, horizontal trace, top), but as soon as the saccade takes place, the target is moved back by a small amount (“Early adaptation” example). Upon saccade completion, there is retinal error, as the target is not on the fovea. With repeated trials, the brain learns to produce a smaller saccade in response to the same change in the target position (“Late adaptation” example). In the early adaptation trial, the saccade is in the contraversive direction, and the IBN cell discharges a few spikes. In the late adaptation trial, the saccade is again in the contraversive direction, but now, the IBN cell produces a large number of spikes. B: as the training proceeds, and saccade gain becomes smaller (that is, amplitude of the saccade is reduced), the latency of the cell discharge for the contraversive saccades becomes earlier. C: activity of 1 IBN neuron (left) during saccades to the right (contraversive) during gain-down adaptation. With adaptation, eye velocity decreases, the number of spikes in the cell increases, and the discharge arrives earlier. From Kojima et al. (2008), with permission.
Together, these results demonstrated that one of the inputs to the ocular motoneurons was from a region that was only responsible for the movement phase: inhibitory and excitatory neurons that precisely controlled the motion of the eyes. During an adaptation task that required reduced saccade amplitude, the inhibitory drive increased. However, once the eyes had arrived on target, these neurons ceased their discharge, relinquishing control to another circuit.
HOLDING THE EYES
If the only signal available to the motoneurons included those generated by the EBNs and IBNs, then as the saccade comes to an end, the eyes would not stay still. Rather, the mechanical properties of the muscles and the eyes would produce a drift of the eyes back to center with a time constant of ~200 ms (Robinson 1964). The neural integrator that Robinson (1970) had envisioned predicted existence of a nucleus that was responsible for generating the tonic discharge that motoneurons needed to hold the eyes after the movement had ended. Cannon and Robinson (1987) discovered one such circuit in the dorsal rostral medulla, a region where neurons project onto the abducens nucleus.
These neurons are part of the nucleus prepositus hypoglossi or in short, the prepositus nucleus and medial vestibular nucleus (Fig. 2A). Cannon and Robinson (1987) injected neurotoxins into this region and observed that the animal could perform saccades in darkness, but after the saccade had ended, the eyes rotated back to near center with a time constant of ~200 ms (Fig. 4A). So, the effect of lesion of prepositus appeared consistent with disruption of the integrator. Along with saccades, they also found deficits in VOR and smooth pursuit. Therefore, with this region partially disabled, the animal could make horizontal eye movements but could not hold the eyes at the desired location, resulting in a slow drift toward the straight-ahead null position.
Fig. 4.
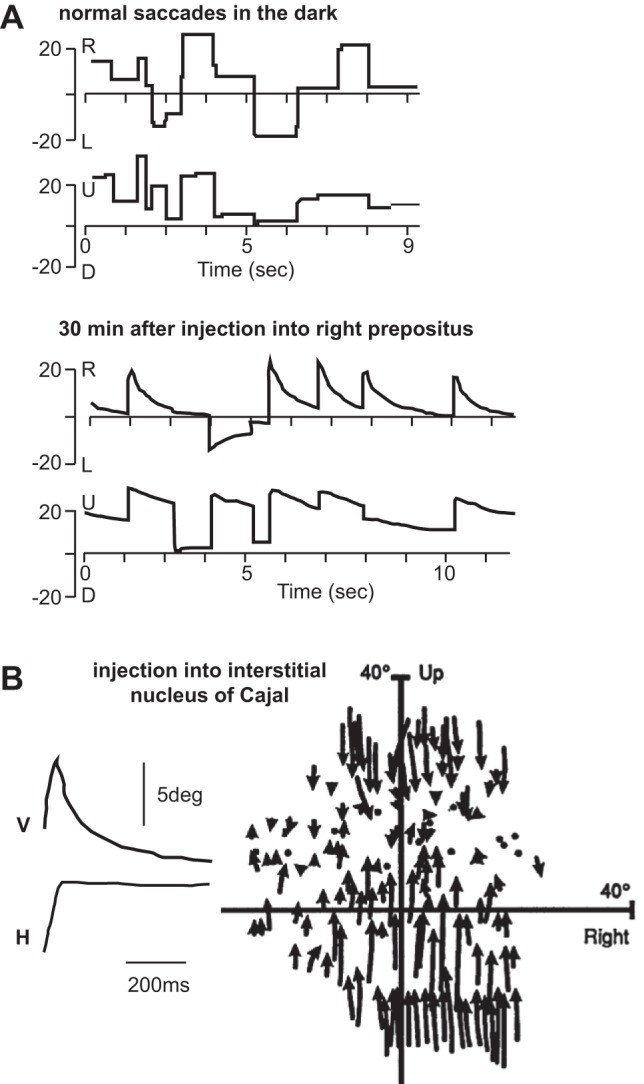
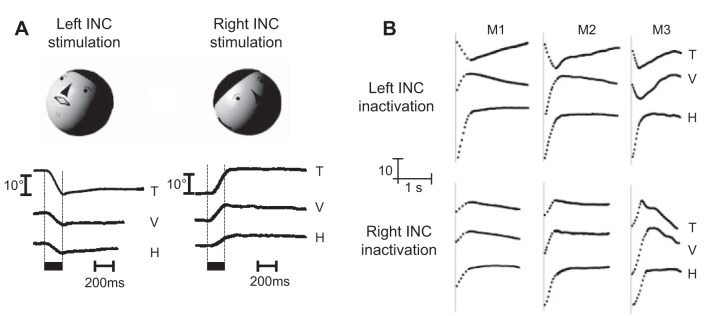
A: spontaneous saccades performed by a monkey in the dark. Despite lack of visual stimulus, the brain can hold the eye in place after the saccade terminates. The bottom shows saccades in the dark following disruption of the prepositus. The horizontal position, in particular, shows a drift back toward the midline. From Cannon and Robinson (1987), with permission. U, up; D, down. B: saccades following disruption of interstitial nucleus of Cajal (INC). V and H, vertical and horizontal eye position, respectively. The subplot on the right shows the direction of eye-position drift during unilateral INC inactivation. Arrows start at the end of the voluntary saccade and end at start of the next saccade, with the arrows indicating direction of drift. From Crawford et al. (1991), with permission.
Crawford et al. (1991) found another neural integrator in the interstitial nucleus of Cajal (INC), which specialized in holding the eyes along the vertical dimension. INC is a small nucleus in the mesencephalic reticular formation in the pons (Fig. 2A). It sends projections to the ocular motoneurons of the third and fourth cranial nerve. Crawford et al. (1991) deactivated the INC via injection of a GABA agonist and found that following vertical saccades, the eyes drifted toward a null location in the vertical plane, ~10° above straight ahead, with a time constant of ~200 ms (Fig. 4B).
These results suggested that the circuit that generated the activity that moved the eyes was anatomically distinct from the circuitry that generated the activity that held the eyes. For horizontal saccades, the move command was generated by the EBNs and IBNs, whereas the hold command was likely generated by a network including the prepositus. To see whether the neural activity of cells in the prepositus resembled a neural integrator, McFarland and Fuchs (1992) recorded from this region. They found that approximately one-half of the cells had a burst-step response for saccades in their on direction (Fig. 5A) and a response that reflected eye position during sinusoidal pursuit (Fig. 5B). Approximately one-quarter of the cells had only a step response for saccades in their on direction (Fig. 5C). For a majority of the neurons, the on direction was ipsiversive, with a discharge that started 8 ms before saccade onset and a duration that linearly increased with saccade duration. For saccades in the off direction, most burst-step neurons did not show a pause but only a step change in activity (Fig. 5A). The number of spikes in the burst correlated with saccade size, and the steady-state firing rates after saccade completion for both types of cells were linearly related to eye position (Fig. 5D). This last result relating the postsaccadic tonic discharge and position of the eye was crucial, as it indicated that neurons in the prepositus nucleus could serve as the source of the tonic input to the abducens motoneurons, holding the eye in place following completion of a saccade.
Fig. 5.
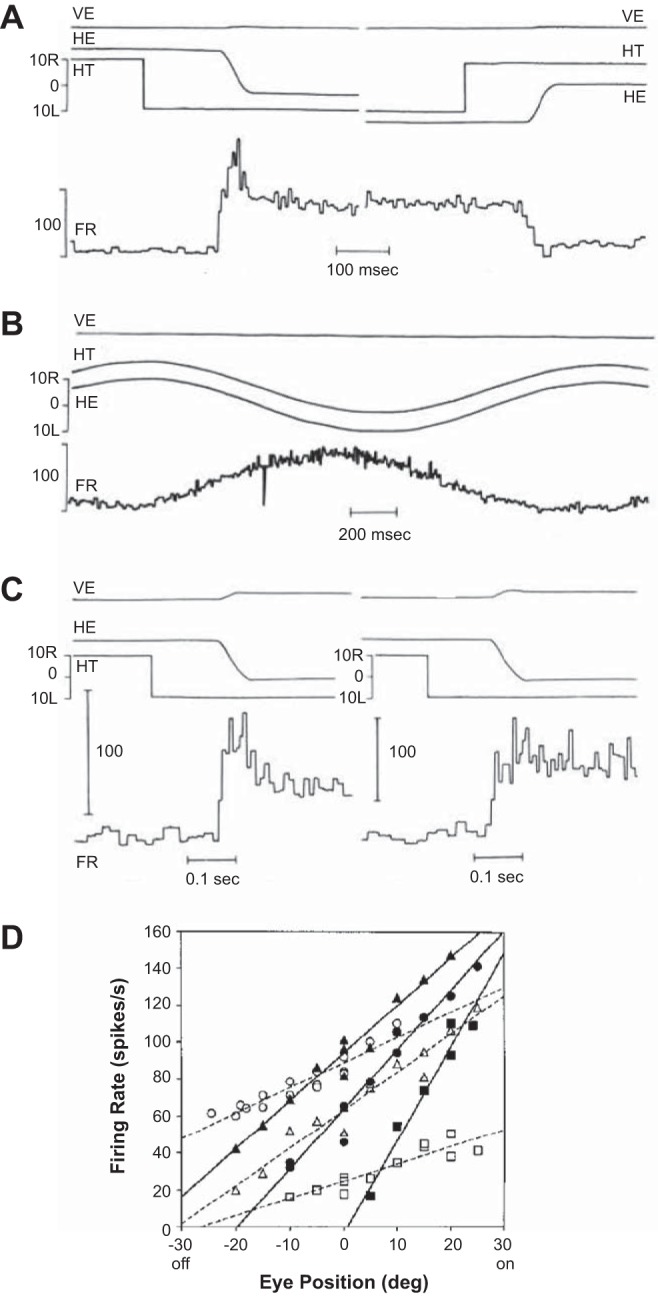
Activity of neurons in the prepositus during saccades. A: a cell in the left prepositus. Vertical and horizontal eye positions are labeled as VE and HE, respectively, and horizontal target position is labeled as HT. The on direction of the cell is for leftward saccades. FR, firing rate. B: activity of the same cell during pursuit. The cell discharge increases as horizontal eye position shifts more leftward. C: activity of 2 different cells, both in the left prepositus. One cell shows a burst-step response, whereas the other shows a step response. D: postsaccade firing rates for 3 burst-step (solid lines) and 3 step-only (dashed lines) neurons. The on direction is ipsilateral. From McFarland and Fuchs (1992), with permission.
Indeed, the prepositus neurons projected to both ipsilateral and contralateral abducens nuclei, with excitatory connections to the ipsilateral side and inhibitory connections to the contralateral side (McCrea and Horn 2006). This implied that following an ipsiversive saccade, there was an increase in the excitatory drive from the ipsilateral prepositus and a decrease in the inhibitory drive from the contralateral prepositus. Together, the two prepositus nuclei provided the tonic drive to the abducens motoneurons, holding the eyes when the saccade ended.
INTERNAL CIRCUITRY OF THE NEURAL INTEGRATOR
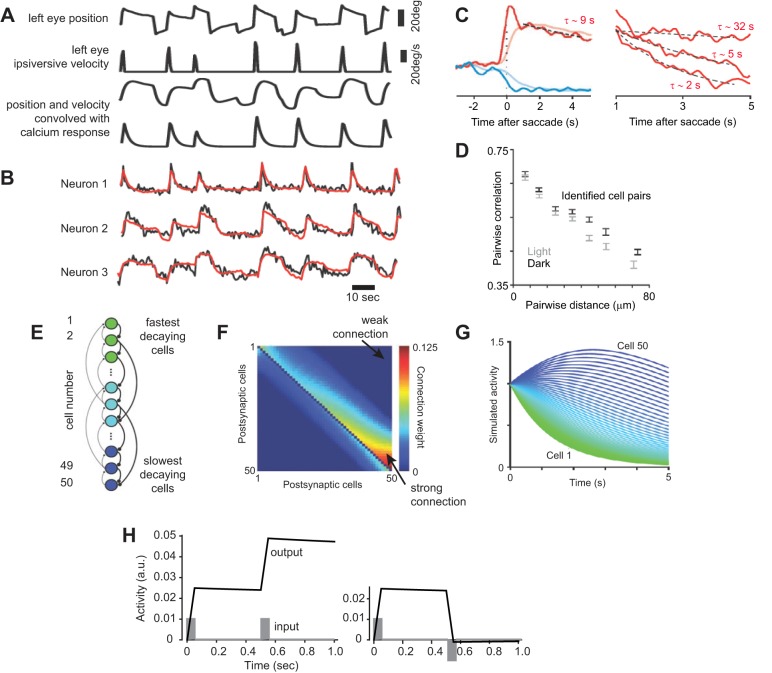
How did neurons in the prepositus transform the burst in the EBNs and IBNs into a quasi-tonic discharge that held the eyes steady? To answer this question, let us step away from saccades in primates and instead consider saccades in a much simpler animal: the zebrafish.
Larval zebrafish have spontaneous eye movements, back and forth scanning of the environment with saccades and fixations (Fig. 6A). Their saccades are slower than primates, but like primates, the zebrafish hold their eyes fairly steady after completion of the saccade (there is a slow drift back toward straight-ahead gaze with a time constant of tens of seconds). Miri et al. (2011) used optical recording to measure calcium changes and inferred firing rates in populations of putative horizontal neural integrator neurons in the medulla. They convolved the eye position and velocity signals with the calcium impulse response function, shown in part of Fig. 6A, and then built a linear model that related the measured calcium response in each neuron (Fig. 6B) with the sum of position and velocity calcium responses (the result of this fit is shown in Fig. 6B). They found that some cells responded strongly to velocity (neuron 1; Fig. 6B), some cells responded mainly to position (neuron 3; Fig. 6B), but most neurons responded to a combination of the two (neuron 2; Fig. 6B). That is, in the larval zebrafish, as in the monkey, the putative neural integrator was composed of a diversity of cells, most responding to a combination of eye velocity and position and some responding predominately to position (Fig. 6C).
Fig. 6.
A: eye position and velocity during spontaneous movements of a larval zebrafish. The position and velocity signals were convolved with the impulse response of calcium (as measured via an optical recording system), resulting in the 2 bottom traces. These 2 traces indicate the expected signal change that would be recorded if a neuron is exclusively encoding velocity or position of the eye. B: calcium signal (noisier trace) recorded from 3 neurons in the horizontal neural integrator circuit of the zebrafish, during the movements shown in A. A linearly weighted sum of position and velocity convolved calcium traces was fitted to the recorded signal. The result of the fit is shown by the smoother (red) trace. Neuron 1 is predominately velocity sensitive, whereas Neuron 3 is predominately position sensitive. C: calcium signal following a saccade in the on and off direction. In the period following the saccade, the data were fitted to an exponential, and the time constant was estimated, as shown for 3 neurons on the right. There was a diversity of time constants. D: pair-wise correlation in the signals recorded from neurons as a function of physical distance between the neurons. Physically close-by neurons exhibited highly correlated activities. E: neural network model of the integrator. Cells are numbered from top to bottom. Cell 1 receives weak connection from Cell 2 and still weaker connections from Cell 3, etc. Cell 50 receives strong connections from Cell 49 and weaker connections from Cell 48, etc. F: the weight matrix, where weight wij represents the weight of the connection from neuron j to neuron i. G: response of each cell in the network following the initial condition of ri = 1. From Miri et al. (2011), with permission. H: simulations of a neural integrator. The weight matrix wij was set to max[0, 20 − (j − i)] for i < j, max[0, 10 − (i − j)] for i > j, and 0 for i = j. Weights were then divided by the sum of all weights in their respective columns and then scaled by 0.21 + 1.6 (i/N), where N = 50. This produced the largest weight of ~1.8 and smallest weight of 0. The network was simulated with 1 ms time steps. The simulations show 2 consecutive saccade-like inputs. The input to the network (each cell) was a pulse of 50 ms in duration, with the amplitude of 0.01. The output of the network was the sum of activities of all neurons.
Next, for each cell, they measured the time constant with which the calcium response decayed following a saccade and found a range from 2 s to tens of seconds (Fig. 6C). Importantly, because larval zebrafish are transparent, the imaging technique allowed them to measure the position of each neuron, as well as record its calcium activity. They found that neurons that were located close to one another tended to have correlated signal changes (Fig. 6D). This suggested that nearby neurons were probably strongly connected—a hypothesis that was recently confirmed when Dale and Cullen (2015) simultaneously recorded electrical activities of multiple neurons in the prepositus of the monkey during saccades.
By building on the recurrent network models proposed by Cannon et al. (1983), Fuchs (1989), and Arnold and Robinson (1997), Miri et al. (2011) presented a mathematical model that could partly reproduce their measured data. In this model, the firing rate of neuron i was represented with ri. This neuron was connected to all other neurons (but not itself), with weight wij representing the weight of the connection from neuron j to neuron i. The neuron’s dynamics were described by
| (1) |
The weight matrix for the network was set so that a cell at the bottom (cell 50) received strong connections from the nearby Cell 49 but only weak connections from the distant Cell 1 (Fig. 6F). On the other hand, a cell at the top (Cell 1) received relatively weak connections from the nearby Cell 2 and still weaker connections from the distant Cell 50. If we pick one of the cells and give it an initial firing rate of ri = 1, then we would find that left to itself with no other inputs, the discharge of the neuron would decay with time constant τ. However, if the other cells also had this same initial firing rate, because of the interconnections between the cells, then the network could sustain its activity for much longer. Indeed, the authors found that despite the fact that all cells had the same time constant, τ = 1 s, in the network, some cells exhibited a fast decay time (Cell 1 in Fig. 6G), and some cells exhibited a rise and then a slow decay (Cell 50 in Fig. 6G). Therefore, this recurrent network had the ability to extend the time constant of a single cell by many folds. As a result, the sum of activities of all cells could sustain an initial input by many seconds.
Let us use this network to simulate the activity needed to hold the eyes during a sequence of saccades. Suppose we wish to produce two saccades of the same amplitude and in the same direction in sequence. For the first saccade, the burst generators (EBNs and IBNs) produce a pulse of some arbitrary magnitude for 50 ms and importantly, produce the same magnitude pulse again at some time later. That is, the pulses encode only the desired displacement vector. If this is the “on” direction of our neural integrator, then we provide the input shown in Fig. 6H to each neuron in the neural integrator (this input would be in addition to the dynamics of Eq. 1). The output of the neural integrator network is the sum of activities of all of its neurons. This output shows a rapid increase when there is input from the burst generators and then a very slow decline when the input is removed (Fig. 6H). Importantly, we note that the result of the second burst adds to the remaining step of the first burst, producing a final step size that is roughly twice as large as the first step and decays slowly.
We can also simulate the making of two saccades in sequence, in which the direction of motion reverses. In this case, the first saccade is in the on direction of the network, and the second saccade is in the off direction. To simulate this condition, we imagine that the pulse input for the first saccade is excitation, and the pulse input for the second saccade is inhibition of the same magnitude (Fig. 6H). The neural integrator produces a step following the first pulse and then brings the step back to near zero following the second pulse. The network translates an input consisting of a pulse that represents a desired displacement into an output that resembles a step, holding the eye in place once the pulse has ended.
This model makes the prediction that stimulation of the burst generators (for example, the EBNs) should not only move the eyes to the ipsilateral direction but also hold the eyes there after cessation of the stimulation. Cohen and Komatsuzaki (1972) inserted an electrode into the PPRF region of the monkey brain stem and stimulated the EBNs in the alert animal. They found that 2.5 ms after the start of stimulation, both eyes moved in the direction ipsilateral to the side of stimulation (Fig. 7A). For a given stimulation frequency, the result was a constant velocity rotation of the eyes that continued until the stimulation ended. Importantly, despite cessation of stimulation, the eyes maintained the new position for several hundred milliseconds, until the animal made a voluntary saccade, usually in the opposite direction. Therefore, stimulation of the premotor neurons for a short period of time not only displaced the eyes but also was sufficient to hold the eyes after the stimulation had stopped.
Fig. 7.
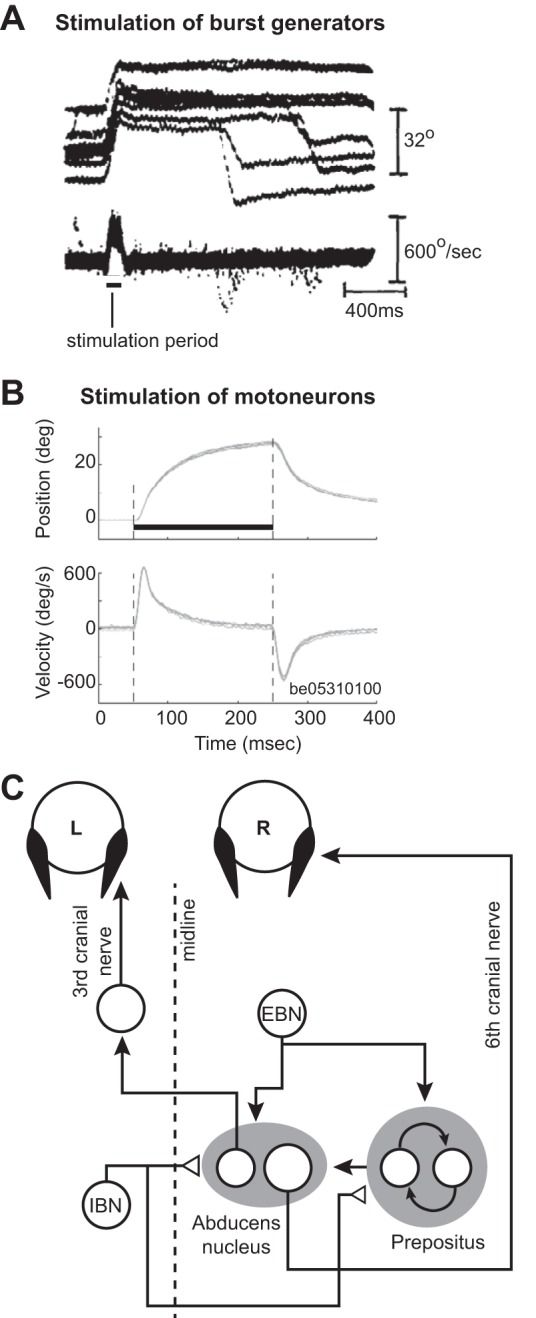
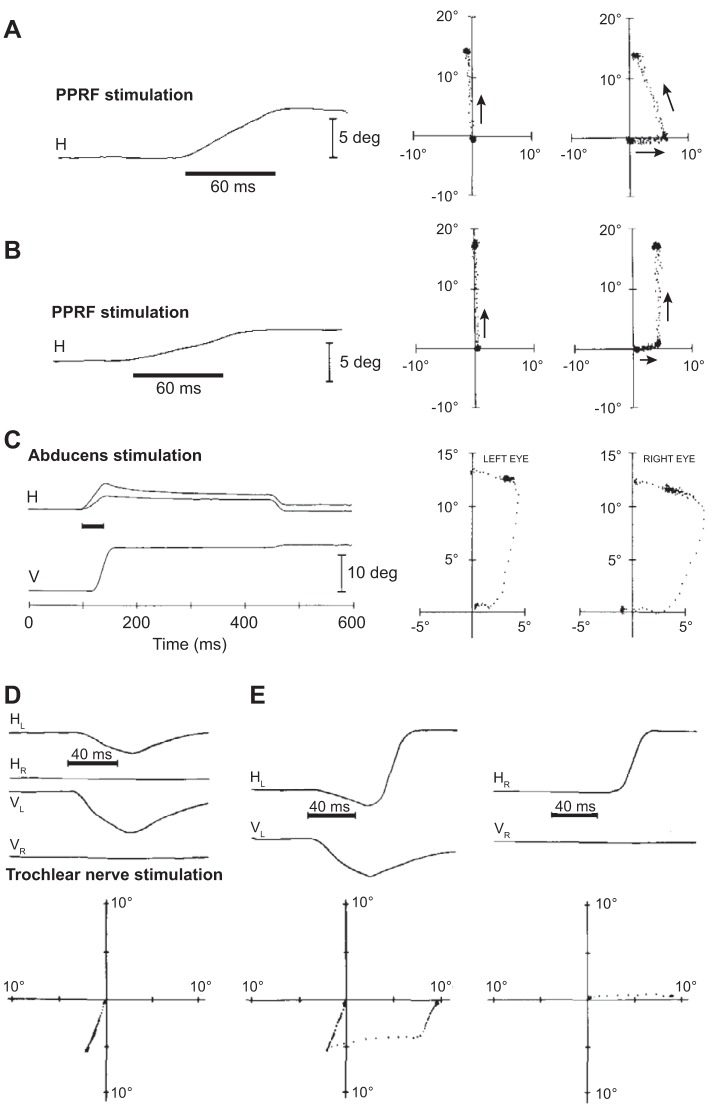
A comparison of eye movements resulting from stimulation of the premotor neurons (burst generators, presumably EBNs) in the PPRF region of the brain stem compared with eye movements resulting from stimulation of the abducens nucleus. A: stimulation of the burst generators. Data collected in darkness. The plot shows eye position and velocity traces aligned to stimulation onset. Upward change in position reflects an ipsilateral movement. The stimulation induces a change in eye position, but the eyes maintain final position, despite termination of the stimulation. On some trials following the stimulation, the animal makes a voluntary saccade in the opposite direction. From Cohen and Komatsuzaki (1972), with permission. B: stimulation of the abducens nucleus. Stimulation period is indicated by the dark, horizontal line. The stimulation produces a change in eye position, but the eye rotates back toward the null position immediately following stimulation end. From Gandhi et al. (2008), with permission. C: schematic of the neural circuitry that produces a rightward horizontal saccade. The burst generators, consisting of the EBNs and IBNs, produce pulse-like activity that drives the neurons in the abducens nucleus, resulting in a rightward motion of the eye. This activity is integrated and sustained by neurons in the prepositus nucleus, producing a step-like input to the abducens neurons, resulting in holding of the eye. Filled arrows depict excitatory connections; open triangles depict inhibitory connections.
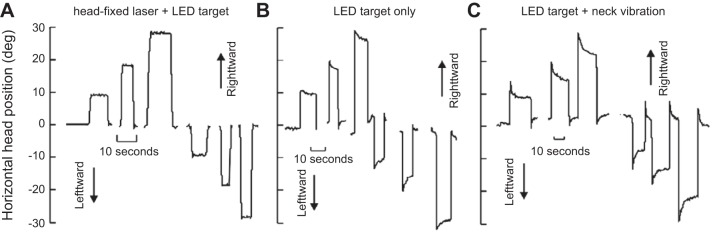
Thirty-six years later, Gandhi et al. (2008) revisited the PPRF and stimulated it during head-free and head-fixed conditions. They confirmed the observations of Cohen and Komatsuzaki (1972), finding that stimulation of the burst generators in the head-fixed condition produced ipsilateral displacement of the eyes, followed by holding of eye position after termination of stimulation. Next, they stimulated the abducens nucleus directly, engaging the motoneurons, and found that stimulation moved the eyes in the ipsilateral direction, but once the stimulation ceased, the eye rotated back toward the null position with a time constant of ~100 ms (Fig. 7B). Therefore, brief stimulation of PPRF not only moved the eyes but also produced sustained activity that held the eyes after the stimulation had stopped.
These results provided further evidence supporting Robinson’s (1973) idea regarding separation of the circuits that moved and held the eyes (Fig. 7C). The circuitry that held the eyes (the neural integrator) appeared to be in series with and received input from the circuitry that moved the eyes (the burst generator).
There are important issues with the specific way that we modeled the neural integrator. First, note that the neurons in our network had zero baseline firing rates, which is inconsistent with reality. However, if the neurons had non-zero baseline rates, then their baseline activities would get integrated, producing a runaway condition. Cannon et al. (1983) approached this problem by providing mutual inhibition between neurons, and Arnold and Robinson (1997) demonstrated that the inhibition in one neural integrator circuit came from the integrator on the contralateral side. Second, the integrator must be adaptive so that it can respond to changes in properties of the eye muscles. If one muscle is damaged, then the activity that this muscle needs to hold the eye in place will need to be changed. As we will see, there is a dedicated circuit in the cerebellum that monitors and controls the activity in the neural integrator, allowing for adaptation of the gaze-holding system. Therefore, the gaze-holding system is not only composed of the neural integrator circuit in the brain stem but also a circuit in the cerebellum.
In summary, the hold circuit was a network of interconnected neurons that accumulated information and sustained it after the input had been removed. When a horizontal saccade took place, the burst generators provided this integrator and the motoneurons with a pulse-like input, describing a displacement vector. This input displaced the eyes to one side, but there was nothing in it to hold the eyes at that location. However, the same input drove the internal dynamics of the circuit dedicated to integration, feeding back the input upon itself to sustain the activity after the input was gone, providing the tonic discharge that the motoneurons need to hold the eyes.
CONTROL OF THE MOVEMENT PHASE VIA INTERNAL FEEDBACK
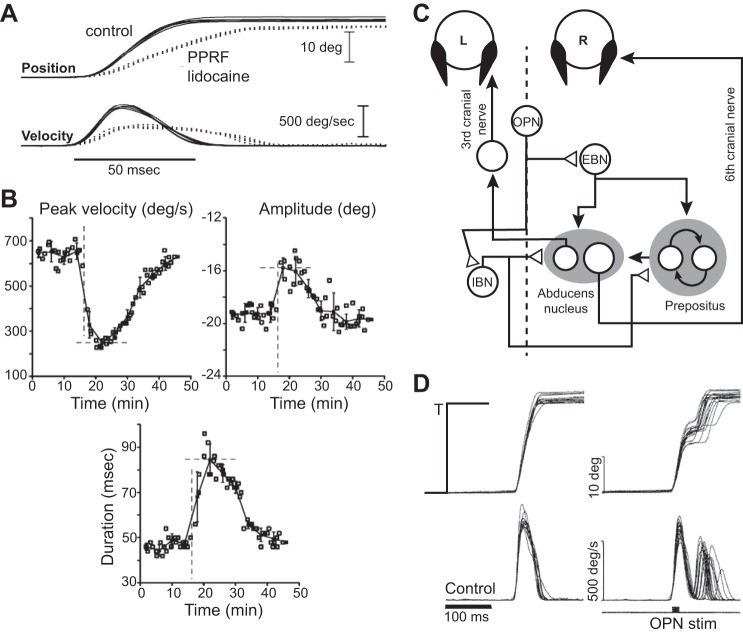
Robinson’s (1973) model (Fig. 1D) had predicted that saccades were not preprogrammed movements but were controlled via internal feedback. To test this idea, Barton et al. (2003) made it more difficult for higher brain regions to engage the neurons that were responsible for generating the move commands (the burst generators). They injected lidocaine into the right PPRF, a region that housed the EBNs. The result was an immediate reduction in the peak velocity of rightward saccades (Fig. 8A). For example, a 20° saccade before the injection had a peak velocity of 650°/s. Immediately after the injection, peak velocity dropped to 250°/s, followed by a 20- to 30-min recovery back to normal (Fig. 8B). This result implied that upon injection, the input to the burst generators became insufficient to drive the eyes with the same velocity as before. In a sense, lidocaine significantly decreased the gain of the burst neurons in response to excitation.
Fig. 8.
Disruption of saccades and control via internal feedback. A: effects of lidocaine injection in the PPRF, left of the midline, partially disabling the EBNs in monkeys. The plot shows position and velocity of saccades to a leftward visual target at 20° before and after injection. B: data are for 20° leftward saccades. Dashed lines indicate time of lidocaine injection. Each point represents a single trial. Injection produced a transient but significant reduction in peak saccade velocity. This coincided with a significant increase in saccade duration, which appeared to compensate partially for the reduced velocity, resulting in relatively small changes in saccade amplitude. From Barton et al. (2003), with permission. C: schematic of the brain stem circuit for generation of horizontal saccades. The burst responsible for moving the eyes during a horizontal saccade is produced by excitatory and inhibitory burst neurons (EBNs and IBNs). The burst neurons project to neurons in the abducens nucleus, as well as the neural integrator circuit in the prepositus. Omnipause neurons (OPNs) inhibit the burst generators. D: normal and interrupted saccades via stimulation of the OPNs. Results of 10 ms stimulation of the OPN region. A target at eccentricity of 25° was presented for 100 ms and then extinguished (T). OPN was stimulated at saccade onset for 10 ms (indicated by black region along the bottom trace of the right-most figure). From Keller et al. (1996), with permission.
If saccades were an open loop, then one would expect that the duration of the movement would remain unchanged, and as a result, the amplitude would fall dramatically. Instead, saccade duration increased immediately following the injection, from 45 to ~90 ms (Fig. 8B). That is, it appeared that a control system monitored the output of the burst generators and compensated for their reduced activity by increasing their duration of discharge. This increase in duration partially compensated for the disruption, although saccade amplitude did decline transiently from 19° to 16°. As a result, lidocaine injection reduced activity of the burst generators, but this was partially compensated for by the rest of the saccade circuitry, increasing the duration of activation, elongating the movement phase.
It is important to note that whereas disruption of the burst generators slowed the saccades, the disruption did not affect the motor commands that were generated during the hold phase: the gaze was held steady following completion of the saccade, as shown by the eye position in Fig. 8A. This result was consistent with the hypothesis that the neural integrator—the system imagined to be responsible for gaze holding via generation of the step-like motor commands—sat downstream from the pulse generator (Fig. 7C).
To explore more directly whether saccades are controlled via internal feedback, one needs to disrupt a single saccade and see whether the brain can correct the movement as it unfolds. One place to induce this disruption is in a brain stem region that houses omnipause neurons (OPNs). OPNs are inhibitory neurons that are located in the PPRF in the nucleus raphe on the midline of the brain stem. They broadly inhibit EBNs and IBNs (Strassman et al. 1987), as illustrated in Fig. 8C. They are active during fixation of the eye but pause during saccades. Their role is to prevent activity in the burst generators during fixation and then by pausing, allow the burst generators to become active when a saccade is about to take place.
Keller and colleagues (1996) stimulated the OPNs as monkeys made saccades of various amplitudes. Figure 8D provides examples of normal 25° saccades and saccades that were interrupted by a 10-ms stimulation of the OPNs, timed with onset of the saccade. In the experiment, the visual target was briefly flashed (for 80–120 ms) and always removed before saccade onset (which was typically 150 ms after onset of the target). As a result, the movement unfolded in darkness so the brain could not rely on any form of visual feedback after the saccade was initiated. Keller et al. (1996) found that whereas the brief stimulation was sufficient to halt the saccade midflight (bringing the eye velocity to ~0), the brain immediately produced a corrective saccade that was accurate to the now-extinguished target. Most interestingly, the final eye position had a mean that was only slightly hypermetric with respect to a normal saccade and an SD that was no different than a normal saccade. When the authors varied the OPN stimulation duration from near 0 to ~100 ms, they found that the resulting end-point error of the saccade was not affected by the stimulation duration: the eyes arrived accurately near the target regardless of the stimulation.
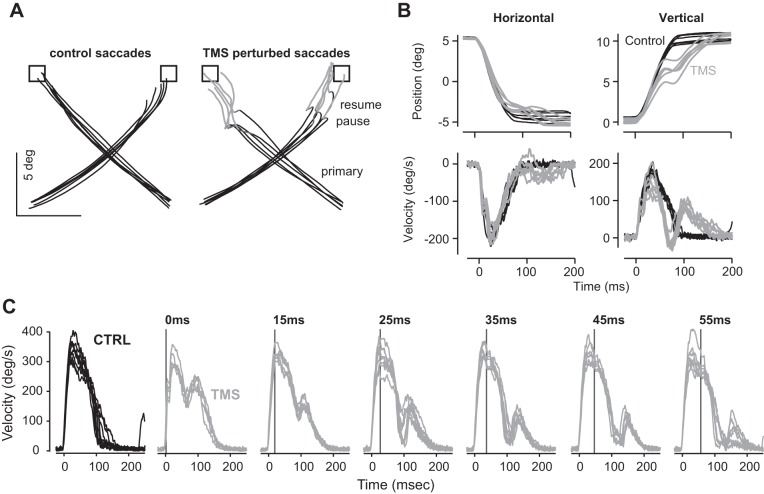
In humans, saccades can be disrupted through application of a single pulse of transcranial magnetic stimulation (TMS) to a region around the cerebellum (Fig. 9). (Stimulation of other areas of the brain also disrupts saccades, but cerebellar stimulation is particularly effective.) Xu-Wilson et al. (2011) asked human subjects to make saccades to visual stimuli and on random trials produced a TMS pulse at saccade onset. The target was removed upon saccade initiation, making it so that there were no visual cues to correct the saccade following its initiation. Examples of normal and interrupted 15° oblique saccades are shown in Fig. 9, A and B. The TMS pulse interrupted the ongoing saccade with a latency of ~45 ms, stopping the eye at 65 ms. Despite the perturbation, the eye trajectory showed a correction with a compensatory saccade that guided the eyes to the now-extinguished target.
Fig. 9.
Disruption of saccades via transcranial magnetic stimulation (TMS). A: examples of normal and interrupted 15° oblique saccades. The visual targets are depicted by the squares and disappear at saccade onset. B: horizontal and vertical position and velocity of the eyes during normal (black) and interrupted (gray) saccades. C: examples of normal and interrupted 30° oblique saccades. The y-axis depicts tangential velocity. In the TMS trials, the vertical line and the text above each figure illustrate time of stimulation. From Xu-Wilson et al. (2011), with permission.
Xu-Wilson et al. (2011) asked whether a within-saccade disruption could be corrected without stopping the eyes. For this question, they considered 30° oblique saccades, as illustrated in Fig. 9C. TMS applied near saccade onset transiently slowed the eyes but did not stop it. The perturbation was corrected immediately with motor commands that followed, steering the eyes to the now-extinguished target.
These experiments showed that the motor commands that moved the eyes were monitored as the movement unfolded. What was the neural mechanism of this internal monitoring?
CONTROL OF THE MOVEMENT PHASE WAS INDEPENDENT OF THE NEURAL INTEGRATOR
Robinson (1973) had proposed that the motor commands that displaced the eyes were controlled by an internal feedback circuit (Fig. 1D). His idea was that the burst generators received an input that was the difference between a desired displacement Δθd and a real-time estimate of the current displacement , written as
| (2) |
The variable θm(t) was the drive to the burst generators, reflecting a real-time measure of displacement to go. The neural integrator provided the estimate . However, there is a theoretical problem with this formulation. For the integrator to provide displacement information, it needs to be reset after each movement. If it were not reset, as in Fig. 6H, then it simply integrates from one command to the next. If it is reset after each movement, then there is a problem with the hold phase: when one makes two consecutive movements in the same direction with the same amplitude, the commands needed to hold the eyes after the first movement are not the same as the second movement, despite the fact that the displacements are the same (to hold the eyes at 20° to the right, you need roughly twice the activity in the right lateral rectus than to hold it at 10° to the right). The integrator could not be both a mechanism for internal feedback (which requires resetting) and a mechanism for providing the motor commands that hold the eyes after the movement ends (which requires integration without resetting).
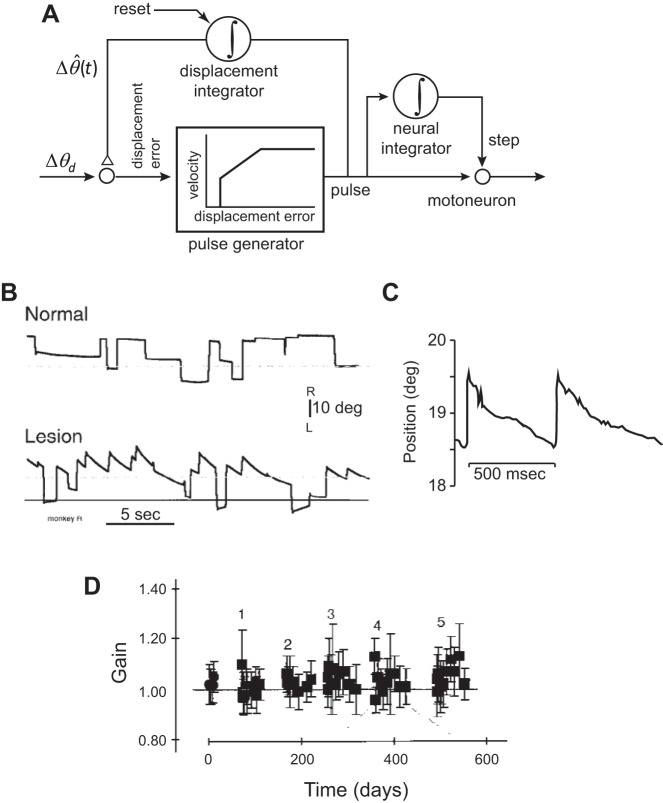
To solve this problem, Jürgens et al. (1981) suggested a modification (Fig. 10A). In the new model, the output of the neural integrator was not fed back and compared with the desired displacement, but rather, a new system, called a “displacement integrator,” monitored the move commands and fed back its estimates. The idea in this new model was that the neural integrator was responsible for generating the commands that held the eyes but was not a part of the feedback circuit, because it did not require resetting. In contrast, the displacement integrator was part of the internal feedback circuit but required resetting after each movement.
Fig. 10.
A: in a model proposed by Jürgens et al. (1981), the output of the neural integrator is used for generating the hold-phase motor commands but not fed back to control the movement phase. The displacement integrator, which needs to be reset after completion of each movement, provides a real-time estimate of the displacement as the movement takes place. This real-time estimate is fed back and compared with the desired displacement. B: bilateral lesion of the prepositus (the hypothesized neural integrator) affects the ability to hold gaze but has little effect on the saccade itself. Spontaneous saccades in the dark. The dashed, horizontal lines are straight ahead, and the bottom solid line is the estimated null position of gaze in the lesion condition. C: post-lesion (141 days) gaze-holding deficits. The target remained lit continuously at 19.3° to the right of midline. The monkey could not maintain eye position at target. D: gain of saccades (saccade amplitude divided by target amplitude). Time refers to days from the initial measurements. Each lesion session is labeled with a number (an additional lesion was performed on each session). Immediately after a lesion, there is no change in saccade gain, although with repeated lesions, there is a tendency for increased gain. From Kaneko (1997), with permission.
If we compare the model of Fig. 10A with that of Fig. 1D, we see that Robinson’s (1973) model (Fig. 1D) predicts that damage to the neural integrator should not only impair the ability to hold the eye in place after a saccade, but it should also lead to hypermetric saccades (because there is no longer an accurate estimate of position to be compared with the desired one). However, Fig. 10A predicts that damage to the neural integrator should affect the hold phase but have no effect on the movement phase of the saccade.
Kaneko (1997) tested this hypothesis by producing a sequence of bilateral lesions in the prepositus nucleus. He found that the monkeys were severely impaired in maintaining gaze, even when they made saccades with the lights on and were provided with a target. Saccades that displaced the eye away from the midline were followed by a drift back toward a null position. This null position was not straight ahead but had shifted to a position contralateral to the site of lesion. That is, following a lesion in the right prepositus, the null position of the eyes shifted to the left of straight ahead. However, the saccades themselves appeared to be only minimally affected. For example, in one of the monkeys (Monkey R) the damage was greater in the right prepositus, and the animal exhibited particular difficulties with gaze holding following saccades to the right (Fig. 10B), drifting with a time constant of ~250 ms (Fig. 10C) toward a null position that was to the left of the midline (Fig. 10B shows null position after the lesion and straight ahead). Note that the drift began almost immediately after saccade termination.
Despite these hold-phase problems, Kaneko (1997) observed that when the animal was given a visual target, it produced a saccade that was nearly as accurate as before the prepositus lesion (Fig. 10D; where gain is the ratio of saccade amplitude to target amplitude). By the fifth lesion, the saccade gain had increased by only ~10%. (It is possible that this small change may not have been a direct consequence of the damage to the integrator but an adaptive response to the damage: the animal learned to produce larger than normal saccades to compensate partially for the drift back toward center that it experienced following each saccade.) Despite the numerous lesions, saccades had peak velocities that were indistinguishable from saccades produced before the lesion, and duration had increased by only a small amount.
Kaneko (1997) had confirmed Cannon and Robinson’s (1987) finding that damage to the prepositus impaired the animal’s ability to hold the eyes steady following the saccade. However, he also found that the saccade itself was only minimally affected. He wrote the following: “Even minor changes in saccade gain were not seen until the prepositus was largely destroyed, whereas fixation was affected immediately after the initial injection.”
Therefore, whereas the neural integrator played a critical role in generating the motor commands that were needed to hold the eyes in place following completion of the saccade, it did not play a significant role in shaping the inputs that moved the eyes. Contrary to what Robinson (1973) had proposed, the neural integrator did not play a significant role in the control system that monitored the pulse as the saccade unfolded. Another system was responsible for the internal feedback.
CONTROL OF THE MOVEMENT PHASE: A ROLE FOR THE CEREBELLUM
Disruptions of the burst generators via pharmacological intervention (Fig. 8A), via stimulation of the OPNs (Fig. 8D), or via TMS (Fig. 9) all produced perturbations that affected the eye’s trajectory during a saccade. However, the brain corrected for these perturbations in real time. Perhaps a copy of the commands produced by the burst generators was sent to another brain region, allowing that region to control the input to the burst generators in real time. What were the properties of this hypothetical feedback circuit?
In the model of Fig. 10A, the displacement integrator would have to be reset after each saccade. If there is a time constant associated with the resetting process, then in cases where two saccades are extremely close in time to each other, the second saccade’s amplitude would be affected by the amplitude of the first saccade (because the displacement made by the first movement leaks onto the second movement). Corneil et al. (1999) and Goossens and Van Opstal (1997) tested this idea by having people make eye and/or eye-head movements to sequence targets. They found that in some cases, the second movement took place almost immediately after completion of the first. Despite this, the accuracy of the second movement was not a function of the interval between completion of the first movement and start of second. This implied that the hypothesized displacement integrator was essentially reset immediately after completion of the gaze shift.
To pinpoint the control circuitry that was responsible for the internal feedback, it was useful to consider perturbations that displaced the eyes but could not be corrected. Sparks et al. (1987) briefly stimulated the PPRF and observed an ipsilateral displacement of the eyes (Fig. 11A). As expected, following termination of the stimulation, the eyes remained in their new position. Would an action that followed the stimulation take into account this displacement?
Fig. 11.
Effects of stimulation of burst generators (PPRF region), stimulation of the abducens nucleus, and stimulation of trochlear nerve on control of saccades. A: a target was presented along the vertical axis for 50 ms. On randomly selected trials, following target offset, the right PPRF was stimulated for 60 ms. This produced a horizontal, rightward displacement of the eyes. At ~50 ms, following stimulation offset, the animal made an oblique saccade to the now-extinguished target. Therefore, the saccade compensated for the stimulation-induced displacement. The thick, horizontal line indicates stimulation period. B: the compensation was missing for some PPRF stimulation sites, as shown in this example. C: the right abducens nucleus was stimulated in the same paradigm as in A. The result was rightward displacement of both eyes, with a greater displacement on the right eye. The stimulation coincided with saccade onset. The stimulation produced horizontal displacement of both eyes, followed by immediate decay toward the null position. The brain could not compensate for the within-saccade abducens stimulation. Similar results were observed when abducens stimulation preceded the saccade. From Sparks et al. (1987), with permission. D: effects of stimulation of the trochlear nerve. Stimulation of the left nerve in the dark displaces the left eye downward, which is then followed by a gradual drift back toward the initial position. The right eye remains stationary. The thick, horizontal line indicates stimulation period. E: a target is flashed for 50 ms at 10° to the right. At stimulus offset, the nerve on the left eye is stimulated for 40 ms. The result is a displacement of the left eye in the downward direction. The saccade generated following this stimulation missed the target by an amount equal to the stimulation-induced displacement. HL, horizontal component of the left eye; HR, horizontal component of the right eye; VL, vertical component of the left eye; VR, vertical component of the right eye. From Sparks and Mays (1983), with permission.
Animals were trained to fixate a center target and then saccade to a target that was flashed along the vertical axis for 50–100 ms. The saccade, in response to presentation of the target, took place at 150–200 ms after target onset. Because the target was flashed for a shorter period than this reaction time, the saccade took place in darkness, and therefore, any corrections could not rely on visual information. Under normal circumstances (no stimulation), the saccade was vertical, arriving at the target location. In a fraction of trials, at target offset, Sparks et al. (1987) stimulated the PPRF for a duration of 60 ms. This resulted in an ipsilateral displacement of the eye (in the case of Fig. 11A, ~5°). Approximately 200 ms after target onset (~50 ms after stimulation offset), the animal made an oblique saccade, rather than a vertical saccade. As a result, for many stimulation sites (15 out of 27), the saccade that followed the displacement had an end point that was at the target (Fig. 11A). That is, for these stimulation sites, the motor commands for the ensuing saccade took into account the displacement caused by the external stimulation. This result suggested that a copy of the commands being produced by the burst generators was being sent to another region and used to control the subsequent inputs to the burst generators.
However, for some PPRF stimulation sites, this compensation was missing. For example, in Fig. 11B, the stimulation at this site displaced the eyes by ~5° to the right, but the ensuing saccade was vertical, not oblique. As a result, the saccade missed the target. For this stimulation site, the motor commands did not compensate for the stimulation-induced displacement. Overall, it appeared that output of some but not all burst generator neurons in the PPRF region was monitored by a feedback control system.
Did the outputs of the motoneurons become part of the efference copy? Sparks et al. (1987) placed their microelectrode in the abducens nucleus and stimulated just before a vertical saccade. The stimulation of the abducens resulted in displacement of the eyes along the horizontal direction (the ipsilateral eye was displaced more than the contralateral eye, as shown in Fig. 11C). However, the saccade that followed the stimulation did not compensate for the stimulation-induced displacement. This suggested that whereas output of some burst generator neurons was monitored by a feedback control system, the output of the motoneurons was not. Consistent with Fig. 10A, the motoneurons were outside of the feedback control loop, and their output was not part of the efference copy.
A particularly striking example of the inability to compensate for a perturbation occurred when the motor nerve that innervated a muscle was stimulated. The trochlear nerve (“pulley” in Latin, 4th cranial nerve) innervates the superior oblique muscle. Its stimulation pulls the eye downward and rotates it toward the nose. Sparks and Mays (1983) stimulated this nerve and observed that the stimulation displaced one eye (in this case, the left eye, as shown in Fig. 11D) in the downward direction, whereas the other eye remained still. Importantly, after the stimulation ended, the displaced eye rotated back toward the null position. Next, they flashed a target briefly (50 ms) and then stimulated the left trochlear nerve at target offset (Fig. 11E). The stimulated muscle (in this case, on the left eye) produced a displacement of the left eye in a downward direction. The motor commands generated by the saccade that followed were appropriate for a horizontal saccade. As a result, the combined effect of the stimulation-induced displacement and the ensuing saccade was an end point on the left eye that missed the target by a large margin. The right eye, however, arrived on target. Following stimulation offset, the left eye drifted toward the now-extinguished target.
The extraocular muscles are endowed with proprioceptive sensors (muscle spindles) (Maier et al. 1974). Therefore, in principle, the compensatory behaviors that we have observed may be not because of efference copy but because of feedback from proprioception. However, if the ability to compensate for displacement of the eye comes about because the brain relies on proprioceptive feedback from the ocular muscles, then we would expect that it should also be able to compensate for the displacement induced by nerve stimulation. Results in Fig. 11E argue against this possibility.
To check for the role of sensory feedback from the eye muscles in a more direct way, Lewis et al. (2001) compared saccades before and after surgical deafferentation of all extraocular muscles. They found that all aspects of saccadic eye movements, including the velocity-amplitude relationship, end-point accuracy, and ability to hold the eyes following the saccade, remained unaffected. Guthrie et al. (1983) surgically deafferented the extraocular muscles, and then they presented the monkey a visual target for 50 ms. However, before the animal could make a saccade, they displaced the eyes away from fixation by stimulating the superior colliculus. The stimulation produced a saccade. Despite the fact that the stimulation had moved the eyes away from the target—50 ms after completion of the stimulation-induced saccade—the animal made a saccade that brought the eyes near the target location. This demonstrated that even without proprioception, the brain could compensate for a stimulation-induced displacement of the eye.
In principle, the within-saccade compensation (as in Figs. 8 and 9) could take place because a region monitored the activity in the burst generators and steered the eyes as the movement took place. The between-saccade compensation (as in Fig. 11A) could take place because another region monitored the activity in the neural integrator, providing an estimate of the current position of the eye, which could then be used to generate the subsequent motor commands that moved the eyes. The locations of these regions remain largely unknown. However, there is some evidence that the cerebellum may be a critical node.
The motor commands that move the eyes during a saccade vary naturally because of the utility that the brain assigns to the action. This utility, consisting of the reward at stake minus the effort required, affects the vigor of the movement (Shadmehr et al. 2010; Shadmehr et al. 2016). For example, saccades have a higher velocity if the goal is to visualize the image of a face compared with an inanimate object (Xu-Wilson et al. 2009b). Saccades have a higher velocity if the movement is associated with acquisition of food (Takikawa et al. 2002) or money (Reppert et al. 2015) but have a lower velocity if people are forced to wait before they are allowed to look at the stimulus (Haith et al. 2012). Saccade velocity declines when subjects are asked to make movements repeatedly to the same stimulus (Fuchs and Binder 1983; Straube et al. 1997). This decline is not because of fatigue but because repetition tends to devalue the stimulus. Golla et al. (2008) and Xu-Wilson et al. (2009a) used this repetition method to introduce variability in the motor commands that moved the eyes. They then examined the ability of people with cerebellar degeneration to respond to this variability and found that repetition of the stimulus coincided with reductions in saccade velocities in both groups (Fig. 12A). This suggested that even with cerebellar damage, reductions in utility of the action coincide with reduced size of motor commands that move the eyes. However, whereas in the healthy subjects, saccade amplitudes remained accurate, in the cerebellar patients, the saccades fell short of the target. It appeared that in healthy people, the variability in the motor commands that initiated the saccade was generally compensated via motor commands that arrived later in the same saccade. However, the compensation was missing in cerebellar subjects.
Fig. 12.
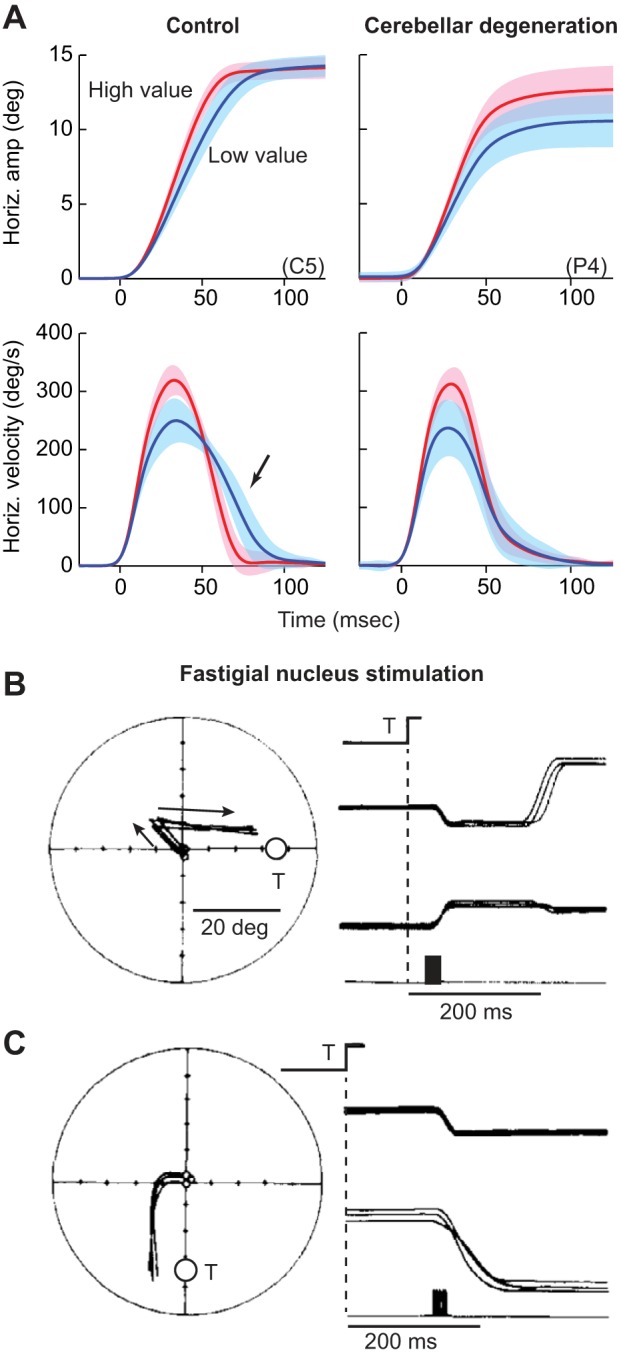
Effects of cerebellar damage or disruption on control of saccades. A: examples of saccades from a healthy subject and a patient with degeneration of the cerebellum. With repeated presentation of a visual target, the saccade target is devalued, and the motor commands that initiate the saccade become smaller, generating smaller velocities in both the healthy subject and the cerebellar patient (blue lines). In response to this variability, the healthy brain produces motor commands late in the saccade (arrow) to maintain accuracy, bringing the eyes to the target. In contrast, the patient with cerebellar damage cannot correct for the reductions in the motor commands that initiate the saccade. As a result, the saccades of the cerebellar patient fall short of the target. From Xu-Wilson et al. (2009a), with permission. B: the brain cannot correct for displacements that are caused by stimulation of the cerebellum. Effects of stimulation of the right caudal fastigial nucleus. The visual target (T) is presented at a random location for 25 ms. At target offset, the right fastigial nucleus is stimulated for 20 ms, resulting in a leftward displacement of the eye. The saccade that follows misses the target by an amount equal to the stimulation-induced displacement. Filled, vertical rectangle on the bottom traces indicate stimulation period. C: fastigial nucleus is stimulated at saccade onset, displacing the eyes as the saccade takes place. The ongoing saccade does not compensate for the stimulation-induced displacement. From Noda et al. (1991), with permission.
If the cerebellum plays a critical role in monitoring the move commands, then it would follow that disruption of the cerebellum should prevent the brain from compensating for inadequacies of these commands. The fastigial nucleus of the cerebellum is critical for “sculpting” the motor commands that are produced by the burst generators. For example, the increase in the IBN discharge displayed during adaptation (Fig. 3) is likely because of increased input from the fastigial nucleus. Indeed, stimulation of the fastigial nucleus engages the IBNs (and perhaps EBNs) on the contralateral side (with respect to the fastigial nucleus), displacing the eye to the contralateral side. Noda et al. (1991) explored the effects of stimulating the fastigial nucleus. They provided the animal with a visual target at a random location for 25 ms. They then stimulated the fastigial nucleus for 20 ms and observed that both eyes were displaced to the contralateral side, as illustrated in Fig. 12B. The stimulation not only produced a displacement, but the eyes were also maintained at the new location with little or no drift back toward the initial position. This suggested that stimulation of the deep cerebellar nucleus engaged the burst generators whose activity not only resulted in displacement of the eye but also engagement of the neural integrator, producing activity that held the eye after the fastigial stimulation had ended.
The saccade that followed this stimulation-induced displacement missed the target by an amount equal to the displacement produced by the stimulation. In a second experiment, Noda et al. (1991) timed the stimulation onset to the onset of the saccade. This stimulation disrupted the ongoing saccade, displacing it to one side, as shown in Fig. 12C. However, the brain could not compensate for this disruption, and the saccade end point missed the target by an amount equal to the stimulation-induced displacement. This is despite the fact that similar disruptions produced by stimulation of other brain regions, such as OPNs (Fig. 8), could be corrected.
The PPRF houses burst generators that produce the pulse-like command that moves the eyes during a horizontal saccade. Data in Fig. 11, A and B, suggest that output of some of these neurons, but not all, was monitored by a control circuitry, allowing for compensation due to perturbation or unexpected output. Importantly, the brain could not compensate for the cerebellum-induced perturbations in the burst generators. This leads to the conjecture that there exists a group of burst generators whose output not only goes to the motoneurons and the neural integrator but is also sent to an internal feedback circuitry that monitors the commands. Perhaps this circuitry is in the oculomotor vermis of the cerebellum. The output of the oculomotor vermis is to the fastigial nucleus, which in turn, projects back to the burst generators. Because stimulation of the fastigial displaced the eyes but was not corrected by internal feedback, it seems likely that the fastigial projects to that subgroup of burst generators whose output is not part of the efference copy. Stimulation of these burst generators would not result in compensation, as in Fig. 11C. In this view, the hypothesized displacement integrator is at least partly dependent on the oculomotor vermis region of the cerebellum.
In summary, the commands that move the eye during a saccade are controlled via an internal feedback circuit that monitors the activity of the burst generators but not the motoneurons. This internal feedback circuit is concerned with control of the movement phase and is distinct from the neural integrator, despite the fact that both systems rely on the activity of the burst generators. The cerebellum appears to be a critical part of the internal feedback circuit because its damage or disruption impairs the ability to compensate for the natural variability that is present in the motor commands.
CONTROL OF THE HOLD PHASE VIA INTERNAL FEEDBACK
A neural network, such as the one that we considered in Fig. 6E, could, in principle, act as an integrator, adding to the pulse motor commands a step-like motor command to hold the eye following the saccade. For horizontal saccades, the network in the prepositus likely plays such a function. For vertical saccades, the network in the INC likely plays such a function. However, the pulse and step would have to be tightly coupled, as a mismatch would result in a slow drift of the eye following completion of the saccade. On occasion, such a scenario does occur, particularly when healthy people are fatigued: following the saccade, the eye may drift back toward an equilibrium position (a backward drift), as if the step was not large enough compared with the pulse. Alternatively, following the saccade, the eye may drift forward (an onward drift), as if the step was too large compared with the pulse.
To check whether the process of generating the step was actively monitored and controlled via internal feedback, Optican and Miles (1985) artificially perturbed the gaze-holding system and looked to see if the brain responded by adapting the commands that described the step. In their experiment, every saccade was followed by a perturbation that produced a drift in the visual scene. As the animal completed a saccade, the computer detected that event and moved the visual field that was displayed on a large screen via an exponential drift that had a time constant of 50 ms and an amplitude of one-half that of the just-completed saccade. In one experiment, the drift was backward with respect to the saccade, whereas in another experiment, the drift was onward.
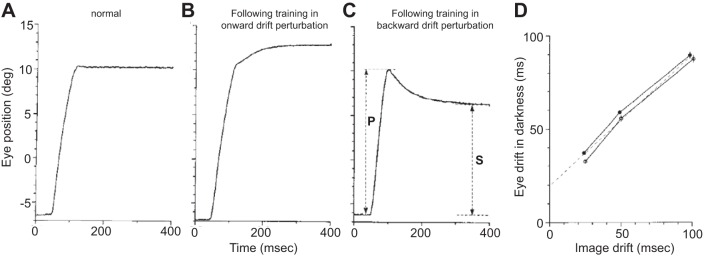
Before this training, spontaneous saccades in the dark exhibited little or no drift (Fig. 13A); the pulse was followed by a step that precisely held the eye. However, following several days of training with the onward perturbation, spontaneous saccades in the dark now exhibited an onward drift (Fig. 13B). Similarly, following several days of training with the backward perturbation, saccades exhibited a backward drift (Fig. 13C). Importantly, this training did not affect the saccade itself but only the motor commands that held the eye after completion of the saccade. Optican and Miles (1985) varied the time constant of the perturbation drift (in separate days of training) from 25 to 50 to 100 ms and found that with training, the animals produced a drift with their eyes that had a time constant that roughly matched the perturbation (Fig. 13D). That is, the brain learned to produce motor commands that no longer held the eye steady following the pulse but made it drift in a way that was consistent with the motion of the visual field.
Fig. 13.
Mismatch between the motor commands that move the eye and commands that hold the eye produces postsaccadic drift. A: spontaneous saccade in the dark under normal conditions. B: adaptation of the gaze-holding system. Spontaneous saccade in the dark following days of training with a perturbation that moved the visual field following completion of each saccade. Each saccade was followed by a drift of the visual image in the direction of the just-completed saccade (onward drift perturbation), with a time constant of 50 ms and amplitude of one-half of the preceding saccade. The saccade in the dark shows an onward postsaccadic drift with a time constant of ~50 ms. C: spontaneous saccade in the dark following days of training in which each saccade was followed by a shift of the visual stimulus in the direction opposite that of the just-completed saccade (backward drift perturbation) with a time constant of 50 ms and amplitude of one-half of the preceding saccade. P, rapid portion of the saccade; S, final amplitude of the saccade. D: time constant of the postsaccadic drift for spontaneous saccades made in the dark following training with perturbations that had 25, 50, or 100 ms time constants. From Optican and Miles (1985), with permission.
This indicated that the normal condition in which gaze was held steady following a saccade was merely a reflection of the fact that the visual world typically did not move when we moved our eyes. If it were to do so, as in the perturbation that Optican and Miles (1985) programmed, then during the postsaccadic period, the brain would produce motor commands that did not hold the eyes steady but moved them in such a way as to match the visual motion, thereby keeping the image constant on the retina. Therefore, the objective of the motor commands that came following the termination of the saccade was not to hold the eyes steady but to hold the visual input on the fovea steady. That is, the aim of the hold circuit was to maintain a constant sensory state, and for the eyes, this meant a stable image on the fovea.
What neural system was critical for this ability to control the motor commands adaptively during the hold phase? Optican et al. (1986) trained monkeys over a 2-day period to make saccades, while the visual field was perturbed with the postsaccadic drift (time constant of 50 ms and an amplitude of one-half that of the saccade). In one experiment, the drift was backward, whereas in another experiment, the drift was onward. To measure the effect on saccades, they measured the pulse amplitude by quantifying the size of the rapid portion of the saccade (P), and then the final amplitude of the saccade (S; Fig. 13C). The P/S ratio for a normal saccade is one. If the animal adapts to the visual displacement in the onward-perturbation experiment, then gain should decrease. In normal, healthy monkeys, the P/S ratio before the experiment started was 0.98. After 2 days of adaptation to the onward task, the ratio dropped to 0.75. For the backward-perturbation experiment, the gain increased to 1.14.
Next, they removed the flocculus region of the cerebellum bilaterally and found that the animals could no longer alter the step height, and P/S ratio remained at near one. This suggested that the adaptive mechanism for control of the hold phase depended on the flocculus region of the cerebellum.
In summary, it appeared that the circuits responsible for generating the motor commands that moved the eyes during a horizontal saccade (burst generators, EBNs, and IBNs) were separate from the circuits responsible for motor commands that held the eyes still following the saccade (prepositus). Whereas both the movement and hold circuits were actively monitored and controlled via internal feedback, damage to the hold circuit produced postsaccadic drift, as well as a change in the null position of the eyes, but largely spared the movement component of the saccade. Figure 14 is a schematic that summarizes these ideas. As we will see, adaptive control of the move commands depends on the oculomotor vermis region of the cerebellum, whereas adaptive control of the hold commands depends on the flocculus region of the cerebellum.
Fig. 14.
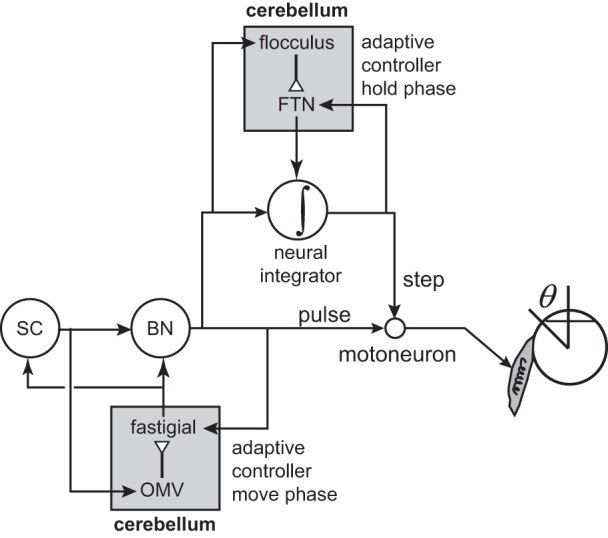
Control of saccades via internal feedback. To generate a saccade, there is a burst of activity in a caudal region of the superior colliculus (SC) corresponding to the saccade vector (simultaneously, there is a pause in the fixation-related neurons in the rostral pole regions of the SC). This input drives the burst generators (BN), producing a pulse-like motor command that activates the motoneurons. This pulse is under control of the oculomotor vermis (OMV) region of the cerebellum. The output of OMV is to the fastigial nucleus, which in turn, acts on the burst generators. The output of the fastigial nucleus is also to the rostral pole region of the superior colliculus (May et al. 1990). With its projections to the rostral pole of SC, the cerebellum may serve to reactivate the fixation-related neurons, helping to end precisely the activity in the caudal colliculus. The pulse is transformed by the neural integrator into a step-like command that holds the eye still following completion of the saccade. The step is also under control of the cerebellum: the flocculus and the flocculus target neurons (FTN) in the vestibular nucleus. Arrows represent excitatory connections, and open triangles represent inhibitory connections.
ADAPTIVE CONTROL OF THE MOVEMENT PHASE AND THE HOLD PHASE
Robinson had suspected that the cerebellum must play a central role in controlling the movement phase of saccades (the pulse), because with his student David Zee, they had examined people with cerebellar degeneration and noted that the patients had enduring saccade dysmetria. He had also suspected that the cerebellum played a critical role in controlling the postsaccadic holding phase (the step), because he had found that following complete removal of the cerebellum (Fig. 15F), the animals were no longer able to hold their gaze steady (Robinson 1974): following a saccade, the eyes drifted toward a null position near the center, with a time constant of ~1.3 s. Indeed, in humans, the clinical manifestation of a deficit in the neural integrator is gaze-evoked nystagmus, a condition where the brain cannot maintain gaze at an eccentric location, and the eyes drift back toward the straight-ahead position. This condition is frequently observed in people who suffer from cerebellar degeneration (Gomez et al. 1997; Tarnutzer et al. 2015). An example is shown in Fig. 15G, where the patient was instructed to fixate gaze to the right or to the left. However, the eyes drifted slowly toward the center (time constant of >1 s), and this was followed by a saccade that corrected the position of the eyes back toward the lateral position. Recall that inactivation of the prepositus had also produced gaze-holding deficits (Fig. 10C), but the resulting time constant of the drift was ~200 ms—the time constant of the mechanical plant of the eyes. Therefore, it appeared that the cerebellum acted together with the prepositus, allowing the visual image to remain stable on the fovea following completion of a saccade.
Fig. 15.
Contributions of the cerebellum to adaptive control of saccades. A: the medial and lateral rectus muscles of one eye were weakened, and then the eye was covered by a patch. Saccades with the weak eye are hypometric and exhibit postsaccadic drift. B: immediately after the patch was removed from the weak eye and placed on the normal eye, the weak eye must make multiple saccades to reach the target, and the normal eye is grossly hypermetric. C: after 5 days with the patch on the normal eye, the brain has learned to compensate for the weak muscles. The weak eye shows normal saccade and normal postsaccadic gaze holding. The patched, normal eye exhibits hypermetria and postsaccadic drift. D: in another animal, the lateral rectus muscles of one eye were weakened and the entire cerebellum removed. Left: the weak eye was patched, and the normal eye had been provided visual experience for 5 days. The normal eye exhibits postsaccadic drift. Right: the patch has been placed on the normal eye, and the weak eye had been provided visual experience for 5 days. E: in another animal, the muscles of one eye were weakened, and the oculomotor vermis and fastigial nuclei were removed. Left: a saccade after the normal eye had been provided visual experience for 13 days. The saccade is hypermetric but without postsaccadic drift. Right: a saccade after the patch was placed on the normal eye, and the weak eye provided visual experience for 19 days. There is no postsaccadic drift on the weak eye. From Optican and Robinson (1980), with permission. F: effects of complete removal of the cerebellum in a cat. The traces show spontaneous saccades made in the dark. Following completion of each saccade, the eye shows a drift with a time constant of 1.3 s. From Robinson (1974), with permission. G: effects of cerebellar degeneration in a human. The subject was instructed to hold gaze at 30° to the right or left of the midline. The gaze showed a slow drift toward the midline, followed by rapid corrective saccades. From Shaikh et al. (2016), with permission.
With his student Lance Optican, Robinson began an investigation of the function of the cerebellum in control of saccades. Optican and Robinson (1980) weakened the lateral and medial rectus muscles of one eye in a monkey and then immediately placed a patch on that eye. The weakening of the muscles made it so that the move command had to be larger than normal to displace the eye, and the hold command needed to be larger to hold the eye at that off-center location. However, because the weak eye was patched, the brain did not have visual feedback from that eye and could not learn from the visual information on the retina that the saccade did not reach the target or that the eye was not held steady following completion of the movement. This is noteworthy, because the muscles of the eyes are well endowed with proprioceptive sensors. Despite this, the visual feedback was necessary to produce significant compensation for the damage to the muscles.
When the monkey made saccades while viewing with the good eye, the resulting saccade, of course, moved both eyes. The good eye arrived on target, whereas the weakened eye fell quite short of the target (Fig. 15A). This was expected, as the pulse generated to move the weakened muscles was not large enough, and as a result, the saccade on the weakened eye was hypometric. However, following completion of the saccade, the weak eye had gaze-holding issues, exhibiting a backward drift (see Fig. 15A). As a result, damage to the muscles had produced both a problem with the movement phase of the task (saccades were hypometric) and a problem with the holding phase (movements had postsaccadic drift). Could the healthy brain adapt the move and hold commands to compensate for the damage to the muscles? To do so, the brain needed visual feedback regarding the consequences of the motor commands to the weak eye.
Optican and Robinson (1980) switched the patch from the normal eye to the weak eye. Now the brain could get visual feedback regarding the hypometric saccades, as well as the drift that was occurring during the postsaccadic period. Initially, the animal needed to make approximately four saccades to place the 10° target on the fovea of the weak eye (Fig. 15B). However, after 5 days of experience with the weak eye, the animal showed a remarkable recovery; it was not only able to make normal saccades with the weak eye, but it was also able to hold the gaze steady following the saccades (Fig. 15C).
This observation suggested two ideas. First, the move circuit and the hold circuit were both under control of an adaptive system that monitored their outputs (Fig. 14). Perhaps efference copy from both the burst generators and the prepositus was used by control circuits to adjust their respective activities. Second, the adjustment could undergo adaptation only if there was visual feedback that indicated the presence of an error. That is, the visual error that arrived following completion of the saccade drove adaptive control of both the movement and the hold circuits.
As the animal adapted to errors it observed with the weak eye, the normal eye was patched. Learning to make a 10° saccade with the weak eye resulted in severely hypermetric saccades with the patched, normal eye; it made a 25° saccade in response to a 10° target (Fig. 15C). Furthermore, in the postsaccadic period, now the normal eye exhibited a drift (onward drift; see Fig. 15C). Therefore, given the visual consequences of the motor commands in the weak eye, the brain adapted both the move and the hold commands, producing a normal amplitude saccade with little or no postsaccadic drift. However, because the normal eye was covered, this adaptation, due to errors observed with the weak eye, generalized to the normal eye, resulting in hypermetria and gaze-holding issues in that eye.
To test whether the cerebellum played a role in the adaptation that had taken place in response to the weakening of muscles, Optican and Robinson (1980) weakened the eye in another animal, kept that eye patched, and then removed the entire cerebellum. Removal of the entire cerebellum produced three fundamental problems. First, the animal had trouble with gaze holding, as evidenced by the drift before the saccade was initiated and after it terminated (Fig. 15D). Second, the saccade, made by the normal eye, was hypermetric, whereas the saccade made by the weakened eye was approximately on target. This suggested that in general, the role of the cerebellum in control of the movement phase was inhibitory, reducing the gain of the system. Removal of the cerebellum made all saccades hypermetric, but because muscles in one eye had been weakened, the combined effect of cerebellar removal (hypermetria) and weakened eye muscle (hypometria) made movements in the weak eye of near-normal amplitude (the combined effects of muscle damage and cerebellum removal on postsaccadic drift were less clear). Third, whereas with a healthy cerebellum, the animal could adapt to the weakened muscle and alter the move, as well as the hold commands, following removal of the cerebellum, this ability was no longer present. When Optican and Robinson (1980) put the patch on the good eye and gave the animal time to adapt, the animal was unable to hold the gaze, despite having many days to adapt to the drifting visual input. The cerebellum was critical for the ability to adapt both the move and hold motor commands in response to the damage in the eye muscles. To perform this adaptation, the cerebellum relied on visual information regarding consequences of motor commands (the covering of the weak eye removed the possibility of adaptation).
Finally, Optican and Robinson (1980) asked whether different parts of the cerebellum were important for control of the move and hold commands. In another animal, after weakening the muscles of one eye, they removed the oculomotor vermis and the underlying fastigial nucleus of the cerebellum. The oculomotor vermis has Purkinje cells that project to the fastigial nucleus, which in turn, projects to the contralateral burst generators. After damage to the oculomotor vermis and fastigial, as well as the muscles of one eye, they put the patch on the weak eye. They observed that for movements with the good eye, this damage to the cerebellum produced saccade hypermetria (particularly for horizontal saccades) but no deficits with postsaccadic gaze holding (Fig. 15E). Therefore, the oculomotor vermis and fastigial were critical for the movement phase of the saccade but not the hold phase. Next, they removed the patch from the weak eye and placed it on the normal eye and allowed the animal 13 days to adapt. They found that during this period, the brain learned to improve the saccades made by the weak eye, particularly in the holding phase (Fig. 15E). That is, when the patch was placed on the normal eye, the gaze holding improved on the weak eye but now became poor on the normal eye.
This was the first experiment to demonstrate that the intact cerebellum was required for adaptive control of both the movement phase and the holding phase of saccades. The weakening of an eye muscle had two effects: it made saccades hypometric and introduced a postsaccade drift. With an intact cerebellum and visual feedback, the animals were able to correct both problems on the damaged eye, albeit at the expense of hypermetria on the normal eye (which was denied visual feedback). With removal of the oculomotor vermis and fastigial regions of the cerebellum, the animals lost the ability to adapt the move commands but could adapt the hold commands and eliminate postsaccadic drift. Together, the results suggested that adaptive control of the move phase relied on the oculomotor vermis of the cerebellum, whereas control of the hold phase was due to another region of the cerebellum.
Zee et al. (1981) refined this view by recording eye movements before and after bilateral removal of the flocculus region of the cerebellum. They found that without the flocculus, the animals were not impaired in generating the move phase: saccade peak velocity appeared normal, and the amplitude of saccades also appeared unaffected. The major impairment caused by damage to the flocculus was postsaccadic drift (Fig. 16A). For horizontal saccades, the postsaccadic drift was usually onward. For vertical saccades, the postsaccadic drift was also usually onward (Fig. 16A) but in some animals, backward (Fig. 16A). With the light off, gaze could not be maintained, exhibiting a drift toward a central location with a time constant of 1.6 s (Fig. 16B).
Fig. 16.
A: effects of bilateral lesion of the flocculus. Plot shows vertical saccades made in response to a visual stimulus with the lights on. Left: saccades in the healthy animal; middle: saccades in 1 animal that had bilateral lesion of the flocculus. The movement phase of the saccade appeared unaffected, but the eyes exhibit postsaccadic drift (onward drift). Right: saccades in another animal that had bilateral lesion of the flocculus. This animal also had normal saccades but exhibited postsaccadic drift (in this case, backward drift). B: following bilateral flocculus removal, animals showed severe gaze-holding deficits in the dark. In the dark, the animal exhibited nystagmus, an inability to maintain gaze. The attractor point for the horizontal component of eye position appears near 0 (straight ahead), whereas the attractor point for the vertical component appears well above 0. From Zee et al. (1981), with permission. C: effects of bilateral lesion of the oculomotor vermis. Saccades are hypometric and exhibit greater end-point variability but do not show postsaccadic drift. D and E: lesion of the oculomotor vermis (OMV) reduces saccade velocities and extends duration. From Takagi et al. (1998), with permission.
In sharp contrast, when Takagi et al. (1998) bilaterally removed the oculomotor vermis (and left the underlying fastigial nucleus intact), they found no deficits in gaze holding, as illustrated in Fig. 16C. Instead, they found that the lesion fundamentally altered the ability of the animal to control the movement phase of the saccade. The saccades became hypometric, with the gain (ratio of eye displacement to target displacement) changing from 0.97 in the healthy animal to 0.51 in the lesioned animal. The lesion made saccade end points more variable, as illustrated by the saccades shown in Fig. 16C. When saccade amplitudes were matched in the normal and lesioned animal, it became apparent that the peak saccade velocity was much lower in the lesioned condition (Fig. 16D). The damage to the oculomotor vermis had reduced the acceleration phase of the movement and in particular, made the velocity profile have a very long tail, as if the movement could no longer end abruptly (Fig. 16E).
In summary, the intact brain could learn to compensate for the damaged muscles of one eye by altering both the commands that moved the eye and the commands that held the eye following saccade completion. With damage to the flocculus region of the cerebellum, the time constant of gaze holding was reduced from a normal 20 s to as little as 1.5 s. This damage also severely impaired the ability of the brain to adapt the motor commands that held the eye steady following the saccade, as evidenced by the inability to adapt in response to perturbations that produced postsaccadic drift of the visual scene. With bilateral damage to the oculomotor vermis region of the cerebellum, horizontal saccades became hypermetric, but there were no significant gaze-holding issues. This damage did not impair the ability to adapt the postsaccadic gaze-holding motor commands but did impair the ability to modify the motor commands that moved the eye during the saccade.
Together, these observations suggested that the oculomotor vermis and the associated fastigial nuclei played a critical role in adaptive control of the motor commands that moved the eye during the saccade, whereas the flocculus and the associated vestibular nuclei played a critical role in adaptive control of the motor commands that held the image steady on the fovea following completion of the saccade (Fig. 14).
MOVING THE HEAD VS. HOLDING IT STILL
The fact that there are distinct circuits in the brain stem for moving the eye during a saccade vs. holding it still (burst generators vs. the prepositus) and that each function may be monitored and supported by a distinct region of the cerebellum (oculomotor vermis vs. flocculus) raises a question: is such a separation of function also present in other kinds of movements?
In principle, the purpose of the move circuit is to produce a change in the sensory state of the body part, whereas the purpose of the hold circuit is to maintain a constant sensory state in that body part. For example, perhaps a hold circuit specializes in maintaining the arm in place after one completes a reach: lifting a cup from the table to our lips requires not only moving the cup upward but also holding it once the reach has completed. Similarly, it is possible that a hold circuit maintains the neck in place after one rotates it away from the straight-ahead configuration. At present, it is not known whether separate systems are responsible for moving a part of the skeletal system and holding it in place or whether a single system does both jobs. However, there are clues that suggest that these two functions may be divided into separate circuits.
During a head movement, for example, one that we might do to look at an object to the left, the saccadic eye movement is followed by head rotation (Fig. 17). To generate the head rotation, neck muscles receive a burst of activity. To maintain the new position, the same muscles receive sustained activity, as illustrated in the recordings that Bizzi et al. (1971) made from the splenius capitis muscle. Activity in the right splenius is sustained when the head is held stationary to the right, and activity in the left splenius is sustained when the head is held stationary to the left (Fig. 17). Just like the eyes, holding of the head steady requires sustained activity in the various neck muscles.
Fig. 17.
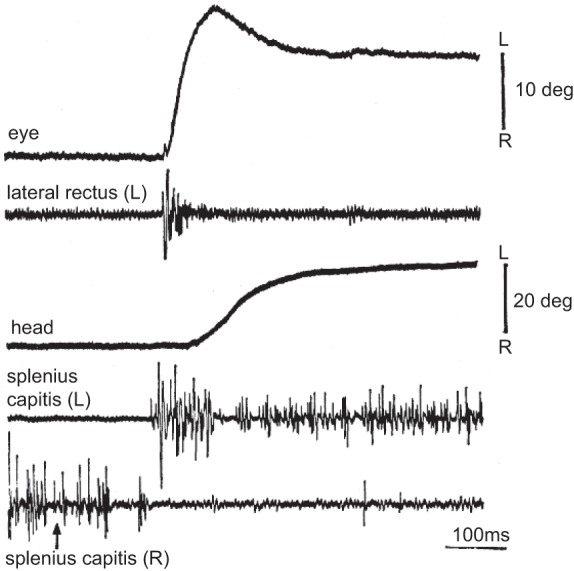
Electromyography (EMG) of eye and neck muscles during a horizontal eye-head turning to the left. The arrow indicates onset of visual stimulus. Note that holding the head steady coincides with sustained activity in the neck muscle. From Bizzi et al. (1971), with permission.
Shaikh et al. (2013) attached a laser to the head of human volunteers and asked them to rotate their head to point the laser at a light-emitting diode target placed on the horizontal axis. Following presentation of the target, the head rapidly rotated toward the target (with peak velocity of ~50°/s) and then remained stationary until the next target was presented (Fig. 18A). This ability to hold the head steady following completion of the movement was aided by two factors: visual input from the laser and proprioceptive input from the neck muscles. Next, they turned off the laser and asked the subjects to perform the same task but now, without the aid of visual feedback. They found that following completion of the head movement, without the visual feedback, the head did not maintain a steady position (Fig. 18B); rather, it drifted back toward the straight-ahead configuration. This drift had a time constant of ~48 s. Finally, they vibrated the neck muscles, attempting to introduce noise in the sensory system that would provide feedback regarding the current position of the head. The vibration increased the rate of decay of the head, reducing the time constant to ~28 s (Fig. 18C). Importantly, they found that the time constant of decay was independent of the position of the head. Regardless of where the head was located following completion of the movement, it rotated back toward the center with a time constant of ~48 s in the intact condition and 28 s in the neck-vibration condition. The data appeared consistent with a “leaky” integrator that responded to the commands that moved the head by producing commands that held it in place.
Fig. 18.
Characteristics of head movements in healthy people. A: horizontal head movements of a healthy subject, who was instructed to point a head-fixed laser to light-emitting diode (LED) targets. The subject holds the head steady following each movement. B: similar movements but without the head-fixed laser. Without the visual cue regarding head position, the head drifts following completion of the movement toward a null position. C: the decay becomes faster when neck muscles are vibrated. From Shaikh et al. (2013), with permission.
If there is a neural integrator for head movements, it should have two properties: first, its stimulation should not only move the head to a new position but also hold it there, despite stimulation offset (much like Fig. 7A and simulations in Fig. 6H). Second, damage to this system should make it so that the subject can make nearly normal head movements but cannot hold the head steady following the movement.
Klier et al. (2002) found a region in the midbrain of the monkey that had these two properties. They focused on the INC, an area that Crawford et al. (1991) had earlier shown was critical for neural integration of vertical oculomotor commands (Fig. 4B). They began by stimulating INC and found that the stimulation produced torsional deviations in head position (Fig. 19A). The stimulation of the right INC produced a rotation of the head opposite that of stimulating the left INC. For example, 200 ms stimulation produced a sluggish rotation of the head (Fig. 19A). However, once the stimulation ended, the head maintained nearly all of its torsional position. Therefore, a brief stimulation not only moved the head but also roughly held it at its new location, despite cessation of stimulation.
Fig. 19.
Effects of stimulation and inactivation of interstitial nucleus of Cajal (INC) on control of head movements. A: brief stimulation of INC produces mostly torsional rotation of the head. The animal maintains head position, despite termination of the stimulation. Stimulation period is shown by the dashed, vertical lines. T, torsional; V, vertical; H, horizontal. B: inactivation of INC. The vertical lines indicate initiation of a voluntary head movement. Following the rapid phase of the movement (dots), the torsional position of the head, in particular, rotates back toward a null position. The drift in the torsional position reverses direction when the opposite INC is deactivated. From Klier et al. (2002), with permission.
Next, Klier et al. (2002) injected muscimol (a GABA agonist) into either the left or right INC. They found that the animal could make head movements but was not able to hold the head steady following completion of the movement (Fig. 19B). Rather, the head rotated back following completion of the movement, as if the hold circuit had been disabled. These results suggested that like eye movements, control of head movements relied on a neural integrator that transformed brief, pulse-like inputs that moved the head into steady, step-like commands that held it in place following completion of the movement.
These results on control of head movements shed light on a clinical disorder, called cervical dystonia (also known as torticollis, Latin for twisted neck), where patients hold their heads not in the straight-ahead, vertical position but in a null position that has the head twisted in a nonvertical configuration. There are beneficial effects of surgery in the region of INC for patients with cervical dystonia (Vasin et al. 1985). A recent review summarized the evidence that links cervical dystonia with disorders of the neural integrator (Shaikh et al. 2016).
DISPLACING THE ARM VS. HOLDING IT STILL: THE MOTOR CORTEX
The M1 in primates is not a uniform structure but one that appears to be separated into two regions: a caudal region that includes the anterior bank and crown of the central sulcus and a rostral region that includes the precentral gyrus and continues to the premotor cortex. This distinction comes from microstimulation and anatomical tracing. Very low current stimulation of the caudal M1 can produce muscle contractions, whereas higher currents are needed to achieve similar contractions from rostral M1 (Kwan et al. 1978). In its Layer V, caudal M1 contains corticomotoneurons that project monosynaptically to the motoneurons in the spinal cord that innervate hand (Rathelot and Strick 2006) and arm (Rathelot and Strick 2009) muscles. In contrast, cells in Layer V of rostral M1 do not project to the motoneurons but project to an intermediate region before reaching the motoneurons (Rathelot and Strick 2009). The caudal M1 corticomotoneurons appear to be specific to primates, as they do not appear in the cat. In this sense, rostral M1 is the old motor cortex in primates—the one that we share with other mammals—whereas caudal M1 is the new motor cortex.
Intriguingly, many cells in the old motor cortex appear to be concerned with motor commands that move the arm but not the commands that hold the arm after the movement has completed. In contrast, many cells in the new motor cortex appear to be concerned with both the movement and the hold components.
Crammond and Kalaska (1996) trained monkeys to hold a light-weight handle at a central location and reach to one of eight targets that were placed at an 8-cm distance. Once at the target, the animals held their hand at that location for 2 s before returning to center. Earlier work by Georgopoulos et al. (1984) had established that holding the arm required sustained activity of various muscles (Fig. 20A). For example, the deltoid was more active when the right arm was maintained steady in a more flexed posture (target at 180°; Fig. 20A). Therefore, like the eyes and the neck, holding the arm steady at a given posture was achieved by sustained activity of muscles.
Fig. 20.
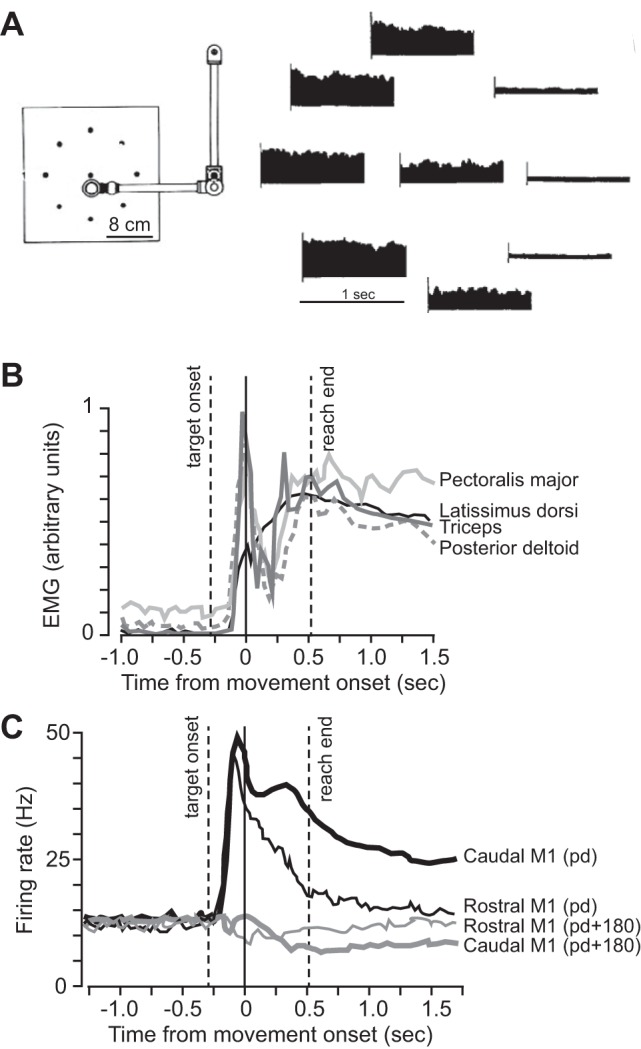
Activity of neurons in the rostral and caudal regions of the primary motor cortex (M1) during reaching. A: experimental setup and activity in the deltoid muscle of the right arm as the hand was held steady at each location. From Georgopoulos et al. (1984). B: activity in a selection of arm muscles during reaching movement in the preferred direction (pd) of each muscle. C: population activity in rostral and caudal M1 cells during a reach in the pd of the cells or the opposite direction (pd + 180). Caudal M1 cells show tonic activity during the hold period but not rostral M1 cells. From Crammond and Kalaska (1996), with permission.
Crammond and Kalaska (1996) reported that if the movement was in the PD of a muscle during the movement phase, that muscle displayed a burst of activity, followed by tonic discharge that was sustained during the hold phase (Fig. 20B). They recorded from a large number of M1 cells (n = 224) and found that in caudal M1, cells (n = 72) had a discharge that was roughly similar to the muscles: when the reach was in the PD of the M1 cell (Fig. 20C), the population exhibited a burst during the reach period, followed by a tonic discharge above the baseline during the hold period. When the reach was in the opposite direction (PD + 180; Fig. 20B), the population exhibited a decline in activity, followed by a tonic discharge below baseline.
In contrast to the cells in caudal M1, Crammond and Kalaska (1996) found that cells in rostral M1 (n = 152) exhibited a burst during the movement phase but lacked the tonic activity during the hold phase (Fig. 20C): despite the strong modulation during the movement phase, rostral M1 cells were largely indifferent to the activity needed to hold the arm once the movement had ended. Cells in dorsal premotor cortex were similar to the cells in rostral M1, generally displaying a modulation only during the movement phase, with little or no tonic activity during the hold phase. Therefore, roughly speaking, rostral M1 cells exhibited activity that carried information about the move period but not the hold period, whereas caudal M1 cells carried information about both periods.
Anatomical findings of Rathelot and Strick (2009) showed that Layer V cells in caudal M1 were more likely to project directly onto the motoneurons, whereas cells in rostral M1 were more likely to project to an intermediate region that in turn, projected onto the motoneurons. They used retrograde transneuronal transport of rabies virus from single muscles of the rhesus monkey to identify corticomotoneuronal cells in M1 and found that in caudal M1, many cells made monosynaptic connections to motoneurons that innervated shoulder, elbow, or finger muscles (Fig. 21A). In contrast, there were much fewer cells in rostral M1 with monosynaptic projections to spinal motoneurons.
Fig. 21.
Corticomotoneuron cells in the primary motor cortex (M1) of the monkey. A: topographic organization of corticomotoneuron cells. The color scale at the right indicates density of labeled neurons as percent relative to peak density. Almost all cells are in the caudal region of M1. The left, dashed lines separate areas 6 and 4; middle, dashed lines are the central sulcus (CS); right, dashed lines separate areas 4 and 3a. B: results of intracortical microstimulation of M1. Colors indicate the movement evoked by threshold stimulation at each site. Symbol size indicates the threshold for each site. Cells in caudal M1 required much lower currents to produce activation of their corresponding muscles than cells in rostral M1. Redrawn in Rathelot and Strick (2009) from data originally collected by Kwan et al. (1978), with permission. C: average activity of corticomotoneurons (CM) during wrist movements in the preferred direction of the cell and the average activity of their target muscle during the same movements. The peak muscle activity occurs 15 ms after neuronal peak, and the activities in both the cells and their target muscles are sustained after movement ends (arrow). From Griffin et al. (2015), with permission.
Kwan and colleagues (1978) had noted that caudal M1 cells required less current during microstimulation to produce motion in single joints of the arm than rostral M1 cells (Fig. 21B). Therefore, it appeared possible that among the caudal M1 cells that Crammond and Kalaska (1996) had recorded were a fair number of corticomotoneurons. If so, identified corticomotoneurons in M1 should exhibit bursting activity during the move phase, as well as tonic activity during the hold phase.
Strick and colleagues (Griffin et al. 2015) considered a task where the animal moved a cursor on the screen by rotating its wrist. Figure 21C shows averaged activity of corticomotoneurons during the wrist movement in the PD of the neuron and the averaged activity of their target muscle during the same movement. The peak muscle activity occurred 15 ms after neuronal peak activity. Importantly, activity in the corticomotoneurons was sustained after movement end, similar to that in the muscles. Therefore, the corticomotoneurons—cells that were located in Layer V of caudal M1—exhibited activity that included both the movement phase and the hold phase of the action.
With the consideration of these results together, we may speculate that in caudal M1, where Layer V cells are more likely to make monosynaptic projections onto spinal motoneurons, the cortical neurons convey commands that control both the movement and the hold phases of the action. However, in the rostral M1, where Layer V neurons project to spinal interneurons or brain stem interneurons, the cortical neurons convey commands for moving the limb but have little involvement in the commands that are responsible for holding the limb after the movement has ended.
TRANSIENT FORCE VS. STEADY FORCE: THE MOTOR CORTEX
Shalit et al. (2012) trained monkeys to hold a force transducer and produce forces in various directions. A cue, consisting of eight circles, with one red color, appeared, instructing the animal of the force vector that was required (Fig. 22A). After a delay period, a Go cue appeared (removal of the central target), instructing the animal to produce the cued force. The animal held the force in the target range for 0.35–1.0 s, after which, the center cue was displayed, and the animal reduced the force back to zero.
Fig. 22.
Activity of neurons in the rostral and caudal primary motor cortex (M1), as well as spinal cord, during isometric force production. A: discharge of a spinal neuron and a rostral M1 neuron during a force production task. The force trace is shown at the bottom (in units of torque, in Newton meters). Whereas activity of the spinal neuron shows a burst, followed by tonic activity during the hold period, the M1 neuron exhibits a burst but is silent during the hold period. Force offset is marked by a green arrow. B: activity of M1 neurons, spinal neurons, and EMG averaged across all directions of force. C: activity of M1 and spinal cells aligned to force onset when target was in the preferred direction (PD). D: activity of M1 and spinal cells aligned to force offset when the center target was in the PD. From Shalit et al. (2012), with permission. E: activity of a caudal M1 neuron during isometric force production. The cell is active during the hold period. F: population averaged activity of caudal M1 neurons during isometric force production, as well as during reaching, toward PD or 180° away from PD. From Sergio et al. (2005), with permission. G: schematic model of projections from the rostral and caudal M1 cells to the spinal cord.
The authors recorded from 285 cells in M1, mostly in the rostral region, as well as 102 cells from the spinal cord (interneurons, spinal segments C5–T1). Examples of single M1 and spinal cells are shown in Fig. 22A. The spinal cell had a discharge that tracked the force, while the M1 cell behaved differently: it first had a weak response to onset of the cue and then a burst near force onset but then silence as the force was maintained. That is, whereas the spinal cell exhibited activity that included both a pulse-like and a step-like component, the M1 cell exhibited only a pulse. When the activity for all M1 and spinal cells was averaged across all directions of force (Fig. 22B), the M1 cells showed a transient response that led electromyography (EMG) but then declined to near baseline long before the end of the hold period. However, the spinal cells produced an activity that rose with the M1 activity and were sustained during the hold period.
Shalit et al. (2012) found that each M1 and spinal cell exhibited a PD of force for which it produced its largest response. They found this PD for each cell during the force-onset period, as well as force-offset period. They then averaged activity of the various cells at force onset when the target was in the PD of each cell and found that activity in M1 rose before that of the spinal cells but then soon declined (Fig. 22C). In contrast, when they examined activity near force offset, they again found a group of M1 cells that showed a transient burst but now, with preferred force direction that was toward the center target. That is, the authors found that among their population of M1 cells, the average activity was a transient burst around force onset with no hold-related activity. In contrast, in the spinal cord, the activity included both a burst and a hold-related activity.
The results of Shalit et al. (2012) were consistent with those reported earlier by Georgopoulos et al. (1992). In those experiments, the monkey was first instructed to maintain a force that countered a bias load. Once this bias force was achieved, the animal was instructed to produce an additional force pulse to one of eight directions. Georgopoulos et al. (1992) found that their collection of M1 cells was tuned to the force pulse, irrespective of the bias force that the animal was producing. They concluded that rather than encoding the total force being produced by the muscles, during the force-pulse period, the M1 cells encoded largely the change in force with respect to the bias force.
In our framework, the cells described by both Georgopoulos et al. (1992) and Shalit et al. (2012) would fall into the category of rostral M1 cells, described by Crammond and Kalaska (1996): sensitive to motor commands in the move phase but not the hold phase. In contrast, the corticomotoneurons described by Griffin et al. (2015) would fall into the category of caudal M1 cells described by Crammond and Kalaska (1996): sensitive to motor commands during both the move phase and the hold phase.
To illustrate this distinction between the caudal and rostral M1 cells, consider the cells recorded by Sergio et al. (2005) from the most caudal part of M1 in the anterior bank of the central sulcus. The animals performed two tasks: in some trials, an isometric force task; in other trials, a reaching task. Unlike the M1 cells reported by Shalit et al. (2012), the caudal M1 cells not only produced a burst aligned with force onset but also tonic activity during the ensuing hold period (Fig. 22E). The same cells were active during both the move phase and the hold phase of a reach task (Fig. 22F).
The potential distinction between M1 cells that were engaged for movement vs. holding was also noted by Kurtzer et al. (2005). These authors trained a monkey in a task in which the animal held its hand at a center location, while an exoskeleton robot pushed on the elbow and/or shoulder joints. During holding, the discharge of some cells was modulated by the direction of the load, whereas other cells appeared insensitive to the varying loads. However, some of the cells that were insensitive to load during holding became active during reaching, exhibiting a burst as the hand reached to various directions. That is, some M1 cells were modulated by force during reaching but were insensitive to similar forces during holding.
With the consideration of these data together, it seems plausible that the cells in rostral M1—the old motor cortex common to all mammals or more generally, M1 cells that have higher stimulation thresholds to produce muscle activity (Kalaska et al. 1989)—are concerned with a change in the state of the limb: increase or decrease of force in an isometric task, move phase of a reaching task. Because the rostral M1 cells lack the sustained activity needed for the hold phase, they may rely on a downstream structure that serves as a neural integrator for the arm (Fig. 22G). In this model, the sum of the rostral M1 activity and this integrated activity is fed to the spinal interneurons. Where might such a neural integrator for the arm be located?
The brain stem houses a host of nuclei that are important for control of limb movements. In the caudal medulla, a nucleus, called medullary reticular nucleus, is noteworthy because of its anatomical projections and its functional contributions to control of reaching (Esposito et al. 2014). The ventral part of this nucleus (MdV) in mice projects monosynaptically to ipsilateral forelimb motoneurons, particularly biceps, with minor projections to triceps or any of the hindlimb motoneurons. In contrast, other brain stem nuclei, including spinal trigeminal nucleus, pontine reticular nucleus, and vestibular nucleus, show monosynaptic projections to forelimb motoneurons that are biased toward triceps. MdV neurons are almost exclusively glutamatergic, providing excitation on forelimb motoneurons. Importantly, MdV receives direct projections from Layer V pyramidal neurons in the contralateral M1, as well as from the medial (fastigial) and interposed nuclei of the cerebellum.
To understand the function of MdV, Esposito et al. (2014) trained mice to reach through a narrow barrier to pick up a food pellet. They divided the task into a reach phase, a grasp phase, and a retrieval phase. In the MdV-damaged animals, the authors reported no deficits in the reaching phase or the retrieval phase. This suggested that MdV damage did not affect the ability to displace the arm. However, MdV-damaged animals had a specific deficit in the phase where they had to place the paw on top of the food and use the fingers to grab the pellet. Given the projections of MdV to biceps and the necessity of biceps activity to hold the limb to use finger muscles to grab the pellet, it is possible that elimination of this part of the control circuit had an effect on the hold phase but not the reach phase. However, it is also possible that the deficit was due to the influence of MdV on motoneurons of the hand required for grasping and not the motoneurons of the arm required for holding still during grasping. Unfortunately, the experiment was not designed to dissociate the control of the move phase from the hold phase.
Suppose it is the case that the new M1 is specific to primates and that the new M1 is capable of producing commands that contribute to both the reach and the hold phases (Fig. 21). In rodents, the new M1 is presumably absent. Is activity of M1 in rodents during reaching similar to the rostral regions in the monkey (the old M1) in the sense that there is bursting during reaching but a paucity of contributions in the hold phase? If so, the understanding of neural control of reaching in rodents becomes particularly important, because in these species, one might have a better chance of answering whether there is a neural integrator for reaching.
A SUPERPOSITION OF MOVE AND HOLD CONTROLLERS FOR REACHING
The hypothesis that control of the move and the hold phases of a reach may be associated with distinct neural circuits was first described by Claude Ghez et al. (2007), Robert Sainburg and coworker (Sainburg and Kalakanis 2000), and Robert Scheidt and colleague (Scheidt and Ghez 2007).
Scheidt and Ghez (2007) trained people to hold the handle of a robotic arm. Whenever the hand was not moving, it experienced random force pulses, thereby training the subjects to co-contract the muscles of the arm and increase stiffness to minimize displacements caused by the random forces. Subjects trained to make out-and-back “slicing” movements from a center location to a target (Fig. 23A). They also made reaching movement from the same center location to the same target, where they were asked to hold the hand. After a period of training in both tasks, a perturbation was introduced during the slicing movements: the visual feedback was rotated counter-clockwise with respect to the direction of the reach. Subjects learned to alter their slicing motion, changing the move-phase commands to produce a clockwise rotation in direction of motion (Fig. 23A). Following adaptation of the slicing movements, subjects reached without visual feedback to the same target. If there were separate systems that controlled the move and the hold phases, then the adaptation that had taken place for moving might not transfer to holding, despite the fact that the targets in the two tasks were identical. They found that in the reach task, after the movement had come to an end, the hand did not stay still but drifted to a location opposite the perturbation, finally ending at a place that would be consistent with little or no adaptation. That is, whereas the motor commands for the move phase of the reaching task had partially adapted to the perturbation that was experienced during the slicing task, the motor commands for the hold phase that followed the reach continued to produce the same (unrotated) attractor as before training.
Fig. 23.
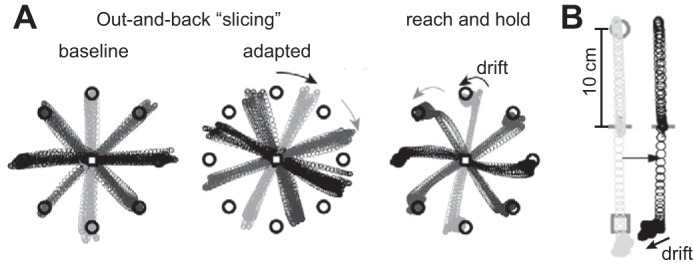
A superposition of controllers for moving and holding during a reaching task. A: subject made out-and-back slicing movements to targets, as well as reaching movements to the same targets. At end of reach, while hand was held at the target, they received random force pulses. Following this training, the slicing movements adapted to a 30° counter-clockwise rotation of the cursor. In the adapted state, the subject was asked to reach and hold at the target without visual feedback. While holding, the hand drifted back in a counter-clockwise pattern toward the original target. From Scheidt and Ghez (2007), with permission. B: subject made a slicing movement from a center position to target at 90° and then back to a target at 270° (gray circles). At end of slicing movement, the hand received random force pulses. The start position of the movement was covertly changed to 4 cm to the right. The subsequent movement (black circles) had a similar hand trajectory, but at movement end, the hand drifted toward the left. From Ghez et al. (2007), with permission.
To test whether the motor commands during the hold phase could be described as an attractor, Ghez et al. (2007) asked subjects to hold a robotic arm and move their hand in a slicing motion from a central location to a target at 90° and then return to a target at 270° (Fig. 23B). Once the hand had stopped at the 270° target, the robot again produced small, random force perturbations, resulting in co-contraction of arm muscles. After the trial had ended, the robot brought the arm back (without visual feedback) to a new start position, 4 cm to the right of the usual start position. Subjects were unaware of this covert manipulation of the starting location, which occurred on occasional trials. The reach that followed was without visual feedback. It had nearly the same pattern of motion, except at the slicing movement’s end, the hand drifted toward the position that the brain had practiced holding against the random perturbations. The muscle activity that was repeatedly produced during the hold phase appeared to persist, despite the fact that the start position of the movement had shifted.
A subsequent examination of muscle activity during reaching and slicing movements of the elbow provided additional support for the idea that the brain used different mechanisms to plan hand trajectories and final positions in point-to-point movements: trajectory planning proceeded independent of the specific limb-impedance values to be implemented at the end-point target (Scheidt et al. 2011). To account for these data, the authors proposed a model that consisted of a superposition of two controllers: one for the move phase and another for the hold phase. However, this reach model was unlike the framework that was established in the oculomotor system. Whereas in the saccadic system, the circuit that controlled the hold phase received a copy of the output generated by the move-phase circuit (Fig. 14), the model proposed for reaching assumed that the two circuits were in parallel (Yadav and Sainburg 2011): the move circuit generated transient activity to displace the arm, whereas the hold circuit independently generated an equilibrium position via coactivation of antagonist muscles. Temporal superposition of these two controllers generated torques that moved and then held the arm.
Sainburg and colleagues (Duff and Sainburg 2007; Sainburg and Kalakanis 2000) have considered this dissociation by comparing reach trajectories in the dominant and nondominant arms. In the dominant arm, the move-phase commands appeared to compensate better for the physics of motion (Sainburg and Kalakanis 2000). This could be interpreted as a better adaptive controller or greater reliance on that controller during the move phase for the dominant arm (Duff and Sainburg 2007). However, more recent work found that adaptation of reaching to a force field was roughly equal between the dominant and nondominant arms (Reuter et al. 2016; Stockinger et al. 2015). At this point, it is not clear whether the dominant arm benefits from a better adaptive controller for the reach phase than the nondominant arm.
Analysis of reach-direction errors and end-point errors has suggested that damage to the left hemisphere of humans (dominant hemisphere in this group of subjects) may particularly affect the ability to predict and account for arm dynamics during the reach phase, whereas right-hemisphere damage (nondominant hemisphere) may particularly affect the ability to place the arm accurately during the hold phase (Mani et al. 2013; Schaefer et al. 2009). The dynamic-dominance hypothesis suggests that in making a reaching movement, the dominant hemisphere is relatively more reliant on the move circuit (compared with the hold circuit), whereas the nondominant hemisphere is relatively more reliant on the hold circuit.
In summary, during reaching, as well as isometric force production, the phylogenetic older region of motor cortex (rostral M1) is particularly concerned with the motor commands that change the state of the arm but may have little or no activity when the motor commands are being generated to maintain a steady state. In contrast, an M1 region that is specific to primates (caudal M1) produces activity that reflects both phases of the action. The motor commands that hold the arm can adapt to noise (random force pulses), producing a more robust equilibrium position for the arm. The motor commands that move the arm can also adapt to perturbations (such as visuomotor rotations or force fields). The two forms of adaptation may rely on different neural structures, as evidenced by the observation of independent signatures of each form of adaptation in the move and hold phases of reaching.
MOVING THE BODY VS. HOLDING IT STILL: THE HIPPOCAMPUS
Finally, let us consider the act of locomotion. It is a curious fact that the representation of the spatial location of the animal during locomotion is distinct from representation of that same location when the animal is holding still. That is, despite the fact that the animal may be in precisely the same physical space, different cells represent that location in space whether the animal is holding still or moving through it.
In the CA1 region of the hippocampus, the principal cells fire when the subject moves through distinct locations in space. The firing in the “place cells” provides an internal representation of where the animal is located in space. For example, Wilson and McNaughton (1993) recorded simultaneously from tens of neurons in the rat hippocampus as the animal foraged in a familiar environment for pieces of food. They found cells that discharged in preferred spatial locations. Importantly, these cells fired as the animal moved through these locations with a rate that increased with the speed of travel: a positive correlation existed between firing rate and speed for place cells in CA1, CA3, and some CA2 cells.
However, Kay et al. (2016) reported another group of neurons—in region CA2 (called N cells)—which fired only when the animal was standing still. For example, when the animal explored a maze, one cell fired when the animal was holding still in one part of the maze, whereas another cell fired when the animal was holding still in another location. Importantly, neural activity in each of these locations was negatively correlated with speed of movement. That is, activity in the CA2 cells was highest when the animal was holding still in the preferred location, whereas activity in the CA1 and CA3 cells was highest when the animal was moving rapidly through the same preferred location.
It thus appears that in the hippocampus, the representation of space is differentiated between moving and holding still. The same location in space is encoded by different cells in potentially different regions of the hippocampus, depending on whether the animal is moving through that space or standing still.
OVERVIEW
We move, i.e., change our state, because the act of moving promises to bring us to a state that has a greater utility than our current state. In contrast, we hold, i.e., maintain our state, because the act of holding promises a greater utility than any change in state that may be available. For example, perhaps we make a change in the state of our eyes (engage the move circuit and make a saccade) because something with greater utility is available than what we currently have on our fovea. We maintain the image on our fovea because that image currently has greater utility than anything available elsewhere in the visual field (engage the hold circuit to fixate or do smooth pursuit to maintain the image on the fovea). In this framework, motor control is characterized by brief periods of transition between states of holding still.
Making a change in the sensory state (bringing a new image on the fovea) and maintaining the sensory state (keeping the image on the fovea) both involve monitoring and adaptive control of the ongoing motor commands. For the saccadic system, distinct premotor circuits in the brain stem are engaged in generating the motor commands needed for changing the state and maintaining the state of the eye, and distinct regions of the cerebellum are involved in adaptive control of these motor commands.
The functional anatomy of the oculomotor system suggests that distinct regions are concerned with providing the transient motor commands needed to make saccades, whereas other regions provide the sustained commands needed to hold the eyes after the movement has ended. The circuitry involved in the hold phase of saccades shares many components of the circuitry involved in smooth pursuit and VOR, suggesting that “holding” does not imply generating commands to keep the eyes still but rather, generating commands to keep the image on the fovea still.
This separation of control between the change-state and maintain-state circuits—what we have termed the move and hold circuits—may not be exclusive to the oculomotor system but also present in control of skeletal movements.
Control of head movements relies on a brain stem circuit (INC) that specializes in maintaining the head’s position once the neural drive for moving it has ended: a transient input to this circuit not only displaces the head but also maintains it at a new posture, despite termination of the input. Therefore, there is at least one neural circuit in the skeletal motor control system that functions similarly to the hold circuit found in the saccadic eye-movement system.
For control of the arm, activity in the rostral region of M1—the phylogenetic older motor cortex—appears to be primarily concerned with the transient input needed to change the state of the arm, lacking the sustained input needed to maintain its state. However, activity of the spinal interneurons, as well as many neurons in the caudal region of M1—the newer motor cortex—reflects both the transient and the sustained inputs. It remains to be seen whether the spinal activity is the sum of inputs from two distinct neural circuits responsible for the change and maintain phases.
For representation of body position during locomotion, activity in the hippocampus appears to be dissociated between regions that represent space when the animal is moving through a location vs. representation of the same location when the animal is holding still.
This view that distinct circuits in the brain stem and perhaps the cortex may be involved in changing of state vs. maintaining of state provides some insights regarding the function of the cerebellum. For control of saccadic eye movements, the oculomotor vermis region of the cerebellum appears to be concerned with monitoring and controlling the movement phase of the eye, whereas the flocculus region of the cerebellum appears to be concerned with monitoring and controlling the hold phase. Damage to the oculomotor vermis produces dysmetria in the movement phase but leaves the ability to hold fixation intact. In contrast, damage to the flocculus leaves intact the movement phase but causes deficits in the hold phase, leading to a condition called gaze-evoked nystagmus, where following a saccade, the eyes drift back toward the center (as well as a host of pursuit and vestibular dysfunctions).
The idea of a hold circuit, a neural integrator, was introduced by Robinson (1970) following his study of VOR, a reflex whose function is to maintain an image on the fovea, despite motion of the head. However, the goal of maintaining a sensory state, despite motion of a body part, is not unique to the oculomotor system but a general problem in motor control. Evolution of a skeletal system introduced this problem, because motion of any joint affected not just the sensory state of that joint but also all other joints. For example, lifting the arm while standing moved the center of mass of the body forward, requiring the muscles of the legs to compensate for this motion to hold the torso still. The use of the right hand to lift a book held by the left hand required the muscles of the left hand to anticipate the motion of the book to keep the left hand still.
These patterns of postural control are generally studied under the rubric of automatic postural adjustments (APAs). However, if we view the hold circuit as a system whose primary function is to maintain a constant sensory state in a body part, then it is not surprising that the circuit that holds the eye steady following a saccade is part of the same circuit that holds the image steady on the fovea in smooth pursuit and VOR. That is, the neural integrator for saccades not only participates in holding the eye still when the saccade ends but also holds the image still on the fovea when the head rotates. Its general function is to maintain state. In this framework, VOR is a member of the APA family.
This view suggests that if there is a hold circuitry for the arm, then its function may not be specific to holding the arm still after it is voluntarily moved but also holding it still when the voluntary action involves motion of another body part. When the left hand is holding the book still, it would be the hold circuit for the left arm that maintains it steady as the right hand picks up the book. The same hold circuit would be engaged when the left arm lifts the book off of the table and holds it to be read.
The necessity of having a hold circuit may have arisen from the need to maintain a constant sensory state in a body part, despite motion of other body parts. For example, if we imagine that a hold circuit accumulates the transient information that is sent by the right rostral M1 cells to displace the left arm and produces the sustained activity that is needed to hold the left arm still after it has been moved, then we can also imagine that this same hold circuit is responsible for the APA that is exhibited by the left arm, holding it still, despite the fact that now it is the right arm that is the moving body part.
Robinson’s (1970) insight in predicting a neural integrator for saccadic eye movements came about because he noticed that VOR required a circuit that translated a velocity-like signal coming from the vestibular afferents into a position-like signal needed by the motoneurons of the eyes. If we view the VOR circuit as a member of the APA family of neural circuits whose job is to maintain a constant sensory state in a body part and note that in control of saccadic eye movements, the act of holding the eyes still after completion of a movement is co-opting an existing circuit whose original function had nothing to do with control of voluntary movements, then we might speculate that in general, circuits that are concerned with voluntary movements may have co-opted existing circuits that specialize in holding still. Whereas the original function of the hold circuitry may have been to maintain a constant sensory state in the context of a postural disturbance, that same circuitry can be used for holding still following a voluntary disturbance—a disturbance that we label a goal-directed movement.
GRANTS
Support for this work was provided by grants from the National Institute of Neurological Disorders and Stroke (5R01NS078311) and the Office of Naval Research (N00014-15-1-2312).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
R.S. conceived and designed research; R.S. prepared figures; R.S. drafted manuscript; R.S. edited and revised manuscript; R.S. approved final version of manuscript.
ACKNOWLEDGMENTS
I am grateful to Robi Soetedjo, Yoshiko Kojima, David Zee, David Herzfeld, and Scott Albert who provided comments on this manuscript.
REFERENCES
- Arnold DB, Robinson DA. The oculomotor integrator: testing of a neural network model. Exp Brain Res 113: 57–74, 1997. doi: 10.1007/BF02454142. [DOI] [PubMed] [Google Scholar]
- Barton EJ, Nelson JS, Gandhi NJ, Sparks DL. Effects of partial lidocaine inactivation of the paramedian pontine reticular formation on saccades of macaques. J Neurophysiol 90: 372–386, 2003. doi: 10.1152/jn.01041.2002. [DOI] [PubMed] [Google Scholar]
- Bizzi E, Kalil RE, Tagliasco V. Eye-head coordination in monkeys: evidence for centrally patterned organization. Science 173: 452–454, 1971. doi: 10.1126/science.173.3995.452. [DOI] [PubMed] [Google Scholar]
- Cannon SC, Robinson DA. Loss of the neural integrator of the oculomotor system from brain stem lesions in monkey. J Neurophysiol 57: 1383–1409, 1987. [DOI] [PubMed] [Google Scholar]
- Cannon SC, Robinson DA, Shamma S. A proposed neural network for the integrator of the oculomotor system. Biol Cybern 49: 127–136, 1983. doi: 10.1007/BF00320393. [DOI] [PubMed] [Google Scholar]
- Cohen B, Komatsuzaki A. Eye movements induced by stimulation of the pontine reticular formation: evidence for integration in oculomotor pathways. Exp Neurol 36: 101–117, 1972. doi: 10.1016/0014-4886(72)90139-2. [DOI] [PubMed] [Google Scholar]
- Corneil BD, Hing CA, Bautista DV, Munoz DP. Human eye-head gaze shifts in a distractor task. I. Truncated gaze shifts. J Neurophysiol 82: 1390–1405, 1999. [DOI] [PubMed] [Google Scholar]
- Crammond DJ, Kalaska JF. Differential relation of discharge in primary motor cortex and premotor cortex to movements versus actively maintained postures during a reaching task. Exp Brain Res 108: 45–61, 1996. doi: 10.1007/BF00242903. [DOI] [PubMed] [Google Scholar]
- Crawford JD, Cadera W, Vilis T. Generation of torsional and vertical eye position signals by the interstitial nucleus of Cajal. Science 252: 1551–1553, 1991. doi: 10.1126/science.2047862. [DOI] [PubMed] [Google Scholar]
- Dale A, Cullen KE. Local population synchrony and the encoding of eye position in the primate neural integrator. J Neurosci 35: 4287–4295, 2015. doi: 10.1523/JNEUROSCI.4253-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff SV, Sainburg RL. Lateralization of motor adaptation reveals independence in control of trajectory and steady-state position. Exp Brain Res 179: 551–561, 2007. doi: 10.1007/s00221-006-0811-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito MS, Capelli P, Arber S. Brainstem nucleus MdV mediates skilled forelimb motor tasks. Nature 508: 351–356, 2014. doi: 10.1038/nature13023. [DOI] [PubMed] [Google Scholar]
- Fuchs AF. The vestibular system. In: Textbook of Physiology: Excitable Cells and Neurophysiology, edited by Patton HD, Fuchs AF, Hille B, Scher AM, Steiner R. Philadelphia: W.B. Saunders Company, 1989, p. 582–607. [Google Scholar]
- Fuchs AF, Binder MD. Fatigue resistance of human extraocular muscles. J Neurophysiol 49: 28–34, 1983. [DOI] [PubMed] [Google Scholar]
- Gandhi NJ, Barton EJ, Sparks DL. Coordination of eye and head components of movements evoked by stimulation of the paramedian pontine reticular formation. Exp Brain Res 189: 35–47, 2008. doi: 10.1007/s00221-008-1401-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos AP, Ashe J, Smyrnis N, Taira M. The motor cortex and the coding of force. Science 256: 1692–1695, 1992. doi: 10.1126/science.256.5064.1692. [DOI] [PubMed] [Google Scholar]
- Georgopoulos AP, Caminiti R, Kalaska JF. Static spatial effects in motor cortex and area 5: quantitative relations in a two-dimensional space. Exp Brain Res 54: 446–454, 1984. doi: 10.1007/BF00235470. [DOI] [PubMed] [Google Scholar]
- Ghez C, Scheidt R, Heijink H. Different learned coordinate frames for planning trajectories and final positions in reaching. J Neurophysiol 98: 3614–3626, 2007. doi: 10.1152/jn.00652.2007. [DOI] [PubMed] [Google Scholar]
- Golla H, Tziridis K, Haarmeier T, Catz N, Barash S, Thier P. Reduced saccadic resilience and impaired saccadic adaptation due to cerebellar disease. Eur J Neurosci 27: 132–144, 2008. doi: 10.1111/j.1460-9568.2007.05996.x. [DOI] [PubMed] [Google Scholar]
- Gomez CM, Thompson RM, Gammack JT, Perlman SL, Dobyns WB, Truwit CL, Zee DS, Clark HB, Anderson JH. Spinocerebellar ataxia type 6: gaze-evoked and vertical nystagmus, Purkinje cell degeneration, and variable age of onset. Ann Neurol 42: 933–950, 1997. doi: 10.1002/ana.410420616. [DOI] [PubMed] [Google Scholar]
- Goossens HH, Van Opstal AJ. Human eye-head coordination in two dimensions under different sensorimotor conditions. Exp Brain Res 114: 542–560, 1997. doi: 10.1007/PL00005663. [DOI] [PubMed] [Google Scholar]
- Griffin DM, Hoffman DS, Strick PL. Corticomotoneuronal cells are “functionally tuned”. Science 350: 667–670, 2015. doi: 10.1126/science.aaa8035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie BL, Porter JD, Sparks DL. Corollary discharge provides accurate eye position information to the oculomotor system. Science 221: 1193–1195, 1983. doi: 10.1126/science.6612334. [DOI] [PubMed] [Google Scholar]
- Haith AM, Reppert TR, Shadmehr R. Evidence for hyperbolic temporal discounting of reward in control of movements. J Neurosci 32: 11727–11736, 2012. doi: 10.1523/JNEUROSCI.0424-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jürgens R, Becker W, Kornhuber HH. Natural and drug-induced variations of velocity and duration of human saccadic eye movements: evidence for a control of the neural pulse generator by local feedback. Biol Cybern 39: 87–96, 1981. doi: 10.1007/BF00336734. [DOI] [PubMed] [Google Scholar]
- Kalaska JF, Cohen DA, Hyde ML, Prud’homme M. A comparison of movement direction-related versus load direction-related activity in primate motor cortex, using a two-dimensional reaching task. J Neurosci 9: 2080–2102, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko CR. Eye movement deficits after ibotenic acid lesions of the nucleus prepositus hypoglossi in monkeys. I. Saccades and fixation. J Neurophysiol 78: 1753–1768, 1997. [DOI] [PubMed] [Google Scholar]
- Kay K, Sosa M, Chung JE, Karlsson MP, Larkin MC, Frank LM. A hippocampal network for spatial coding during immobility and sleep. Nature 531: 185–190, 2016. doi: 10.1038/nature17144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller EL, Gandhi NJ, Shieh JM. Endpoint accuracy in saccades interrupted by stimulation in the omnipause region in monkey. Vis Neurosci 13: 1059–1067, 1996. doi: 10.1017/S0952523800007719. [DOI] [PubMed] [Google Scholar]
- Klier EM, Wang H, Constantin AG, Crawford JD. Midbrain control of three-dimensional head orientation. Science 295: 1314–1316, 2002. doi: 10.1126/science.1067300. [DOI] [PubMed] [Google Scholar]
- Kojima Y, Iwamoto Y, Robinson FR, Noto CT, Yoshida K. Premotor inhibitory neurons carry signals related to saccade adaptation in the monkey. J Neurophysiol 99: 220–230, 2008. doi: 10.1152/jn.00554.2007. [DOI] [PubMed] [Google Scholar]
- Kurtzer I, Herter TM, Scott SH. Random change in cortical load representation suggests distinct control of posture and movement. Nat Neurosci 8: 498–504, 2005. [DOI] [PubMed] [Google Scholar]
- Kwan HC, MacKay WA, Murphy JT, Wong YC. Spatial organization of precentral cortex in awake primates. II. Motor outputs. J Neurophysiol 41: 1120–1131, 1978. [DOI] [PubMed] [Google Scholar]
- Leigh RJ, Zee DS. The Neurology of Eye Movements. Oxford, UK: Oxford Univ. Press, 2015. doi: 10.1093/med/9780199969289.001.0001. [DOI] [Google Scholar]
- Lewis RF, Zee DS, Hayman MR, Tamargo RJ. Oculomotor function in the rhesus monkey after deafferentation of the extraocular muscles. Exp Brain Res 141: 349–358, 2001. doi: 10.1007/s002210100876. [DOI] [PubMed] [Google Scholar]
- Maier A, DeSantis M, Eldred E. The occurrence of muscle spindles in extraocular muscles of various vertebrates. J Morphol 143: 397–408, 1974. doi: 10.1002/jmor.1051430404. [DOI] [PubMed] [Google Scholar]
- Mani S, Mutha PK, Przybyla A, Haaland KY, Good DC, Sainburg RL. Contralesional motor deficits after unilateral stroke reflect hemisphere-specific control mechanisms. Brain 136: 1288–1303, 2013. doi: 10.1093/brain/aws283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PJ, Hartwich-Young R, Nelson J, Sparks DL, Porter JD. Cerebellotectal pathways in the macaque: implications for collicular generation of saccades. Neuroscience 36: 305–324, 1990. doi: 10.1016/0306-4522(90)90428-7. [DOI] [PubMed] [Google Scholar]
- McCrea RA, Horn AK. Nucleus prepositus. Prog Brain Res 151: 205–230, 2006. doi: 10.1016/S0079-6123(05)51007-0. [DOI] [PubMed] [Google Scholar]
- McFarland JL, Fuchs AF. Discharge patterns in nucleus prepositus hypoglossi and adjacent medial vestibular nucleus during horizontal eye movement in behaving macaques. J Neurophysiol 68: 319–332, 1992. [DOI] [PubMed] [Google Scholar]
- Miri A, Daie K, Arrenberg AB, Baier H, Aksay E, Tank DW. Spatial gradients and multidimensional dynamics in a neural integrator circuit. Nat Neurosci 14: 1150–1159, 2011. doi: 10.1038/nn.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda H, Murakami S, Warabi T. Effects of fastigial stimulation upon visually-directed saccades in macaque monkeys. Neurosci Res 10: 188–199, 1991. doi: 10.1016/0168-0102(91)90056-5. [DOI] [PubMed] [Google Scholar]
- Optican LM, Miles FA. Visually induced adaptive changes in primate saccadic oculomotor control signals. J Neurophysiol 54: 940–958, 1985. [DOI] [PubMed] [Google Scholar]
- Optican LM, Robinson DA. Cerebellar-dependent adaptive control of primate saccadic system. J Neurophysiol 44: 1058–1076, 1980. [DOI] [PubMed] [Google Scholar]
- Optican LM, Zee DS, Miles FA. Floccular lesions abolish adaptive control of post-saccadic ocular drift in primates. Exp Brain Res 64: 596–598, 1986. doi: 10.1007/BF00340497. [DOI] [PubMed] [Google Scholar]
- Rathelot JA, Strick PL. Muscle representation in the macaque motor cortex: an anatomical perspective. Proc Natl Acad Sci USA 103: 8257–8262, 2006. doi: 10.1073/pnas.0602933103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathelot JA, Strick PL. Subdivisions of primary motor cortex based on cortico-motoneuronal cells. Proc Natl Acad Sci USA 106: 918–923, 2009. doi: 10.1073/pnas.0808362106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppert TR, Lempert KM, Glimcher PW, Shadmehr R. Modulation of saccade vigor during value-based decision making. J Neurosci 35: 15369–15378, 2015. doi: 10.1523/JNEUROSCI.2621-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter EM, Cunnington R, Mattingley JB, Riek S, Carroll TJ. Feedforward compensation for novel dynamics depends on force field orientation but is similar for the left and right arms. J Neurophysiol 116: 2260–2271, 2016. doi: 10.1152/jn.00425.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DA. Models of the saccadic eye movement control system. Kybernetik 14: 71–83, 1973. doi: 10.1007/BF00288906. [DOI] [PubMed] [Google Scholar]
- Robinson DA. Oculomotor unit behavior in the monkey. J Neurophysiol 33: 393–403, 1970. [DOI] [PubMed] [Google Scholar]
- Robinson DA. The effect of cerebellectomy on the cat’s bestibulo-ocular integrator. Brain Res 71: 195–207, 1974. doi: 10.1016/0006-8993(74)90961-5. [DOI] [PubMed] [Google Scholar]
- Robinson DA. The mechanics of human saccadic eye movement. J Physiol 174: 245–264, 1964. doi: 10.1113/jphysiol.1964.sp007485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL, Kalakanis D. Differences in control of limb dynamics during dominant and nondominant arm reaching. J Neurophysiol 83: 2661–2675, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer SY, Haaland KY, Sainburg RL. Hemispheric specialization and functional impact of ipsilesional deficits in movement coordination and accuracy. Neuropsychologia 47: 2953–2966, 2009. doi: 10.1016/j.neuropsychologia.2009.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidt RA, Ghez C. Separate adaptive mechanisms for controlling trajectory and final position in reaching. J Neurophysiol 98: 3600–3613, 2007. doi: 10.1152/jn.00121.2007. [DOI] [PubMed] [Google Scholar]
- Scheidt RA, Ghez C, Asnani S. Patterns of hypermetria and terminal cocontraction during point-to-point movements demonstrate independent action of trajectory and postural controllers. J Neurophysiol 106: 2368–2382, 2011. doi: 10.1152/jn.00763.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scudder CA, Fuchs AF, Langer TP. Characteristics and functional identification of saccadic inhibitory burst neurons in the alert monkey. J Neurophysiol 59: 1430–1454, 1988. [DOI] [PubMed] [Google Scholar]
- Sergio LE, Hamel-Pâquet C, Kalaska JF. Motor cortex neural correlates of output kinematics and kinetics during isometric-force and arm-reaching tasks. J Neurophysiol 94: 2353–2378, 2005. doi: 10.1152/jn.00989.2004. [DOI] [PubMed] [Google Scholar]
- Shadmehr R, Huang HJ, Ahmed AA. A representation of effort in decision-making and motor control. Curr Biol 26: 1929–1934, 2016. doi: 10.1016/j.cub.2016.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, Orban de Xivry JJ, Xu-Wilson M, Shih TY. Temporal discounting of reward and the cost of time in motor control. J Neurosci 30: 10507–10516, 2010. doi: 10.1523/JNEUROSCI.1343-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh AG, Wong AL, Zee DS, Jinnah HA. Keeping your head on target. J Neurosci 33: 11281–11295, 2013. doi: 10.1523/JNEUROSCI.3415-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh AG, Zee DS, Crawford JD, Jinnah HA. Cervical dystonia: a neural integrator disorder. Brain 139: 2590–2599, 2016. doi: 10.1093/brain/aww141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalit U, Zinger N, Joshua M, Prut Y. Descending systems translate transient cortical commands into a sustained muscle activation signal. Cereb Cortex 22: 1904–1914, 2012. doi: 10.1093/cercor/bhr267. [DOI] [PubMed] [Google Scholar]
- Sparks DL, Barton EJ, Gandhi NJ, Nelson J. Studies of the role of the paramedian pontine reticular formation in the control of head-restrained and head-unrestrained gaze shifts. Ann N Y Acad Sci 956: 85–98, 2002. doi: 10.1111/j.1749-6632.2002.tb02811.x. [DOI] [PubMed] [Google Scholar]
- Sparks DL, Mays LE. Spatial localization of saccade targets. I. Compensation for stimulation-induced perturbations in eye position. J Neurophysiol 49: 45–63, 1983. [DOI] [PubMed] [Google Scholar]
- Sparks DL, Mays LE, Porter JD. Eye movements induced by pontine stimulation: interaction with visually triggered saccades. J Neurophysiol 58: 300–318, 1987. [DOI] [PubMed] [Google Scholar]
- Stockinger C, Thürer B, Focke A, Stein T. Intermanual transfer characteristics of dynamic learning: direction, coordinate frame, and consolidation of interlimb generalization. J Neurophysiol 114: 3166–3176, 2015. doi: 10.1152/jn.00727.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassman A, Evinger C, McCrea RA, Baker RG, Highstein SM. Anatomy and physiology of intracellularly labelled omnipause neurons in the cat and squirrel monkey. Exp Brain Res 67: 436–440, 1987. doi: 10.1007/BF00248565. [DOI] [PubMed] [Google Scholar]
- Strassman A, Highstein SM, McCrea RA. Anatomy and physiology of saccadic burst neurons in the alert squirrel monkey. I. Excitatory burst neurons. J Comp Neurol 249: 337–357, 1986. doi: 10.1002/cne.902490303. [DOI] [PubMed] [Google Scholar]
- Straube A, Fuchs AF, Usher S, Robinson FR. Characteristics of saccadic gain adaptation in rhesus macaques. J Neurophysiol 77: 874–895, 1997. [DOI] [PubMed] [Google Scholar]
- Sylvestre PA, Cullen KE. Quantitative analysis of abducens neuron discharge dynamics during saccadic and slow eye movements. J Neurophysiol 82: 2612–2632, 1999. [DOI] [PubMed] [Google Scholar]
- Takagi M, Zee DS, Tamargo RJ. Effects of lesions of the oculomotor vermis on eye movements in primate: saccades. J Neurophysiol 80: 1911–1931, 1998. [DOI] [PubMed] [Google Scholar]
- Takikawa Y, Kawagoe R, Itoh H, Nakahara H, Hikosaka O. Modulation of saccadic eye movements by predicted reward outcome. Exp Brain Res 142: 284–291, 2002. doi: 10.1007/s00221-001-0928-1. [DOI] [PubMed] [Google Scholar]
- Tarnutzer AA, Weber KP, Schuknecht B, Straumann D, Marti S, Bertolini G. Gaze holding deficits discriminate early from late onset cerebellar degeneration. J Neurol 262: 1837–1849, 2015. doi: 10.1007/s00415-015-7773-9. [DOI] [PubMed] [Google Scholar]
- Van Gisbergen JA, Robinson DA, Gielen S. A quantitative analysis of generation of saccadic eye movements by burst neurons. J Neurophysiol 45: 417–442, 1981. [DOI] [PubMed] [Google Scholar]
- Vasin Nla, Medzhidov MR, Shabalov VA. [Stereotaxic destruction of the interstitial nucleus of Cajal on spastic torticollis]. Zh Vopr Neirokhir Im N N Burdenko 3–7, 1985. [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. Dynamics of the hippocampal ensemble code for space. Science 261: 1055–1058, 1993. doi: 10.1126/science.8351520. [DOI] [PubMed] [Google Scholar]
- Xu-Wilson M, Chen-Harris H, Zee DS, Shadmehr R. Cerebellar contributions to adaptive control of saccades in humans. J Neurosci 29: 12930–12939, 2009a. doi: 10.1523/JNEUROSCI.3115-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu-Wilson M, Tian J, Shadmehr R, Zee DS. TMS induced startle perturbs saccade trajectories and unmasks the internal feedback controller. J Neurosci 31: 11537–11546, 2011. doi: 10.1523/JNEUROSCI.1584-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu-Wilson M, Zee DS, Shadmehr R. The intrinsic value of visual information affects saccade velocities. Exp Brain Res 196: 475–481, 2009b. doi: 10.1007/s00221-009-1879-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav V, Sainburg RL. Motor lateralization is characterized by a serial hybrid control scheme. Neuroscience 196: 153–167, 2011. doi: 10.1016/j.neuroscience.2011.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zee DS, Yamazaki A, Butler PH, Gücer G. Effects of ablation of flocculus and paraflocculus of eye movements in primate. J Neurophysiol 46: 878–899, 1981. [DOI] [PubMed] [Google Scholar]